Abstract
Hepatocyte growth facor activator (HGFA) is a serine protease that converts hepatocyte growth factor (HGF) into its active form. Our previous study demonstrated that tumor–stromal interaction under hypoxia augments the aggressive invasive features of pancreatic cancer line PK8 through activated HGF/c‐Met signaling. The present study investigated whether or not hypoxia increases HGFA expression in PK8 cells and promotes the processing of HGF, and leads to c‐Met activation. Moreover, HGFA promoter assays were performed to define whether hypoxia inducible factor‐1 alpha (HIF‐1α) directly activates the HGFA promoter in a hypoxia‐dependent fashion. As a result, hypoxia induced the HGFA mRNA and protein expression in PK8 and the elevation under hypoxia was inhibited by the transfection of HIF‐1α siRNA, thus indicating HIF‐1α‐dependent induction of HGFA. The transfection of siRNA against HGFA to PK8 cells suppressed the conversion to the active HGF, which is secreted from fibroblast MRC5. Furthermore, the phosphorylation of c‐Met and cancer invasion of PK8 cells were decreased by the transfection of HGFA siRNA under hypoxia. Using the luciferase reporter system, HIF‐1α was shown to transactivate the HGFA promoter under hypoxia. These experiments demonstrated for the first time that HGFA is a novel HIF‐1 target gene. Under hypoxia, HGFA might be overexpressed and secreted from pancreatic cancer cells, which contributes to accelerate processing of HGF from fibroblast, resulting in the activation of the c‐Met pathway. HGF/HGFA/c‐Met recruited between cancer‐stromal fibroblasts is activated under hypoxic conditions and therefore might play a central role in the aggressive invasion of pancreatic cancer. (Cancer Sci 2008; 99: 1341–1347)
Hepatocyte growth factor (HGF) is a multifunctional cytokine produced from stromal cells, and it functions as a mitogen, motogen, and morphogen, and in angiogenesis.( 1 , 2 , 3 ) The various effects of HGF are mediated through binding to the specific receptor, c‐Met receptor tyrosine kinase, which is expressed on the cell surfaces of epithelial origin. HGF is mainly produced in cells of mesenchymal origin and secreted as a single‐chain precursor form (pro‐HGF). The proteolytic conversion of pro‐HGF to the two‐chain heterodimeric active form (mature HGF) is essential in its biological activity.( 4 ) The most potent enzyme involved in the activation of pro‐HGF is HGF activator (HGFA), a serine protease discovered in serum that is related to coagulation factor XIIa.( 5 ) The liver is the main source of HGFA, and produces HGFA as an inactive form (pro‐HGFA), which is converted to the active form by thrombin.( 6 ) The extrahepatic expression of HGFA has also been reported, and recent studies have demonstrated HGFA to be involved in the activation of pro‐HGF in several tumors.( 7 , 8 , 9 , 10 )
Hypoxia is a common feature of various solid tumors due to inadequacies in their vasculature.( 11 ) The hypoxic environment, in which hypoxia‐inducible factor‐1 (HIF‐1) plays a key role, might be associated with both malignant progression and a poor response to various treatments.( 12 , 13 ) HIF‐1 is a heterodimeric basic helix‐loop‐helix PER/ARNT/SIM (HLH‐PAS) transcription factor that consists of HIF‐1α and a constitutively expressed aryl hydrocarbon receptor nuclear translocator known as HIF‐1β. Under normoxic conditions, HIF‐1α is bound to the tumor suppressor Von Hippel–Lindau protein. This protein complex causes HIF‐1α to be targeted by proteasomes, thus leading to rapid protein degradation. In contrast, under hypoxic condition, HIF‐1α is stabilized and dimerized with HIF‐1β, translocates to the nucleus, and transactivates expressions of various genes.( 14 , 15 ) The hypoxia‐responsive element (HRE) consists of a pair of consecutive transcription factor binding sites, and at least one of which contains the core sequence 5′‐RCGTG‐3′ and is recognized by HIF‐1.( 16 ) At present, the expression of over 70 genes is known to be HIF‐1 regulated.( 17 )
Pancreatic cancer is one of the most lethal malignancies in industrialized countries.( 18 ) Most patients with pancreatic cancer have a poor outcome due to difficulties in its early diagnosis, and its highly invasive and metastatic features. Several studies have revealed that tumor hypoxia exists within pancreatic cancer tissue,( 19 ) and HIF‐1α overexpression has also been reported to be significantly associated with a poor prognosis in pancreatic cancer patients.( 20 ) Pancreatic cancer is also characterized by abundant stroma, thus indicating that tumor–stromal interactions play an important role in the progression of this malignancy. We have revealed that tumor–stromal cell interaction under hypoxia increases the invasiveness of pancreatic cancer cells through the HGF/c‐Met pathway( 21 ) and therefore negatively affects the prognosis in patients with pancreatic cancer.( 22 ) To date, no studies have focused on whether or not the hypoxic environment plays an essential role in the activation of HGF.
The present study investigated the regulation of HGFA gene expression in pancreatic cancer cells under hypoxic condition to determine whether or not the HGF processing by HGFA plays an important role in possessing the highly invasive character of this tumor that exists in a hypoxic microenvironment.
