Abstract
Inotuzumab ozogamicin (CMC‐544), an antibody‐targeted chemotherapeutic agent composed of an anti‐CD22 antibody conjugated to calicheamicin, a potent cytotoxic antibiotic, specifically targets the CD22 antigen present in >90% of B‐lymphoid malignancies, rendering it useful for treating patients with B‐cell non‐Hodgkin lymphoma (B‐NHL). This phase I study evaluated the safety, tolerability, efficacy, and pharmacokinetics of inotuzumab ozogamicin in Japanese patients. Eligible patients had relapsed or refractory CD22‐positive B‐NHL without major organ dysfunction. Inotuzumab ozogamicin was administered intravenously once every 28 days (dose escalation: 1.3 and 1.8 mg/m2). All 13 patients had follicular lymphoma, were previously treated with ≥1 rituximab‐alone or rituximab‐containing chemotherapy, and were enrolled into two dose cohorts (1.3 mg/m2, three patients; 1.8 mg/m2, 10 patients). No patient had dose‐limiting toxicities, and the maximum tolerated dose, previously determined in non‐Japanese patients (1.8 mg/m2), was confirmed. Drug‐related adverse events (AEs) included thrombocytopenia (100%), leukopenia (92%), lymphopenia (85%), neutropenia (85%), elevated AST (85%), anorexia (85%), and nausea (77%). Grade 3/4 drug‐related AEs in ≥15% patients were thrombocytopenia (54%), lymphopenia (31%), neutropenia (31%), and leukopenia (15%). The AUC and Cmax of inotuzumab ozogamicin increased dose‐dependently with pharmacokinetic profiles similar to non‐Japanese. Seven patients had complete response (CR, 54%) including unconfirmed CR, four patients had partial response (31%), and two patients had stable disease (15%). The overall response rate was 85% (11/13). Inotuzumab ozogamicin was well tolerated at doses up to 1.8 mg/m2 and showed preliminary evidence of activity in relapsed or refractory follicular lymphoma pretreated with rituximab‐containing therapy, warranting further investigations. This trial was registered in ClinicalTrials.gov (NCT00717925). (Cancer Sci 2010)
The successful use of monoclonal antibodies (mAbs) in the treatment of human diseases has been growing steadily in the past decade. Rituximab, a human‐mouse chimeric anti‐CD20, unconjugated antibody, was approved in 1997 in the USA as the first mAb for antilymphoma therapy. It is now most commonly used in combination with chemotherapy for first and subsequent lines of therapy in B‐cell non‐Hodgkin lymphoma (B‐NHL), such as diffuse large B‐cell lymphoma (DLBCL) and follicular lymphoma (FL).( 1 , 2 , 3 , 4 , 5 , 6 ) However, a subgroup of patients does not respond, and early relapses occur in patients with initial response, thus indicating rituximab resistance. This indicates a clear unmet need to explore alternative antibodies non‐cross resistant to rituximab as a therapy for B‐NHL. One alternative is inotuzumab ozogamicin (CMC‐544), an antibody‐targeted chemotherapy agent that specifically targets CD22. Inotuzumab ozogamicin is composed of a recombinant engineered humanized IgG4 anti‐CD22 antibody G544 conjugated to calicheamicin, a potent cytotoxic antibiotic derivative.( 7 )
CD22 is a potential therapeutic target for B‐NHL because it is expressed in >90% of B‐NHL cells.( 8 ) In addition, CD22 is expressed in mature B cells, but not in their precursor or memory B cells, which may potentially minimize the adverse effect of CD22‐targeted treatment on long‐term immune function. Moreover, when antibodies bind to the CD22 antigen, the antigen is internalized, that is it is not shed into the extracellular environment.( 9 )
Both inotuzumab ozogamicin and unconjugated calicheamicin showed potent cytotoxic activity in vitro against CD22‐positive B cells in preclinical studies.( 7 ) In addition, the unconjugated form of inotuzumab ozogamicin, G544, did not demonstrate any antitumor activity in preclinical studies.( 7 ) Inotuzumab ozogamicin inhibited the growth and the establishment of B‐cell lymphomas and induced the regression of large B‐cell lymphomas in mouse xenograft models.( 7 ) Furthermore, in preclinical models of disseminated B‐NHL in which rituximab was ineffective, treatment with inotuzumab ozogamicin lead to a significant tumor regression and an improvement in survival.( 10 ) This potent cytotoxic activity in preclinical murine models of B‐cell lymphomas in which rituximab had failed as a therapeutic agent( 11 ) establishes support for the clinical investigation of inotuzumab ozogamicin for the treatment of CD22‐positive B‐NHL.
