Abstract
Thymic epithelial cells can produce many kinds of cytokines, and interleukin (IL)‐6‐producing thymic carcinoma cases have been reported. However, a cytokine‐producing human thymic tumor cell line has not previously been established. In this paper, we report a novel, multiple inflammatory cytokine‐productive cell line that was established from a patient with thymic carcinoma. This cell line, designated ThyL‐6, positively expressed epithelial membrane antigen, cytokeratins, vimentin intermediate filament and CD5, although hematological markers were not present in the cells. Cytokine antibody array analysis showed that the cells secreted several cytokines including IL‐1α, IL‐6, IL‐8, RANTES, soluble TNFα‐receptor 1, VEGF and CTLA into the culture medium. The addition of ThyL‐6‐cultured supernatant supported the growth of human myeloma ILKM‐3 cells, which require the presence of IL‐6 in the culture medium for the maintenance of cell growth, suggesting that the secreted IL‐6 from ThyL‐6 cells was biologically active. Chromosome analysis demonstrated that ThyL‐6 cells had complex karyotype anomalies, including der(16)t(1;16); the latter has been recognized in thymic squamous cell carcinoma and thymic sarcomatoid carcinoma cases, as well as in several other kinds of malignancies. Heterotransplantation of the cells into nude mice showed tumorigenesis with neutrophil infiltration and liquefactive necrosis. These findings suggest that ThyL‐6 cells will provide us with a new experimental tool for investigating not only the pathogenesis, biological behavior, chromo‐somal analysis and therapeutic reagents of human thymic carcinoma, but also for studying cytokine–chemokine network systems. (Cancer Sci 2008; 99: 1778–1784)
Abbreviations:
- CK
cytokeratin
- CTLA
cytotoxic T lymphocyte antigen
- CRP
C‐reactive protein
- EIA
enzyme immuno assay
- IL
interleukin
- LCA
leukemia common antigen
- RANTES
regulated upon activation, normal T‐cell expressed, and secreted
- sTNFR
soluble TNFα‐receptor
- VEGF
vascular endothelial growth factor
The thymus is known to develop multiple types of neoplasms including thymomas, germ cell tumors, neurogenic tumors, lymphoblastic leukemias/lymphomas and other tumors. Thymic epithelial tumors are rare, but are the most common neoplasm of the anterior mediastinum. Thymoma shows an indolent course, thymic carcinoma exhibits a heterogeneous group of aggressive epithelial malignancies,( 1 , 2 ) and the epithelial tumors are divided into 11 subtypes in the World Health Organization classification.( 3 ) Biologically, human thymic epithelial cells have been shown to produce many cytokines, including interleukin (IL) –1α, IL‐3, IL‐6, IL‐7, IL‐8, granulocyte macrophage‐colony stimulating factor (GM‐CSF), G‐CSF, M‐CSF, leukemia inhibitory factor and transforming growth factor α, that are likely to contribute to thymocyte differentiation.( 4 , 5 ) This ability carries with it a risk of developing various cytokine‐producing tumors from the thymic epithelial neoplasms. In fact, several case reports have described the existence of IL‐6 producing thymic carcinomas.( 6 , 7 ) On the other hand, no cytokine‐producing human thymic epithelial tumor cell line has yet been reported, only a mouse thymic stromal cell line that produced M‐CSF and IL‐6.( 8 )
Recently, we established and characterized a new inflammatory cytokine‐producing cell line, ThyL‐6, derived from a patient with thymic carcinoma. Because the establishment of cell lines is important for the investigation of the pathophysiological characteristics of diseases, biochemical analysis and drug discovery, as well as the production of biosources, the establishment of a ThyL‐6 cell line would contribute to the investigation of the pathogenesis and biological behavior of thymic carcinoma, therapeutic reagents against it and the study of cytokine–chemokine network systems. To our knowledge, ThyL‐6 is the first cytokine productive human thymic carcinoma cell line.
Materials and Methods
Patient. A 57‐year‐old male without a history of smoking was admitted to The University of Fukui Hospital because of a mediastinal tumor and leukocytosis. Histological inspection was performed by his primary doctor, and he was pathologically diagnosed as having undifferentiated thymic carcinoma. His complete blood cell counts showed leukocytosis with a 27 200/µL white blood cell (WBC) count with 90% mature neutrophils, normocytic anemia with a 2.35 × 106/µL red blood cell count, and thrombocytosis with 63.2 × 104/µL of platelets, respectively. Body temperature was elevated above 38°C and serum C‐reactive protein (CRP) was increased to 32.6 mg/dL. Serum uric acid level was low, in the range between 1.7 and 2.3 mg/dL, whereas the serum creatinine level was within the normal range. A uric acid clearance test( 9 ) showed an excess of uric acid excretion, with a urate clearance (Cua) of 18.2 mL/min, an excretion of uric acid (Eua) of 0.438 mg/kg/h and a creatinine clearance (Ccr) of 122 mL/min. Serum IL‐6 levels measured by Enzyme Immuno Assay (EIA) were increased to 298 pg/mL. The serum levels were dependent on the tumor size because the value was reduced after chemotherapy. Despite several courses of antineoplastic chemotherapies, the tumor progressed and the patient died of pleuritis carcinomatosa. Informed consent was obtained from a family member of the patient for this research work, and this research was approved by the ethics/institutional review board of The University of Fukui Hospital and conforms to the provisions of the declaration of Helsinki in 1995.