Materials and Methods
Cell culture and exposure to hypoxia. The human pancreatic cancer cell lines PK8, PK1, and KP4 were purchased from the Institute of Development, Aging, and Cancer, Tohoku University (Sendai, Japan). The MRC5 human fibroblast cell line was purchased from the Riken Cell Bank (Ibaragi, Japan). The cells were cultured in RPMI‐1640 medium (Sigma, St. Louis, MO, USA), supplemented with 10% heat‐inactivated fetal bovine serum (Sigma) and 100 µg/mL kanamycin (Meiji, Tokyo, Japan), and incubated at 37°C in a humidified atmosphere containing 20% O2 and 5% CO2 in air. When the culture of pancreatic cancer cells reached semiconfluence, the medium was removed and replaced by fresh medium with 1% fetal bovine serum; half of the cells remained in the same conditions (referred to as normoxia), while the remaining half was moved to a hypoxic chamber (ASTEC, Fukuoka, Japan) containing 1% O2, 5% CO2, and 94% N2, and this hypoxia chamber was maintained at 37°C (referred to as hypoxia). The serum‐free conditioned medium from MRC5 fibroblasts was collected following 24 h cultivation under normoxia and was used in experiments as a MRC5 conditioned medium (MRC5 CM).
Quantitative reverse transcription–polymerase chain reaction (RT‐PCR) assay. PK8, PK1, and KP4 cells were incubated under normoxia and hypoxia up to 24 h and total RNA was isolated from each cell line using an Isogen RNA extraction kit (Nippongene, Toyama, Japan). The reverse transcriptase reaction was carried out using the RNA LA PCR Kit (AMV) version 1.1 (Takara Biochemicals, Shiga, Japan), in which each 1 µg of RNA from the three cell lines were converted into cDNA. To quantitatively estimate the expression level of the HGFA mRNA in PK8, PCR was performed on a Light‐Cycler instrument system (Roche, Mannheim, Germany) using the Light‐Cycler‐FastStart DNA Master SYBR green I Kit (Roche) according to the manufacturer's instructions. After a denaturing step at 95°C for 3 min, PCR amplification was performed with 50 cycles of 15 s denaturing at 95°C, 5 s annealing at 60°C, and 10 s extension at 72°C. Melting curves were obtained according to the protocol under the following conditions: 0 s denaturation period at 95°C, starting temperature at 65°C, ending temperature at 95°C, and a rate of temperature increase of 0.1°C/s. The sequences of the PCR primer pair were 5′‐GAATCCCTCACCAGAGTCCA‐3′ and 5′‐AGCTGTCCCCGAT‐GTAGATG‐3′ for HGFA and 5′‐TTAAGGAGAAGCTGTGCTACG‐3′ and 5′‐GTTGAAGGTAGTTTCGTGGAT‐3′ for β‐actin (as an internal control). These experiments were carried out in triplicate and the mean value was calculated. Finally, the quantitative value was normalized based on the β‐actin expression. Using two other pancreatic cancer cell lines PK1 and KP4, HGFA expression under hypoxic conditions was investigated with conventional RT‐PCR. The PCR conditions were as follows: initial denaturation at 94°C for 2 min followed by 32 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 60 s. PCR for β‐actin served as an internal standard. The PCR product was separated on 1% agarose gel, stained with ethidium bromide, and visualized under UV illumination.
Western blot analysis and immunoprecipitation. PK8 cells cultured under normoxia and hypoxia were lyzed in lysis buffer composed of 150 mM NaCl, 50 mM Tris‐HCl (pH 7.6), 0.5% Triton X‐100, and a protease inhibitor cocktail mix (Roche). The aliquots of each cell extract containing 30 µg of protein were separated by 4–12% sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS‐PAGE) and the separated extracts were electrophoretically transferred onto Hybond nitrocellulose–enhanced chemiluminescence membranes (Amersham Pharmacia Biotech, Buckinghamshire, UK) in a transfer buffer. The primary antibodies used in the Western blot analysis were anti‐HIF‐1α antibody (clone HI‐67, 1:1000 dilution; Novus Biologicals, Littleton, CO, USA), anti‐HGFA antibody (clone AF1514, 1:500 dilution; R&D Systems, Oxford, UK), anti‐HGF antibody (clone BAF294, 1:500 dilution; R&D Systems), anti‐c‐Met antibody (clone C‐28 1:200 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA), and anti‐β‐actin antibody (clone AC‐15, 1:10 000 dilution; Sigma). After incubation with the corresponding secondary antibodies, the signals were finally developed using an enhanced chemiluminescence detection kit (Amersham Pharmacia Biotech).
For immunoprecipitation, the cell lysates were prepared in a lysis buffer composed of 20 mM Tris‐HCl (pH 7.4), 5 mM EDTA, 10 mM Na4P2O7, 100 mM NaF, 2 mM Na3VO4, 1% NP‐40, and protease inhibitor cocktail mix (Roche). Immunoprecipitation was performed using 500 µg of cell extracts incubated with anti‐c‐Met polyclonal antibody (clone C‐28; Santa Cruz) with 20 µL of protein A–agarose beads (Sigma). After the beads were washed and boiled at 95°C for elution, the sample was subsequently subjected to a Western blot analysis using anti‐c‐Met monoclonal antibody (clone B‐2, 1:100 dilution; Santa Cruz) and anti‐p‐Tyr antibody (clone PY99, 1:1000 dilution; Santa Cruz).
