Abstract
Melanosis is frequently observed in the upper aerodigestive tract of Japanese alcoholic men, and the prevalences of squamous cell dysplasia and SCC in the upper aerodigestive tract of Japanese alcoholic men are high. This study evaluated associations between melanosis and both neoplasms of the upper aerodigestive tract and factors contributing to the development of melanosis in Japanese alcoholic men. Endoscopic screening of 643 Japanese alcoholic men (aged 50–79 years) was combined with oropharyngolaryngeal inspection and esophageal iodine staining, and ALDH2 genotyping was carried out in 425 of them. Melanosis was frequently (20.8%) observed in the upper aerodigestive tract. The palate was the most common site of melanosis (11.2%), followed by the pharynx (9.5%), and by the esophagus (7.0%). The incidence of melanosis was higher in those with esophageal dysplasia (31/126, 24.6%), esophageal SCC (19/42, 45.2%), and oropharyngolaryngeal SCC (8/14, 54.1%) than in cancer‐ and dysplasia‐free controls (69/437, 15.8%). The presence of melanosis was associated with a higher risk of esophageal dysplasia, esophageal SCC, and oropharyngolaryngeal SCC (OR 1.69, 4.03, and 6.61, respectively). Multivariate analysis showed that older age, heavier smoking, and heterozygosity for inactive ALDH2 were positively associated with the presence of melanosis. The presence of melanosis indicates a high risk for neoplasms in the upper aerodigestive tract of Japanese alcoholic men. Melanosis and neoplasms have the same causes, including older age, heavy smoking, and high acetaldehyde exposure. (Cancer Sci 2006; 97: 905–911)
Abbreviations:
- ALDH2
aldehyde dehydrogenase‐2
- CI
confidence interval
- DIUL
distinct iodine‐unstained lesion, OR, odds ratio
- SCC
squamous cell carcinoma.
Melanosis in the upper aerodigestive tract is characterized by flat, dark‐pigmented (greenish‐black) areas and can be detected endoscopically. Endoscopic screening by esophageal iodine staining at the National Hospital Organization Kurihama Alcoholism Center (Yokosuka, Japan)( 1 ) has recently demonstrated esophageal melanosis in many Japanese alcoholic men,( 2 ) and those with esophageal melanosis have been found to be at higher risk of non‐cancerous DIULs, mainly squamous cell dysplasia, and SCC in the upper aerodigestive tract.( 2 ) That finding is consistent with the results of an earlier pathological study that found high frequency and density of melanocytes in surgical specimens of Japanese esophageal carcinoma patients.( 3 )
ALDH2 is a key enzyme in eliminating the acetaldehyde generated by alcohol metabolism.( 4 ) However, when the mutant allele ALDH2*2, which is prevalent in East Asians, is present, the enzyme is inactive, leading to excessive accumulation of acetaldehyde. Alcoholics who are heterozygous for inactive ALDH2*2/*1 are at much higher risk of DIUL( 2 ) and SCC of the esophagus and oropharyngolarynx,( 5 , 6 ) and at higher risk for esophageal melanosis.( 2 ) Thus, esophageal melanosis and squamous neoplasms in the upper aerodigestive tract have a common cause, exposure to high acetaldehyde levels.
We have also noted frequent concomitant melanosis of the palate and pharynx in alcoholics with esophageal melanosis. Intrigued by the esophageal melanosis‐related risk of neoplasms, since 2003 we have been paying particular attention to the detection of melanosis in the palate and pharynx as well as in the esophagus. This study was designed to evaluate the association between melanosis of the above areas and dysplasia or SCC in the upper aerodigestive tract, and to attempt to identify factors, including inactive ALDH2*1/*2, that might contribute to the development of melanosis in these areas in Japanese alcoholic men.
Materials and Methods
At the Kurihama Alcoholism Center we routinely use a regimented cancer‐screening program consisting of endoscopy combined with oropharyngolaryngeal inspection and esophageal iodine staining in male alcoholic patients. More than 90% of the patients fully informed of the purpose of the screening have not refused the examination. The screening program and diagnostic procedure used in the present study have been described in our previous report.( 1 ) The proposal for this study was reviewed by the ethics committee of the Center (approval document number 11–5), and written informed consent was obtained from all participating patients.
