Abstract
CHOP combined with rituximab (R‐CHOP) is regarded as one of the most effective treatments for indolent B‐cell non‐Hodgkin lymphoma (B‐NHL), however, its optimal combination schedule remains unknown. We performed a randomized phase II study to explore a more promising schedule in untreated, advanced indolent B‐NHL. Patients were randomized to receive either six courses of CHOP concurrently with rituximab (Arm C), or six courses of CHOP followed by six courses of weekly rituximab (Arm S). A total of 69 patients received the concurrent (n = 34) or sequential (n = 35) regimen. Overall response rate (ORR) in Arm C was 94% (95% confidence interval [CI], 79 to 99), including a 66% complete response (CR) compared with 97% (95% CI, 85–100), including a 68% CR in Arm S. Patients in Arm C experienced more grade 4 neutropenia (85%versus 70%) and experienced more grade 3 or greater non‐hematological toxicities (21%versus 12%). Both arms were tolerated well. With a median follow‐up of 28.2 months, the median progression‐free survival (PFS) time was 34.2 months in Arm C, and was not reached in Arm S. R‐CHOP is highly effective in untreated indolent B‐NHL, either concurrent or in a sequential combination. Both combination schedules deserve further investigation. (Cancer Sci 2006; 97: 305 – 312)
Indolent non‐Hodgkin lymphomas (NHLs), in which the representative type of lymphoma is follicular lymphoma (FL), are characterized by an advanced stage at presentation, lack of symptoms associated with the disease, and indolent behavior in terms of the time to symptomatic disease progression.( 1 , 2 ) Although many chemotherapeutic agents and combination therapies are used in the treatment of patients with FL, a large majority of these patients remain incurable.( 3 , 4 , 5 ) Thus, more effective strategies are needed to overcome the current therapeutic limitations. Rituximab is a chimeric monoclonal anti‐CD20 antibody that can deplete malignant B cells through complement‐dependent cytotoxicity, antibody‐dependent cell‐mediated cytotoxicity (ADCC),( 6 ) and apoptotic mechanisms.( 7 ) It has also been shown to sensitize lymphoma cell lines resistant to cytotoxic drugs.( 8 ) In recent years, it was demonstrated that rituximab is an active agent against indolent B‐NHL and has become a standard component of first‐line therapy, either as a single agent or in combination with chemotherapy.( 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 ) Recently, the addition of rituximab to the cyclophosphamide, doxorubicin, vincristine and prednisolone (CHOP) regimen or cyclophosphamide, vincristine and prednisolone (CVP) regimen was demonstrated to improve the clinical outcome in patients with previously untreated advanced FL, without increased toxicity. Czuczman et al. conducted the first phase II study on the combination of rituximab with CHOP in mostly untreated patients with low‐grade B‐NHL or FL.( 14 ) They treated the patients with six cycles of standard CHOP given at 3‐week intervals along with rituximab administered twice before, during and after the six cycles of CHOP therapy. All treated patients (n = 38) responded with a complete response (CR) rate of 87%, and the median time to progression (TTP) was 82.3 months.( 15 ) Marcus et al. reported significant superiority of CVP plus rituximab (R‐CVP) over CVP for previously untreated patients with advanced FL in a randomized phase III study.( 18 ) From the viewpoint of the possible synergistic effect between rituximab and chemotherapeutic drugs, it seems to be reasonable that rituximab be delivered in combination with chemotherapeutic drugs concurrently. Whereas, from the viewpoint of enhancing the ADCC effect, which is one of the putative antitumor mechanisms of rituximab, it seems reasonable that rituximab be administered in situations in which effector cells such as macrophages, natural killer cells and neutrophils are intact, in other words, there are no cytotoxic or immunosuppressive effects of chemotherapeutic drugs. Thus, to maximize the possible ADCC effect, it might be preferable that rituximab be delivered to patients after recovery from the toxic or immunosuppressive effect of chemotherapy. However, the optimal schedule for the combined use of rituximab and chemotherapy remains unclear. To explore a more promising regimen of rituximab combined with CHOP therapy for the treatment of indolent B‐cell NHL, we conducted a randomized phase II trial.
Materials and Methods
Patients
Between July 1999 and July 2000, 69 patients with newly diagnosed indolent B‐cell NHLs were enrolled. Eligibility criteria included: aged between 20 and 70 years; a histopathological diagnosis of indolent B‐NHL according to the Revised European‐American Lymphoma (REAL) classification( 19 ) (including small lymphocytic lymphoma, lymphoplasmacytic lymphoma, FL or marginal zone B‐cell lymphoma); no previous treatment; stages III or IV disease according to the Ann Arbor staging system;( 20 ) CD20 positive lymphomas confirmed by immunohistochemistry or flow cytometry; an Eastern Cooperative Oncology Group (ECOG)( 21 ) performance status (PS) of 0, 1 or 2; negative for the hepatitis B virus surface antigen, hepatitis C virus antibody or human immunodeficiency virus antibody; having no other malignancies and normal renal, pulmonary and hepatic function. Approval was obtained from the local institutional review boards of all participating institutions. Informed consent was obtained from all patients before enrollment in accordance with the Declaration of Helsinki.
Study design
This randomized phase II study was designed as a two arm parallel phase II study. The expected overall response rate (ORR) (P1) for either arm was set at 95% based on the phase II study by Czuczman et al. where CHOP was combined with rituximab,( 14 ) while the threshold response rate (P0) was set at 75%, based on previous reports on CVP or COP, CHOP or CHOP‐like studies.( 22 ) The number of patients required for this study was 27 per arm, calculated in accordance with Fleming's two‐stage testing procedure,( 23 ) at α = 0.05 (two‐side) and 1‐β = 0.8. Assuming that up to 20% of patients might be ineligible due to inaccurate histopathological diagnosis at participating institutions, we planned to enroll at least 34 patients per arm. From the viewpoint of selection design by Simon et al.,( 24 ) the selection of one arm showing a 15% higher percentage CR at 90% probability would be possible with this number of patients, if the percentage CR of both arms would achieve at least 65%.