A phase I dose escalation study was previously conducted in the USA and the European Union in patients with relapsed or refractory B‐NHL (both FL and DLBCL).( 12 ) In this study, intravenous administration of the drug demonstrated clinical activity in patients with relapsed or refractory B‐NHL with clinically manageable thrombocytopenia as the main toxicity. The maximum tolerated dose (MTD) in this non‐Japanese patient population was determined to be 1.8 mg/m2 once every 4 weeks.
The objectives of the present study were to assess the safety, toleralility, efficacy, and pharmacokinetics of inotuzumab ozogamicin in Japanese patients with relapsed or refractory B‐NHL who had received prior treatment with rituximab.
Materials and Methods
Study design. The present trial was an open‐label multi‐center phase I study in which inotuzumab ozogamicin was administered intravenously (IV) as a single agent to patients with CD22‐positive B‐NHL once every 28 days (±2 days, 1 cycle) for at least four doses provided that the drug was well tolerated with no evidence of progressive disease (PD). The protocol was approved by the Institutional Review Board of each participating institution, and it conformed to the provisions of the Declaration of Helsinki in 1995 (as revised in Tokyo, 2004). All the patients gave written informed consent.
Patients. Patients were eligible for enrollment if they had a diagnosis of CD22‐positive B‐NHL, according to the World Health Organization (WHO) classification, version 3.( 13 ) Patients were included if they had progressed after at least one prior chemotherapy regimen for indolent B‐NHL, or after one or two chemotherapy regiments, which included anthracyline or anthraquinone for aggressive B‐NHL. Other inclusion criteria were age ≥20 and <75 years, a performance status of one or better on the Eastern Cooperative Oncology Group Scale, life expectancy ≥12 weeks, an absolute neutrophil count (ANC) ≥1.5 × 109/L and platelet count ≥100 × 109/L, serum creatinine ≤1.5 × upper limit of normal (ULN), urine protein‐to‐creatinine ratio of ≤0.2, total bilirubin ≤1.5 × ULN, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) ≤2.5 × ULN, and at least one measurable lesion ≥1.5 cm in at least one dimension by computer tomography (CT) at inclusion, in an area of no prior radiation therapy, or clear progression in an area that had been previously irradiated.
Dose escalation and toxicity criteria. Dose escalation decisions were based on the toxicities observed in the first 28 days after the administration of the first dose. Patients (three and 10 patients per cohort) could receive more than the four planned doses of inotuzumab ozogamicin if they experienced at least stable disease and tolerated treatment. The starting dose was 1.3 mg/m2 administered IV once every 28 days, and dose escalation was performed up to the MTD of 1.8 mg/m2 administered IV once every 28 days. Both the starting dose and the MTD were based on information from a previous clinical trial.( 12 ) The dose escalation in subsequent cohorts was based on the toxicity assessed in the first 28 days after the first dose. Dose escalation continued until three or more patients in a cohort experienced a dose‐limiting toxicity (DLT).
A DLT was defined as any of the following that were at least possibly related to inotuzumab ozogamicin during the first 28 days after the first dose: any grade 3 or 4 (National Cancer Institute Common Terminology Criteria for Adverse Events [NCI CTC], version 3.0) nonhematologic toxicity (except grade 3 alopecia, nausea, or vomiting unless the patient was receiving optimal medical therapy); febrile neutropenia (grade 4 ANC ≥3‐day duration and temperature ≥38.0°C); grade 4 ANC ≥7‐day duration; grade 4 thrombocytopenia ≥3‐day duration, or any bleeding episode requiring platelet transfusion; or delayed recovery (to grade 1 or baseline, except alopecia or grade 2 nausea or vomiting unless the patient was receiving optimal medical therapy) from a toxicity related to inotuzumab ozogamicin that delayed the initiation of the next dose by more than 3 weeks. Patients who experienced a DLT had the subsequent doses of inotuzumab ozogamicin reduced by one dose level, the maximum allowed dose reduction per patient. Patients who experienced toxicities other than DLTs could receive additional doses of inotuzumab ozogamicin at the same dose if they met the following criteria: recoveries to ≤grade 1 (nonhematologic), or baseline toxicity except alopecia; ANC ≥1.5 × 109/L; platelet count ≥75 × 109/L; serum creatinine ≤1.5 × ULN, and urine protein‐to‐creatinine ratio of ≤0.2. The maximum number of doses of inotuzumab ozogamicin was 8 for 1.3 mg/m2 and 7 for 1.8 mg/m2.
Pharmacokinetics. Timed blood samples for pharmacokinetic analysis were collected for cycles 1–3 at 0 (pre‐dose), 1, 4 (cycles 1 and 3 only), 24, 48, 120, 168, 216, 336, and 504 h relative to the start of infusion for each dosing period and at pre‐dose only for cycle 4. If the patient received four doses, then the sample had to be drawn before cycle 5. The serum concentrations of inotuzumab ozogamicin and total calicheamicin were determined using a validated enzyme‐linked immunosorbent assay.