Reagents. Roswell Park Memorial Institute medium (RPMI)‐1640 medium, fetal bovine serum (FBS), 10% trypsin–ethylenediametetraacetic acid (EDTA) and an 3‐(4,5‐dmethylthiazole‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT) assay kit were purchased from Sigma (St. Louis, MO, USA). A TranSignal Human Cytokine Antibody Array 3.0 kit was purchased from Panomics (Redwood City, CA, USA). Recombinant human IL‐6, IL‐8 and RANTES were purchased from PeproTech EC (London, UK). An antihuman rabbit polyclonal IL‐6 antibody, a monoclonal mouse IL‐8 antibody (6217.11) and a goat polyclonal RANTES antibody were obtained from Sigma. Anti‐human monoclonal carcinoembryonic antigen (CEA) (Clone; II‐7), epithelial membrane antigen (EMA) (E29), pan‐cytokeratin (Pan CK) (AE1/AE3), CK 5/6 (D5/16 B4), CK 7 (OV‐TL 12/13), CK 8 (35βH11), CK 10 (DE‐K10), CK 18 (DC 10), CK 19 (BA17), CK 20 (Ks20.8), melanosome (HMB‐45), placental alkaline phosph‐atase (PLAP) (8A9), vimentin (V9), CD5 (CD5/54/F6), CD20cy (L26), CD30 (Ber‐H2), CD45 (leukemia common antigen, LCA) (2B11 + PO7/26), CD45R0 (UCHL‐1), polyclonal CD117 and S100 antibodies were acquired from DAKO (Kyoto, Japan). Secondary horseradish peroxidase (HRP) conjugated against mouse, goat and rabbit immunoglobulin G were obtained from Santa Cruz (Santa Cruz, CA, USA) and a BM Chemiluminescence Western Blotting Kit was purchased from Roche (Indianapolis, IN, USA). For immunohistochemical staining, a HISTOFINE SAB‐PO kit (Nichirei, Tokyo, Japan) was used for the secondary reaction. Other general reagents were of the highest purity commercially available.
Animal. Six‐week‐old male nude mice (BALB/c nu/nu, Sankyo Labo, Tokyo) were subcutaneously inoculated in the back with 1 × 106 ThyL‐6 cells. Two weeks later, the mice were sacrificed and pathological sections were prepared as described previously.( 10 ) The slide sections were stained by hematoxylin and eosin dye,( 11 ) and the histological findings were evaluated by two different pathologists. Immunohistochemical staining of paraffin‐embedded sections was performed as described previously.( 12 )
Cell culture. Tumor cells were harvested from the pleural effusion and were cultured in RPMI‐1640 medium supplemented with 10% FBS, penicillin and streptomycin in a 5% CO2 atmosphere at 37°C. The established cell line was designated as ThyL‐6. Cell growth was evaluated by trypan‐blue dye exclusion assay or MTT assay according to manufacturer's protocol. The exponentially growing cells were used for several experiments. To determine cytokine secretion into the culture medium from the cells, the supernatants were collected at the confluent growth phase and stored at –80°C until analysis. A human multiple myeloma ILKM‐3 cell line (kindly provided by Dr S. Shimizu, Shimane Prefectural Central Hospital),( 13 ) which requires IL‐6 for cell growth, was used for the biological assay.
Immunohistochemical staining. To explore the characteristics of the ThyL‐6 cells, cells attached to an eight‐chamber slide (Nalge Nunc International, Tokyo, Japan) were fixed using 10% formaldehyde for 8 min. The fixed cells were incubated for 30 min with Tris‐buffered saline (TBS; pH 7.6) in the presence of 10% FBS and 5% bovine serum albumin (BSA) to protect them from the non‐specific binding of antibodies. The cells were incubated with antihuman primary antibodies and visualized using a HISTOFINE SAB‐PO kit according to the manufacturer's instructions.
Chromosome analysis. For identification of the chromosomes in the ThyL‐6 cells, the exponentially growing cells were harvested and their karyotypes were analyzed according to the standard protocol of the Giemsa‐banding technique.
Human cytokine antibody array. A TranSignal Human Cytokine Antibody Array 3.0 kit was used to investigate the secreted cytokine profiles of the ThyL‐6 cells. The array membrane was incubated for 2 h at room temperature in the presence of 2 mL of the supernatant in which the ThyL‐6 cells had been cultured for 4 days. The membrane was reacted with biotin‐conjugated antihuman cytokine antibodies and then incubated with streptavidine‐conjugated HRP. Secreted cytokines were visualized by a Chemiluminescence Image Analyzer (Alpha Innotech, San Leandro, CA, USA) and a BM Chemiluminescence Western Blotting Kit. Quantitative determination of cytokines in the culture medium was performed by EIA using the standard protocol.
Western blotting. For Western blotting analysis, the supernatant was directly loaded onto a 10% or 15% of sodium dodecylsulfate–polyacrylamide gel electrophoresis (SDS‐PAGE) gel with 2 × SDS‐PAGE sample buffer (100 mM Tris‐HCl pH 6.8, 4% SDS, 20% glycerol, 20%β‐mercaptoethanol, and 0.02% bromophenol blue). In some experiments, the supernatant was concentrated by an Ultrafree‐MC 10000 NMWL Filter Unit (Millipore, Bedford, MA, USA) before sample loading. Separated proteins were transferred onto Immobilon‐P 0.45 µm polyvinylidene fluoride (PVDF) membrane (Millipore) and probed with specific antibodies in TBS‐T (25 mM Tris, pH 8.4 supplemented with 130 mM NaCl, 5 mM potassium phosphate, 5% non‐fat dry milk, and 0.1% Tween 20) as described previously.( 14 , 15 ) Secondary antibodies conjugated to HRP and a BM Chemiluminescence Western Blotting Kit were used to develop images in a Chemiluminescence Image Analyzer.
Results
ThyL‐6 cell line shows epithelial cell differentiation. Thymic neoplasms include thymomas, germ cell tumors, carcinoid tumors, lymphoblastic leukemias/lymphomas, neuronal tumors and other tumors. To clarify the origin of the cell line, an immunohistochemical stain was performed. As shown in Fig. 1, the cells expressed the epithelial cell markers EMA, Pan CK, CK5/6, CK 7, CK19, CK20, mesenchymal filament vimentin and CD5, whereas hematological antigens including CD20cy, CD30, CD45 and CD45R0, as well as CEA, melanosome and PLAP antigens were not detected in the cells. Immunostaining data from the ThyL‐6 cells with additional antibodies is summarized in Table 1.
Figure 1.