Patients
The subjects were 643 Japanese alcoholic men (aged 50–79 years) who underwent cancer screening at the Center for the first time between July 2003 and April 2005, and who had no history of cancer of the oropharyngolarynx, esophagus, or stomach. Information regarding the patients’ drinking and smoking habits was obtained from the patients themselves and, when available, their partners. Daily alcohol consumption during the preceding year was expressed in grams of ethanol per day by using a standard conversion for alcoholic beverages. Beer was assumed to be 5% ethanol (v/v); wine, 12%; sake, 16%; shochu, 25%; and whiskey, 40%. All of the patients met the criteria for alcohol dependence as defined in the third revised edition of the Diagnostic and Statistical Manual of Mental Disorders.( 7 )
Endoscopic assessment of melanosis
Endoscopy was carried out with an Olympus Q240, Q240Z, or XQ230 panendoscope (Olympus Optical, Tokyo, Japan). First we asked the patients to open their mouth wide and examined the palate, especially the lateral areas of the transition zone between the soft and hard palate with the endoscope. After insertion of the endoscope into the pharynx, the hypopharynx and the posterior wall of the oropharynx were carefully examined while secretions were removed by suction. After insertion of the endoscope past the upper esophageal sphincter, the esophageal mucosa was flushed with 40 mL of water through the biopsy port. During the first passage of the esophagus, particular attention was given to detect melanosis. We used the term “melanosis” to describe the flat, dark‐pigmented (greenish‐black) lesions (1, 2, 3).
Figure 1.
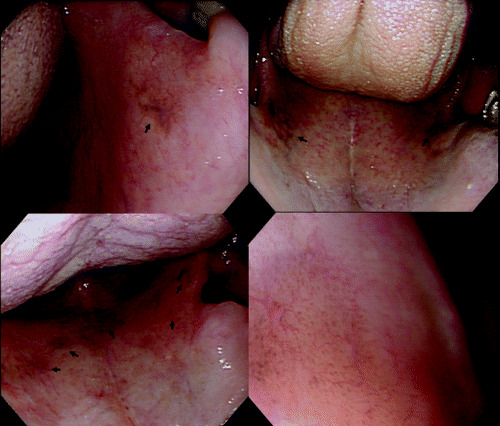
Endoscopic views of melanosis of the palate showing flat, dark‐pigmented (greenish‐black) areas (arrows). The most common site of palatal melanosis is the lateral transition zone between the soft and hard palates. Magnifying endoscopy shows a cluster of minute pigmented granules (right, bottom, original magnification ×80).
Figure 2.
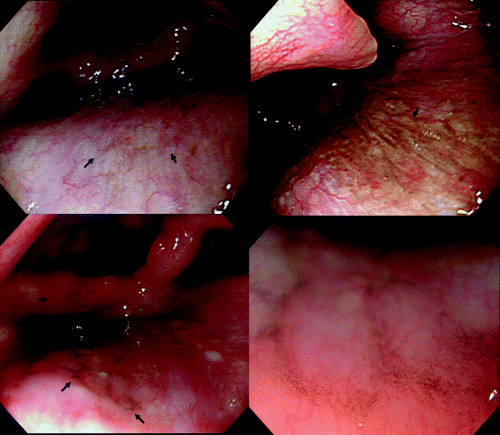
Endoscopic views of melanosis of the pharynx showing flat, dark‐pigmented (greenish‐black) areas (arrows). Magnifying endoscopy shows a cluster of minute pigmented granules (right, bottom, original magnification ×80).
Figure 3.
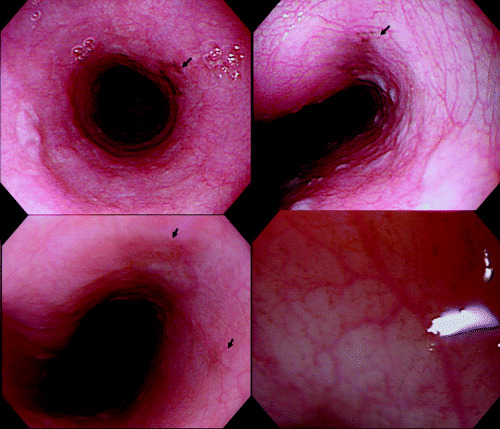
Endoscopic views of melanosis of the esophagus showing flat, dark‐pigmented (greenish‐black) areas (arrows). Magnifying endoscopy shows a cluster of minute pigmented granules (right, bottom, original magnification ×80).
Histological diagnosis of neoplasms
Mucosal biopsy specimens were taken from DIUL with diameters measuring 5 mm or more. Each specimen was embedded in paraffin, and five deep serial sections were obtained for staining with hematoxylin–eosin. All the sections were viewed by one of the authors (T.H.), who is an expert pathologist in the field of neoplasia of the gastrointestinal tract. Oropharyngolaryngeal and esophageal SCC were histologically confirmed also in specimens resected by endoscopic mucosectomy( 1 , 8 ) or radical surgery.