The noncompartmental pharmacokinetic parameters of inotuzumab ozogamicin and total calicheamicin were estimated using the WinNonlin (version 4.1) program. The parameters which were determined included the following: end‐of‐infusion peak concentration (Cmax), area under the concentration‐time curve (AUC), clearance (CL), apparent steady‐state volume of distribution (Vss), and the terminal‐phase elimination half‐life (t1/2).
Safety. An AE was considered to be treatment emergent if its onset occurred between the first and the last dose, plus a lag of 28 days provided the following criteria were met: (i) the AE was not present before the start of the first dose and did not occur in the patient as a chronic condition; (ii) the AE was present before the start of the first dose or was part of the patient’s medical history, but the severity or frequency increased after the start of the first dose.
Efficacy. Patients were evaluable for efficacy if they received ≥2 doses of inotuzumab ozogamicin, had a baseline tumor CT scan and had undergone at least one tumor assessment for response after baseline assessment. In addition, patients with documented PD prior to receiving two doses of inotuzumab ozogamicin were considered evaluable for efficacy. Tumor response was assessed according to the International Workshop Response Criteria for Non‐Hodgkin Lymphoma.( 14 ) The overall response rate (ORR) was defined as the percentage of patients meeting the criteria for complete response (CR), unconfirmed complete response (CRu), or partial response (PR). Stable disease (SD) was measured from the start of the treatment until the criteria for PD were met, taking as the reference the smallest measurements recorded since the initiation of treatment.
Statistical analysis. The sample size for this study was determined by clinical rather than statistical considerations. The probabilities of detecting at least one AE of grade ≥3 with six patients receiving inotuzumab ozogamicin were 0.469, 0.822, and 0.984 when the true rates were 0.10, 0.25, and 0.50, respectively. The probabilities of detecting at least one such event in 10 patients receiving treatment were 0.651, 0.944, and 0.999, respectively.
With cohort sizes of three to six patients, if the true underlying rates of DLT were 0.1, 0.2, 0.3, 0.4, and 0.5, there would be a 0.985, 0.905, 0.754, 0.558, and 0.359 chance, respectively, of escalating to the next full dose. The ORR was estimated using an exact confidence interval (CI) approach.
Results
Patients. From March 2007 to July 2008, a total of 13 patients were enrolled in the study; three patients enrolled in the 1.3 mg/m2 dose cohort and 10 patients in the 1.8 mg/m2 dose cohort. The summary of demographic and other baseline characteristics for all patients is presented in Table 1. There were seven males and six females, all with a median age of 49 years (range, 43–72 years). All 13 patients had FL. The median number of prior treatment regimens was 1 (range, 1–13). All 13 patients had previous rituximab treatment (monotherapy or in combination with chemotherapy). Patients were categorized in low (38.5%), intermediate (42%), and high (15%) risk groups according to Follicular Lymphoma International Prognostic Index (FLIPI).( 15 )
Table 1.
Demographic and baseline characteristics, safety population
| Characteristics | Inotuzumab ozogamicin treatment | ||
|---|---|---|---|
| 1.3 mg/m2 (n = 3) | 1.8 mg/m2 (n = 10) | Total (n = 13) | |
| Median age, years (range) | 57 (51–66) | 48 (43–72) | 49 (43–72) |
| Sex, n (%) | |||
| Female | 2 (67) | 4 (40) | 6 (46) |
| Male | 1 (33) | 6 (60) | 7 (54) |
| ECOG performance status, n (%) | |||
| 0 | 3 (100) | 10 (100) | 13 (100) |
| Primary diagnosis, n (%) | |||
| Follicular lymphoma | 3 (100) | 10 (100) | 13 (100) |
| FLIPI risk groups, n (%) | |||
| Low | 2 (67) | 3 (30) | 5 (39) |
| Intermediate | 1 (33) | 5 (50) | 6 (46) |
| High | 0 | 2 (20) | 2 (15) |
| Number of prior chemo‐/immunotherapy regimens, n (%) | |||
| 1 | 2 (67) | 6 (60) | 8 (62) |
| 2 | 0 | 0 | 0 |
| 3 | 0 | 1 (10) | 1 (8) |
| ≥4 | 1 (33) | 3 (30) | 4 (31) |
ECOG, Eastern Cooperative Oncology Group; FLIPI, Follicular Lymphoma International Prognostic Index.
Safety. In dose escalation, no patients had DLTs, and the MTD previously determined in non‐Japanese patients (1.8 mg/m2) was confirmed for Japanese patients in this study. The most common drug‐related AEs were thrombocytopenia (100% patients); leukopenia (92%); neutropenia, elevated AST, anorexia, and lymphopenia (85%, each); elevated blood fibrinogen (69%); nausea (77%); elevated ALT, elevated alkaline phosphatase, and decreased hemoglobin (54%, each); malaise, elevated blood bilirubin, and headache (46%, each; Table 2(a)).