Small interfering RNA (siRNA) treatment and transfection. The sense and antisense siRNA oligonucleotides corresponding to the following cDNA sequences were purchased from iGENE (Ibaragi, Japan): CCAGCAGACUCAAAUACAAGAACCUAG for HIF‐1α, UGAUCCGGCUGAAGAAGA‐AAGGGGAAG for HGFA, and GAGAGCUGAUUUACGGAUGUAGAAGAG for scrambled controls. The pancreatic cancer cells were transfected with MicroPorator (Digital Bio Technology, Suwon, Korea) according to the manufacturer's instructions in the presence of siRNA. A protein expression analysis, gene expression analysis, immunoprecipitation, and in vitro invasion assay were all performed 48 h after siRNA transfection.
In vitro invasion assay. The in vitro invasion activities were examined as reported previously using a gel matrix (Matrigel; Beckton Dickinson, Franklin Lakes, NJ, USA) in 24‐well plates.( 23 ) Briefly, 6.5‐mm diameter polycarbonate filters (8‐µm pore size) of the Falcon Transwell chemotaxis chambers (Beckton Dickinson) were coated with 50 µL (0.25 mg/mL) of Matrigel biomatrix in cold RPMI‐1640 medium and dried overnight. Suspensions of 5 × 105 transfected or untransfected PK8 cells in 200 µL of complete RPMI‐1640 medium were placed in the upper compartments of the chamber, whereas the lower compartments were filled with 800 µL conditioned medium from MRC5 fibroblasts. These culture units were incubated for 24 h at 37°C under normoxia and hypoxia. Non‐invasive cells on the upper surface of the filters were then removed completely with a cotton swab. Any viable invasive cells, which infiltrated onto the lower surface of the filter, were fixed in 70% ethanol and the nuclei were stained using hematoxylin. Next, the number of invasive cells was counted. These experiments were carried out in triplicate and independently repeated at least three times.
HGF processing assay. HIF‐1α, HGFA, and scrambled siRNA were transfected into PK‐8. The serum‐free conditioned medium from the PK8 cells (PK8 CM) was collected following the 24 h cultivation under normoxia or hypoxia. The PK8 CM was mixed with MRC5 CM and incubated at 37°C for 8 h. To detect pro‐ and mature HGF expression in the mixed CM, protein A–agarose beads along with antihuman HGF capture antibody (clone 24516; R&D Systems) was added to the mixed CM and incubated overnight at 4°C. Next, the supernatants were discarded by centrifugation at 740 g and the beads were washed, followed by boiling at 95°C for elution. Finally, the eluted samples were subjected to a Western blot analysis using anti‐HGF antibody (Clone BAF294, 1:500 dilution; R&D Systems).
Construction of reporter plasmid. To generate plasmids P1 and P2, the sequences of the human HGFA gene between –329 and +38 (P1) or between –1908 and +258 (P2) relative to the transcriptional start site were amplified by PCR from genomic DNA, along with KOD‐Plus‐DNA polymerase (Toyobo, Osaka, Japan) and oligonucleotides 5′‐CGCCCGTAGTGGCTCTCATCA‐3′ and 5′‐TCCAG‐AGCGGCCAAGGCAGGT‐3′ for P1, and 5′‐AAGTGCCCCAGGGTAAGGTCA‐3′ and 5′‐TGCGACAAGGTCAGTGCTCAC‐3′ for P2. The PCR product was purified, blunted, and connected upstream of luciferase in the pGL3‐basic vector (Promega, Madison, WI, USA). Direct sequencing confirmed that there was no mutation in the insert. The empty pGL3‐basic vector without the insert was used as a control (control plasmid).
The HIF‐1α expression vector was kindly provided by Dr Yoshiaki Fujii (Laboratory of Genomics and Proteomics, Faculty of Pharmacy and Pharmaceutical Science, Fukuyama University, Japan). As a control, an empty pcDNA3.1 vector (Promega) was used.
Transient expression assay. Pancreatic cancer cells (1 × 105 cells/well) were transiently transfected by electroporation with a MicroPorator (Digital Bio Technology) according to the manufacturer's instructions in the presence of P1 plasmid, P2 plasmid, Control plasmid, HIF‐1α expression plasmid, or empty pcDNA3.1 vector at 1 µg. The transfected cells were preincubated in six‐well culture dishes containing 2 mL of antibiotics‐free medium for 24 h, and then exposed to normoxia or hypoxia for another 24 h after replacing by fresh medium with 1% fetal bovine serum. After the incubation, the cells were washed in phosphate‐buffered saline (PBS), and lyzed by reporter lysis buffer (Promega). The luciferase activities were measured using the luciferase assay system (Promega) according to the manufacturer's instructions. Finally, luciferase activities were normalized by β‐galactosidase activity from a cotransfected pSVβ‐galactosidase vector (Promega) at 1 µg. These experiments were examined in triplicate and repeated at least three times.
Statistical analysis. The values were expressed as the mean ± SD. Comparisons between the two groups were analyzed by Student's t‐test and Fisher's exact test. P‐values less than 0.05 were considered to be statistically significant.
Results
Induction of the HGFA gene expression under hypoxia. In PK8 cells, quantitative RT‐PCR revealed that the HGFA mRNA level was up‐regulated under hypoxia (1% O2) in a time‐dependent manner (Fig. 1a). The protein expression of the HGFA was also elevated under hypoxia in comparison to normoxia (20% O2) (Fig. 1b).
Figure 1.