Treatment schedule
Patients fulfilling the inclusion criteria were randomly assigned to either the concurrent arm (Arm C) or sequential arm (Arm S) at the independent randomization center, thereby minimizing the bias between the arms regarding PS, clinical stage and institution. All patients were treated with six courses of standard CHOP chemotherapy (cyclophosphamide 750 mg/m2, i.v., day 1; doxorubicin 50 mg/m2 i.v., day 1; vincristine 1.4 mg/m2[capped at 2 mg] i.v., day 1; and prednisolone 100 mg, p.o., days 1–5) every 3 weeks. In addition, patients allocated to Arm C received rituximab (375 mg/m2 i.v.) 2 days prior to each CHOP cycle, whereas patients allocated to Arm S received rituximab (375 mg/m2, weekly six times, i.v.) 4 weeks after completion of the sixth cycle of CHOP. Rituximab was given intravenously based on the preceding phase I study in Japan.( 25 )
Patient evaluation, end‐points and response criteria
Patients were observed until the progression of lymphomas or death. Tumor restaging was performed at approximately 3‐monthly intervals for the first 12 months and every 4 to 6 months thereafter.
The primary end‐point of this study was an ORR in all eligible patients, that is, the percentage of patients achieving a CR, CRu, or partial response (PR), evaluated according to the International Workshop Response Criteria for NHL.( 26 ) CR required the disappearance of all detectable clinical and radiographic evidence of disease, disappearance of disease‐related symptoms, and normalization of biochemical abnormalities. Adenopathy on computed tomography (CT) scans must have regressed to normal size (1.5 cm or less in the greatest transverse diameter). CRu was defined as complete disappearance of all detectable clinical and radiographic abnormalities of the disease, with the exception of the presence of a residual adenopathy larger than 1.5 cm, as long as the sum of products of the greatest diameters (SPDs) of the adenopathy had decreased by more than 75%. Residual bone marrow abnormalities, that included increased number or size of lymphoid aggregates without definite cytological evidence of persistent lymphoma, could also be present in patients in the CRu response category. PR was defined as a greater than 50% decrease in the SPDs of the largest dominant nodes or nodal masses. Stable disease patients were defined as having any response that was less than a PR or an increase in the SPDs by less than 25%, with no new lesions appearing. Progressive disease was defined by an increase of more than 25% in the size of the SPDs of the measured lesions, or the appearance of new lesions. All cases were centrally reviewed radiographically using CT films.
Secondary end‐points were percentage CR, including percentage CRu and a progression‐free survival (PFS) for all eligible patients, as an interval from the day of enrollment to the first day when tumor progression or death due to any cause was observed. The response to the combined regimen and PFS period for each patient was evaluated until at least 2 and a half years after the completion of treatment.
Adverse events (AEs) were graded according to the toxicity criteria of the Japan Clinical Oncology Group,( 27 ) an expanded version of the Common Toxicity Criteria of the National Cancer Institute (version 1.0).
Human antichimeric antibody assay and pharmacokinetics of rituximab
Serum human antichimeric antibody (HACA) levels were monitored at 8 and 10 months after treatment initiation using an enzyme‐linked immunosorbent assay (ELISA), as described previously.( 28 )
Serum rituximab levels were monitored using ELISA for patients who signed another informed consent form to participate in this pharmacokinetic (PK) study. The PK parameters were calculated using WinNonlin PK software (WinNonlin Standard Japanese Edition, version 1.1; Scientific Consulting, Apex, NC, USA).
Statistical methods
The ORR, percentage CR, and their 95% confidence intervals (CIs) were calculated with per protocol sets (PPS) of data for all eligible patients and full analysis sets (FAS) of data for all enrolled patients under the F‐distribution. The median PFS time, time to CR (TTCR) and time to response (TTR), and their 95% CIs were estimated for all eligible and evaluative patients using the method of Kaplan and Meier, and were compared using the log–rank test. In addition, pretreatment factors affecting the ORR and PFS were analyzed for all eligible and evaluative patients by univariate and multivariate analyses using Fisher's exact test, Wilcoxon's rank sum test, the log–rank test, the logistic regression model or Cox's proportional hazard regression model.
All statistical analyses were performed using SAS software (version 6.12; SAS Institute, Cary, NC, USA). Data used for theses analyses were finally confirmed on March 31, 2004.
Results
Patient characteristics
A total of 69 patients were enrolled from 21 institutions (see Appendix I); 34 patients were allocated to Arm C and 35 patients to Arm S. Patient characteristics at study entry are summarized in Table 1. The median age was 52 years (range, 26–69 years). The major characteristics of the two arms were very similar in both the enrolled and eligible patients. Retrospectively, we analyzed the Follicular Lymphoma International Prognostic Index (FLIPI) in all patients.( 29 ) FLIPI was equally distributed between the two arms. Twenty‐eight patients (82%) in Arm C and 30 patients (86%) in Arm S were judged to belong to the low, or low‐intermediate risk group categorized by FLIPI. Three patients were judged ineligible by an extramural review committee, because two of them had concomitant active cancer and one had a history of prior chemotherapy, including doxorubicin for the treatment of breast cancer. Sixty‐five patients (94%) were confirmed to have FL in the central pathology review.
Table 1.
