Abstract
Tumor angiogenesis is necessary for solid tumor progression and metastasis. Tumor blood vessels have been shown to differ from their normal counterparts, for example, by changes in morphology. An important concept in tumor angiogenesis is that tumor endothelial cells are assumed to be genetically normal, even though these endothelial cells are structurally and functionally abnormal. To date, many anti‐angiogenic drugs have been developed, but, their therapeutic efficacy is not dramatic and they have also been reported to cause toxic side effects. To develop ideal antiangiogenic therapies, understanding tumor endothelial cell abnormalities is important. We have isolated tumor endothelial cells from mouse tumor xenografts and have shown that tumor‐associated endothelial cells are abnormal. Tumor‐associated endothelial cells upregulate many genes, such as epidermal growth factor receptor (EGFR). Tumor‐associated endothelial cells are also more sensitive to EGF. They also have relatively large, heterogeneous nuclei. Unexpectedly, tumor endothelial cells are cytogenetically abnormal. Fluorescence in situ hybridization (FISH) analysis showed that freshly isolated uncultured tumor endothelial cells were aneuploid and had abnormal multiple centrosomes. The degree of aneuploidy was exacerbated by passage in culture. In marked contrast, freshly isolated normal skin and adipose endothelial cells were diploid. They had normal centrosomes and remained cytogenetically stable in culture even up to 20 passages. We conclude that tumor endothelial cells can acquire cytogenetic abnormalities while in the tumor microenvironment. Questions as to whether or not tumor endothelial cells become resistant to antiangiogenic drugs are thus raised. Our preliminary data show that tumor endothelial cells are more resistant to certain chemotherapeutic drugs. Studies to evaluate the mechanism for cytogenetic abnormalities in tumor endothelial cells are underway. It is becoming quite clear that the tumor vasculature is much more complex and unpredictable than initially perceived. Here, we provide an overview of the current studies on tumor endothelial cell abnormalities. (Cancer Sci 2008; 99: 459–466)
Tumor blood vessels have been recognized as an important target for cancer therapy, after the concept that tumor growth is dependant on angiogenesis was originated by Folkman.( 1 ) Angiogenesis, the process of new blood vessel growth, is necessary for tumor progression and metastasis. Tumor blood vessels provide nutrition and oxygen, and get rid of waste from tumor tissue, resulting in tumor progression. Tumor vessels act as gatekeepers for tumor cells to metastasize to distant organs.( 2 )
Thus, the attempt to target tumor endothelial cells with angiogenic inhibitors (anti‐angiogenic therapy) has been an important strategy for cancer therapy, and many anti‐angiogenic drugs have been discovered and tested.( 3 )
Tumor Angiogenesis as a Target for Cancer Therapy
Traditional concepts in anti‐angiogenic therapy have been that: (i) one tumor endothelial cell supports many tumor cells, thus, targeting endothelial cells might be a much more effective strategy than targeting tumor cells; (ii) tumor endothelial cells are the same among all tumor types, thus, an ideal anti‐angiogenic drug could be useful in treating all cancers; and (iii) until recently, tumor endothelial cells have been believed to be genetically stable, so they might not acquire drug resistance, unlike tumor cells. However, recent studies suggest that tumor endothelial cells might be different from normal endothelial cells and might also be heterogeneous among organs or tumor types. Furthermore, the therapeutic efficacy of anti‐angiogenic therapy is not dramatic but rather marginal. Contrary to the presumption that anti‐angiogenic drugs are not as toxic as cytotoxic drugs, they are reported to cause severe side‐effects such as lethal hemoptysis( 4 , 5 ) and perforation of intestines.( 6 , 7 )
To develop ideal tumor anti‐angiogenic therapies, it is very important to understand the biology of tumor endothelial cells (Fig. 1).
Figure 1.
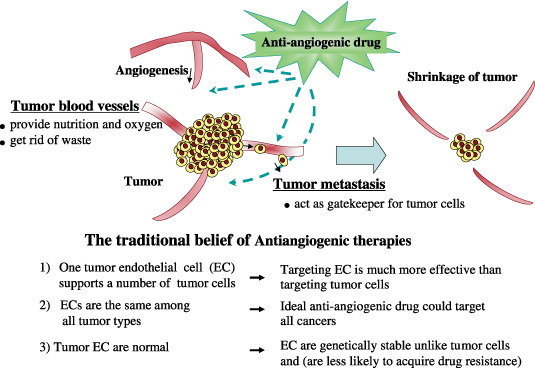
Generally accepted concept of tumor angiogenesis and anti‐angiogenic therapy. Tumor blood vessels are important for tumor progression. They provide nutrition and oxygen to tumor tissue and get rid of waste. For tumor metastasis, tumor blood vessels are gatekeepers. Anti‐angiogenic therapy has been developed to target these blood vessels and inhibit new vascularization. Anti‐angiogenic therapy has been considered to have several advantages: (i) targeting endothelial cells might be a much more effective strategy than targeting tumor cells; (ii) tumor endothelial cells are the same among all tumor types, therefore an ideal anti‐angiogenic drug could be useful in treating all cancers; and (iii) tumor endothelial cells were, until recently, believed to be genetically stable. Thus, tumor endothelial cells might not acquire drug resistance, unlike tumor cells. However, recent studies suggest that tumor endothelial cells might be different from normal endothelial cells and might also be heterogeneous among organs or tumor types.