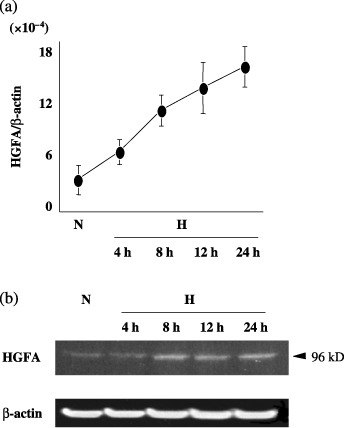
(a) The levels of hepatocyte growth factor activator (HGFA) mRNA in pancreatic cancer cells were quantitatively estimated under hypoxia. H, hypoxia (1% O2); N, normoxia (20% O2). (b) The protein expression of HGFA was analyzed by Western blotting. β‐actin protein levels were used as an internal marker. H, hypoxia; N, normoxia.
The elevated expression of the HGFA gene under hypoxia was blocked by HIF‐1a siRNA. In order to clarify whether or not the HGFA induction under hypoxia was regulated by HIF‐1α, the knockdown effect of HIF‐1α siRNA was examined in PK8 cells. Figure 2a shows that HIF‐1α was not expressed under normoxia in PK8 cells. Under hypoxic condition, HIF‐1α protein was induced in PK8 cells, which transfected by siRNA (control). In constast, the transfection of HIF‐1α siRNA dramatically decreased HIF‐1α expression in a dose‐dependent manner. Figure 2b shows that the transfection of HIF‐1α siRNA at 50 nM diminished the elevated expression of the HGFA mRNA level under hypoxia in comparison to the scrambled siRNA (control). In addition, at the protein level, the hypoxic induction of 96 kDa‐sized HGFA protein was suppressed by HIF‐1α transfection in a dose‐dependent manner (Fig. 2c).
Figure 2.
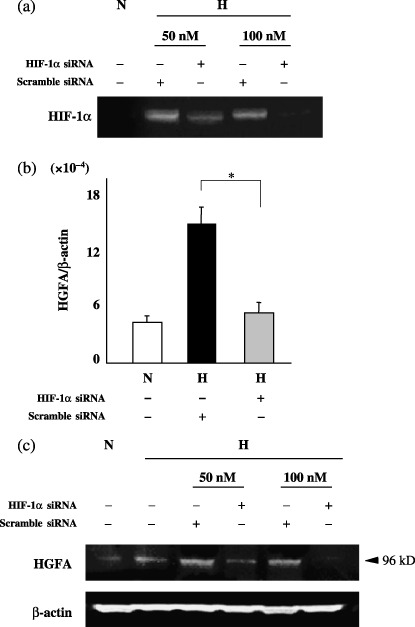
(a) A Western blot analysis for hypoxia inducible factor‐1 alpha (HIF‐1α) in pancreatic cancer cells transfected with HIF‐1α or scrambled siRNA under normoxia or hypoxia. H, hypoxia; N, normoxia. (b) The levels of HGFA mRNA were presented as the relative yield of polymerase chain reactio (PCR) product from the target sequence in comparison to that from the β‐actin gene with HIF‐1α or scrambled siRNA under normoxia or hypoxia. N, normoxia; H, hypoxia. (c) The protein expressions of HGFA in pancreatic cancer cells transfected with HIF‐1α or scrambled siRNA under normoxia or hypoxia were analyzed by Western blotting. H, hypoxia; N, normoxia.
HGF processing under hypoxia. To evaluate whether or not the processing of HGF polypeptide was affected by the HGFA expression in PK8, the MRC5 CM was incubated with PK8 CM at 37°C and subjected to a Western blot analysis (Fig. 3). Pro‐HGF (94 kDa) was highly converted to the mature HGF (62 kDa) in CM from PK8 exposed under hypoxia, in comparison to the CM under normoxia. Furthermore, the acceleration of the HGF processing under hypoxia was significantly inhibited when HIF‐1α or HGFA siRNA was transfected into the PK8 cells (Fig. 3).
Figure 3.
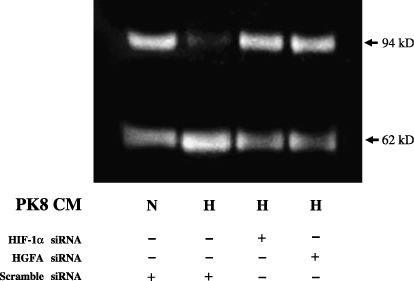
Hepatocyte growth factor (HGF) processing in MRC5 conditioned medium mixed with normoxic or hypoxic PK8 conditioned medium, which were transfected with hypoxia inducible factor‐1 alpha (HIF‐1α), hepatocyte growth factor activator (HGFA), or scrambled siRNA. CM, conditioned medium; H, hypoxia; N, normoxia.
Expression and phosphorylation of c‐Met protein under hypoxia. The next experiments investigated whether the activation of c‐Met, a cognate receptor for HGF, is influenced by knockdown of HGFA as well as HIF‐1α. Figure 4 showed c‐Met, phosphorylated c‐Met (p‐Tyr), expressions in Western blot analysis (Fig. 4a) and the expression level of these two proteins, which were normalized by β‐actin (Fig. 4b). Hypoxic stimulation augmented the expression as well as the phosphorylation of c‐Met in PK8 cells (lane 2 in Fig. 4a,b). Elevated expression of c‐Met protein under hypoxia was decreased by the transfection of HIF‐1α siRNA in comparison to scrambled siRNA (control) (lanes 3,4 in Fig. 4a,b). The transfection of HGFA siRNA had little effect on the expression of c‐Met protein. However, the elevated phosphorylation of c‐Met protein under hypoxia decreased after the transfection of HGFA siRNA in comparison to scrambled siRNA (control) (lanes 5,6 in Fig. 4a,b).