Table 2.
Inotuzumab ozogamicin‐related adverse events, (a) all grades in ≥4 patients (b) grades ≥3
| Adverse event, n (%) | Inotuzumab ozogamicin treatment | ||
|---|---|---|---|
| 1.3 mg/m2 (n = 3) | 1.8 mg/m2 (n = 10) | Total (n = 13) | |
| (a) all grades in ≥4 patients | |||
| Thrombocytopenia | 3 (100) | 10 (100) | 13 (100) |
| Leukopenia | 3 (100) | 9 (90) | 12 (92) |
| Lymphopenia | 3 (100) | 8 (80) | 11 (85) |
| Neutropenia | 3 (100) | 8 (80) | 11 (85) |
| Aspartate aminotransferase increased | 3 (100) | 8 (80) | 11 (85) |
| Anorexia | 3 (100) | 8 (80) | 11 (85) |
| Nausea | 3 (100) | 7 (70) | 10 (77) |
| Blood fibrinogen increased | 2 (67) | 7 (70) | 9 (69) |
| Alanine aminotransferase increased | 1 (33) | 6 (60) | 7 (54) |
| Blood alkaline phosphatase increased | 1 (33) | 6 (60) | 7 (54) |
| Hemoglobin decreased | 1 (33) | 6 (60) | 7 (54) |
| Malaise | 3 (100) | 3 (30) | 6 (46) |
| Blood bilirubin increased | 2 (67) | 4 (40) | 6 (46) |
| Headache | 2 (67) | 4 (40) | 6 (46) |
| Constipation | 1 (33) | 4 (40) | 5 (39) |
| Influenza | 1 (33) | 4 (40) | 5 (39) |
| Blood lactate dehydrogenase increased | 2 (67) | 3 (30) | 5 (39) |
| Fibrin D dimer increased | 0 | 5 (50) | 5 (39) |
| Hyperglycemia | 1 (33) | 4 (40) | 5 (39) |
| Stomach discomfort | 1 (33) | 3 (30) | 4 (31) |
| Fatigue | 0 | 4 (40) | 4 (31) |
| Hypercholesterolemia | 1 (33) | 3 (30) | 4 (31) |
| Hypokalemia | 2 (67) | 2 (20) | 4 (31) |
| Somnolence | 2 (67) | 2 (20) | 4 (31) |
| Epistaxis | 0 | 4 (40) | 4 (31) |
| Rash | 1 (33) | 3 (30) | 4 (31) |
| (b) grades ≥3 | |||
| Thrombocytopenia | 2 (67) | 5 (50) | 7 (54) |
| Lymphopenia | 0 | 4 (40) | 4 (31) |
| Neutropenia | 1 (33) | 3 (30) | 4 (31) |
| Leukopenia | 0 | 2 (20) | 2 (15) |
| Blood bilirubin increased | 1 (33) | 0 | 1 (8) |
| Hypokalemia | 1 (33) | 0 | 1 (8) |
A summary of drug‐related grade 3 or higher AEs is shown in Table 2(b). At least one drug‐related grade ≥3 AEs was reported in nine of the 13 (69%) patients. Drug‐related grade ≥3 AEs were thrombocytopenia (7 patients, 54%), lymphopenia and neutropenia (4, 31% each), leukopenia (2, 15%), and elevated blood bilirubin and hypokalemia (1, 8% each). Although neither lymphopenia nor leukopenia was reported for the 1.3 mg/m2 cohort, the overall incidence of drug‐related grade ≥3 AEs was comparable between the two cohorts. There were no patients who died during the study.
A total of four patients experienced dose delays, one (33%) patient in the 1.3 mg/m2 cohort and three (30%) patients in the 1.8 mg/m2 cohort (Table 3). Each had one delay. The AEs leading to dose delays were neutropenia (3 patients, 23%) and thrombocytopenia (2, 15%). Two (20%) patients in the 1.8 mg/m2 cohort had one dose reduction (Table 4). Adverse events (AEs) leading to the dose reduction were thrombocytopenia and pleural effusion (1 patient, 8% each). There were no dose reductions in the 1.3 mg/m2 cohort.
Table 3.
Number (%) of patients reporting adverse events leading to dose delays, safety population
| Parameter, n (%) | Inotuzumab ozogamicin treatment | ||
|---|---|---|---|
| 1.3 mg/m2 (n = 3) | 1.8 mg/m2 (n = 10) | Total (n = 13) | |
| No. of patients with dose delays | |||
| No dose delays | 2 (67) | 7 (70) | 9 (69) |
| One or more dose delays | 1 (33) | 3 (30) | 4 (31) |
| No. of dose delays per patient* | |||
| One | 1 (100) | 3 (100) | 4 (31) |
| Any adverse event leading to dose delay† | 1 (33) | 3 (30) | 4 (31) |
| Neutropenia | 1 (33) | 2 (20) | 3 (23) |
| Thrombocytopenia | 1 (33) | 1 (10) | 2 (15) |
*Percentages are based on number of patients with ≥1 inotuzumab ozogamicin dose delay in each treatment group. †Totals at a higher level are not necessarily the sum of those at the lower levels since a patient was able to report two or more different adverse events within the higher level category.