Patient characteristics
| Factor | Enrolled (n = 69) | Eligible (n = 66) | ||||
|---|---|---|---|---|---|---|
| Arm C | Arm S | Total | Arm C | Arm S | Total | |
| Sex | ||||||
| Female | 18 | 18 | 36 | 17 | 18 | 35 |
| Male | 16 | 17 | 33 | 15 | 16 | 31 |
| Age (years) | ||||||
| Median | 53 | 50 | 52 | 54.5 | 49.5 | 52.5 |
| Range | 36–65 | 26–69 | 26–69 | 36–65 | 26–69 | 26–69 |
| Performance status (ECOG) | ||||||
| 0 | 29 | 30 | 59 | 28 | 29 | 57 |
| 1 | 5 | 5 | 10 | 4 | 5 | 9 |
| Histopathology (REAL) † | ||||||
| Follicular, grade 1 | 12 | 11 | 23 | 11 | 11 | 22 |
| Follicular, grade 2 | 21 | 19 | 40 | 20 | 19 | 39 |
| Follicular, grade 3 | 0 | 2 | 2 | 0 | 2 | 2 |
| Marginal zone B‐cell | 1 | 0 | 1 | 1 | 0 | 1 |
| Low grade B‐NHL, NOS ‡ | 0 | 2 | 2 | 0 | 2 | 2 |
| No specimen submitted§ | 0 | 1 | 1 | 0 | 0 | 0 |
| Clinical stage (Ann Arbor) | ||||||
| III | 14 | 15 | 29 | 13 | 14 | 27 |
| IV | 20 | 20 | 40 | 19 | 20 | 39 |
| B‐symptoms | ||||||
| Absent | 30 | 33 | 63 | 29 | 32 | 61 |
| Present | 4 | 2 | 6 | 3 | 2 | 5 |
| LDH | ||||||
| Normal | 32 | 31 | 63 | 31 | 30 | 61 |
| Elevated | 2 | 4 | 6 | 1 | 4 | 5 |
| No. of extranodal sites | ||||||
| 0–1 | 25 | 26 | 51 | 24 | 25 | 49 |
| ≤2 | 9 | 9 | 18 | 8 | 9 | 17 |
| International Prognostic Index | ||||||
| Low | 21 | 21 | 42 | 21 | 20 | 41 |
| Low‐intermediate | 12 | 12 | 24 | 10 | 12 | 22 |
| High‐intermediate | 1 | 1 | 2 | 1 | 1 | 2 |
| High | 0 | 1 | 1 | 0 | 1 | 1 |
| Follicular Lymphoma International Prognostic Index | ||||||
| Low | 16 | 15 | 31 | 16 | 15 | 31 |
| Intermediate | 12 | 15 | 27 | 10 | 14 | 25 |
| High | 6 | 5 | 11 | 5 | 5 | 10 |
According to the diagnosis by the central pathology review.
Low‐grade B‐cell non‐Hodgkin lymphoma (NHL) not otherwise specified. ‡Specimen was not submitted to the central pathology review. LDH, lactic dehydrogenase.
Response to treatment and survival
Sixty‐six eligible patients (Arm C, 32 patients; Arm S, 34 patients) were evaluated with PPSs of data, and 69 patients (Arm C, 34 patients; Arm S, 35 patients) with FASs of data. One patient allocated to Arm C could not be evaluated for response because the first cycle of chemotherapy given was not CHOP (doxorubicin in the CHOP regimen was erroneously replaced with daunorubicin). Two patients (one patient eligible and one ineligible) allocated to Arm S could not be evaluated because they had withdrawn from the study before starting treatment.
As shown in Table 2, similar results of the ORRs and the percentage CRs were obtained in Arm C and Arm S. The ORRs and percentage CRs calculated with PPSs and FASs were similar. Kaplan‐Meier curves of TTR and TTCR were plotted for eligible and evaluative patients in each arm, as shown in Fig. 1. Although the median TTRs for patients in Arm C and Arm S were not different (61 days versus 62 days, respectively), the 75th percentile TTRs for patients were shorter in Arm C (66 days) than Arm S (127 days), with no statistical difference (P = 0.0994, log–rank test). The median TTCRs were similar in Arm C and Arm S (136 days and 140 days, respectively). As shown in Fig. 2, the median PFS time for patients in Arm C (n = 32) was 34.2 months (95%CI, 27.1 months – inestimable), whereas that for patients in Arm S (n = 33) had not yet been reached, with a median follow‐up time of 28.2 months. One patient (#38) in Arm S died of tumor progression 730 days after the first treatment. No other patients died within approximately 3 years of observation.
Table 2.
Response to therapy
| Arm | n | No. of patients achieving response | Response rate (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CR | CRu | PR | SD | PD | NE | %CR | ORR | |||
| Arm C | Eligible | 32 | 19 | 2 | 9 | 1 | 0 | 1 | 66% (47–81%) | 94% (79–99%) |
| 21 | ||||||||||
| 30 | ||||||||||
| Enrolled | 34 | 21 | 2 | 10 | 1 | 0 | 0 | 68% (50–83%) | 97% (85–100%) | |
| 23 | ||||||||||
| 33 | ||||||||||
| Arm S | Eligible | 34 | 22 | 1 | 10 | 0 | 0 | 1 | 68% (50–83%) | 97% (85–100%) |
| 23 | ||||||||||
| 33 | ||||||||||
| Enrolled | 35 | 21 | 1 | 10 | 0 | 0 | 2 | 66% (44–81%) | 94% (81–99%) | |
| 23 | ||||||||||
| 33 | ||||||||||
Response to each therapy was evaluated according to the International Workshop Criteria for Non‐Hodgkin's Lymphoma. CI, confidence interval; CR, complete response; CRu, complete response/unconfirmed; NE, not evaluative due to insufficient follow‐up; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease.