Tumor Blood Vessels are Abnormal
It is well documented that tumor blood vessels differ morphologically from normal vessels (Fig. 2). Tumor vessels are disorganized whereas the normal vasculature shows a hierarchal branching pattern of arteries, veins, and capillaries.( 8 ) Tumor endothelial cells do not form regular monolayers and thus do not have a normal barrier function.( 9 ) Tumor endothelial cell basement membranes have structural abnormalities including loose associations with endothelial cells, and varying thicknesses of type IV collagen layers that are usually not seen in normal endothelial cells.( 10 ) Pericytes are present on tumor endothelial cells, but have abnormally loose associations with these cells and extend cytoplasmic processes deep into the tumor tissue.( 11 ) These abnormalities result in leakiness. Tumor blood vessels are often tortuous in appearance with uneven vessel diameters due in part to compression of the immature vessel wall by tumor cells. Tumor vessels have chaotic blood flow and vessel leakiness due to loose endothelial cell interconnections.( 12 ) The high interstitial fluid pressure in a tumor causes blood vessel collapse and impedes blood flow. This is one reason why tumor tissue is usually under a hypoxic condition, even though it is highly vascularized. This sometimes causes resistance to radiation therapy.( 13 )
Figure 2.
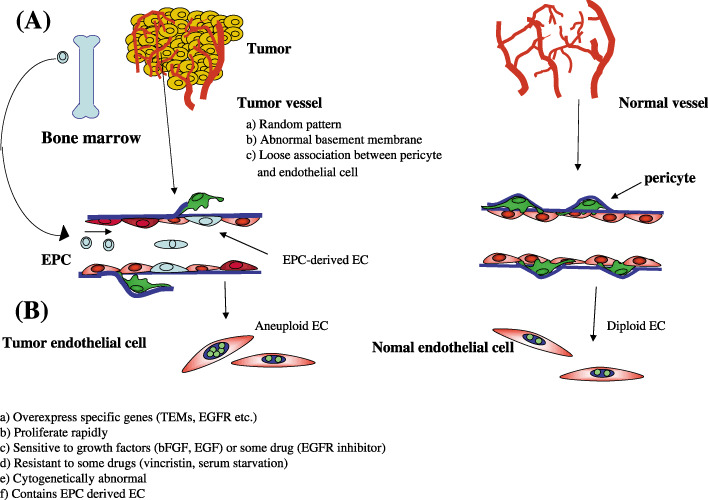
Differences in blood vessels and endothelial cells between tumors and normal tissues. (A) Tumor vessels are disorganized, whereas normal vasculature show a hierarchal branching pattern of arteries, veins, and capillaries. Tumor endothelial cell basement membranes have structural abnormalities including loose associations with endothelial cells, and various thicknesses of type IV collagen layers that are not usually seen in normal endothelial cells. Pericytes have abnormally loose associations with endothelial cells and extend cytoplasmic processes deep into the tumor tissue. Tumor vessels have chaotic blood flow and vessel leakiness due to loose endothelial cell interconnections. (B) Tumor endothelial cells differ from normal endothelial cells. (a) Tumor endothelial cells (EC) overexpress specific genes, such as tumor endothelial markers and epidermal growth factor receptors (EGFR); (b) tumor EC proliferate more rapidly and (c) are sensitive to growth factors such as basic fibroblast growth factor, EGF and vascular endothelial growth factor, or some drugs like EGFR inhibitors; (d) tumor EC are resistant to apoptotic stimuli such as serum starvation or chemotherapeutic drugs and (v) have cytogenetic abnormalities; (e) there are some endothelial progenitor cell‐derived endothelial cells in tumors.
Differences between Tumor Endothelial Cells and Normal Endothelial Cells
The morphological abnormalities in tumor blood vessels compared to normal vessels raises questions as to whether there are phenotypical differences at the molecular and functional levels between tumor and normal endothelial cells. To address this question, tumor endothelial cells isolated from tumor tissue were required. However, there have been few reports about isolation of tumor endothelial cells until recently. In fact, for a long time, most studies on tumor angiogenesis were carried out using normal endothelial cells such as human umbilical vein endothelial cells (HUVEC), human dermal microvascular endothelial cells, or bovine aortic endothelial cells. To isolate tumor endothelial cells for global analysis of gene expression has been difficult because endothelial cells are usually enmeshed in a complex tissue consisting of vessel wall components, stromal cells, and tumor cells, and only a small fraction of cells within these tissues are endothelial cells. Besides technical difficulties, there might have been concerns about trials to isolate tumor endothelial cells themselves, because they were sometimes considered to lose their specific phenotype soon after being isolated from tumor tissue. In the first report about tumor endothelial‐specific markers, St Croix et al. succeeded in isolating endothelial cells from colon carcinoma and normal colonic mucosa and compared the gene expression profiles between tumor and normal endothelial cells in a relatively low number of cells. They identified the specific genes for tumor endothelial cells and designated them as tumor endothelial markers (TEMs) using serial analysis of gene expression (SAGE). SAGE revealed that there are 46 TEMs.( 14 ) Some of them (TEM1, TEM5, TEM7, and TEM8) are transmembrane proteins and are also conserved in mice.( 15 , 16 ) Very recently, they showed that these TEMs, except TEM8, are also overexpressed during physiological angiogenesis, as well as in tumor endothelial cells. Instead, they identified 13 novel cell surface proteins as TEMs.( 17 ) Other studies about the gene profile of tumor endothelial cells using global analysis have been published recently (Table 1). Buckanovich et al. identified 12 ovarian tumor vascular markers from vascular cells captured by laser‐capture microdissection and some tumor vascular markers correlated with the prognosis of patients. However, they commented that these markers are not strictly specific to tumor endothelial cells, because laser‐capture microdissection‐captured cells contain not only endothelial cells but also mural cells such as pericytes or smooth muscle cells.( 18 ) Ovarian tumor endothelial cells were also isolated with magnetic beads and 23 TEMs were identified by DNA microarray.( 19 ) Among the 23 markers, several genes are involved in the pro‐angiogenic pathway. Colon carcinoma endothelial cell markers were also identified by SAGE.( 17 , 20 ) These studies on the gene profiling of tumor endothelial cells are listed in Table 1. However, tumor endothelial cells were not cultured in these studies and the biological phenotype in tumor endothelial cells remains to be clarified. Another study, based on cultured tumor endothelial cells, found that human renal cell carcinoma endothelial cells did not undergo the senescence that is typical of normal endothelial cells, and were resistant to apoptotic stimuli such as serum‐starvation and vincristine. They showed higher proliferation rates in low serum, enhanced Akt activation, and decreased expression of the tumor suppressor, PTEN.