ALDH2 genotyping
Before the endoscopic screening, we systematically genotyped the ALDH2 of the 405 patients who were admitted to the Center's detoxification unit on Tuesdays or Thursdays. For the other 20 patients with SCC in the upper aerodigestive tract, genotyping were added after the SCC diagnosis. We genotyped ALDH2 in lymphocyte DNA samples of the patients’ blood by the polymerase chain reaction–restriction fragment length polymorphism method of Harada and Zhang( 9 ) with slight modifications.( 5 )
Statistical analysis
We used the screened patients who had no DIUL or cancer as a control group and calculated the risk of esophageal dysplasia, esophageal SCC, oropharyngolaryngeal SCC, and upper aerodigestive tract SCC associated with the presence of melanosis and other factors with a multiple logistic regression model, and expressed the risks as the OR and 95% CI. The relationships between the presence of melanosis and other factors were also analyzed using a multiple logistic regression model. Allele frequency was determined by direct counting. We used the exact test to analyze for deviations of the genotype distribution from the Hardy–Weinberg equilibrium and Fisher's exact test to compare group statistics. All analyses were carried out using Statistical Analysis System software (version 8.2; SAS Institute, Cary, NC, USA).
Results
Screening of the 643 patients resulted in the diagnosis of 200 cases of esophageal DIUL with diameters of at least 5 mm. Biopsy specimens were taken from 173 patients, and they were diagnosed as SCC in 42 patients, dysplasia in 126, and non‐neoplastic lesions in 5. Biopsy was not done in 27 patients with DIUL measuring 5–8 mm that were purely flat, and in whom there were no other findings suggestive of SCC. The main reasons for not taking a biopsy were the presence of bleeding tendency and/or liver cirrhosis. Fourteen patients had oropharyngolaryngeal SCC (hypopharyngeal in 11, tongue in 2, and oropharyngeal SCC in 1), and 11 patients had gastric adenocarcinoma. Ten patients had synchronous primary cancers in two organs (esophageal and oropharyngolaryngeal in 7, oropharyngolaryngeal and gastric in 2, and esophageal and gastric cancers in 1). The 437 patients without DIUL or cancer served as a control group.
Esophageal SCC was confined to the epithelium in 19 patients and had invaded the proper mucosal layer in 15, the submucosa in 4, and the proper muscle layer or deeper in 4. Oropharyngolaryngeal SCC was limited to the epithelium in 11 patients and to the subepithelium in 2, but it extended to the proper muscle layer or deeper in 1.
Melanosis of the palate was observed in 72 of the 643 patients (11.2%). Almost all the patients with palatal melanosis had a pigmented area in at least one lateral area of the transition zone between the soft and hard palates (Fig. 1). Melanosis was detected in the hypopharynx or posterior wall of the oropharynx in 61 patients (9.5%) (Fig. 2), and in the esophagus in 45 (7.0%) (Fig. 3). One hundred and thirty four patients (20.8%) had melanosis of either the palate, pharynx, or esophagus, and 38 (5.9%) patients had melanosis at two or three of these sites. Magnifying endoscopy revealed that the melanosis was a cluster of minute pigmented granules in all three organs.
We observed more smoking among the patients with SCC in the esophagus or upper aerodigestive tract than among the controls (Table 1). The frequency of inactive ALDH2*1/*2 was markedly higher in SCC patients than in the controls.
Table 1.
Basic characteristics of 643 Japanese alcoholic men who participated in this study
| Esophageal dysplasia (n = 126) | Esophageal SCC (n = 42) | Oropharyngo‐ laryngeal SCC (n = 14) | Esophageal/ oropharyngo‐ laryngeal SCC (n = 49) | Controls(n = 437) | |
|---|---|---|---|---|---|
| Age (years) | |||||
| 50–59 | 44% | 43% | 71% | 47% | 53% |
| 60–69 | 41% | 43% | 21% | 41% | 37% |
| 70–79 | 15% | 14% | 7% | 12% | 9% |
| P, vs. controls † | 0.073 | 0.34 | 0.42 | 0.58 | – |
| Alcohol intake (drinks/day) ‡ | |||||
| <6 | 21% | 26% | 7% | 22% | 16% |
| 6–8.9 | 31% | 43% | 71% | 47% | 30% |
| 9–11.9 | 22% | 17% | 7% | 14% | 28% |
| 12 + | 26% | 14% | 14% | 16% | 25% |
| P, vs. controls § | 0.59 | 0.068 | 0.016 | 0.027 | – |
| Smoking (cigs/day) | |||||
| <20 | 39% | 19% | 21% | 16% | 44% |
| 20–29 | 33% | 52% | 50% | 51% | 35% |
| 30 + | 29% | 29% | 29% | 33% | 22% |
| P, vs. controls § | 0.078 | 0.003 | 0.34 | 0.0004 | – |
| ALDH2genotype | (n = 86) †† | (n = 42) | (n = 14) | (n = 49) | (n = 273) †† |
| *1/*1 | 83% | 57% | 50% | 57% | 91% |
| *1/*2 | 17% | 43% | 50% | 43% | 9% |
| *2/*2 | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| P, vs. controls § | 0.072 | <0.0001 | < 0.0001 | < 0.0001 | – |
| P for HWE ¶ | 1.0 | 0.16 | 0.51 | 0.091 | 1.0 |
Fisher's exact test;
1 drink = 12 g ethanol;
Cochran–Mantel–Haenszel test for homogeneity adjusted for age;
Exact P‐value for deviation from the Hardy–Weinberg equilibrium (HWE);
Genotype data were available only for those admitted to the Center on Tuesday or Thursday. –, not applicable.