Table 4.
Number (%) of patients reporting adverse events leading to dose reduction, safety population
| Parameter, n (%) | Inotuzumab ozogamicin treatment | ||
|---|---|---|---|
| 1.3 mg/m2 (n = 3) | 1.8 mg/m2 (n = 10) | Total (n = 13) | |
| No. of patients with dose reductions | |||
| No dose reductions | 3 (100) | 8 (80) | 11 (85) |
| One or more dose reductions | 0 | 2 (20) | 2 (15) |
| No. of dose reductions per patient* | |||
| One | 0 | 2 (100) | 2 (15) |
| Any adverse event leading to dose reduction† | 0 | 2 (20) | 2 (15) |
| Thrombocytopenia | 0 | 1 (10) | 1 (8) |
| Pleural effusion | 0 | 1 (10) | 1 (8) |
*Percentages are based on number of patients with ≥1 dose reduction in each treatment group. †Totals at a higher level are not necessarily the sum of those at the lower levels since a patient was able to report two or more different adverse events within the higher level category.
Seven patients discontinued treatment due to AEs: one patient because of grade 2 rash, one patient because of grade 2 urticaria, and five patients because of AEs that required treatment delays of >3 weeks (two patients with prolonged thrombocytopenia, one patient with prolonged thrombocytopenia and neutropenia, one patient with neutropenia and elevated alkaline phosphatase, and one patient with prolonged neutropenia and elevated total bilirubin).
Pharmacokinetics. Pharmacokinetic data after the first dosing were obtained for all 13 patients. The two patients who received 1.8 mg/m2 inotuzumab ozogamicin and had a dose reduction after cycle 1 were excluded from pharmacokinetic assessments for cycle 2 and thereafter. The mean ± SD serum concentrations of inotuzumab ozogamicin and total calicheamicin versus time for patients who received 1.8 mg/m2 are shown in 1, 2, respectively. The peak concentration of inotuzumab ozogamicin was generally observed at or shortly after the termination of infusion with moderate intersubject variability. The peak total calicheamicin concentrations were observed typically within 4 h after the start of inotuzumab ozogamicin infusion with small intersubject variability.
Figure 1.
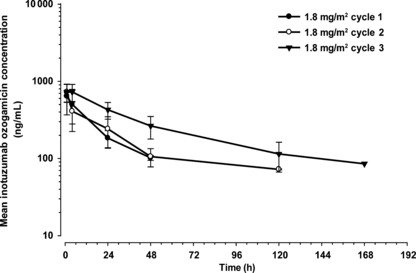
Mean (SD) serum concentrations of inotuzumab ozogamicin after 1.8 mg/m2 infusion of inotuzumab ozogamicin once every 4 weeks. Closed circle, cycle 1 (day 1, n = 10); open circle, cycle 2 (day 29, n = 8); closed triangle, cycle 3 (day 57, n = 5).
Figure 2.
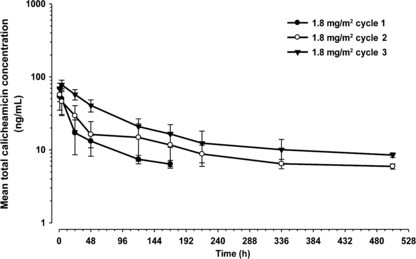
Mean (SD) serum concentrations of total calicheamicin after 1.8 mg/m2 infusion of inotuzumab ozogamicin once every 4 weeks. Closed circle, cycle 1 (day 1, n = 10); open circle, cycle 2 (day 29, n = 8); closed triangle, cycle 3 (day 57, n = 5).
The mean pharmacokinetic parameters for inotuzumab ozogamicin and total calicheamicin are shown in 5, 6, respectively. The AUC of inotuzumab ozogamicin tended to increase with increased dose and period. The t1/2 was prolonged with repeated treatment cycles. These were reflected by substantial decreases in clearances.
Table 5.