( 21 ) Murine Lewis lung carcinoma was characterized by elongated morphology, and upregulated adhesion molecules such as CD31 or ICAM‐1. They required a tumor‐specific matrix to maintain their characteristics. Sca‐1 expression was also elevated in these cells, suggesting the presence of circulating endothelial progenitors in their tumor endothelial cells.( 22 ) We have also purified tumor endothelial cells in an attempt to better understand the effects of the tumor microenvironment on endothelial cell properties.( 23 ) Human tumor xenograft models in nude mice were established as sources of mouse tumor endothelial cells. Murine tumor (melanoma and liposarcoma) endothelial cells and normal (skin and adipose) endothelial cell counterparts were isolated with high purity by magnetic bead cell sorting( 24 ) (Fig. 3). As it is known that heparin‐binding epidermal growth factor‐like growth factor (HB‐EGF) is a receptor of diphtheria toxin (DT) in human cells, but not mouse cells, and DT binds to human cells expressing HB‐EGF‐like growth factor and is toxic to them while mouse cells are resistant to DT,( 25 ) we used DT in tumor endothelial cell isolation.( 24 ) To remove any human tumor cell contamination which might have overgrown in the endothelial cell culture, DT was added to the tumor endothelial cell subculture to kill human cells and normal endothelial cells for technical consistency. The mouse tumor endothelial cells expressed typical endothelial cell markers such as CD31 and vascular endothelial growth factor (VEGF) receptors, and upregulated several tumor endothelial markers that have already been reported, such as TEMs( 24 ) or aminopeptidase N (CD13) (Matsuda et al. unpublished data, 2007). From these data, tumor endothelial cells retain their specificity for tumor endothelial cells (at least some) even in culture. After this publication, we isolated two more tumor endothelial cells from oral carcinoma and renal carcinoma (data not shown). Microarray analysis showed several genes were overexpressed in four different tumor endothelial cells commonly compared to normal skin endothelial cells. There were approximately 50 genes that were expressed 10‐fold higher in tumor endothelial cells than in normal endothelial cells (data not shown). These genes are now under investigation to identify novel tumor endothelial cell‐specific markers. Our tumor endothelial cells retained endothelial cell properties at least up to 20 passages and the cells could be maintained in culture for at least 50 passages. Tumor endothelial cells grew faster, had a lower serum requirement, and were more responsive to angiogenic growth factors such as basic fibroblast growth factor (bFGF) and VEGF compared to normal counterpart endothelial cells.( 23 ) Furthermore, Amin et al. have found that tumor endothelial cells express high levels of epidermal growth factor receptor (EGFR), not usually expressed in normal endothelial cells such as HUVEC.( 26 ) EGF can induce phosphorylation of tumor endothelial cell EGFR and stimulate tumor endothelial cell proliferation. EGFR tyrosine kinase inhibitors inhibit EGF‐induced EGFR activation and proliferation of tumor endothelial cells. Thus, it was suggested that EGFR kinase inhibitors might target not only tumor cells, but also tumor endothelial cell EGFR. This data has clinical significance. Anti‐EGFR therapy could target tumor vasculature specifically. Moreover, this therapy can be applied to any cancer in which tumor cells do not express, or express a low level of, EGFR.
Table 1.
Studies on isolated tumor endothelial cells (EC)
| Host | Origin of tumor EC | Isolation method | Antigen used for EC isolation | Findings | Culture of tumor EC | Publication |
|---|---|---|---|---|---|---|
| Human | Human primary colon carcinoma | Magnetic beads selection | P1H12 (CD146) | Identified nine TEMs by SAGE | _ | St Croix B et al. Science 2000; 289: 1197–202 |
| Human | Human primary renal carcinoma | Magnetic beads selection | CD105 | Tumor EC are resistant to apoptotic stimuli, upregulate Akt, downregulate PTEN | + | Bussolati B et al. FASEB J 2003; 17: 1159–61 |
| Mouse | Mouse lung carcinoma cell line | Magnetic beads selection | CD31 | Upregulate adhesion molecules such as ICAM‐1 and Sca‐1 | + | Allport JR, Weissleder R. Neoplasia 2003; 5: 205–17 |
| Mouse | Human melanoma cell line, primary liposarcoma | Magnetic beads selection | CD31, BS1‐B4 lectin | Tumor EC are sensitive to growth factor, upregulate EGFR, TEMs, have chromosomal abnormality | + | Hida K et al. Cancer Res 2004; 64: 8249–55; Amin DN et al. Cancer Res 2006; 66: 2173–80 |
| Human | Human primary B‐cell lymphoma | Magnetic beads selection | CD31 | Have same chromosomal abnormality as lymphoma cell | + | Streubel B et al. N Engl J Med 2004; 351: 250–9 |
| Human | Human primary colon carcinoma | Magnetic beads selection | CD31, CD34 | Identified 17 tumor EC markers by SAGE | _ | van Beijnum JR et al. Blood 2006; 108: 2339–48 |
| Human | Human primary ovarian carcinoma | Magnetic beads selection | P1H12 (CD146) | Identified 23 tumor EC markers, using RNA amplification and DNA microarray | _ | Lu C et al. Cancer Res 2007; 67: 1757–68 |
| Human | Human primary ovarian carcinoma | Immunohistochemistry‐ guided laser‐capture microdissection | CD31+ P1H12 (CD146) | Identify 12 tumor vascular markers using RNA amplification and DNA microarray | _ | Buckanovich RJ et al. J Clin Oncol 2007; 25: 852–61 |
| Mouse | Human mammary/colon carcinoma cell line, mouse lung/colon carcinoma cell line | Magnetic beads selection | CD105 | Identify 13 novel TEMs by SAGE | _ | Seaman S et al. Cancer Cell 2007; 11: 539–54 |
EGFR, epidermal growth factor receptor; SAGE, serial analysis of gene expression; TEM, tumor endothelial marker.