Melanosis lesions in the palate, pharynx, esophagus, and at any of the sites were more frequently observed in patients with esophageal SCC, oropharyngolaryngeal SCC, and upper aerodigestive tract SCC than in the control patients (Table 2). After adjustment for age and daily alcohol and cigarette consumption, the analysis showed that the presence of melanosis in the palate, pharynx, esophagus, and any one of them was associated with significantly higher risk of esophageal SCC (OR = 2.62, 3.49, 7.50, and 4.03, respectively), oropharyngolaryngeal SCC (OR = 5.96, 5.25, 11.67, and 6.61, respectively) and upper aerodigestive tract SCC (OR = 3.19, 3.94, 7.45, and 4.71, respectively).
Table 2.
Risk of esophageal dysplasia and SCC in the esophagus, oropharyngolarynx, and upper aerodigestive tract according to the presence of melanosis in Japanese alcoholic men
| Disease | Prevalence of melanosis | P, vs controls † | OR ‡ | 95% CI | ||
|---|---|---|---|---|---|---|
| n, total | n | % | ||||
| In the palate | ||||||
| Esophageal dysplasia | 126 | 15 | 11.9% | 0.30 | 1.44 | 0.75–2.75 |
| Esophageal SCC | 42 | 9 | 21.4% | 0.0104 | 2.62 | 1.11–6.18 |
| Oropharyngolaryngeal SCC | 14 | 5 | 35.7% | 0.0010 | 5.96 | 1.25–47.21 |
| Esophageal/oropharyngolaryngeal SCC | 49 | 12 | 24.5% | 0.0006 | 3.19 | 1.46–6.98 |
| Controls | 437 | 38 | 8.7% | |||
| In the pharynx | ||||||
| Esophageal dysplasia | 126 | 10 | 7.9% | 0.94 | 0.98 | 0.46–2.08 |
| Esophageal SCC | 42 | 10 | 23.8% | 0.0005 | 3.49 | 1.49–8.14 |
| Oropharyngolaryngeal SCC | 14 | 5 | 35.7% | 0.0003 | 5.25 | 1.20–29.04 |
| Esophageal/oropharyngolaryngeal SCC | 49 | 13 | 26.5% | <0.0001 | 3.94 | 1.81–8.60 |
| Controls | 437 | 33 | 7.6% | |||
| In the esophagus | ||||||
| Esophageal dysplasia | 126 | 13 | 10.3% | 0.013 | 2.52 | 1.17–5.43 |
| Esophageal SCC | 42 | 12 | 28.6% | <0.0001 | 7.50 | 3.04–18.48 |
| Oropharyngolaryngeal SCC | 14 | 4 | 28.6% | <0.0001 | 11.67 | 1.88–108.40 |
| Esophageal/oropharyngolaryngeal SCC | 49 | 14 | 28.6% | <0.0001 | 7.45 | 3.15–17.58 |
| Controls | 437 | 18 | 4.1% | |||
| At any of the above sites | ||||||
| Esophageal dysplasia | 126 | 31 | 24.6% | 0.030 | 1.69 | 1.03–2.77 |
| Esophageal SCC | 42 | 19 | 45.2% | <0.0001 | 4.03 | 2.00–8.12 |
| Oropharyngolaryngeal SCC | 14 | 8 | 57.1% | <0.0001 | 6.61 | 1.83–40.54 |
| Esophageal/oropharyngolaryngeal SCC | 49 | 24 | 49.0% | <0.0001 | 4.71 | 2.44–9.09 |
| Controls | 437 | 69 | 15.8% | |||
Cochran–Mantel–Haenszel test adjusted for age;
OR of melanosis (present vs absent) for each of esophageal dysplasia and cancers, adjusted for age, smoking, and alcohol drinking by a logistic regression analysis. An exact logistic regression analysis was carried out for oropharyngolaryngeal SCC.
Melanosis lesions in the esophagus and at any of the sites was more frequently observed in the patients with esophageal dysplasia than in the controls, and they were associated with a significantly higher risk of esophageal dysplasia (OR = 2.52 and 1.69, respectively).
Multivariate analysis showed that the inactive ALDH2*1/*2 genotype, older age, and heavier smoking were positively associated with the presence of melanosis (Table 3).
Table 3.