Figure 4.
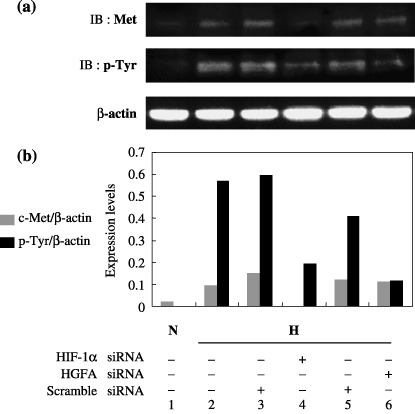
(a) The expression and tyrosine phosphorylation of c‐Met protein in pancreatic cancer cells PK8, transfected with hypoxia inducible factor‐1 alpha (HIF‐1α), hepatocyte growth factor activator (HGFA), or scrambled siRNA, for comparison under normoxia and hypoxia. The β‐actin protein levels were used as an internal marker. CM, conditioned medium; H, hypoxia; IB, immunoblotting; N, normoxia. (b) Band intensities of c‐Met or p‐Tyr Met observed in (a) were quantitatively assessed by LAS3000 and normalized by that of β‐actin. The expression level of c‐Met and p‐Tyr Met was demonstrated in the histogram.
Invasive activity of PK8 cells under hypoxia was inhibited by HIF‐1a or HGFA siRNA. The invasive activity of PK8 cells significantly increased under hypoxic conditions in comparison to that in normoxia (lanes 1, 4, 7, and 10 in Fig. 5). The transfection of HIF‐1α siRNA did not affect the invasive activity of PK8 cells under normoxia (lanes 2,3 in Fig. 5). In contrast, transfection of HIF‐1α siRNA resulted in the significant suppression of the invasive activity of PK8 cells under hypoxia in comparison to the cells transfected with scrambled siRNA (control) (lanes 5,6 in Fig. 5). HGFA siRNA suppressed the invasive activity of PK8 cells under normoxia; however, the difference was not statistically significant (P = 0.102) (lanes 8,9 in Fig. 5). Moreover, the transfection of HGFA siRNA under hypoxia also led to the significant inhibition of the invasion of PK8 cells in comparison to the cells transfected with scrambled siRNA (control) (lanes 11,12 in Fig. 5).
Figure 5.
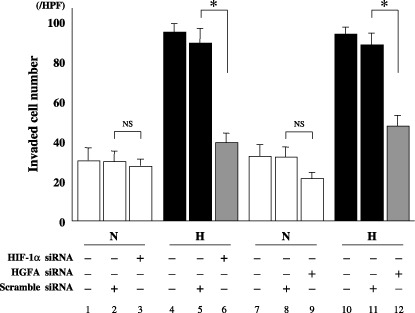
The invasive ability of pancreatic cancer cells PK8, transfected with hypoxia inducible factor‐1 alpha (HIF‐1α), hepatocyte growth factor activator (HGFA), or scrambled siRNA, and then analyzed by an in vitro invasion assay. Data are presented as mean ± SD of the triplicate measurements. H, hypoxia; HPF, high power field; N, normoxia; NS, not significant; *P < 0.05.
Increased promoter activity of the HGFA gene under hypoxia. To examine the promoter activity of HGFA, the 5′‐flanking region of the human HGFA gene was cloned and connected upstream to the luciferase plasmid. Based on a homology search, there are two putative HIF‐1α binding sites (the core consensus sequence is ‘ACGTG’). Two reporter plasmids were constructed, where the P2 reporter plasmid has the two putative HIF‐1α binding sites (A, B), and the P1 reporter plasmid has only one site (B; Fig. 6a). Hypoxic stimulation significantly increased the relative luciferase activity in both P1 and P2 reporters to the same degree (Fig. 6b). In addition, the cotransfection of the P1 plasmid along with the HIF‐1α expression plasmid resulted in the significant elevation of the promoter activity from P1 in comparison to the empty pcDNA3.1 vector (Fig. 6c).
Figure 6.
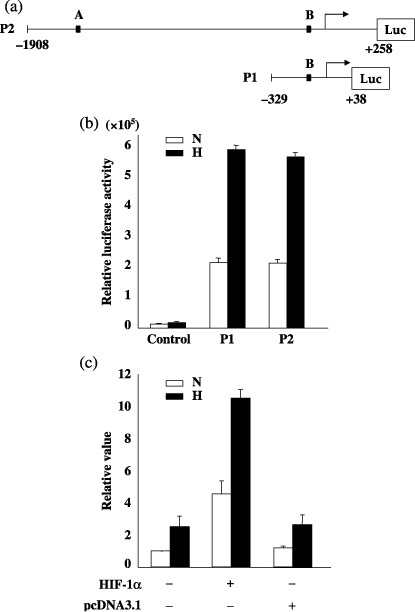
(a) The luciferase (Luc) reporter plasmids harboring two (P2) or one putative hypoxia inducible factor‐1 alpha (HIF‐1α) binding site (P1). (b) PK8 cells were transfected with pGL3‐basic vector alone (control), or P1 or P2 plasmids under normoxia and hypoxia. Luciferase activities were normalized to β‐galactosidase activities and shown as mean ± SD of the triplicate measurements. H, hypoxia; N, normoxia. (c) PK8 cells were transfected by the P1 plasmid along with the HIF‐1α expression vector or pcDNA3.1 vector under normoxia and hypoxia. The each value of luciferase activity was determined relative to the normoxic controls without the HIF‐1α expression vector and pcDNA3.1 vector, which was set equal to 1. H, hypoxia; N, normoxia.