Figure 3.
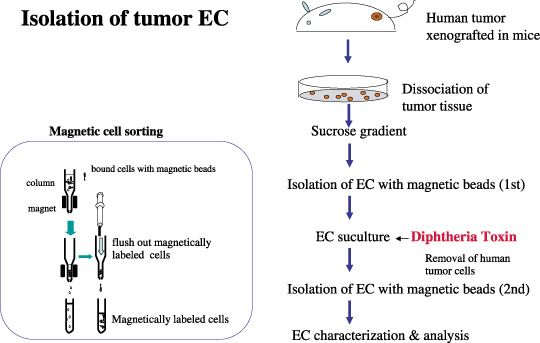
Isolation of tumor endothelial cells. To isolate tumor endothelial cells (EC) from human tumor xenograft in nude mice, excised tissue is minced and digested with collagenase. After blood cells are removed by a single sucrose step‐gradient centrifugation, endothelial cells are isolated using a magnetic cell sorting (MACS) system using an anti‐CD31 antibody. Diphtheria toxin is added to tumor EC cultures to remove human tumor cells. After subculture for approximately 2 weeks, EC are purified by a second MACS using fluorescein‐isothiocyanate‐BS1‐B4 lectin. After EC purity is confirmed, the cells are characterized and used for analysis.
Taking the in vivo and in vitro studies together, there is mounting evidence that there are distinct differences between tumor and normal blood vessels and their endothelial cells in terms of biology, morphology, and gene profile (Fig. 2).
Cytogenetic Abnormalities in Tumor Endothelial Cells
Tumor endothelial cells had relatively larger nuclei than normal endothelial cells, indicating they had more DNA content.( 24 ) Strikingly, tumor endothelial cells were cytogenetically abnormal. Tumor endothelial cells were karyotypically aneuploid, whereas normal endothelial cells grown under the same conditions were diploid. In addition, they had structural aberrations such as non‐reciprocal translocations, missing chromosomes, marker chromosomes, and double minutes by multicolor fluorescent in situ hybridization (FISH) analysis.( 24 ) Thus, tumor endothelial cells have the hallmarks of chromosomal instability. To avoid possible artifacts due to culture conditions, freshly isolated, uncultured endothelial cells were analyzed by FISH. CD31 staining was used to confirm endothelial cell identity. Approximately 16% of liposarcoma endothelial cells and 34% of melanoma endothelial cells were aneuploid by FISH using a mouse chromosome 17 probe.( 24 ) After this report, we investigated the aneuploidy of other types of tumor endothelial cells. Approximately 35% of oral carcinoma endothelial cells (Fig. 4B) and 54% of renal carcinoma endothelial cells (Fig. 4C) were also aneuploid, even when uncultured. Significantly, the degree of aneuploidy of tumor endothelial cells almost doubled in culture in each tumor endothelial cell. In contrast, freshly isolated, uncultured skin and adipose endothelial cells were diploid with 3% abnormalities. The 1–4% abnormalities were observed in other normal cells such as normal murine lymphocytes when the probe was tested before analysis. The 92% of normal skin endothelial cells remained diploid when cultured until passage 19. In karyotype analysis, these abnormal skin endothelial cells were noted to be precisely tetraploid; this finding is not uncommon in cultures of normal cells and is not interpreted to be reflective of genetic instability in these normal endothelial cells. Tumor endothelial cells were noted to have much more complex abnormal karyotype. These results of aneuploidy in cultured and uncultured endothelial cells are summarized in Fig. 4A. These results suggest that tumor endothelial cells, unlike normal endothelial cells, have chromosomal instability.
Figure 4.