Multivariate relationships between melanosis and selected factors in Japanese alcoholic men
| Risk factors of melanosis Categories | Site of melanosis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n, total | In the palate | In the pharynx | In the esophagus | In any site | |||||||||
| n | OR | 95% CI | n | OR | 95% CI | n | OR | 95% CI | n | OR | 95% CI | ||
| ALDH2 genotype † | |||||||||||||
| *1/*1 | 347 | 37 | 1.00 | Referent | 33 | 1.00 | Referent | 17 | 1.00 | Referent | 66 | 1.00 | Referent |
| *1/*2 | 58 | 13 | 2.64 | 1.25–5.60 | 12 | 1.70 | 0.72–3.99 | 16 | 5.82 | 2.43–13.95 | 29 | 3.46 | 1.84–6.49 |
| Age (years) | |||||||||||||
| 50–59 | 222 | 25 | 1.00 | Referent | 22 | 1.00 | Referent | 13 | 1.00 | Referent | 47 | 1.00 | Referent |
| 60–69 | 148 | 17 | 1.25 | 1.03–5.60 | 19 | 1.56 | 0.76–3.17 | 15 | 1.53 | 0.61–3.86 | 34 | 1.16 | 0.67–2.01 |
| 70–79 | 35 | 8 | 3.06 | 1.05–8.95 | 4 | 1.41 | 0.35–5.65 | 5 | 3.67 | 0.85–15.95 | 14 | 3.06 | 1.26–7.43 |
| Alcohol intake (drinks/day) | |||||||||||||
| <6 | 65 | 6 | 1.00 | Referent | 6 | 1.00 | Referent | 8 | 1.00 | Referent | 17 | 1.00 | Referent |
| 6–8.9 | 132 | 20 | 2.49 | 0.82–7.58 | 19 | 1.42 | 0.50–4.00 | 10 | 0.59 | 0.17–2.04 | 32 | 1.06 | 0.49–2.29 |
| 9–11.9 | 101 | 11 | 1.85 | 0.57–6.01 | 9 | 0.71 | 0.22–2.32 | 7 | 0.63 | 0.18–2.23 | 18 | 0.74 | 0.32–1.71 |
| 12 + | 107 | 13 | 2.23 | 0.69–7.24 | 11 | 1.11 | 0.37–3.35 | 8 | 0.55 | 0.14–2.10 | 28 | 1.42 | 0.64–3.15 |
| Smoking (cigs/day) | |||||||||||||
| <20 | 156 | 15 | 1.00 | Referent | 10 | 1.00 | Referent | 5 | 1.00 | Referent | 25 | 1.00 | Referent |
| 20–29 | 147 | 19 | 1.74 | 0.81–3.74 | 21 | 2.31 | 1.01–5.32 | 14 | 2.95 | 0.89–9.84 | 40 | 2.17 | 1.18–4.00 |
| 30 + | 102 | 16 | 1.69 | 0.71–4.01 | 14 | 2.39 | 0.94–6.07 | 14 | 8.41 | 2.40–29.52 | 30 | 2.59 | 1.31–5.11 |
OR were estimated by simultaneously entering all the variables into a multiple logistic regression model. †Genotype data were available only for 405 subjects who were admitted to the Center on Tuesday or Thursday.
Discussion
Melanosis was frequently (20.8%) observed in the upper aerodigestive tract of Japanese alcoholic men in this study. The palate, especially the lateral transition zone between the soft and hard palates, was the most common site of melanosis (11.2%), followed by the pharynx (9.5%) and the esophagus (7.0%). This study demonstrated a strong association between the presence of melanosis and increased risk of SCC of the upper aerodigestive tract. Although the magnitude of the increase in risk was not very high, the presence of melanosis was also related to a higher risk of esophageal dysplasia. These findings seem to support the idea that melanosis and neoplasms of the entire upper aerodigestive tract have the same causes, including alcoholism.
The present study indicates that melanosis in the upper aerodigestive tract is added to the list of high‐risk biomarkers for neoplasms. The palate can be examined for melanosis by visual inspection during routine medical or dental examinations, or even by the patient. Pharyngeal melanosis might also be detected during laryngological examinations. Higher sensitivity is crucial for a biomarker identifying susceptible individuals. When melanosis at all three sites was combined, melanosis was observed in 24.6% of patients with esophageal dysplasia, 45.2% of patients with esophageal SCC, 57.1% of patients with oropharyngeal SCC, and 49.0% of patients with upper aerodigestive tract SCC. The proportion of neoplasm‐free controls who were melanosis‐free (specificity) was 85.2%.
This study confirmed associations between inactive heterozygous ALDH2 and risk of both melanosis and SCC in the upper aerodigestive tract.( 5 , 6 ) The carcinogenicity of acetaldehyde has been adequately demonstrated in experimental animals.( 10 ) Acetaldehyde has been found to interact covalently with DNA to form DNA adducts in human, and their levels have been reported to be much higher in alcoholics than in nonalcoholics.( 11 ) These results lend support to the concept that acetaldehyde plays an important role in the development of melanosis and neoplasms in the upper aerodigestive tract.