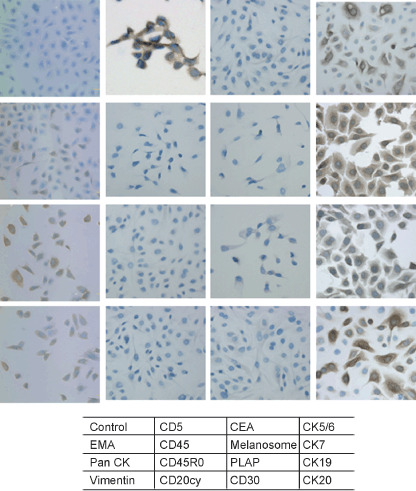
Immunohistochemical staining of ThyL‐6 cells. To investigate the expression profiles of ThyL‐6 cells, the cells were attached to an eight‐chamber slide and were treated with several primary antibodies and then visualized using a HISTOFINE SAB‐PO kit according to the manufacturer's protocol. CEA: carcinoembryonal antigen; CK: cytokeratin; EMA: epithelial membrane antigen; PLAP: placental alkaline phosphatase.
Table 1.
Immunohistochemical staining of ThyL‐6 cells
| Antigen | Clone | Specificity | ThyL‐6 |
|---|---|---|---|
| Pan‐Cytokeratin | AE1/AE3 | Epithelial cells | ++ |
| Cytokeratin 5/6 | D5/16/B4 | Epithelial cells | ++ |
| Cytokeratin 7 | OV‐TL 12/30 | Epithelial cells | +++ |
| Cytokeratin 8 | 35βH11 | Epithelial cells | + |
| Cytokeratin 10 | DE‐K10 | Epithelial cells | – |
| Cytokeratin 18 | DC 10 | Epithelial cells | +++ |
| Cytokeratin 19 | BA17 | Epithelial cells | ++ |
| Cytokeratin 20 | Ks20.8 | Epithelial cells | + |
| Epithelial Membrane Antigen (EMA) | E29 | Epithelial cells | + |
| Carcinoembryonic antigen (CEA) | II‐7 | Ductal cells | – |
| Melanosome | HMB‐45 | Melanocytes | – |
| Placental alkaline phosphatase (PLAP) | 8A9 | Placenta | – |
| S100 | Glial cells | – | |
| Vimentin | V9 | Mesenchymal cells | + |
| CD5 | CD5/54/F6 | T lymphocytes, Thymus + | |
| CD20cy | L26 | B lymphocytes | – |
| CD30 | Ber‐H2 | Ki‐1 lymphocytes | – |
| CD45 (LCA) | 2B11 + PO7/26 | Leukocytes | – |
| CD45R0 | UCHL‐1 | T lymphocytes | – |
| CD117 (c‐Kit) | c‐Kit | – | |
| Interleukin‐6 (IL‐6) | IL‐6 producing cells | +++ | |
| Interleukin‐8 (IL‐8) | 6217.11 | IL‐8 producing cells | +++ |
| RANTES | Rantes producing cells | +++ |
RANTES: regulated upon activation, normal T‐cell expressed, and secreted.
ThyL‐6 produces multiple inflammatory cytokines and chemokines. Interleukin 6 is associated with many reactions including the induction of inflammation, fever generation, thrombocytosis, increase of CRP and promotion of uric acid excretion.( 16 , 17 , 18 , 19 ) The patient had the following clinical symptoms: high body temperature, thrombocytosis, high CRP value, hypouricemia and high serum IL‐6 level, suggesting that the established thymic carcinoma cells were also producing inflammatory cytokines, especially IL‐6. Therefore, we investigated the profile of cytokine production using a cytokine antibody array. After incubating the cells in culture medium for 96 h, the resulting supernatant was found to contain IL‐6, IL‐8, RANTES, IL‐1α, VEGF, soluble TNFα‐receptor I (sTNFR I) and small amounts of eotoxin and cytotoxic T lymphocyte antigen (CTLA) (Fig. 2). We then measured the cytokine concentrations of IL‐6, IL‐8, RANTES, IL‐1α, VEGF, sTNFR I and eotoxin by EIA. The concentrations of IL‐6, IL‐8, RANTES, IL‐1α, VEGF, and sTNFR I were 88 800 ± 3111 pg/mL (normal serum level (nsl): ≤2.41 pg/mL), 88 350 ± 15 203 pg/mL (nsl: <8.0 pg/mL), 526 ± 237 pg/mL (nsl: not determined), 502 ± 132 pg/mL (nsl: <7.8 pg/mL), 9625 ± 1803 pg/mL (nsl: ≤38.3 pg/mL) and 542 ± 57 pg/mL (nsl: 434–930 pg/mL), respectively, whereas eotoxin was not detected by EIA (Table 2). In addition to EIA, we stained IL‐6, IL‐8 and RANTES using immunohistochemical techniques and found that these cytokines were localized in the cytoplasm (Fig. 3). Although Apo1/Fas, eotoxin, IP‐10, leptin, MMP3, ICAMP‐1, IL‐4, IL‐7 and IL‐12 were faintly detected by the cytokine antibody array, we considered them as being background levels already present in either the culture medium or FBS. GM‐CSF, EGF, MIP1α, MIP 1β, MIP‐4, MIP‐5, TGF β, IFN γ, TNFR II, VCAMP‐1, IL‐1β, IL‐1Rα, IL‐2, IL‐3, IL‐5, IL‐6R, IL‐10, IL‐15 and IL‐17 were not detected by the cytokine antibody array (Fig. 2).
Figure 2.