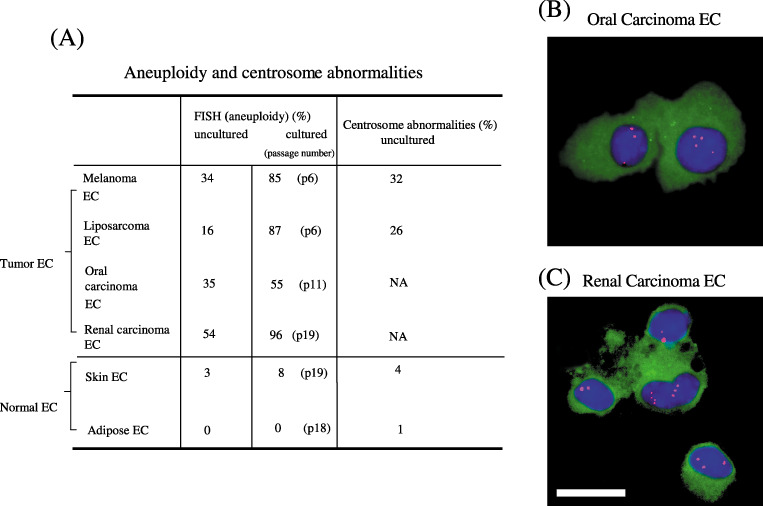
Cytogenetically abnormal tumor endothelial cells. (A) Quantitative analysis of cytogenetic abnormalities in endothelial cells. Tumor endothelial cells (melanoma, liposarcoma, oral carcinoma, and renal carcinoma endothelial cells) are aneuploid even before cultured and the degree of aneuploidy increases in culture, whereas uncultured normal endothelial cells are diploid and stay nearly diploid in culture. The centrosome abnormality was also detected in tumor endothelial cells. Mouse oral carcinoma endothelial cells (B) and renal carcinoma endothelial cells (C) were isolated and cytospun onto glass slides, followed by immunostaining with an anti‐CD31 antibody and fluorescent in situ hybridization with a chromosome 17 probe. Representative aneuploid endothelial cells are shown. Green, CD31; red; chromosome 17; blue, 4′,6′‐diamidino‐2‐phenylindole dihydrochloride. Scale bar, 10 µm.
Aneuploid tumor endothelial cells were also detected on frozen tumor sections by FISH. Tumor endothelial cells also have abnormal centrosomes. The normal function of centrosomes is to establish cell polarity and to properly segregate chromosomes. Defects in centrosome function correlate with loss of polarity and chromosome mis‐segregation in malignant tumors that contribute to aneuploidy.( 27 , 28 ) In addition, our studies showed a correlation between the number of tumor endothelial cells with extra chromosomes and those with multiple centrosomes, suggesting a potential causal relationship (Fig. 4A).
As tumor endothelial cells continue to proliferate in culture, it appears that these cells, like tumor cells, lack the normal cell cycle checkpoints that inhibit mitosis in response to chromosomal abnormalities.
There are some other reports about chromosomal abnormalities in tumor endothelial cells in hematopoietic tumors such as leukemia( 29 ) and lymphoma.( 30 ) In chronic myeloid leukemia, for example, circulating endothelial cells had leukemia‐specific translocations.( 29 ) In B‐cell lymphomas, 37% of endothelial cells were shown to harbor lymphoma‐specific chromosomal translocations, suggesting that lymphoma and lymphoma endothelial cells might both be derived from hemangioblastic cells.( 30 ) In addition, circulating endothelial cells in multiple myeloma had the same translocation as myeloma cells, indicating the possibility that both cells were originally from the same multipotent hemangioblast.( 31 )
Furthermore, a recent study reported that neuroblastoma endothelial cells had a varying proportion of microvascular endothelial cells that showed MYCN amplification, which are typically amplified in neuroblastoma, suggesting these tumor endothelial cells are dedifferentiated from their tumor origin.( 32 )
Significance of Tumor Endothelial Cell Aneuploidy
An abnormal chromosome number, aneuploidy, is a common characteristic of tumor cells. In addition, it has been proposed for nearly 100 years that aneuploidy causes tumorigenesis. However, this remains unproven as there have been controversial reports that aneuploidy is merely a benign side‐effect of transformation or a contributor to tumor progression, but not to tumor initiation.( 33 ) Recently, Weaver et al. generated aneuploid cells and animals by reduction of centromere‐associated protein‐E. In their study, aneuploidy was shown to promote spontaneous tumorigenesis in aged animals, but at a modest frequency. However, an increased rate of aneuploidy was shown to inhibit tumorigenesis.( 34 )
To return to the subject of tumor endothelial cells, do aneuploid tumor endothelial cells have tumorigenicity? Melanoma and liposarcoma endothelial cells were plated in soft agar to monitor anchorage‐independent growth. However, these tumor endothelial cells did not form colonies in soft agar, whereas a mouse endothelial cell line (MS1) immortalized by an SV40 T antigen formed colonies in soft agar. When injected into nude mice subcutaneously, tumor endothelial cells did not form tumors in mice, whereas MS1 cells did form hemangioma in mice, consistent with a previous report( 35 ) (data not shown). These data are still preliminary and many further studies should be done before concluding that aneuploid tumor endothelial cells are transformed or tumorigenic.
In any case, the aneuploidy of tumor endothelial cells is significant. Tumor endothelial cells have been considered to be genetically normal, unlike tumor cells, for a long time. However, aneuploid tumor endothelial cells could be a different matter. Tumor endothelial cells might develop drug resistance like tumor cells, contrary to past beliefs. It has been shown previously that tumor endothelial cells in culture are more resistant to vincristine than normal endothelial cells.( 21 ) Our studies also showed tumor endothelial cells were more resistant to 5‐FU than normal endothelial cells (Muraki et al. unpublished data, 2005).
Some anti‐angiogenic drugs have been shown to lose their effectiveness over time, possibly due to acquired resistance. For example, as a mechanism of resistance to anti‐angiogenic therapy, Kerbel et al. suggested that survival factors such as cytokines or growth factors, which are rich in the tumor microenvironment, might cause epigenetic changes not only in tumor cells, but also in tumor endothelial cells.( 36 ) For example, bFGF was reported to inhibit apoptosis signal kinase 1 activity, inducing chemoresistance in HUVEC.( 37 )
Taken together, the possibility that aneuploid tumor endothelial cells are chemotherapy‐resistant (or sensitive to some drugs) warrants further investigation.