The upper aerodigestive tract can be exposed to high local levels of acetaldehyde through several pathways. Ethanol is metabolized by mucosal enzymes, including strong expression of high Km alcohol dehydrogenase‐4 (previously called ADH7) and extremely weak expression of ALDH2.( 12 , 13 ) Chronic alcohol consumption leads to induction of cytochrome P‐4502E1, which metabolizes ethanol to acetaldehyde.( 4 ) Ethanol‐induced P4502E1 has been demonstrated in the oropharyngeal mucosa of alcoholics with cancer.( 14 ) Alcoholic beverages themselves contain high levels of acetaldehyde.( 15 ) After ethanol ingestion by healthy volunteers, their salivary acetaldehyde levels rise to 10–20 times higher than their blood acetaldehyde levels.( 16 ) The salivary acetaldehyde levels of heterozygotes for inactive ALDH2 are 2–3 times higher than those of homozygotes for active ALDH2.( 17 ) Normal oral microflora also produce acetaldehyde from ethanol and contribute to the acetaldehyde levels in saliva.( 16 )
Exposure to high local levels of acetaldehyde might be involved in the etiology of the epithelial hyperproliferation in the upper aerodigestive tract that has been demonstrated in experimental animals.( 18 ) Proliferating keratinocytes might play a regulatory role in melanogenesis through paracrine effects.( 19 ) Acetaldehyde‐induced epithelial proliferation might be at least in part related to increased melanogenesis throughout the upper aerodigestive tract. Histological examination of melanosis in the upper aerodigestive tract has shown that melanin is produced by proliferating melanocytes located in the basal layer of the epithelium and transferred via dendrites to surrounding keratinocytes and to macrophages and fibroblasts in the mucosal layer.( 2 , 20 , 21 ) The mechanisms of the influence of acetaldehyde on each step of melanogenesis in the mucosa are new foci of future research.
Smoking precipitates oral pigmentation, and studies in Western and Asian populations have shown that ‘smoker's melanosis’ occurs in about 20% of smokers.( 22 ) It tends to increase with the level of tobacco use,( 23 ) and many alcoholic patients are heavy smokers. Alcohol drinking and tobacco smoking synergistically enhance the risk of SCC in the upper aerodigestive tract,( 24 ) and the results of the present study revealed that heavy smoking is also positively associated with ‘alcoholic's melanosis’. The melanosis might act as a protective barrier against noxious components in tobacco smoke, either through stimulation of melanin production or melanin binding to the toxic products, including polycyclic amines and free radicals.( 25 ) Acetaldehyde is one of the major chemical constituents of tobacco smoke,( 26 ) and salivary acetaldehyde levels markedly increase during active smoking.( 27 ) In contrast to the oral cavity and pharynx, the esophagus is not directly exposed to tobacco smoke. Saliva, however, is transported from the mouth to the esophagus, and alcohol acts as an efficient solvent and enhances the penetration of the components of inhaled tobacco smoke through the entire mucosa.( 28 )
Other lifestyle or hereditary factors that contribute to the development of neoplasms might be associated with the development of melanosis. Chronic alcohol consumption and metabolism result in the generation of several classes of DNA‐damaging molecules, including reactive oxygen species and lipid peroxidation products.( 25 ) Melanosis in the upper aerodigestive tract might function as an antioxidant defense, because melanogenesis in the epidermis is involved in neutralizing reactive oxygen species and prevents damage to cell lipids, proteins, and DNA.( 29 )
Among several potential limitations of our study is the fact that the melanosis was diagnosed on the basis of the endoscopic findings alone and lacked histological confirmation. Nevertheless, the conventional and magnifying endoscopic features of melanosis in the palate, pharynx, and esophagus were very similar, and our previous study showed that all biopsy specimens from 15 consecutive cases of endoscopically diagnosed melanosis were confirmed histologically,( 2 ) suggesting the reliability of our diagnostic procedure. The prevalence of esophageal melanosis in our control patients was much higher than previously reported in Japanese general populations,( 21 , 30 ) and it was double the prevalence in our previous study of alcoholics.( 2 ) In addition to the difference in populations, improvement in endoscopes must have contributed to the high detection rate of melanosis. The Olympus Q240 and Q240Z panendoscopes mainly used in this study provided much clearer images than the Olympus P20 model used in previous Japanese studies of nonalcoholics,( 21 , 30 ) and sharper images than obtained with the Olympus XQ220 and XQ230 models mainly used in our earlier study.( 2 )
Because this was a cross‐sectional study, it is difficult to show the time sequence of emergence of melanosis and neoplasms. Melanosis might develop earlier than neoplasms, or vice versa. Follow‐up studies are important to clarify whether or to what extent the presence of melanosis influences future cancer development.