Figure 1.
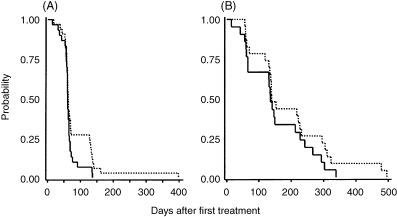
(A) Time to response (TTR) and (B) time to complete response (TTCR). Medians were estimated by the Kaplan‐Meier method. A total of 63 patients (Arm C [‐], 30; Arm S [‐‐‐], 33) were analyzed for TTR, and 44 patients (Arm C, 21; Arm S, 23) for TTCR with per protocol sets of data. Median TTRs in Arm C and Arm S were 61 days (95% confidence interval [CI] 59 to 65 days) and 62 days (95% CI 60–70 days), respectively. The 75th percentile TTRs in Arm C and Arm S were 66 days (95% CI 63 to 76 days) and 140 days (95% CI 66–135 days), respectively (P = 0.0994, log–rank test). Median TTCRs in Arm C and Arm S were 136 days (95% CI 65 to 213 days) and 140 days (95% CI 134–227 days), respectively. The 75th percentile TTCRs in Arm C and Arm S were 228 days (95% CI 141 to 293 days) and 295 days (95% CI 153–323 days), respectively (P = 0.2201, log–rank test).
Figure 2.
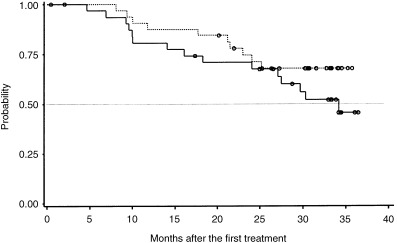
Progression‐free survival (PFS). Medians were estimated by the Kaplan‐Meier method. The upper limit of the 95% confidence interval (CI) for Arm C has not yet been determined. A total of 65 patients (Arm C, 32; Arm S, 33) were analyzed with per protocol sets of data. The median PFS time for patients in Arm C (‐) was 34.2 months (95%CI, 27.1 months, inestimable), whereas that for patients in Arm S (…) had not yet been reached, with a median follow‐up time of 28.2 months. Log–rank test, P = 0.220. (o) Censored.
Adverse events
Information about AEs was available for 67 patients (Arm C, 34 patients; Arm S, 33 patients) who received protocol treatment. Hematological toxicity was documented at its highest grade throughout the study period. As shown in Table 3, major hematological toxicity was neutropenia; grade 3 or greater neutropenia was observed in 32 patients (94%) in Arm C and in 33 patients (100%) in Arm S; grade 4 neutropenia was seen in 29 patients (85%) in Arm C and in 23 patients (70%) in Arm S. All hematological toxicities were controllable and reversible, although some patients required hematopoietic cytokines.
Table 3.
Hematological toxicity
| Toxicity | Arm | n | Grade 0–2 | Grade 3 | Grade 4 |
|---|---|---|---|---|---|
| Any hematological toxicity | Arm C | 34 | 2 (6%) | 3 (9%) | 29 (85%) |
| 32 (94%) | |||||
| Arm S | 33 | 0 (0%) | 10 (30%) | 23 (70%) | |
| 33 (100%) | |||||
| Leukopenia | Arm C | 34 | 5 (15%) | 16 (47%) | 13 (38%) |
| 29 (85%) | |||||
| Arm S | 33 | 3 (9%) | 23 (70%) | 7 (21%) | |
| 30 (91%) | |||||
| Neutropenia | Arm C | 34 | 2 (6%) | 3 (9%) | 29 (85%) |
| 32 (94%) | |||||
| Arm S | 33 | 1 (3%) | 9 (27%) | 23 (70%) | |
| 32 (97%) | |||||
| Thrombocytopenia | Arm C | 34 | 32 (94%) | 1 (3%) | 1 (3%) |
| 2 (6%) | |||||
| Arm S | 33 | 33 (100%) | 0 (0%) | 0 (0%) | |
| 0 (0%) | |||||
| Anemia | Arm C | 34 | 31 (91%) | 3 (9%) | – |
| Arm S | 33 | 31 (94%) | 2 (6%) | – |
Hematological toxicity was evaluated according to the JCOG Toxicity Criteria, an expanded version of the NCI‐CTC version 1.0. All hematological toxicities (possibly related to rituximab, or unknown relationship to rituximab) observed during the treatment and follow‐up period (for 6 months after the last cycle of CHOP for Arm C, and for 4 months after the last rituximab infusion for Arm S) are listed.
Grade 3 or greater non‐hematological AEs observed during treatment and initial follow‐up periods are listed in Table 4. A total of 11 patients (Arm C, seven patients, 21%; Arm S, four patients, 12%) developed 14 events of grade 3 or greater non‐hematological adverse events. All non‐hematological toxicities were reversible. There was no therapy‐related death.
Table 4.