Possible Mechanisms of Tumor Aneuploidy
The mechanisms that result in tumor endothelial cell aneuploidy are not yet understood. Unraveling this mystery would be a significant breakthrough in understanding how endothelial cells or other tumor stroma cells become cytogenetically abnormal and unstable in the tumor microenvironment. This information would allow insights into how to block this process. Possible mechanisms include the following.
Tumor cell transdifferentiation In the case of hematopoietic tumors such as human B‐cell lymphomas and multiple myeloma, a common progenitor targeted by transformation can differentiate in tumor cells or endothelial cells( 29 , 31 ) (Fig. 5a). In addition, tumor cells might dedifferentiate to endothelial cells (Fig. 5b). This mechanism could be applied to neuroblastoma endothelial cells that harbor the same chromosome amplication as tumor cells.( 32 ) However, these are not likely in our cases that are solid tumors of non‐hematopoietic origin. In addition, in our cross‐species tumor model, the mouse origin of endothelial cells was shown relatively easily as the probe for FISH or multicolor FISH is mouse‐specific.
Figure 5.
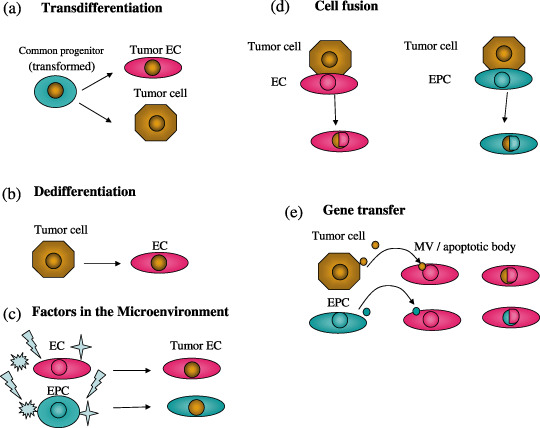
Possible mechanisms causing tumor endothelial cytogenetic abnormalities include the following. (a) Tumor cell transdifferentiation. In the case of hematopoietic tumors, a common progenitor targeted by transformation can differentiate in tumor cells or endothelial cells. (b) Dedifferentiation. Tumor cells might also dedifferentiate to endothelial cells. (c) Tumor microenvironment. Factors such as growth factors or cytokines in the tumor microenvironment could cause genetic instability. Hypoxia in tumors is known to cause genetic changes, for example, upregulation of survival factors, not only in tumor cells. Thus, the tumor microenvironment can induce genetic instability not only tumor cells, but also endothelial cells. (d) Cell fusion. Malignant tumor cells can fuse with normal endothelial cells or circulating endothelial progenitor cells (EPC). (e) Uptake of oncogene or gene transfer. Endothelial cells can uptake human tumor oncogenes released from EPC or tumor cells by phagocytosis of apoptotic bodies or microvesicles (MV).
Tumor microenvironment Factors such as growth factors or cytokines in the tumor microenvironment might cause genetic instability. For example, VEGF, bFGF, and EGFR ligands expressed in tumor and stromal cells are anti‐apoptotic survival factors that activate the survival signal transduction pathway and upregulate oncogene expression, causing genetic instability.( 37 , 38 ) In addition, the hypoxic condition in tumor tissue is known to cause genetic changes, for example, upregulation of survival factors, in tumor cells.( 39 ) Thus, the tumor microenvironment can induce genetic instability in not only tumor cells, but also endothelial cells (Fig. 5c).
Cell fusion Malignant tumor cells can fuse with normal endothelial cells or circulating endothelial progenitor cells (Fig. 5d). In a human breast carcinoma xenograft model in mice, the fibroblasts were aneuploid harboring both mouse and human chromosomes, suggesting these cells are fused with tumor cells.( 40 ) In the case of our cultured tumor endothelial cells, this is not applicable, as the mouse aneuploid tumor endothelial cells were not hybridized to a human genome probe for FISH (data not shown).
Uptake of oncogene or gene transfer Endothelial cells can uptake human tumor oncogenes by phagocytosis of apoptotic bodies.( 41 ) Furthermore, it has been suggested that genes can be transferred by tumor‐derived microvesicles (MV).( 42 ) Furthermore, it was reported that endothelial progenitor cells (EPC) released microvesicles that can activate endothelial cell angiogenic properties( 43 ) (Fig. 5e).
Stem cells Adult stem cells are reported to fuse with mature cells such as hepatocytes, and show aneuploidy.( 44 ) Tumor stem cells might receive signals in the tumor microenvironment, triggering transdifferentiation. Furthermore, it has been suggested that EPC, a subset of stem cells derived from bone marrow, can be incorporated into tumor blood vessels.( 45 , 46 , 47 ) In particular, EPC plays an important role in early neovascularization.( 48 ) It is possible that some of the aneuploid tumor endothelial cells are EPC‐derived. Our tumor endothelial cells overexpressed Sca‐1, a stem cell marker, suggesting that they are differentiated from bone marrow‐derived EPC at least in part. The mechanisms such as cell fusion or gene transfer described above might also be applicable in EPC. It is also possible that these EPC‐derived endothelial cells have been selected in culture and show an increased rate of aneuploidy.
Conclusion
As reviewed in this article, tumor endothelial cells are different from normal endothelial cells in gene profile and behavior, as well as the morphological changes described previously. Furthermore, the endothelial cells, even in non‐hematopoietic solid tumors, also have cytogenetic abnormalities, contrary to the assumption that endothelial cells in tumors are genetically stable and thus not drug‐resistant. It is speculated that drug‐resistance could possibly develop and compromise the effectiveness of anti‐angiogenesis therapies. Whatever mechanism underlies tumor endothelial abnormality, it is important to understand that even stroma cells can be abnormal in the tumor microenvironment. Recent studies suggest that both tumor cells and cells in the tumor microenvironment are a target for cancer therapy. Studies on tumor endothelial cell abnormalities will help to develop ideal anti‐angiogenic therapies and also to understand how tumor tissues are orchestrated by various cell types.