HGFA induction under hypoxia in other pancreatic cancer cells. To investigate whether or not the increased HGFA expression under hypoxia is observed in other pancreatic cancer cells, RT‐PCR analysis of HGFA was performed in PK1 and KP4 cells. As shown in Figure 7, HGFA expression under hypoxia was elevated with time dependency.
Figure 7.
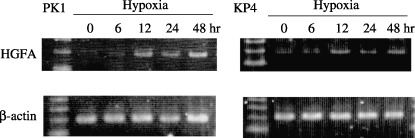
Expression of hepatocyte growth factor activator (HGFA) mRNA was estimated by reverse transcription–polymerase chain reaction using pancreatic cancer cell lines PK‐1 and KP‐4 under hypoxic stimulation.
Discussion
Pancreatic cancer is a solid tumor in which the hypoxic microenvironment plays a crucial role in tumor progression.( 19 ) In addition, the characteristic abundant stroma is reported to contribute to the malignant behavior of this cancer.( 22 ) Our previous study revealed that the aggressively invasive features of pancreatic cancer are caused by tumor–stromal interactions under hypoxia through the binding of HGF (from MRC‐5) to c‐Met (on PK8), thereby leading to the highly invasive character and the poor outcome of this cancer.( 21 , 22 ) The conversion from pro‐HGF to mature HGF is an essential step in such tumor tissue and HGF possess multiple biological functions. To date, several proteinases have been thought to be essential in the activation of HGF, such as HGFA, factor XIIa, matriptase, and urokinase‐type plasminogen activator. Among these factors, HGFA is reported to exhibit the most potent activity in the processing of pro‐HGF to mature HGF.( 24 , 25 ) The present study hypothesized that the hypoxic environment might affect HGFA expression in pancreatic cancer cells and activate the HGF/c‐Met pathway through increased mature HGF production.
The initial experiments examined whether hypoxia affects HGFA gene expression in the pancreatic cancer cell line PK8. The HGFA mRNA and protein expression both significantly increased under hypoxia, and the transfection of HIF‐1α siRNA suppressed elevated HGFA expression under hypoxia. These findings indicated that increased HGFA expression under hypoxia depended on HIF‐1α expression. HGFA is activated by proteolysis, leading to a 66 kDa long chain and 32 kDa light chain.( 26 ) The light chain at N terminus exhibits the enzymatic activity and the long chain is further cleaved by proteases. The present study revealed 96 kDa of pro‐HGFA but not a 32 kDa light chain in Western blot analysis. The goat polyclonal HGFA antibody used in this study recognizes the epitope between 370 and 655 amino acids at C terminal region of pro‐HGFA. Thus the light chain at 32 kDa might not be detectable by a Western blot analysis. On the other hand, matriptase, which is one of the activators of HGF, has been also reported in the expression in several tumors.( 27 , 28 ) Ihara et al. showed that matriptase might contribute to tumor progression by the remodeling of the extracellular matrix.( 29 ) However, current studies reveal no difference in its expression between under normoxia and hypoxia (data not shown).
In HGF processing, it has been revealed that the siRNA against HIF‐1α and HGFA repressed the conversion of pro‐HGF to mature HGF in PK8 cells under hypoxia (Fig. 3). These results indicate that HGFA in PK8 cells is induced by HIF‐1α under hypoxia and the increased HGFA expression accelerates the processing of HGF polypeptide. Our previous report demonstrated that hypoxic stimulation elevated not only c‐Met, but also matrix metalloproteinase‐2 (MMP2), MMP7, and MT1‐MMP expression in PK8 cells, whereas the hypoxia increased HGF secretion from MRC‐5.( 21 ) It was further revealed that the MMP2 activity depended on HGF/c‐Met signaling, because the removal of HGF from MRC‐5CM caused the reduction in not only c‐Met phosphorylation, but also the enzyme activity of MMP2. These findings demonstrated that tumor–stromal cell interaction under hypoxia increases the invasiveness of PK8 through HGF/c‐Met signaling, which is connected to MMP activation. In the present study, knockdown of HIF‐1α expression by siRNA transfection resulted in decrease of c‐Met expression and its phosphorylation (Fig. 4), thus indicating HIF‐1α‐dependent induction of c‐Met under hypoxia. Currently, one recent report suggests that HIF‐1α directly regulates c‐Met expression in trophoblasts, which supports the present data.( 30 )
Furthermore, the knockdown of HGFA in PK8 cells under hypoxia caused the attenuation in c‐Met phosphorylation, but had no effect on the expression (Fig. 4). This result indicated that the unsuccessful HGF processing, which originated from the suppressed HGFA expression, lowered the activated c‐Met. As shown in Figure 5, HIF‐1α knockdown significantly reduced the invasiveness of PK8 cells under hypoxic conditions. This suggests that c‐Met as well as HGFA expression was reduced by HIF‐1α siRNA transfection, thus leading to both insufficient processing of HGF and a decrease in the c‐Met expression. Through this mechanism, HIF‐1α siRNA possibly suppressed the invasion of PK‐8 cells under hypoxia. However, the blockade at the HGF processing by HGFA knockdown also inhibited the invasion under hypoxia to the same degree in comparison to the HIF‐1α knockdown. This indicates that the blockade at the HGF processing step by HGFA knockdown might therefore be capable of completely inhibiting HGF/c‐Met signaling.