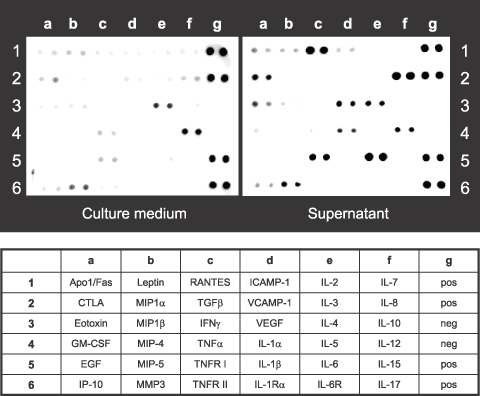
Cytokine secretion profiles in ThyL‐6 cell line detected by a cytokine antibody array. The supernatant in which ThyL‐6 cells had been cultured for 4 days was collected and the secreted cytokine profiles were detected using a TranSignal Human Cytokine Antibody Array 3.0 kit. Roswell Park Memorial Institute medium (RPMI)‐1640 with 10% fetal bovina serum (FBS) was used as an untreated control. The lower panel represents the list of cytokines plotted on the array membrane. neg: negative control; pos: positive control.
Table 2.
Secreted cytokine concentrations of ThyL‐6 cells
| Cytokine | Concentration (pg/mL) |
|---|---|
| Interleukin (IL)‐6 | 88 800 ± 3111 |
| IL‐8 | 88 350 ± 15 203 |
| RANTES | 526 ± 237 |
| sTNFα‐receptor I | 542 ± 57 |
| IL‐1α | 502 ± 132 |
| VEGF | 9625 ± 1803 |
| Eotoxin | <3.9 |
Data represents mean ± SD from 2 different experiments. RANTES: regulated upon activation, normal T‐cell expressed, and secreted; VEGF, vascular endothelial growth factor.
Figure 3.
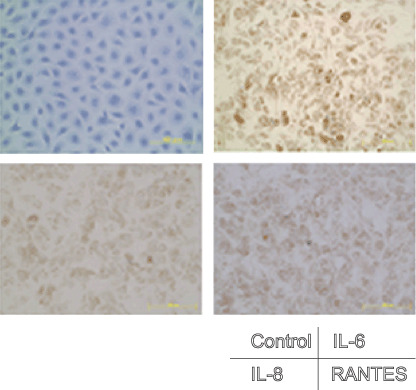
Immunohistochemical staining of inflammatory cytokines. Cells attached to an eight‐chamber slide were fixed with 10% formaldehyde, and the cells were stained with antihuman interleukin (IL)‐6, IL‐8 or regulated upon activation, normal T‐cell expressed, and secreted (RANTES) antibodies, respectively, and then visualized using a HISTOFINE SAB‐PO kit.
Chromosomal analysis of ThyL‐6 cells. In the cytogenetic analysis of ThyL‐6 cells, 20 metaphases were investigated using the Giemsa‐band technique. This cell line had two modes of chromosomes, with the number of chromosomes being 51. The consistent karyotype aberrations were 51, XY, +3, i(3) (q10), +7, add(7) (q11), dic(7)t(7;17)(p11;p11), +8, add(8)(p11), i(8)(q10), –9, –13, +15, i(15)(q10), der(16)t(1;16)(q11;q24), der(16)t(9;16)(q11;q24), add(21)(p11), der(?)t(?;q11), +2mar [18]/51, idem, ‐der(?)t (?;q11), +mar [2] (Fig. 4).
Figure 4.
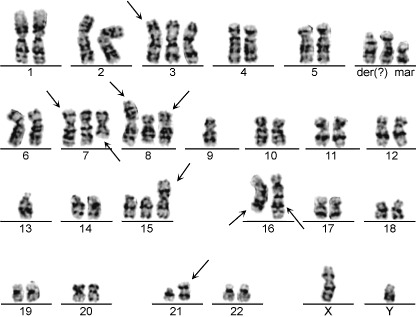
Chromosome analysis of ThyL‐6 cells. The typical karyotype of ThyL‐6 cells was 51, XY, +3, i(3) (q10), +7, add(7)(q11), dic(7)t(7;17) (p11;p11), +8, add(8)(p11), i(8)(q10), –9, –13, +15, i(15)(q10), der(16)t(1;16) (q11;q24), der(16)t(9;16)(q11;q24), add(21)(p11), der(?)t(?;q11), +2mar [18]/51, idem, ‐der(?)t(?;q11), + mar [2].
Cell growth of ThyL‐6 is associated with the autocrine mechanism of IL‐6. The growth rate of the ThyL‐6 cells was evaluated by the trypan‐blue dye exclusion method, and the doubling time was calculated at 26.3 h (Fig. 5a). Several cytokines including IL‐6, G‐CSF and GM‐CSF can stimulate cell proliferation of normal marrow cells as well as several cancer cell lines by autocrine or paracrine mechanisms. To investigate the relevance of autocrine growth, ThyL‐6 cells were cultured in the presence of neutralizing antihuman IL‐6 or IL‐8 antibody, and the growth inhibition was evaluated using an MTT assay. As shown in Fig. 5(b), the growth of ThyL‐6 cells was dose‐dependently inhibited in the presence of the anti‐IL‐6 or IL‐8 antibody, and the final volume increase of 10% when the IL‐6 antibody was added to the medium exhibited a significant growth inhibition (35.7 ± 5.2% inhibition of untreated control, P < 0.05), indicating that cell proliferation was partially dependent on the autocrine stimulation of IL‐6.
Figure 5.
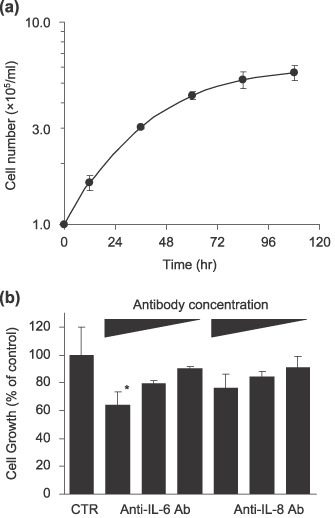
(a) Cell growth curve of ThyL‐6 cells. 1 × 105 cells/mL of ThyL‐6 cells were cultured in Roswell Park Memorial Institute (RPMI)‐1640 medium with 10% fetal bovine serum (FBS), and the cell growth was evaluated by the trypan‐blue dye exclusion method. Data represent the mean ± SD of triplicate cultures. (b) Growth inhibition of ThyL‐6 cells in the presence of neutralizing antibodies. Trypsinized exponential growing cells were seated on a 96‐well plate at a concentration of 1 × 105 cells and incubated overnight. Following the preincubation, the cells were washed three times with PBS and resuspended in fresh medium including neutralizing interleukin (IL)‐6 or IL‐8 antibodies at a final concentration of 10%, 1%, or 0.1% of culture medium. The cells were incubated for an additional 24 h, and the growth inhibition was investigated using an MTT assay. Data represent the mean ± SD of triplicate cultures. *indicates growth rate significantly decreased (P < 0.05) in comparison with that of the untreated control cells. CTR: control; Ab: antibody.