Grade 3 or greater‐non‐hematological adverse events
| Arm | Patient | Serious adverse event † | Grade ‡ | Onset timing | Relating drug (causative) |
|---|---|---|---|---|---|
| Arm C (n = 32) | #04 | Hyperglycemia | 3 | 6th cycle (day 4) | CHOP (diabetes) |
| #07 | Hyperglycemia | 3 | 4th cycle (day 2) | CHOP, rituximab | |
| #13 | Hypertension | 3 | 1st cycle (day 3) | CHOP, rituximab | |
| #21 | Total bilirubin elevation | 3 | 2nd cycle (day 5) | – (constitutional) | |
| #23 | Abdominal pain | 3 | 1st cycle (day 9) | CHOP, rituximab | |
| #58 | Acute cholangitis with elevated AST and ALT | 3 | 3rd cycle (day 10) | CHOP, rituximab | |
| #59 | Hyperglycemia, hypertension | 3 | 5th cycle (day 6) | CHOP, rituximab | |
| Arm S (n = 33) | #25 | Total bilirubin elevation | 3 | 6th cycle (day 132) | – (constitutional) |
| #56 | Diarrhea | 4 | 1st cycle (day 13) | – (alimentary) | |
| Febrile neutropenia | 3 | 3rd cycle (day 12) | CHOP | ||
| Interstitial pneumonia | 3 | 3rd cycle (day 15) | CHOP | ||
| #62 | Total bilirubin elevation | 3 | 4th cycle (day 7) | CHOP | |
| #69 | AST and ALT elevation | 3 | 1st cycle (day 10) | CHOP | |
| 2nd cycle (day 8) | CHOP | ||||
| 6th cycle (day 29) | CHOP |
Grade 3 or greater adverse events other than hematological toxicities that were observed during the treatment and follow‐up period (for 6 months after the last cycle of CHOP for Arm C, and for 4 months after the last rituximab infusion for Arm S).
‡ JCOG Toxicity Criteria, an expanded version of the NCI‐CTC, version 1.0.
Prognostic factors
Pretreatment factors affecting ORR and PFS were analyzed. Because the sample size of each arm was small, analyses were not performed separately for the two arms, but results were pooled (n = 64). There were two factors affecting ORR when analyzed by the Wilcoxon's rank sum test. Patients with PS 0 (41CR, 13PR, 1NC, 0 PD) demonstrated a superior response to those with PS 1 (3CR, 6PR, 0NC, 0PD) (P = 0.0182, Wilcoxon's rank‐sum). Patients with a tumor size <5 cm (32CR, 6PR, 1NC, 0 PD) had a superior response to those with tumors equal to 5 cm (12CR, 13PR, 0NC, 0 PD) (P = 0.0066, Wilcoxon's rank‐sum).
However, no factor significantly affected PFS. Multivariate analyses were also performed using the same factors, excluding IPI. There was no factor that independently affected ORR and PFS.
HACA and pharmacokinetics of rituximab
Out of 67 patients who received rituximab, HACA assays were performed for 65 patients (Arm C, 33; Arm S, 32) at 8 months after treatment, and for 64 patients (Arm C, 33; Arm S, 31) at 10 months after treatment. No patient developed HACA. For all 27 patients (Arm C, 14; Arm S 13) who received four rituximab infusions and whose planned monitoring of serum rituximab levels were completed, pharmacokinetic parameters were calculated throughout the four infusions. As shown in Table 5, Arm S showed higher values for the parameters of area under the curve (AUC), maximum concentration (Cmax), elimination half‐life (T1/2), mean residence time (MRT), and volume of distribution (Vd).
Table 5.
Pharmacokinetic parameters of rituximab
| Arm | Dose (mg/day) | AUC (µg. h/mL) | Cmax † (µg/mL) | T1/2 (h) | Clearance ‡ (litter/h) | MRT (h) | Vd (litter) | |
|---|---|---|---|---|---|---|---|---|
| Arm C | Mean | 593.9 | 372 498.9 | 262.5 | 232.3 | 0.0259 | 335.1 | 4.49 |
| (n = 14) | SD | 51.1 | 111 660.4 | 73.2 | 113.8 | 0.0301 | 164.2 | 0.66 |
| Arm S | Mean | 596.4 | 418 901.3 | 433.5 | 356.9 | 0.0128 | 514.9 | 5.57 |
| (n = 13) | SD | 82.6 | 107 002.6 | 134.9 | 163.4 | 0.0077 | 235.9 | 1.95 |
Actual measured value.
Calculated under the one‐compartment model. Time points for serum collection were as follows; Arm C: before, and 10 min and 2 days after each rituximab infusion, and 1 week, 1, 4 and 6 months after the sixth rituximab infusion. Arm S: before, 10 min after each rituximab infusion and 2 days, 1 and 2 weeks, and 1 and 4 months after the sixth rituximab infusion. AUC, area under the curve; Cmax, maximum concentration; T1/2, elimination half‐life; MRT, mean residence time; Vd, volume of distribution.
Discussion
In this randomized phase II trial, we have demonstrated that the combined use of rituximab and CHOP yielded an ORR of 94% and 97%, and a percentage CR of 66% and 68% in the concurrent arm and the sequential arm, respectively. These ORRs and percentage CRs are superior to those reported for combination chemotherapy regimens containing anthracycline without rituximab, which were conducted after stringent clinical staging with CT. The percentage CR obtained by six to eight cycles of CHOP chemotherapy in untreated patients (n = 83) with FL was reported to be 36% (90%CI, 27–46%).( 30 ) The ORR and percentage CR of CHOP chemotherapy obtained by Kimby et al. in their randomized study comparing chlorambucil plus prednisone versus CHOP in symptomatic low‐grade NHL (n = 127), were 60% and 18%, respectively.( 31 )
Data of the present study was comparable to the preceding study on CHOP combined with rituximab in patients with indolent B‐NHL regarding efficacy and tolerability. Although the precise schedule of the administration of rituximab in the first phase II study of R‐CHOP reported by Czuczman et al. was not the same as that of the present study, the concept of concurrent use is identical between their trial and Arm C in the present study.( 14 ) However, the percentage CR of Arm C is less than that of Czuczman et al.'s trial, and the median PFS of Arm C appears to be shorter in the present study, although more than 82% of all enrolled patients in our study were in the low or low‐intermediate risk group by FLIPI. In Czuczman et al.'s trial, as the last two infusions of rituximab were administered 1 month after the sixth CHOP cycle, like in our sequential arm, the design of Czuczman et al.'s trial had characteristics of both the concurrent arm and the sequential arm. So it is possible that the higher percentage CR and longer PFS in Czuczman et al.'s trial compared to our concurrent arm were partly due to the mixed design of the administration schedule of rituximab, in addition to the possible selection bias in phase II studies.