Finally, this study investigated whether or not HIF‐1α directly acts on the HGFA promoter using a luciferase reporter system. According to the homology search, there are 2 putative hypoxia response elements (5′‐RCGTG‐3′) within 2000 bp upstream of the transcription initiation site. A promoter reporter assay revealed that the luciferase activity from P1 plasmid, which contains one HRE (B), was approximately three‐fold increased under hypoxia in comparison to normoxia. The relative luciferase activity in PK8 cells under normoxia as well as hypoxia was almost same between P1 and P2 plasmids (Fig. 6). These results demonstrated that the 5′‐flanking region in the P1 plasmid in which HRE (B) is included is essential to induce HGFA expression under hypoxic stimulation. As shown in Figure 6c, the ectopic HIF‐1α expression in PK8 augmented the luciferase activity under normoxia as well as hypoxia. This result further indicated that HIF‐1α directly acted on the promoter region, possibly mediated through HRE (B) in the P1 plasmid. Although the experiments did not demonstrate that HIF‐1α binds to HRE (B), they did show that HGFA is transcriptionally up‐regulated by HIF‐1α in PK8 cells under hypoxic exposure. Hypoxic stimulation elevated HGFA expression in other pancreatic cancer cell lines PK1 and KP4 (Fig. 7). This result indicates that the HGFA induction in hypoxia is a common event in human pancreatic cancer cells, and is not restricted to PK8 cells.
In conclusion, the present study clearly showed that HGFA expression is induced by HIF‐1α under hypoxia in pancreatic cancer cell PK‐8 and thus plays an important role in the processing of HGF, which is originally secreted from fibroblast MRC‐5. The hypoxic region exists in pancreatic cancer tissue. Under this microenvironment, the three components of HGF, HGFA, and c‐Met might be activated through cancer fibroblast cell interaction and strongly accelerate the invasion activity of this malignancy (Fig. 8). Some drugs abrogating the HGF/HGFA/c–Met interaction or targeting HIF‐1α could be used to overcome pancreatic cancer cells that are highly invasive in character.
Figure 8.
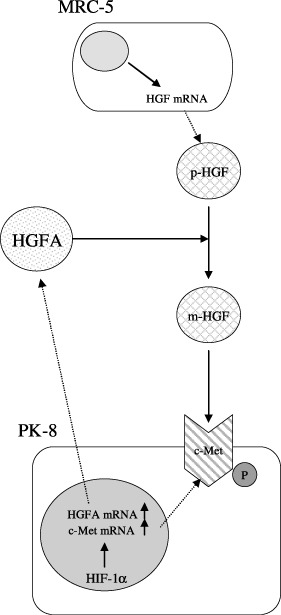
Schematic presentation of pancreatic cancer invasion through cancer–fibroblast cell interaction under hypoxia. Hypoxia induced hepatocyte growth factor activator (HGFA) as well as c‐Met expression via hypoxia inducible factor‐1 alpha (HIF‐1α) in pancreatic cancer cell line PK‐8. Under the microenvironments, pro‐HGF (p‐HGF) from MRC‐5 was processed into mature HGF (m‐HGF) by the overexpressed HGFA, leading to the activation of the over expressed c‐Met on PK‐8 cells. The HGF/HGFA/c‐Met activation under hypoxia caused pancreatic cancer cells to become highly invasive in character. P, phosphorylated.
References
- 1. Nakamura T, Nishizawa T, Hagiya M et al . Molecular cloning and expression of human hepatocyte growth factor. Nature 1989; 342: 440–3. [DOI] [PubMed] [Google Scholar]
- 2. Montesano R, Matsumoto K, Nakamura T, Orci L. Identification of a fibroblast‐derived epithelial morphogen as hepatocyte growth factor. Cell 1991; 67: 901–8. [DOI] [PubMed] [Google Scholar]
- 3. Matsumoto K, Nakamura T. Emerging multipotent aspects of hepatocyte growth factor. J Biochem 1996; 119: 591–600. [DOI] [PubMed] [Google Scholar]
- 4. Gak E, Taylor WG, Chan AML, Rubin JS. Processing of hepatocyte growth factor to the heterodimeric form is required for biological activity. FEBS Lett 1992; 311: 17–21. [DOI] [PubMed] [Google Scholar]
- 5. Miyazawa K, Shimomura T, Kitamura N. Activation of hepatocyte growth factor in the injured tissues is mediated by hepatocyte growth factor activator. J Biol Chem 1996; 271: 3615–18. [DOI] [PubMed] [Google Scholar]
- 6. Miyazawa K, Shimomura T, Daiji N, Kitamura N. Proteolytic activation of hepatocyte growth factor in response to tissue injury. J Biol Chem 1994; 269: 8966–70. [PubMed] [Google Scholar]
- 7. Kataoka H, Hamasuna R, Itoh H, Kitamura N, Koono M. Activation of hepatocyte growth factor/scatter in colorectal carcinoma. Cancer Res 2000; 60: 6148–59. [PubMed] [Google Scholar]
- 8. Tjin EP, Derksen PW, Kataoka H, Spaargaren M, Pals ST. Multiple myeloma cells catalyze hepatocyte growth factor (HGF) activation by secreting the serine protease HGF‐activator. Blood 2004; 104: 2172–5. [DOI] [PubMed] [Google Scholar]
- 9. Parr C, Watkins G, Mansel RE, Jiang WG. The hepatocyte growth factor regulatory factors in human breast cancer. Clin Cancer Res 2004; 10: 202–11. [DOI] [PubMed] [Google Scholar]