Whether the present findings can be extended to the Japanese general population is another important question, because of their potential role in early detection and prevention of a high mortality cancer in the upper aerodigestive tract. There are striking differences in the prevalence of melanosis between ethnic groups. There have been few case reports of esophageal melanosis as a distinct clinical entity detected by endoscopy in Western nations.( 31 , 32 ) Oral melanosis is much more common among Indians, Thais, and Malaysians than in Japanese or Europeans.( 22 ) The extent to which the present findings are applicable must be investigated by additional studies in various populations.
The impact of melanosis on the risk of neoplasms has an intriguing implication. Esophageal dysplasia is seldom diagnosed without esophageal iodine staining, and more than half of the cases of intraepithelial or mucosal SCC in the esophagus would be missed without it.( 2 ) Detection of superficial SCC in the oropharyngolarynx requires careful endoscopic inspection. Treatment of early esophageal SCC by endoscopic mucosectomy has become a widespread practice in Japan,( 1 , 33 ) and has succeeded in improving the outcome of this high mortality cancer.( 33 ) Application of endoscopic mucosectomy has been extended to SCC of the oropharyngolarynx,( 8 ) and melanosis in the upper aerodigestive tract could be useful for identifying patients at high risk for the neoplasms in these regions. The presence of melanosis might not only encourage endoscopists to use esophageal iodine staining and to examine the oropharyngolaryngeal sites carefully, but also help persuade high risk individuals to change their lifestyle choices to avoid or prevent the neoplasms.
Acknowledgments
We thank Yumi Kitagawa (Kurihama Alcoholism Center) for her expert technical assistance. This work was supported by a Grant‐in‐Aid for Cancer Research from the Ministry of Health, Labor and Welfare of Japan.
References
- 1. Yokoyama A, Ohmori T, Makuuchi H et al. Successful screening for early esophageal cancer in alcoholics using endoscopy and mucosa iodine staining. Cancer 1995; 76: 928–34. [DOI] [PubMed] [Google Scholar]
- 2. Yokoyama A, Omori T, Yokoyama T et al. Esophageal melanosis, an endoscopic finding associated with squamous cell neoplasms of the upper aerodigestive tract, and inactive aldehyde dehydrogenase‐2 in alcoholic Japanese men. J Gastroenterol 2005; 40: 676–84. [DOI] [PubMed] [Google Scholar]
- 3. Ohashi K, Kato Y, Kanno J, Kasuga T. Melanocytes and melanosis of the esophagus in Japanese subjects – analysis of factors affecting their increase. Virchows Arch A Path Anat Histol 1990; 417: 137–43. [DOI] [PubMed] [Google Scholar]
- 4. Lieber CS. Metabolism of alcohol. Clin Liver Dis 2005; 9: 1–35. [DOI] [PubMed] [Google Scholar]
- 5. Yokoyama A, Muramatsu T, Omori T et al. Alcohol and aldehyde dehydrogenase gene polymorphisms and oropharyngolaryngeal, esophageal and stomach cancers in Japanese alcoholics. Carcinogenesis 2001; 22: 433–9. [DOI] [PubMed] [Google Scholar]
- 6. Yokoyama A, Omori T. Genetic polymorphisms of alcohol and aldehyde dehydrogenases and risk for esophageal and head and neck cancers. Jpn J Clin Oncol 2003; 33: 111–21. [DOI] [PubMed] [Google Scholar]
- 7. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, 3rd rev. edn. Washington: American Psychiatric Association, 1987. [Google Scholar]
- 8. Inoue H, Sato Y, Inui M et al. Endoscopic strategy for superficial middle and hypopharyngeal tumors. I Cho 2005; 40: 1270–6. (In Japanese with English abstract.) [Google Scholar]
- 9. Harada S, Zhang S. New strategy for detection of ALDH2 mutant. Alcohol Alcohol Suppl 1993; 1A: 11–3. [DOI] [PubMed] [Google Scholar]
- 10. International Agency for Research on Cancer. Allyl compounds, aldehydes, epoxides and peroxides. In: IARC Monographs on the Evaluation of Carcinogenic Risks of Chemicals to Humans, Vol. 36. IARC: Lyon, 1985; 101–32. [Google Scholar]
- 11. Fang JL, Vaca CE. Detection of DNA adducts of acetaldehyde in peripheral white blood cells of alcohol abusers. Carcinogenesis 1997; 18: 627–32. [DOI] [PubMed] [Google Scholar]