Serum pharmacokinetic parameters of inotuzumab ozogamicin
| Dose (Once/4 weeks) | Treatment Day (n) | Number of cycles | Cmax (ng/mL) (%) | t1/2 (h) (%) | AUC (ng h/mL) (%) | CL (L/h) (%) | Vss (L) (%) |
|---|---|---|---|---|---|---|---|
| 1.3 mg/m2 | 1 (3) | 1 | 463 (8) | NC | NC | NC | NC |
| 29 (3) | 2 | 610 (17) | 29.7 (30) | 24166 (29) | 0.08 (32) | 3.27 (11) | |
| 57 (3) | 3 | 524 (18) | 43.6 (18) | 31642 (21) | 0.06 (22) | 3.79 (12) | |
| 1.8 mg/m2 | 1 (10) | 1 | 657 (41) | 13.0 (30) | 14266 (32) | 0.24 (40) | 4.06 (21) |
| 29 (8) | 2 | 727 (27) | 35.8 (43) | 34518 (46) | 0.11 (54) | 4.40 (20) | |
| 57 (5) | 3 | 763 (20) | 44.0 (32) | 39677 (41) | 0.09 (56) | 4.89 (19) |
Data are expressed as mean, and percent coefficient of variance is expressed in parentheses. AUC, total area under the concentration‐time curve; CL, clearance; Cmax, peak concentration; NC, not calculated; t1/2, terminal‐phase elimination half‐life (0.693/λz); Vss, steady‐state volume of distribution.
Table 6.
Serum pharmacokinetic parameters of total calicheamicin
| Dose (Once/4 weeks) | Treatment Day (n) | Number of cycles | Cmax (ng/mL) (%) | t1/2 (h) (%) | AUC (ng h/mL) (%) | CL (L/h) (%) | Vss (L) (%) |
|---|---|---|---|---|---|---|---|
| 1.3 mg/m2 | 1 (3) | 1 | 44.6 (17) | 17.0 (39) | 987 (44) | 2.35 (58) | 49.44 (13) |
| 29 (3) | 2 | 52.4 (22) | 150.6 (45) | 5754 (40) | 0.38 (48) | 62.86 (30) | |
| 57 (3) | 3 | 56.6 (26) | 216.3 (55) | 8060 (37) | 0.27 (43) | 60.17 (25) | |
| 1.8 mg/m2 | 1 (10) | 1 | 59.0 (31) | 49.6 (77) | 2329 (51) | 1.61 (54) | 72.3 (28) |
| 29 (8) | 2 | 59.4 (15) | 162.4 (34) | 7100 (48) | 0.54 (62) | 89.18 (41) | |
| 57 (5) | 3 | 78.2 (15) | 172.7 (48) | 9225 (32) | 0.37 (44) | 68.37 (26) |
Data are expressed as mean, and percent coefficient of variance is expressed in parentheses. AUC, total area under the concentration‐time curve; CL, clearance; Cmax, peak concentration; NC, not calculated; t1/2, terminal‐phase elimination half‐life (0.693/λz); Vss, steady‐state volume of distribution.
The mean total calicheamicin Cmax appeared to increase with dose. The AUC of total calicheamicin increased with increased dose and period. No antibodies to inotuzumab ozogamicin were detectable in patients’ serum during the course of the study. The pharmacokinetics data indicate that the disposition of inotuzumab ozogamicin and total calicheamicin following IV treatment was nonlinear with dose or number of doses.
Efficacy. The best tumor response is presented in Table 7. Antitumor activity was observed at both dose levels. In the 1.3 mg/m2 cohort, two out of three patients had CR, and one patient had CRu for an ORR of 100% (95% CI, 29–100%). In the 1.8 mg/m2 cohort, one out of 10 patients had CR, three patients had CRu, and four patients had PR for an ORR of 80% (95% CI, 44–98%).
Table 7.
The best tumor response during treatment: number (%) of patients in efficacy population
| Best tumor response | Inotuzumab ozogamicin treatment | ||
|---|---|---|---|
| 1.3 mg/m2 (n = 3) | 1.8 mg/m2 (n = 10) | Total (n = 13) | |
| CR, CRu | 3 (100) | 4 (40) | 7 (54) |
| PR | 0 | 4 (40) | 4 (31) |
| OR | 3 (100) | 8 (80) | 11 (85) |
| SD | 0 | 2 (20) | 2 (15) |
CR, complete response; CRu, unconfirmed complete response; OR, overall response (CR + CRu + PR); PR, partial response; SD, stable disease.
Discussion
To improve the clinical outcome of patients with B‐NHL who were pretreated with rituximab or rituximab‐containing regimens, a number of new agents including antibodies, small molecule, targeted agents, and chemotherapeutic drugs have been developed. However, new treatment modalities with improved toxicity profiles and better responses are needed. Inotuzumab ozogamicin (CMC‐544), an antibody‐targeted chemotherapy agent, has demonstrated an acceptable toxicity profile and high activity against relapsed or refractory patients with FL who were pretreated with rituximab or rituximab‐containing treatment.
In a recent phase I, multicenter, open‐label, dose escalation study of inotuzumab ozogamicin administered IV as a single agent in the USA and the European Union, inotuzumab ozogamicin was found to be reasonably well‐tolerated with the MTD of 1.8 mg/m2 administered every 4 weeks and with the major toxicity of grade 3 or greater thrombocytopenia, which was manageable with careful monitoring and platelet transfusion. Response rates of 69% in patients with FL and 33% in patients with DLBCL in the expanded cohort of this trial were observed.( 12 )
In the present phase I dose escalation study in Japanese patients with relapsed or refractory FL, who were pretreated with rituximab, the MTD of inotuzumab ozogamicin was determined to be 1.8 mg/m2 administered once every 28 days, a value that was the same as that observed for non‐Japanese patients.