- 10. Uchinokura S, Miyata S, Fukushima T et al . Role of hepatocyte growth factor activator (HGF activator) in invasive growth of human glioblastoma cells in vivo. Int J Cancer 2006; 118: 583–92. [DOI] [PubMed] [Google Scholar]
- 11. Vaupel P, Thews O, Hoeckel M. Treatment resistance of solid tumors: role of hypoxia and anemia. Med Oncol 2001; 18: 243–59. [DOI] [PubMed] [Google Scholar]
- 12. Rice GC, Hoy C, Schimke RT. Transient hypoxia enhances the frequency of dihydrofolate reductase gene amplification in Chinese hamster ovary cells. Proc Natl Acad Sci USA 1986; 83: 5978–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res 1996; 56: 4509–15. [PubMed] [Google Scholar]
- 14. Jaakkola P, Mole DR, Tian YM et al . Targeting of HIF‐1α to the von Hippel‐Lindau ubiquitylation complex by O2‐regulated prolyl hydroxylation. Science 2001; 292: 468–72. [DOI] [PubMed] [Google Scholar]
- 15. Semenza GL. Targeting HIF‐1 for cancer therapy. Nat Rev 2003; 3: 721–32. [DOI] [PubMed] [Google Scholar]
- 16. Kaelin WG. How oxygen makes its presence felt. Genes Dev 2002; 16: 1441–5. [DOI] [PubMed] [Google Scholar]
- 17. Semenza GL. Hydroxylation of HIF‐1: oxygen sensing at the molecular level. Physiology (Bethesda) 2004; 19: 176–82. [DOI] [PubMed] [Google Scholar]
- 18. Warshaw AL, Fernandez‐del Castillo C. Pancreatic carcinoma. N Engl J Med 1992; 326: 455–65. [DOI] [PubMed] [Google Scholar]
- 19. Koong AC, Mehta VK, Le QT et al . Pancreatic tumors show high levels of hypoxia. Int J Radiat Oncol Biol Phys 2000; 48: 919–22. [DOI] [PubMed] [Google Scholar]
- 20. Shibaji T, Nagao M, Ikeda N et al . Prognostic significance of HIF‐1α overexpression in human pancreatic cancer. Anticancer Res 2003; 23: 4721–8. [PubMed] [Google Scholar]
- 21. Ide T, Kitajima Y, Miyoshi A et al . Tumor–stromal cell interaction under hypoxia increases the invasiveness of pancreatic cancer through the hapatocyte growth factor/c‐Met pathway. Int J Cancer 2006; 119: 2750–9. [DOI] [PubMed] [Google Scholar]
- 22. Ide T, Kitajima Y, Miyoshi A et al . The hypoxic environment in tumor‐stromal cells accelerates pancreatic cancer progression via the activation of paracrine hepatocyte growth factor/c‐Met signaling. Ann Surg Oncol 2007; 14: 2600–7. [DOI] [PubMed] [Google Scholar]
- 23. Miyoshi A, Kitajima Y, Sumi K et al . Snail and SIP1 increase cancer invasion by upregulating MMP family in hepatocellular carcinoma cells. B J Cancer 2004; 90: 1265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miyazawa K, Shimomura T, Kitamura A, Kondo J, Morimoto Y, Kitamura N. Molecular cloning and sequence analysis of the cDNA for a human serine protease responsible for activation of hepatocyte growth factor. J Biol Chem 1993; 268: 10 024–8. [PubMed] [Google Scholar]
- 25. Shimomura T, Miyazawa K, Komiyama Y et al . Activation of hepatocyte growth factor by two homologous proteases, blood‐coagulation factor XIIa and hepatocyte growth factor activator. Eur J Biochem 1995; 229: 257–61. [DOI] [PubMed] [Google Scholar]
- 26. Mukai T, Fukushima T, Naka D, Tanaka H, Osada Y, Kataoka H. Activation of hepatocyte growth factor activator zymogen (pro‐HGFA) by human kallikrein 1‐related peptidases. FEBS J 2008; 275: 1003–17. [DOI] [PubMed] [Google Scholar]
- 27. Kang JY, Dolled‐Filhart M, Ocal IT et al . Tissue microarray analysis of hepatocyte growth factor/Met pathway components reveals a role for Met, matriptase, and hepatocyte growth factor activator inhibitor 1 in the progression of node‐negative breast cancer. Cancer Res 2003; 63: 1101–5. [PubMed] [Google Scholar]
- 28. Oberst MD, Johnson MD, Dickson RB et al . Expression of the serine protease matriptase and its inhibitor HAI‐1 in epithelial ovarian cancer. correlation with clinical outcome and tumor clinicopathological parameters. Clin Cancer Res 2002; 8: 1101–7. [PubMed] [Google Scholar]
- 29. Ihara S, Miyoshi E, Ko JH et al . Prometastatic effect of N‐acetylglucosaminyltransferase V is due to modification and stabilization of active matriptase by adding β1‐6G1cNAc branching. J Biol Chem 2002; 277: 16960–7. [DOI] [PubMed] [Google Scholar]
- 30. Hayashi M, Sakata M, Takeda T et al . Up‐regulation of c‐Met protooncogene product expression through hypoxia‐inducible factor‐1alpha is involved in trophoblast invasion under low‐oxygen tension. Endocrinology 2005; 146: 4682–9. [DOI] [PubMed] [Google Scholar]