The produced IL‐6 was biologically active. Because the produced IL‐6 was likely to be biologically active, we first compared the molecular weight of the produced IL‐6 with that of recombinant human IL‐6 using Western blotting. As shown in Fig. 6(a), the molecular weight of the produced IL‐6 was slightly higher than the recombinant protein. When the expressions of IL‐8 and RANTES were also compared, the molecular weights of these chemokines were almost the same as those of the recombinant proteins (data not shown). Next, we analyzed the growth of a human multiple myeloma cell line, ILKM‐3, because the proliferation of the cells depends on the existence of IL‐6 in the culture medium. When the supernatant of the cultured ThyL‐6 cells was added to RPMI‐1640 medium, the level of growth of the ILKM‐3 cells was found to depend on the dose of the culture medium added (Fig. 6b).
Figure 6.
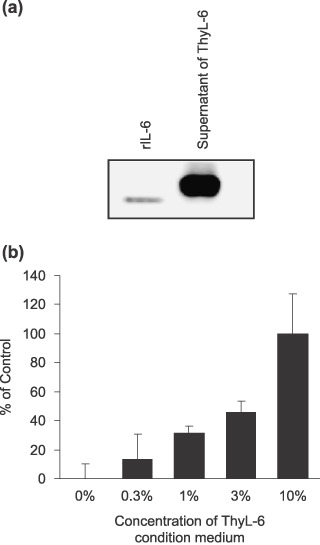
(a) Western blotting detection of interleukin (IL)‐6 in the cultured supernatant of ThyL‐6 cells. The supernatant was resolved with 15% of sodium dodecylsulfate–polyacrylamide gel electrophoresis (SDS‐PAGE), and the separated proteins were transferred onto polyvinylidene difluoride membrane. The membrane was incubated with antihuman IL‐6 monoclonal antibody. Secondary antibodies conjugated to horseradish peroxidase (HRP) and a BM Chemiluminescence Western Blotting Kit were used to develop images in a Chemiluminescence Image Analyzer. (b) Cell growth of IL‐6‐dependent ILKM‐3 cells in the presence of condition medium of ThyL‐6 cells. Cells were cultured in Roswell Park Memorial Institute (RPMI)‐1640 medium with 10% fetal bovine serum (FBS) in the presence or absence of appropriate concentrations of ThyL‐6 condition medium. Cell growth was detected by an MTT assay kit. The percentage of ThyL‐6 condition medium shown represents the final concentration in the culture medium. Data represent the mean ± SD from three independent experiments.
Histological findings of ThyL‐6 cells in nude mice. To evaluate the tumorigenesis in vivo, 1 × 106 of ThyL‐6 cells were inoculated subcutaneously into 11 nude mice. Two weeks later, tumors developed in all of them (Fig. 7a,b). The mice were sacrificed and both paraffin‐embedded sections and frozen sections were prepared. As shown in Fig. 7(c,d), the polymorphic cells had round or oval nuclei with a large round nucleolus and these cells invasively proliferated into the backs of the mice with small amounts of interstitial fibrous connective tissue and scattered liquefactive necrosis present. In addition, neutrophils also infiltrated into the tumor nests independent of necrotic tissue. Similar histological findings containing mature granulocytes had been found in the original biopsy sections of the patient (data not shown), suggesting that the ThyL‐6 cell line is characterized by the induction of neutrophils‐infiltration into the tumor nest.
Figure 7.
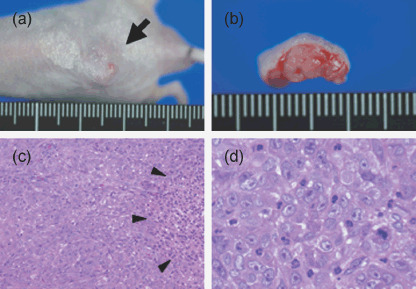
Heterotransplantation of ThyL‐6 cells into a nude mouse. (a) A representative view of tumor (arrow) in the back of a nude mouse. (b) Cross‐section of subcutaneous tumor mass lesion in a nude mouse. (c) A microscopic examination of the tumor produced in a nude mouse (low power magnification view) with hematoxylin and eosin staining. Tumor cells had proliferated with small amount of interstitial fibrous connective tissue. Liquefactive necrotic tissue was seen in the tumor mass (arrow head). (d) High power magnification view of the established tumor. Polymorphic cells with round or oval nuclei and a large round nucleolus had proliferated by mitosis, and neutrophils had infiltrated into the tumor nests.