Most common inotuzumab ozogamicin related adverse events were thrombocytopenia, leukopenia, lymphopenia, neutropenia, elevated AST, anorexia, and nausea, a finding that was very similar to the non‐Japanese study. Adverse events (AEs) leading to dose delays were neutropenia and thrombocytopenia.
The pharmacokinetic profiles of inotuzumab ozogamicin and total calicheamicin indicated that disposition was non‐linear and was associated with increases in drug exposure with increasing dose or number of doses. The pharmacokinetic profiles of inotuzumab ozogamicin and total calicheamicin in Japanese patients were similar to the values for non‐Japanese patients. The study population was very limited, thus no definite conclusion can be made for Japanese patients. However, nonlinearities in drug disposition are known for antibodies( 16 ) and had been observed previously for gemtuzumab ozogamicin.( 17 ) Saturable binding with target antigen is thought to influence antibody disposition, potentially leading to nonlinear distribution and elimination.
Potent antitumor activity for inotuzumab ozogamicin was observed at both the 1.3 and 1.8 mg/m2 dose levels. In the 1.3 mg/m2 cohort, all three patients had CR or CRu for an ORR of 100%. In the 1.8 mg/m2 cohort, one out of 10 patients had CR, three patients had CRu, and four patients had PR for an ORR of 80%. Although the number of patients was limited, our preliminary ORR was greater in comparison to other reported antibody‐based agents in the treatment of patients with FL and prior exposure to rituximab‐containing regimens. For example, in a recent phase I/II study, veltuzumab, a humanized second‐generation anti‐CD20 monoclonal antibody, was reported to have an ORR of 44%.( 18 ) In another phase I/II, single‐agent, dose escalation study, galiximab, an anti‐CD80 antibody, demonstrated an ORR of only 11%.( 19 ) Fludarabine phosphate, one of the most effective drugs in the treatment of indolent B‐NHL, had an ORR of 65%, when administered as a single agent.( 20 )
The FLIPI scores in this study were good predictors of favorable outcome. Of the five patients who had low scores (low risk) two demonstrated CR, two had CRu, and one had PR. Of the six patients who had intermediate scores, one had CR, two had CRu, one had PR, and two had SD. The two patients with high FLIPI scores demonstrated only PR.
In conclusion, the results from this phase I study suggest that inotuzumab ozogamicin is safe, well tolerated, and shows promising efficacy in Japanese patients with relapsed or refractory FL pretreated with rituximab‐containing therapy. In addition, pharmacokinetics and efficacy in this study are comparable with those in preceding studies in non‐Japanese patients. These results therefore warrant further investigation of inotuzumab ozogamicin in relapsed or refractory B‐NHL.
Disclosure Statement
This study was funded by Wyeth which was acquired by Pfizer, Inc., in October 2009. Dr. Junko Ohata was an employee of Wyeth K.K. at the time of the study. Dr. Chiho Ono is an employee of Wyeth K.K. No other potential conflict of interest relevant to the article is reported.
Acknowledgments
The authors thank the patients, doctors, nurses and staff members who participated in this multicenter trial for their excellent cooperation. Participating institutions of this phase I study: Nagoya Daini Red Cross Hospital, National Cancer Center Hospital, and Cancer Institute Hospital. Protocol Committee members: Drs Michinori Ogura, Kensei Tobinai, and Kiyohiko Hatake. The authors also thank Joseph Ramcharan, PhD, and Tricia Gooljarsingh, PhD, for writing assistance that was funded by Pfizer, Inc.
Present affiliations: Teruhisa Azuma, Internal Medicine, Tenri Hospital, Tenri; Junko Ohata, Chugai Pharmaceutical Co., Ltd., Tokyo, Japan.