The South‐west Oncology Group (SWOG) in the USA studied six cycles of CHOP followed by four weekly infusions of rituximab in newly diagnosed patients with FL at advanced stages (31% with bulky disease and 30% with B‐symptoms). Sixteen (19%) of the 84 evaluative patients had an improved tumor response after rituximab treatment, with an ORR of 72%, including 54% with a CR or CRu. The PFS was 76% at the median follow‐up of 2.7 years.( 32 ) The PFS data of the sequential arm in our trial is similar to that of the SWOG trial.
Cancer and Leukemia Group B (CALGB) conducted a randomized phase II study to explore a more suitable administration schedule of rituximab with fludarabine in previously untreated chronic lymphocytic leukemia (CLL) patients.( 33 ) Patients randomly received either six monthly courses of fludarabine concurrently with rituximab followed 2 months later by four weekly doses of rituximab for consolidation therapy, or fludarabine alone followed 2 months later by rituximab consolidation therapy. The ORR with the concurrent regimen was 90% compared to 77% with the sequential regimen. With a median follow‐up time of 23 months, the number of relapsed patients was 18 (35%) in the concurrent regimen and 15 (28%) in the sequential regimen. Although PFS and survival appeared to be somewhat longer with the sequential treatment, CALGB concluded that the concurrent use of rituximab and fludarabine was superior. Our randomized phase II study for indolent B‐cell NHLs showed similar percentage ORRs and percentage CRs between the two arms, and a seemingly longer PFS in the sequential arm. Because patients in the concurrent arm in the CALGB study received consolidated administration of rituximab after induction therapy, the concurrent arm in the CALGB study had characteristics of the concurrent arm and sequential arm of our present study.
In a randomized phase III study that compared eight cycles of R‐CVP to CVP for previously untreated patients with advanced FL, a significantly prolonged TTP of R‐CVP was reported (median 32 months versus 15 months for CVP; P < 0.0001).( 18 ) The median TTP of R‐CVP was similar to the median PFS of Arm C in our study. As the toxicity is stronger in CHOP than CVP, it is worthwhile to conduct a randomized phase III trial to compare R‐CHOP to R‐CVP.
The maintenance use of rituximab after first‐line rituximab therapy was also reported to prolong PFS or event‐free survival (EFS).( 34 , 35 ) Future trials to explore the role of maintenance use of rituximab after first‐line rituximab containing chemotherapy like Arm C are warranted.
About 25% of patients in Arm S did not achieve a response (PR or higher) before the initiation of rituximab treatment, despite the completion of six cycles of CHOP. In Arm C, more than 90% of patients showed a response after the six cycles of CHOP plus rituximab. The same tendency was also shown in the TTCR, as shown in Fig. 1B. The TTCR of each patient in Arm C was relatively shorter than that in Arm S.
While grade 3 or greater non‐hematological AEs were observed in 11 patients (Arm C, seven patients, 21%; Arm S, four patients, 13%), both arms were well tolerated. Two patients were withdrawn from the study before completion of the planned treatment by AE. One patient in Arm C developed acute cholangitis after the third cycle of CHOP plus rituximab. The other patient in Arm S developed interstitial pneumonia after the third cycle of CHOP. Both patients fully recovered. Hematological toxicities were observed in all treated patients; grade 4 neutropenia was frequent and was observed in 85% of patients in Arm C and in 70% in Arm S. However, these hematological toxicities were manageable with or without supportive care using hematopoietic growth factor. No patient was withdrawn from the study due to hematological toxicity. Grade 3 or greater thrombocytopenia was rare in Arm C and absent in Arm S. Although hematological and non‐hematological toxicities were slightly more frequent in Arm C, toxicities were clinically acceptable in both arms.
In conclusion, CHOP combined with rituximab was highly effective in untreated patients with indolent B‐NHL, especially FL, either in a concurrent or sequential combination, with acceptable toxicities. Although the time to achieve a response was more rapid with the concurrent combination than the sequential combination, PFS appeared to be slightly longer with the sequential combination, although the difference was not statistically significant. We conclude that both combination schedules deserve further investigation. Considering the promising results of rituximab maintenance therapy reported by other investigators, it would be worthwhile to conduct future trials to establish the role of rituximab maintenance after concurrent and sequential combinations of rituximab plus CHOP therapy.
Acknowledgments
This study was supported by Zenyaku Kogyo, Tokyo, Japan. We thank all the investigators, including the physicians, nurses and laboratory technicians, in the participating institutions of this multicenter trial. We are grateful to Dr K. Oshimi (Juntendo University School of Medicine, Tokyo), Dr K. Toyama (Tokyo Medical College, Tokyo), and Dr S. Shirakawa (Koudoukai Hospital, Osaka) for their critical review of the clinical data as members of the Independent Monitoring Committee. We are grateful to Dr S. Nakamura (Aichi Cancer Center Hospital, Nagoya), Dr Y. Matsuno (National Cancer Center Hospital, Tokyo), Dr S. Nawano (National Cancer Center Hospital East, Kashiwa), and Dr M. Matsusako (St. Luke's International Hospital, Tokyo) for their central pathological or radiographical review as members of the Central Pathological Review Committee and the Central Response‐Evaluating Committee. We also acknowledge Y. Arita, K. Endo, T. Uesugi, M. Tachikawa, Y. Ikematsu, T. Itoh, H. Iimura, K. Inatomi, M. Ikenami, Y. Koide and T. Kayo (Zenyaku Kogyo) for their help with data collection and statistical and pharmacological analyses.