Discussion
In the present study, we established and characterized a new cancer cell line derived from a patient with undifferentiated thymic carcinoma who had markedly high levels of leukocytosis and thrombocytosis, a high CRP level, a low serum uric acid value and a high serum IL‐6 level. The epithelial nature of ThyL‐6 cells was supported by the presence of cellular cytokeratins and EMA, which are known to be specifically expressed in epithelial cells.( 4 , 20 ) Several multiple cytokine‐producing cell lines have been previously reported involving G‐SCF, GM‐SCF, IL‐6, IL‐8 and other cytokines.( 21 , 22 , 23 , 24 , 25 ) Regarding inflammatory cytokines, several cell lines secrete both IL‐6 and IL‐8,( 26 , 27 ) and human cervical epithelial cell lines immortalized in retroviral constructs are known to produce IL‐6, IL‐8 and RANTES.( 28 ) Similarly, we found that ThyL‐6 cells expressed cytoplasmic IL‐6, IL‐8 and RANTES. In addition, this cell line also secreted IL‐1α, sTNFR I, VEGF and CTLA into the culture medium. Importantly, human thymic epithelial cells have been shown to produce many cytokines, including IL‐1α, IL‐3, IL‐6, IL‐7, IL‐8, G‐CSF, GM‐CSF, M‐CSF, leukemia inhibitory factor and transforming growth factor α, that are likely to contribute to thymocyte differentiation.( 4 , 5 ) Several reports mention that c‐Kit (CD117) is a promising diagnostic marker with 80% of thymic carcinomas positive for it.( 29 , 30 ) In contrast, ThyL‐6 cells do not express the marker. However, the histology of the tumor in nude mice corresponded well to that of the Ty‐82 thymic carcinoma cells and was compatible with the morphological diagnostic criteria of thymic carcinoma, i.e. with large cell size, prominent nucleoli, high nuclear/cytoplasmic ratio, abundant mitosis and multifocal confluent necrosis.( 10 ) In addition, the patient had never smoked. These data suggest that ThyL‐6 is a multiple inflammatory cytokine‐producing thymic‐carcinoma‐derived cell line.
Overproduction or injection of IL‐6 leads to acute inflammatory reactions, including fever and a high CRP level. Our patient suffered from a hyper CRP value of more than 30 mg/dL, documented fever, hyperleukocytosis and thrombocytosis, which allowed us to surmise that the patient had an increased serum IL‐6 level. In fact, serum IL‐6 level was significantly increased in this patient. Using EIA, immunohistochemical staining, and Western blotting, the established ThyL‐6 cells were also verified as being able to produce IL‐6. Moreover, the secreted IL‐6 was shown to be biologically active because the growth of IL‐6‐dependent ILKM‐3 cells was supported in the presence of the supernatant in which the ThyL‐6 cells were cultured, suggesting that ThyL‐6 can produce a bioavailable grade of human IL‐6.
Cytogenetic studies on thymic epithelial tumors are few, and the significance of chromosomal abnormalities is unknown, except for t(15;19)(q15;p13), which is possibly related to thymic carcinoma in children and young adults.( 31 ) A thymic cancer cell line with t(15;19)(q15;p13), Ty‐82, has been established from a patient with undifferentiated thymic carcinoma( 10 ) and the cell line exhibits EMA without showing any epithelial markers including cytokeratins. Whereas, ThyL‐6 cells showed several epithelial markers including EMA, Pan CK, CK 5/6, 7, 8, 18, 19 and 20. In the CK, CK 5/6 is preferentially expressed in epithelial cells differentiating toward squamous cells.( 32 , 33 ) In addition, ThyL‐6 cells represented CD5, which is expressed in thymic carcinoma, including thymic squamous cell carcinomas and rare cases of thymoma. Recently, several reports mention that some pulmonary squamous cell carcinoma cases also express CD5.( 29 , 30 ) Taken together with the results, the expression of this molecule in ThyL‐6 cells suggests that this cell line may have squamous cell potency.
Genetic analysis, based on comparative genomic hybridization of a relatively large number of thymoma and thymic carcinomas, has demonstrated that aggressive‐type B3 thymoma and thymic squamous cell carcinoma preferentially show aberrance karyotypes with a gain of chromosome 1q.( 34 ) In the present case, ThyL‐6 cells had complex chromosome abnormalities and showed a 1q gain with the der(16)t(1;16)(q11;q24) chromosome in them. Abnormalities of 1q, including trisomy or partial gain of the arm, have been demonstrated to be the most frequent genetic changes in malignant cells( 35 , 36 ) and potentially important growth promoting genes have been demonstrated to map to 1q.( 35 ) Chromosome 16 is involved in unbalanced whole arm translocations as a counterpart of chromosome 1,( 36 ) and often constitutes a derivative chromosome, der(16)t(1;16). Der(16)t(1;16) abnormality has been recognized in several tumors including a multiple myeloma,( 37 ) myelodysplastic syndrome,( 38 ) Wilms’ tumor,( 39 ) myxoid/round cell liposarcoma,( 40 ) breast carcinoma,( 41 ) Ewing's sarcoma,( 42 ) alveolar rhabdomyosarcoma( 36 ) and malignant peripheral nerve sheath tumor with rhabdomyoblastic differentiation.( 43 ) Regarding thymocytic tumors, this aberrant derivative chromosome formation has been reported in thymic squamous cell carcinoma and thymic sarcomatoid carcinoma cases.( 44 , 45 ) Thus, these results suggest that der(16)t(1;16) may also play a significant role in the pathophysiology of thymic carcinoma as well as many kinds of malignancies. In addition, cell growth of ThyL‐6 cells showed a dose‐dependent inhibition after the addition of neutralizing IL‐6 antibody. The ThyL‐6 cells had another chromosomal translocation on chromosome 17p11, which involves a pseudogene of human gp130 transducer chain gene of IL‐6.( 46 ) Therefore, this cell line may also be useful for studying the autocrine growth mechanisms of IL‐6, the IL‐6 receptor or reactivation of the pseudogene.
In conclusion, we established a new multiple inflammatory cytokine‐producing cell line. To our knowledge, this is the first such human thymic carcinoma cell line that produces inflammatory cytokines. Although further study is needed, this cell line could provide us with a new experimental tool for investigating not only the pathogenesis, biological behavior, chromosomal analysis and therapeutic reagents of thymic carcinoma, but also for studying cytokine–chemokine network systems.