Acknowledgments
We thank our collaborators, Dr Klagsbrun for fruitful discussion, Dr Morton for discussion on cytogenetical analysis, Dr Munger for discussion on centrosome analysis, and Drs Shii, Tsuchiya, Muraki, Akino, Ohga, Matsuda, Kurosu, Onodera, and Fujie. This work was supported by grants‐in aid for scientific research from the Ministry of Education, Science, and Culture of Japan and The Haraguchi Memorial Foundation for Cancer Research.
References
- 1. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 1971; 285: 1182–6. [DOI] [PubMed] [Google Scholar]
- 2. Folkman J, Kerbel R. Role of angiogenesis in tumor growth and metastasis Clinical translation of angiogenesis inhibitors. Semin Oncol 2002; 29: 15–18. [DOI] [PubMed] [Google Scholar]
- 3. Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov 2007; 6: 273–86. [DOI] [PubMed] [Google Scholar]
- 4. Johnson DH, Fehrenbacher L, Novotny WF et al . Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non‐small‐cell lung cancer. J Clin Oncol 2004; 22: 2184–91. [DOI] [PubMed] [Google Scholar]
- 5. Keedy VL, Sandler AB. Inhibition of angiogenesis in the treatment of non‐small cell lung cancer. Cancer Sci 2007; 98: 1825–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kindler HL, Friberg G, Singh DA et al . Phase II trial of bevacizumab plus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol 2005; 23: 8033–40. [DOI] [PubMed] [Google Scholar]
- 7. Saif MW, Elfiky A, Salem RR. Gastrointestinal perforation due to bevacizumab in colorectal cancer. Ann Surg Oncol 2007; 14: 1860–9. [DOI] [PubMed] [Google Scholar]
- 8. McDonald DM, Choyke PL. Imaging of angiogenesis: from microscope to clinic. Nat Med 2003; 9: 713–25. [DOI] [PubMed] [Google Scholar]
- 9. Hashizume H, Baluk P, Morikawa S et al . Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol 2000; 156: 1363–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer 2003; 3: 422–33. [DOI] [PubMed] [Google Scholar]
- 11. Baluk P, Hashizume H, McDonald DM. Cellular abnormalities of blood vessels as targets in cancer. Curr Opin Genet Dev 2005; 15: 102–11. [DOI] [PubMed] [Google Scholar]
- 12. McDonald DM, Baluk P. Significance of blood vessel leakiness in cancer. Cancer Res 2002; 62: 5381–5. [PubMed] [Google Scholar]
- 13. Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 2005; 307: 58–62. [DOI] [PubMed] [Google Scholar]
- 14. St Croix B, Rago C, Velculescu V et al . Genes expressed in human tumor endothelium. Science 2000; 289: 1197–202. [DOI] [PubMed] [Google Scholar]
- 15. Carson‐Walter EB, Watkins DN, Nanda A, Vogelstein B, Kinzler KW, St Croix B. Cell surface tumor endothelial markers are conserved in mice and humans. Cancer Res 2001; 61: 6649–55. [PubMed] [Google Scholar]
- 16. Nanda A, St Croix B. Tumor endothelial markers: new targets for cancer therapy. Curr Opin Oncol 2004; 16: 44–9. [DOI] [PubMed] [Google Scholar]
- 17. Seaman S, Stevens J, Yang MY, Logsdon D, Graff‐Cherry C, St Croix B. Genes that distinguish physiological and pathological angiogenesis. Cancer Cell 2007; 11: 539–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Buckanovich RJ, Sasaroli D, O’Brien‐Jenkins A et al . Tumor vascular proteins as biomarkers in ovarian cancer. J Clin Oncol 2007; 25: 852–61. [DOI] [PubMed] [Google Scholar]
- 19. Lu C, Bonome T, Li Y et al . Gene alterations identified by expression profiling in tumor‐associated endothelial cells from invasive ovarian carcinoma. Cancer Res 2007; 67: 1757–68. [DOI] [PubMed] [Google Scholar]
- 20. Van Beijnum JR, Dings RP, Van Der Linden E et al . Gene expression of tumor angiogenesis dissected: specific targeting of colon cancer angiogenic vasculature. Blood 2006; 108: 2339–48. [DOI] [PubMed] [Google Scholar]
- 21. Bussolati B, Deambrosis I, Russo S, Deregibus MC, Camussi G. Altered angiogenesis and survival in human tumor‐derived endothelial cells. FASEB J 2003; 17: 1159–61. [DOI] [PubMed] [Google Scholar]
- 22. Allport JR, Weissleder R. Murine Lewis lung carcinoma‐derived endothelium expresses markers of endothelial activation and requires tumor‐specific extracellular matrix in vitro . Neoplasia 2003; 5: 205–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hida K, Klagsbrun M. A new perspective on tumor endothelial cells: unexpected chromosome and centrosome abnormalities. Cancer Res 2005; 65: 2507–10. [DOI] [PubMed] [Google Scholar]
- 24. Hida K, Hida Y, Amin DN et al . Tumor‐associated endothelial cells with cytogenetic abnormalities. Cancer Res 2004; 64: 8249–55. [DOI] [PubMed] [Google Scholar]