Participating institutions and principal investigators of the IDEC‐C2B8 Study Group included: Sapporo National Hospital (K. Aikawa, M. Nakata), Sapporo Hokuyu Hospital (M. Kasai, Y. Kiyama), Tochigi Cancer Center (Y. Kano, M. Akutsu), International Medical Center of Japan (A. Miwa, N. Takesako), National Cancer Center Hospital East (K. Itoh, T. Igarashi, K. Ishizawa), National Cancer Center Hospital (K. Tobinai, Y. Kobayashi, T. Watanabe), Tokyo Medical University (K. Ohyashiki, T. Tauchi), Tokai University School of Medicine (T. Hotta, T. Sasao), Hamamatsu University School of Medicine (K. Ohnishi), Aichi Cancer Center Hospital (Y. Morishima, M. Ogura, Y. Kagami), Nagoya University School of Medicine (T. Kinoshita, T. Murate, H. Nagai), Nagoya National Hospital (K. Tsushita, H. Ohashi), Mie University School of Medicine (S. Kageyama, M. Yamaguchi), Kyoto Prefectural University of Medicine (M. Taniwaki), Kyoto University School of Medicine (H. Ohno, T. Ishikawa), Shiga Medical Center for Adults (T. Suzuki), Center for Cardiovascular Diseases and Cancer, Osaka (A. Hiraoka, T. Karasuno), Hyogo Medical Center for Adults (T. Murayama), Hiroshima University School of Medicine (A. Sakai), National Kyushu Cancer Center (N. Uike), Nagasaki University School of Medicine (T. Maeda, K. Tsukasaki).
Present address: The Department of Hematology, Nagoya Daini Red Cross Hospital, 2‐9, Myokencho, Showaku, Nagoya 466‐8650, Japan.
Portions of this study were presented at the Annual Meeting of the American Society of Clinical Oncology, New Orleans, 2004.
References
- 1. Rohatiner A, Lister TA. Follicular lymphoma, in Magrath IT (ed.): The Non‐Hodgkin's Lymphomas. London: Oxford University Press, 1997: 867–96. [Google Scholar]
- 2. Berger F, Felman P, Sonet A et al. Nonfollicular small B‐cell lymphomas: a heterogeneous group of patients with distinct clinical features and outcome. Blood 1994; 83: 2829–35. [PubMed] [Google Scholar]
- 3. Horning SJ. Natural history of and therapy for the indolent non‐Hodgkin's lymphomas. Semin Oncol 1993; 20: 75–88. [PubMed] [Google Scholar]
- 4. Solal‐Celigny PH. Management of histologically indolent non‐Hodgkin's lymphomas. Baillieres Clin Hematol 1996; 9: 669–87. [DOI] [PubMed] [Google Scholar]
- 5. Aisenberg AC. Coherent view of non‐Hodgkin's lymphoma [review]. Clin Oncol 1995; 13: 2656–75. [DOI] [PubMed] [Google Scholar]
- 6. Reff M, Carner K, Chambers K et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood 1994; 83: 435–45. [PubMed] [Google Scholar]
- 7. Taji H, Kagami Y, Okada Y et al. Inhibition of CD20‐positive B lymphoma cell lines by IDEC‐C2B8 anti‐CD20 monoclonal antibody. Jpn J Cancer Res 1998; 89: 748–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Demidem A, Lam T, Alas S, Hariharan K, Hanna N, Bonavida B. Chimeric anti‐CD20 (IDEC‐C2B8) monoclonal antibody sensitizes a B cell lymphoma cell line to cell killing by cytotoxic drugs. Cancer Biother Radiopharm 1997; 12: 177–86. [DOI] [PubMed] [Google Scholar]
- 9. McLaughlin P, Grillo‐Lopez AJ, Link BK et al. Rituximab chimeric anti‐CD20 monoclonal antibody therapy for relapsed indolent lymphoma: Half of patients respond to a four‐dose treatment program. J Clin Oncol 1998; 16: 2825–33. [DOI] [PubMed] [Google Scholar]
- 10. Foran JM, Gupta RK, Cunningham D et al. A UK multicentre phase II study of rituximab (chimaeric anti‐CD20 monoclonal antibody) in patients with follicular lymphoma, with PCR monitoring of molecular response. Br J Haematol 2000; 109: 81–8. [DOI] [PubMed] [Google Scholar]
- 11. Hainsworth JD, Burrism HA, Morrissey LH et al. Rituximab monoclonal antibody as initial systemic therapy for patients with low grade non‐Hodgkin's lymphoma. Blood 2000; 95: 3052–6. [PubMed] [Google Scholar]
- 12. Colombat P, Salles G, Brousse N et al. Rituximab (anti‐CD20 monoclonal antibody) as single first‐line therapy for patients with follicular lymphoma with a low tumor burden: Clinical and molecular evaluation. Blood 2001; 97: 101–6. [DOI] [PubMed] [Google Scholar]
- 13. Igarashi T, Kobayashi Y, Ogura M et al. Factors affecting toxicity, response and progression‐free survival in relapsed patients with indolent B‐cell lymphoma and mantle cell lymphoma treated with rituximab: a Japanese phase II study. Ann Oncol 2002; 13: 928–43. [DOI] [PubMed] [Google Scholar]
- 14. Czuczman MS, Grillo‐Lopez AJ, White CA et al. Treatment of patients with low‐grade B‐cell lymphoma with the combination of chimeric anti‐CD20 monoclonal antibody and CHOP chemotherapy. J Clin Oncol 1999; 17: 268–76. [DOI] [PubMed] [Google Scholar]