References
- 1. Detterbeck FC, Egan TM. Thoracoscopy using a substernal handport for palpation. Ann Thorac Surg 2004; 78: 1031–6. [DOI] [PubMed] [Google Scholar]
- 2. Duwe BV, Sterman DH, Musani AI. Tumors of the mediastinum. Chest 2005; 128: 2893–909. [DOI] [PubMed] [Google Scholar]
- 3. Travis W, Brambilla E, Muller‐Hermelink H, Harris C. Pathology and Genetics of Tumours of the Lung. Thymus and Heart Lyon: IARC Press, 2005. [Google Scholar]
- 4. Fernandez E, Vicente A, Zapata A et al . Establishment and characterization of cloned human thymic epithelial cell lines. Analysis of adhesion molecule expression and cytokine production. Blood 1994; 83: 3245–54. [PubMed] [Google Scholar]
- 5. Haynes BF, Denning SM, Le PT, Singer KH. Human intrathymic T cell differentiation. Semin Immunol 1990; 2: 67–77. [PubMed] [Google Scholar]
- 6. Ikeda T, Kawakami K, Fujita J, Bandoh S, Yamadori I, Takahara J. Thymic carcinoma associated with a high serum level of interleukin 6 diagnosed through the evaluation for asymptomatic elevation of acute‐phase reactants. Intern Med 1998; 37: 414–16. [DOI] [PubMed] [Google Scholar]
- 7. Matsumura N, Shiiki H, Saito N et al . Interleukin‐6‐producing thymic squamous cell carcinoma associated with Castleman's disease and nephrotic syndrome. Intern Med 2002; 41: 871–4. [DOI] [PubMed] [Google Scholar]
- 8. Lee CK, Kim JK, Kim K, Han SS. A mouse thymic stromal cell line producing macrophage‐colony stimulating factor and interleukin‐6. Arch Pharm Res 2000; 23: 252–6. [DOI] [PubMed] [Google Scholar]
- 9. Ichida K, Hosoyamada M, Hisatome I et al . Clinical and molecular analysis of patients with renal hypouricemia in Japan‐influence of URAT1 gene on urinary urate excretion. J Am Soc Nephrol 2004; 15: 164–73. [DOI] [PubMed] [Google Scholar]
- 10. Kuzume T, Kubonishi I, Takeuchi S et al . Establishment and characterization of a thymic carcinoma cell line (Ty‐82) carrying t (15;19)(q15;p13) chromosome abnormality. Int J Cancer 1992; 50: 259–64. [DOI] [PubMed] [Google Scholar]
- 11. Inai K, Iwasaki H, Noriki S et al . Frequent detection of multidrug‐resistant pneumonia‐causing bacteria in the pneumonia lung tissues of patients with hematological malignancies. Int J Hematol 2007; 86: 225–32. [DOI] [PubMed] [Google Scholar]
- 12. Jin Y, Sun A, Noriki S, Imamura Y, Fukuda M. Detection of cancer clones in human colorectal adenoma as revealed by increased DNA instability and other bio‐markers. Eur J Histochem 2007; 51: 1–10. [PubMed] [Google Scholar]
- 13. Shimizu S, Yoshioka R, Hirose Y, Sugai S, Tachibana J, Konda S. Establishment of two interleukin 6 (B cell stimulatory factor 2/interferon beta 2) ‐dependent human bone marrow‐derived myeloma cell lines. J Exp Med 1989; 169: 339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ostapkowicz A, Inai K, Smith L, Kreda S, Spychala J. Lipid rafts remodeling in estrogen receptor‐negative breast cancer is reversed by histone deacetylase inhibitor. Mol Cancer Ther 2006; 5: 238–45. [DOI] [PubMed] [Google Scholar]
- 15. Inai K, Tsutani H, Yamauchi T, Huberman E, Nakamura T, Ueda T. Differentiation induction in non‐lymphocytic leukemia cells upon treatment with mizoribine. Int J Hematol 1997; 66: 335–44. [DOI] [PubMed] [Google Scholar]
- 16. Akira S, Kishimoto T. IL‐6 and NF‐IL6 in acute‐phase response and viral infection. Immunol Rev 1992; 127: 25–50. [DOI] [PubMed] [Google Scholar]
- 17. Yoshimura A. Signal transduction of inflammatory cytokines and tumor development. Cancer Sci 2006; 97: 439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tsutani H, Yoshio N, Ueda T. Interleukin 6 reduces serum urate concentrations. J Rheumatol 2000; 27: 554. [PubMed] [Google Scholar]
- 19. Urano W, Yamanaka H, Tsutani H et al . The inflammatory process in the mechanism of decreased serum uric acid concentrations during acute gouty arthritis. J Rheumatol 2002; 29: 1950–3. [PubMed] [Google Scholar]
- 20. Sun TT, Bonitz P, Burns WH. Cell culture of mammalian thymic epithelial cells: growth, structural, and antigenic properties. Cell Immunol 1984; 83: 1–13. [DOI] [PubMed] [Google Scholar]
- 21. Baba M, Hasegawa H, Nakayabu M et al . Establishment and characteristics of a gastric cancer cell line (HuGC‐OOHIRA) producing high levels of G‐CSF, GM‐CSF, and IL‐6: the presence of autocrine growth control by G‐CSF. Am J Hematol 1995; 49: 207–15. [DOI] [PubMed] [Google Scholar]
- 22. Asahi Y, Kubonishi I, Imamura J et al . Establishment of a clonal cell line producing granulocyte colony‐stimulating factor and parathyroid hormone‐related protein from a lung cancer patient with leukocytosis and hypercalcemia. Jpn J Cancer Res 1996; 87: 451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kondo K, Okada T, Matsui T et al . Establishment and characterization of a human B cell line from the lung tissue of a patient with scleroderma; extraordinary high level of IL‐6 secretion by stimulated fibroblasts. Cytokine 2001; 13: 220–6. [DOI] [PubMed] [Google Scholar]
- 24. Li A, Varney ML, Valasek J, Godfrey M, Dave BJ, Singh RK. Autocrine role of interleukin‐8 in induction of endothelial cell proliferation, survival, migration and MMP‐2 production and angiogenesis. Angiogenesis 2005; 8: 63–71. [DOI] [PubMed] [Google Scholar]