References
- 1. McLaughlin P, Grillo‐López AJ, Link BK et al. Rituximab chimeric anti‐CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four‐dose treatment program. J Clin Oncol 1998; 16: 2825–33. [DOI] [PubMed] [Google Scholar]
- 2. Czuczman MS, Grillo‐López AJ, White CA et al. Treatment of patients with low‐grade B‐cell lymphoma with the combination of chimeric anti‐CD20 monoclonal antibody and CHOP chemotherapy. J Clin Oncol 1999; 17: 268–76. [DOI] [PubMed] [Google Scholar]
- 3. Marcus R, Imrie K, Belch A et al. CVP chemotherapy plus rituximab compared with CVP as first‐line treatment for advanced follicular lymphoma. Blood 2005; 105: 1417–23. [DOI] [PubMed] [Google Scholar]
- 4. Hiddemann W, Kneba M, Dreyling M et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced‐stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low‐Grade Lymphoma Study Group. Blood 2005; 106: 3725–32. [DOI] [PubMed] [Google Scholar]
- 5. Coiffier B, Lepage E, Briere J et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large‐B‐cell lymphoma. N Engl J Med 2002; 346: 235–42. [DOI] [PubMed] [Google Scholar]
- 6. Pfreundschuh M, Trümper L, Osterborg A et al. CHOP‐like chemotherapy plus rituximab versus CHOP‐like chemotherapy alone in young patients with good‐prognosis diffuse large‐B‐cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol 2006; 7: 379–91. [DOI] [PubMed] [Google Scholar]
- 7. DiJoseph JF, Armellino DC, Boghaert ER et al. Antibody‐targeted chemotherapy with CMC‐544: a CD22‐targeted immunoconjugate of calicheamicin for the treatment of B‐lymphoid malignancies. Blood 2004; 103: 1807–14. [DOI] [PubMed] [Google Scholar]
- 8. Leonard JP, Coleman M, Ketas JC et al. Epratuzumab, a humanized anti‐CD22 antibody, in aggressive non‐Hodgkin’s lymphoma: phase I/II clinical trial results. Clin Cancer Res 2004; 10: 5327–34. [DOI] [PubMed] [Google Scholar]
- 9. Vaickus L, Ball ED, Foon KA. Immune markers in hematologic malignancies. Crit Rev Oncol Hematol 1991; 11: 267–97. [DOI] [PubMed] [Google Scholar]
- 10. DiJoseph JF, Goad ME, Dougher MM et al. Potent and specific antitumor efficacy of CMC‐544, a CD22‐targeted immunoconjugate of calicheamicin, against systemically disseminated B‐cell lymphoma. Clin Cancer Res 2004; 10: 8620–9. [DOI] [PubMed] [Google Scholar]
- 11. DiJoseph JF, Dougher MM, Kalyandrug LB et al. Antitumor efficacy of a combination of CMC‐544 (inotuzumab ozogamicin), a CD22‐targeted cytotoxic immunoconjugate of calicheamicin, and rituximab against non‐Hodgkin’s B‐cell lymphoma. Clin Cancer Res 2006; 12: 242–9. [DOI] [PubMed] [Google Scholar]
- 12. Fayad L, Patel H, Verhoef G et al. Clinical activity of the immunoconjugate CMC‐544 in B‐cell malignancies: preliminary report of the expanded maximum tolerated dose (MTD) cohort of a phase I study. Blood 2006; 108: abs 2711. [Google Scholar]
- 13. Harris NL, Jaffe ES, Diebold J et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting‐Airlie House, Virginia, November 1997. J Clin Oncol 1999; 17: 3835–49. [DOI] [PubMed] [Google Scholar]
- 14. Cheson BD, Horning SJ, Coiffier B et al. Report of an International Workshop to Standardize Response Criteria for Non‐Hodgkin’s Lymphomas. J Clin Oncol 1999; 17: 1244–53. [DOI] [PubMed] [Google Scholar]
- 15. Solal‐Céligny P, Roy P, Colombat P et al. Follicular lymphoma international prognostic index. Blood 2004; 104: 1258–65. [DOI] [PubMed] [Google Scholar]
- 16. Lobo ED, Hansen RJ, Balthasar JP. Antibody pharmacokinetics and pharmacodynamics. J Pharm Sci 2004; 93: 2645–68. [DOI] [PubMed] [Google Scholar]
- 17. Dowell JA, Korth‐Bradley J, Liu H, King S, Berger MS. Pharmacokinetics of gemtuzumab ozogamicin, an antibody‐targeted chemotherapy agent for the treatment of patients with acute myeloid leukemia in first relapse. J Clin Pharmacol 2001; 41: 1206–14. [DOI] [PubMed] [Google Scholar]
- 18. Morschhauser F, Leonard JP, Fayad L et al. Humanized anti‐CD20 antibody, veltuzumab, in refractory/recurrent non‐Hodgkin’s lymphoma: phase I/II results. J Clin Oncol 2009; 27: 3346–53. [DOI] [PubMed] [Google Scholar]
- 19. Czuczman MS, Thall A, Witzig TE et al. Phase I/II study of galiximab, an anti‐CD80 antibody, for relapsed or refractory follicular lymphoma. J Clin Oncol 2005; 23: 4390–8. [DOI] [PubMed] [Google Scholar]
- 20. Tobinai K, Watanabe T, Ogura M et al. Phase II study of oral fludarabine phosphate in relapsed indolent B‐cell non‐Hodgkin’s lymphoma. J Clin Oncol 2006; 24: 174–80. [DOI] [PubMed] [Google Scholar]