- 15. Czuczman MS, Weaver R, Alkuzweny B, Berlfein J, Grillo‐Lopez AJ. Prolonged clinical and molecular remission in patients with low‐grade or follicular non‐Hodgkin's lymphoma treated with rituximab plus CHOP chemotherapy: 9‐year follow‐up. J Clin Oncol 2004; 22: 4711–6. [DOI] [PubMed] [Google Scholar]
- 16. Forstpointner R, Dreyling M, Repp R et al. The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: results of a prospective randomized study of the German Low‐Grade Lymphoma Study Group. Blood 2004; 104: 3064–71. [DOI] [PubMed] [Google Scholar]
- 17. Czuczman MS, Koryzna A, Mohr A et al. Rituximab in combination with fludarabine chemotherapy in low‐grade or follicular lymphoma. J Clin Oncol 2005; 23: 694–704. [DOI] [PubMed] [Google Scholar]
- 18. Marcus R, Imrie K, Belch A et al. CVP chemotherapy plus rituximab compared with CVP as first‐line treatment for advanced follicular lymphoma. Blood 2005; 105: 1417–23. [DOI] [PubMed] [Google Scholar]
- 19. Harris NL, Jaffe ES, Stein H et al. A revised European‐American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood 1994; 84: 1361–92. [PubMed] [Google Scholar]
- 20. Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the committee on Hodgkin's disease staging classification. Cancer Res 1971; 31: 1860–1. [PubMed] [Google Scholar]
- 21. Oken MM, Creech RH, Tormey DC et al. Toxicity and response criteria of Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5: 649–55. [PubMed] [Google Scholar]
- 22. Hiddemann W. Current status and future perspectives in the treatment of low‐grade non‐Hodgkin's lymphomas. Blood Rev 1994; 8: 225–33. [DOI] [PubMed] [Google Scholar]
- 23. Fleming TR. One sample multiple testing procedure for phase II clinical trials. Biometrics 1982; 38: 143–51. [PubMed] [Google Scholar]
- 24. Simon R, Thall PF, Ellenberg SS. New designs for the selection of treatments to be tested in randomized clinical trials. Stat Med 1994; 13: 417–29. [DOI] [PubMed] [Google Scholar]
- 25. Tobinai K, Kobayashi Y, Narabayashi M et al. Feasibility and pharmacokinetic study of a chimeric anti‐CD20 monoclonal antibody (IDEC‐C2B8, rituximab) in relapsed B‐cell lymphoma. Ann Oncol 1998; 9: 527–34. [DOI] [PubMed] [Google Scholar]
- 26. Cheson BD, Horning SJ, Coiffier B et al. Report of an International Workshop to standardize response criteria for non‐Hodgkin's lymphoma. J Clin Oncol 1999; 17: 1244–53. [DOI] [PubMed] [Google Scholar]
- 27. Tobinai K, Kohno A, Shimada Y et al. Toxicity grading criteria of the Japan Clinical Oncology Group (JCOG). Jpn J Clin Oncol 1993; 23: 250–7. [PubMed] [Google Scholar]
- 28. Maloney DG, Grillo‐Lopez AJ, Bodkin DJ et al. IDEC‐C2B8: Results of a phase I multiple‐dose trial in patients with relapsed non‐Hodgkin's lymphoma. J Clin Oncol 1997; 15: 3266–74. [DOI] [PubMed] [Google Scholar]
- 29. Solal‐Celigny P, Roy P, Colombat P et al. Follicular lymphoma international prognostic index. Blood 2004; 104: 1258–65. [DOI] [PubMed] [Google Scholar]
- 30. Freedman A, Gribben J, Neuberg D et al. High‐dose therapy and autologous bone marrow transplantation in patients with follicular lymphoma during first remission. Blood 1996; 88: 2780–6. [PubMed] [Google Scholar]
- 31. Kimby E, Björkholm M, Gahrton G et al. Chlorambucil/prednisone vs. CHOP in symptomatic low‐grade non‐Hodgkin's lymphomas: a randomized trial from the Lymphoma Group of Central Sweden. Ann Oncol 1994; 5 (Suppl. 2): 67–71. [PubMed] [Google Scholar]
- 32. Maloney DG, Press OW, Braziel RM et al. A phase II trial of CHOP followed by rituximab chimeric monoclonal anti‐CD20 antibody for treatment of newly diagnosed follicular non‐Hodgkin's lymphoma: SWOG 9800 (Abstract). Blood 2001; 98: 843a. [Google Scholar]
- 33. Byrd JC, Peterson BL, Morrison VA et al. Randomized phase 2 study of fludarabine with concurrent versus sequential treatment with rituximab in symptomatic, untreated patients with B‐cell chronic lymphocytic leukemia: results from Cancer and Leukemia Group B 9712 (CALGB 9712). Blood 2003; 101: 6–14. [DOI] [PubMed] [Google Scholar]
- 34. Hainsworth JD, Litchy S, Burris HA III et al. Rituximab as first‐line and maintenance therapy for patients with indolent non‐Hodgkin's lymphoma. J Clin Oncol 2002; 20: 4261–7. [DOI] [PubMed] [Google Scholar]
- 35. Ghielmini M, Schmitz SFH, Cogliatti SB et al. Prolonged treatment with rituximab in patients with follicular lymphoma significantly increases event‐free survival and response duration compared with the standard weekly 4 schedule. Blood 2004; 103: 4416–23. [DOI] [PubMed] [Google Scholar]