- 25. Inoue Y, Tsushima H, Ando K et al . Chemokine expression in human erythroid leukemia cell line AS‐E2: macrophage inflammatory protein‐3alpha/CCL20 is induced by inflammatory cytokines. Exp Hematol 2006; 34: 19–26. [DOI] [PubMed] [Google Scholar]
- 26. Sekido Y, Sato M, Usami N et al . Establishment of a large cell lung cancer cell line (Y‐ML‐1B) producing granulocyte colony‐stimulating factor. Cancer Genet Cytogenet 2002; 137: 33–42. [DOI] [PubMed] [Google Scholar]
- 27. Tachibana M, Miyakawa A, Nakashima J et al . Autocrine growth promotion by multiple hematopoietic growth factors in the established renal cell carcinoma line KU‐19‐20. Cell Tissue Res 2000; 301: 353–67. [DOI] [PubMed] [Google Scholar]
- 28. Fichorova RN, Anderson DJ. Differential expression of immunobiological mediators by immortalized human cervical and vaginal epithelial cells. Biol Reprod 1999; 60: 508–14. [DOI] [PubMed] [Google Scholar]
- 29. Hishima T, Fukayama M, Fujisawa M et al . CD5 expression in thymic carcinoma. Am J Pathol 1994; 145: 268–75. [PMC free article] [PubMed] [Google Scholar]
- 30. Nakagawa K, Matsuno Y, Kunitoh H, Maeshima A, Asamura H, Tsuchiya R. Immunohistochemical KIT (CD117) expression in thymic epithelial tumors. Chest 2005; 128: 140–4. [DOI] [PubMed] [Google Scholar]
- 31. Sait SN, Brooks JJ, Ashraf M, Zhang PJ. A novel t(1;8)(p13;p11) in a thymic carcinoma with unusual giant cell features and renal metastasis. Cancer Genet Cytogenet 2001; 124: 140–3. [DOI] [PubMed] [Google Scholar]
- 32. Chu PG, Weiss LM. Expression of cytokeratin 5/6 in epithelial neoplasms: an immunohistochemical study of 509 cases. Mod Pathol 2002; 15: 6–10. [DOI] [PubMed] [Google Scholar]
- 33. Kaufmann O, Fietze E, Mengs J, Dietel M. Value of p63 and cytokeratin 5/6 as immunohistochemical markers for the differential diagnosis of poorly differentiated and undifferentiated carcinomas. Am J Clin Pathol 2001; 116: 823–30. [DOI] [PubMed] [Google Scholar]
- 34. Zettl A, Strobel P, Wagner K et al . Recurrent genetic aberrations in thymoma and thymic carcinoma. Am J Pathol 2000; 157: 257–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Knuutila S, Bjorkqvist AM, Autio K et al . DNA copy number amplifications in human neoplasms: review of comparative genomic hybridization studies. Am J Pathol 1998; 152: 1107–23. [PMC free article] [PubMed] [Google Scholar]
- 36. McManus AP, Min T, Swansbury GJ, Gusterson BA, Pinkerton CR, Shipley JM. Der(16)t(1;16)(q21;q13) as a secondary change in alveolar rhabdomyosarcoma. A case report and review of the literature. Cancer Genet Cytogenet 1996; 87: 179–81. [DOI] [PubMed] [Google Scholar]
- 37. Mugneret F, Sidaner I, Favre B et al . Der(16)t(1;16)(q10;p10) in multiple myeloma: a new non–random abnormality that is frequently associated with Burkitt's–type translocations. Leukemia 1995; 9: 277–81. [PubMed] [Google Scholar]
- 38. Mugneret F, Dastugue N, Favre B et al . Der(16)t(1;16)(q11;q11) in myelodysplastic syndromes: a new non–random abnormality characterized by cytogenic and fluorescence in situ hybridization studies. Br J Haematol 1995; 90: 119–24. [DOI] [PubMed] [Google Scholar]
- 39. Sheng WW, Soukup S, Bove K, Gotwals B, Lampkin B. Chromosome analysis of 31 Wilms’ tumors. Cancer Res 1990; 50: 2786–93. [PubMed] [Google Scholar]
- 40. Birch NC, Antonescu CR, Nelson M et al . Inconspicuous insertion 22;12 in myxoid/round cell liposarcoma accompanied by the secondary structural abnormality der(16)t(1;16). J Mol Diagn 2003; 5: 191–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tsuda H, Takarabe T, Fukutomi T, Hirohashi S. Preferential occurrence of breast carcinomas with loss of chromosome 16q and der(16)t(1;16)/der (1;16) in middle‐aged patients with hyperplasia of mammary glands. Jpn J Cancer Res 2000; 91: 692–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mugneret F, Lizard S, Aurias A, Turc‐Carel C. Chromosomes in Ewing's sarcoma. II. Nonrandom additional changes, trisomy 8 and der(16)t(1;16). Cancer Genet Cytogenet 1988; 32: 239–45. [DOI] [PubMed] [Google Scholar]
- 43. Velagaleti GV, Miettinen M, Gatalica Z. Malignant peripheral nerve sheath tumor with rhabdomyoblastic differentiation (malignant triton tumor) with balanced t(7;9)(q11.2;p24) and unbalanced translocation der(16)t(1;16) (q23;q13). Cancer Genet Cytogenet 2004; 149: 23–7. [DOI] [PubMed] [Google Scholar]
- 44. Sonobe H, Ohtsuki Y, Nakayama H, Asaba K, Nishiya K, Shimizu K. A thymic squamous cell carcinoma with complex chromosome abnormalities. Cancer Genet Cytogenet 1998; 103: 83–5. [DOI] [PubMed] [Google Scholar]
- 45. Eimoto T, Kitaoka M, Ogawa H et al . Thymic sarcomatoid carcinoma with skeletal muscle differentiation: report of two cases, one with cytogenetic analysis. Histopathology 2002; 40: 46–57. [DOI] [PubMed] [Google Scholar]
- 46. Rodriguez C, Grosgeorge J, Nguyen VC, Gaudray P, Theillet C. Human gp130 transducer chain gene (IL6ST) is localized to chromosome band 5q11 and possesses a pseudogene on chromosome band 17p11. Cytogenet Cell Genet 1995; 70: 64–7. [DOI] [PubMed] [Google Scholar]