- 25. Arbiser JL, Raab G, Rohan RM et al . Isolation of mouse stromal cells associated with a human tumor using differential diphtheria toxin sensitivity. Am J Pathol 1999; 155: 723–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Amin DN, Hida K, Bielenberg DR, Klagsbrun M. Tumor endothelial cells express epidermal growth factor receptor (EGFR) but not ErbB3 and are responsive to EGF and to EGFR kinase inhibitors. Cancer Res 2006; 66: 2173–80. [DOI] [PubMed] [Google Scholar]
- 27. Duensing S, Munger K. Human papillomaviruses and centrosome duplication errors: modeling the origins of genomic instability. Oncogene 2002; 21: 6241–8. [DOI] [PubMed] [Google Scholar]
- 28. Storchova Z, Pellman D. From polyploidy to aneuploidy, genome instability and cancer. Nat Rev Mol Cell Biol 2004; 5: 45–54. [DOI] [PubMed] [Google Scholar]
- 29. Gunsilius E, Duba HC, Petzer AL et al . Evidence from a leukaemia model for maintenance of vascular endothelium by bone‐marrow‐derived endothelial cells. Lancet 2000; 355: 1688–91. [DOI] [PubMed] [Google Scholar]
- 30. Streubel B, Chott A, Huber D et al . Lymphoma‐specific genetic aberrations in microvascular endothelial cells in B‐cell lymphomas. N Engl J Med 2004; 351: 250–9. [DOI] [PubMed] [Google Scholar]
- 31. Rigolin GM, Fraulini C, Ciccone M et al . Neoplastic circulating endothelial cells in multiple myeloma with 13q14 deletion. Blood 2006; 107: 2531–5. [DOI] [PubMed] [Google Scholar]
- 32. Pezzolo A, Parodi F, Corrias MV, Cinti R, Gambini C, Pistoia V. Tumor origin of endothelial cells in human neuroblastoma. J Clin Oncol 2007; 25: 376–83. [DOI] [PubMed] [Google Scholar]
- 33. Marx J. Debate surges over the origins of genomic defects in cancer. Science 2002; 297: 544–6. [DOI] [PubMed] [Google Scholar]
- 34. Weaver BA, Silk AD, Montagna C, Verdier‐Pinard P, Cleveland DW. Aneuploidy acts both oncogenically and as a tumor suppressor. Cancer Cell 2007; 11: 25–36. [DOI] [PubMed] [Google Scholar]
- 35. Arbiser JL, Larsson H, Claesson‐Welsh L et al . Overexpression of VEGF 121 in immortalized endothelial cells causes conversion to slowly growing angiosarcoma and high level expression of the VEGF receptors VEGFR‐1 and VEGFR‐2 in vivo . Am J Pathol 2000; 156: 1469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kerbel RS, Yu J, Tran J et al . Possible mechanisms of acquired resistance to anti‐angiogenic drugs: implications for the use of combination therapy approaches. Cancer Metastasis Rev 2001; 20: 79–86. [DOI] [PubMed] [Google Scholar]
- 37. Alavi AS, Acevedo L, Min W, Cheresh DA. Chemoresistance of endothelial cells induced by basic fibroblast growth factor depends on Raf‐1‐mediated inhibition of the proapoptotic kinase, ASK1. Cancer Res 2007; 67: 2766–72. [DOI] [PubMed] [Google Scholar]
- 38. Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med 2004; 10: 789–99. [DOI] [PubMed] [Google Scholar]
- 39. Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature 2005; 438: 967–74. [DOI] [PubMed] [Google Scholar]
- 40. Jacobsen BM, Harrell JC, Jedlicka P, Borges VF, Varella‐Garcia M, Horwitz KB. Spontaneous fusion with, and transformation of mouse stroma by, malignant human breast cancer epithelium. Cancer Res 2006; 66: 8274–9. [DOI] [PubMed] [Google Scholar]
- 41. Bergsmedh A, Szeles A, Henriksson M et al . Horizontal transfer of oncogenes by uptake of apoptotic bodies. Proc Natl Acad Sci USA 2001; 98: 6407–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Baj‐Krzyworzeka M, Szatanek R, Weglarczyk K et al . Tumour‐derived microvesicles carry several surface determinants and mRNA of tumour cells and transfer some of these determinants to monocytes. Cancer Immunol Immunother 2006; 55: 808–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Deregibus MC, Cantaluppi V, Calogero R et al . Endothelial progenitor cell‐derived microvesicles activate an angiogenic program in endothelial cells by an horizontal transfer of mRNA. Blood 2007; 110: 2440–8. [DOI] [PubMed] [Google Scholar]
- 44. Wang X, Willenbring H, Akkari Y et al . Cell fusion is the principal source of bone‐marrow‐derived hepatocytes. Nature 2003; 422: 897–901. [DOI] [PubMed] [Google Scholar]
- 45. Duda DG, Cohen KS, Kozin SV et al . Evidence for incorporation of bone marrow‐derived endothelial cells into perfused blood vessels in tumors. Blood 2006; 107: 2774–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Takakura N. Role of hematopoietic lineage cells as accessory components in blood vessel formation. Cancer Sci 2006; 97: 568–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bertolini F, Shaked Y, Mancuso P, Kerbel RS. The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nat Rev Cancer 2006; 6: 835–45. [DOI] [PubMed] [Google Scholar]
- 48. Nolan DJ, Ciarrocchi A, Mellick AS et al . Bone marrow‐derived endothelial progenitor cells are a major determinant of nascent tumor neovascularization. Genes Dev 2007; 21: 1546–58. [DOI] [PMC free article] [PubMed] [Google Scholar]