- 12. Dong YJ, Peng TK, Yin SJ. Expression and activities of class IV alcohol dehydrogenase and class III aldehyde dehydrogenase in human mouth. Alcohol 1996; 13: 257–62. [DOI] [PubMed] [Google Scholar]
- 13. Yin SJ, Chou FJ, Chao SF et al. Alcohol and aldehyde dehydrogenases in human esophagus: comparison with the stomach enzyme activities. Alcohol Clin Exp Res 1993; 17: 376–81. [DOI] [PubMed] [Google Scholar]
- 14. Baumgarten G, Waldherr R, Stickel F, Simanowski UA, Ingelmann‐Sundverg M, Seitz HK. Enhanced expression of cytochrome p450 2E1 in the oropharyngeal mucosa in alcoholics with cancer (Abstract). Alcohol Clin Exp Res 1996; 20 (Suppl. 2): 80A. [Google Scholar]
- 15. Visapaa JP. Calvados‐related upper‐intestinal tract cancer; further evidence for the local carcinogenic activity of acetaldehyde (Abstract). Gut 2001; 49 (Suppl. 4): abstract no. 2299. [Google Scholar]
- 16. Homann N, Jousimies‐Somer H, Jokelainen K, Heine R, Salaspuro M. High acetaldehyde levels in saliva after ethanol consumption: methodological aspects and pathological implications. Carcinogenesis 1997; 18: 1739–43. [DOI] [PubMed] [Google Scholar]
- 17. Väkeväinen S, Tillonen J, Agarwal DP, Srivastava N, Salaspuro M. High salivary acetaldehyde after a moderate dose of alcohol in ALDH2 deficient subjects: strong evidence for local carcinogenetic action of acetaldehyde. Alcohol Clin Exp Res 2000; 24: 873–7. [PubMed] [Google Scholar]
- 18. Homann N, Karkkainen Koivisto T, Nosova T, Jokelainen K, Salaspuro M. Effects of acetaldehyde on cell regeneration and differentiation of the upper gastrointestinal tract mucosa. J Natl Cancer Inst 1997; 89: 1692–7. [DOI] [PubMed] [Google Scholar]
- 19. Gordon PR, Mansur CP, Gilchrest BA. Regulation of human melanocyte growth, dendricity, and melanization by keratinocyte derived factors. J Invest Dermatol 1989; 92: 565–72. [DOI] [PubMed] [Google Scholar]
- 20. Buchner A, Hansen LS. Melanotic macule of the oral mucosa. A clinicopathologic study of 105 cases. Oral Surg Oral Med Oral Pathol 1979; 48: 244–9. [DOI] [PubMed] [Google Scholar]
- 21. Yamazaki K, Ohmori T, Kumagai Y, Makuuchi H, Eyden B. Ultrastructure of oesophageal melanocytosis. Virchows Arch a Path Anat Histol 1991; 418: 515–22. [DOI] [PubMed] [Google Scholar]
- 22. Hedin CA, Axell H. Oral melanin pigmentation in 467 Thai and Malaysian people with special emphasis on smoker's melanosis. J Oral Pathol Med 1991; 20: 8–12. [DOI] [PubMed] [Google Scholar]
- 23. Araki S, Murata K, Ushio K, Sakai R. Dose–response relationship between tobacco consumption and melanin pigmentation in the attached gingiva. Arch Environ Health 1983; 138: 375–8. [DOI] [PubMed] [Google Scholar]
- 24. International Agency for Research on Cancer. Alcohol drinking. In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 44. IARC: Lyon, 1988; 153–246. [PMC free article] [PubMed] [Google Scholar]
- 25. Brooks PJ. DNA damage, DNA repair, and alcohol toxicity‐a review. Alcohol Clin Exp Res 1997; 21: 1073–82. [PubMed] [Google Scholar]
- 26. International Agency for Research on Cancer. Tobacco smoking. In: IARC Monographs on the Evaluation of Carcinogenic Risks of Chemicals to Humans, Vol. 38. Lyon: IARC, 1986; 83–126. [Google Scholar]
- 27. Salaspuro V, Salaspuro M. Synergistic effect of alcohol drinking and smoking on in vivo acetaldehyde concentration in saliva. Int J Cancer 2004; 111: 480–3. [DOI] [PubMed] [Google Scholar]
- 28. Kuratsune M, Kohchi S, Horie A. Carcinogenesis in the esophagus. I: penetration of benzo (a) pyrene and other hydrocarbons into the esophageal mucosa. Gann 1965; 56: 177–87. [PubMed] [Google Scholar]
- 29. Wood JM, Jimbow K, Boissy RE et al. What's the use of generating melanin? Exp Dermatol 1999; 8: 153–64. [DOI] [PubMed] [Google Scholar]
- 30. Makuuchi H, Mitomi T. Esophageal melanosis. Shokakika 1986; 4: 492–9. (In Japanese.) [Google Scholar]
- 31. Dumas O, Barthelemy C, Billard F et al. Isolated melanosis of the esophagus: systematic endoscopic diagnosis. Endoscopy 1990; 22: 94–5. [DOI] [PubMed] [Google Scholar]
- 32. Bogomoletz WV, Lecat M, Amoros F. Melanosis of the oesophagus in a Western patient. Histopathology 1997; 30: 498–9. [DOI] [PubMed] [Google Scholar]
- 33. Makuuchi H. Endoscopic mucosal resection for mucosal cancer in the esophagus. Gastrointest Endosc Clin N Am 2001; 11: 445–58. [PubMed] [Google Scholar]


