Abstract
Primary gastric diffuse large B‐cell lymphomas are generally well controlled by non‐surgical treatment with combination chemotherapy followed by radiotherapy. We have previously reported that over 90% of patients achieved complete response (CR) with this therapeutic strategy: three cycles of cyclophosphamide, adriamycin, vincristine and prednisone followed by radiotherapy (40.5 Gy). Although the CR rate was very high, some patients still showed resistance to this combination therapy. In order to clarify the factors related to therapy resistance, we examined the relationship between Epstein–Barr virus (EBV), which was examined using an in situ hybridization technique, and the patients’ clinical courses. Out of the 50 patients, four were EBV positive; over half of lymphoma cells were positive for EBV by in situ hybridization. Of the three EBV‐positive patients, two showed progressive disease and one achieved partial response (PR). Two of the patients died of disease progression. The other patient achieved CR, but the lymphoma recurred with distant metastasis in the cerebellum 3 months after remission. In the present study, eight patients did not achieve CR or they relapsed, four patients showed progressive disease, one patient achieved PR, and three patients achieved CR with recurrence. Therefore, half of these unfavorable patients were EBV positive. This finding strongly indicated that EBV‐associated gastric diffuse large B‐cell lymphomas frequently show resistance to standard chemoradiotherapy, although some other adverse factors remain unclear. (Cancer Sci 2006; 97: 163 –166)
Primary gastric lymphoma (PGL) is the most common extranodal non‐Hodgkin's lymphoma. The majority of PGL are mucosa‐associated lymphoid tissue (MALT) lymphomas and diffuse large B‐cell lymphomas (DLBCL). Eradication of Helicobacter pylori is effective for treating most gastric MALT lymphomas without t(11;18)(q21;q21).( 1 ) Primary gastric DLBCL (PGDL) patients are usually treated surgically, but others are treated with surgical resection followed by chemotherapy with or without radiotherapy (RT).( 2 , 3 , 4 , 5 ). Miller et al. have reported that most patients with extranodal and localized DLBCL can frequently achieve complete response (CR) after treatment with a combination chemotherapy of cyclophosphamide, adriamycin, vincristine and prednisone (CHOP) for three cycles followed by RT.( 6 ) We previously carried out a prospective trials for the applicability of CHOP to PGDL cases, and over 90% of patients went into CR even though some patients were resistant to this therapeutic strategy.( 7 ) In order to clarify the factors related to therapy resistance, we examined the relationship between Epstein–Barr virus (EBV) and the patients’ clinical courses.
Patients and Methods
Patients
Fifty patients with PGDL were examined. The clinical charactersistics of 49 of the patients were reported previously.( 7 ) Consensus diagnosis was made by five pathologists (TYos, SN, YM, AO, TYok) according to the World Health Organization classification.( 8 ) Lymphoma cells had large nuclei, as shown in Fig. 1a, and were positive for CD79a and negative for CD3. All of the cases were in clinical stage I–II1 (Lugano staging system for gastrointestinal [GI] lymphomas),( 9 ) had performance status (based on the Eastern Cooperative Oncology Group scale) 0–1, had no prior therapy, and had adequate organ functions. All patients gave written informed consent in accordance with our institutional review boards.
Figure 1.
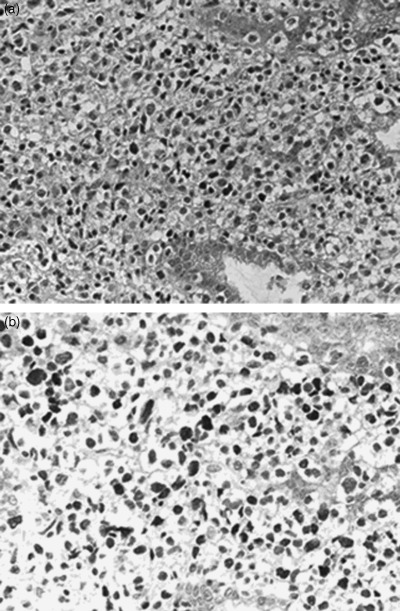
(a) Histology of a case of gastric diffuse large B‐cell lymphoma associated with Epstein–Barr virus (case no. 24). Lymphoma cells had a large nucleus and very little cytoplasm. Magnification of the objective lens was ×62. (b) Lymphoma cells of case no. 24 were positive for Epstein–Barr virus by in situ hybridization. Over half of the lymphoma cells showed nuclear positivity. Magnification of the objective lens was ×75.
Treatment details
All patients were treated with three cycles of CHOP (cyclophosphamide 750 mg/m2 day 1, doxorubicin 50 mg/m2 day 1, vincristine 1.4 mg/m2[capped at 2 mg] day 1, and oral prednisone 100 mg days 1–5) every 3 weeks. RT was started 3–4 weeks after the third cycle of CHOP. The primary tumor and the metastatic lymph nodes were irradiated for a total dose of 40.5 Gy in 27 fractions over 5.5 weeks. Details of other irradiation protocols were as described previously.( 7 )
Follow‐up evaluation and response assessment
The following evaluations were carried out until disease progression every 3 months for the first year after the completion of the protocol treatment, every 4 months for the second year, and every 6 months thereafter: physical examination, complete blood count, serum chemistries, gastroscopy with biopsy, and computed tomography (CT) scan of the abdomen. Endoscopic ultrasound of the stomach and CT scan of the chest were optional.
For the primary tumor in the stomach, CR was defined endoscopically as complete disappearance of all lesions with negative biopsy lasting for ≥4 weeks. Progressive disease (PD) was defined as gross tumor progression or appearance of any new lesion. Partial response (PR) was defined as all other cases where partial tumor regression was observed. The patients were followed up for 4–57 months (mean 37 months).
Epstein–Barr virus in situ hybridization and immunohistochemistory
Epstein–Barr virus‐encoded small RNA‐1 (EBER‐1) in situ hybridization was carried out using a single‐stranded 30‐bp fluorescein isothiocyanate‐labeled oligonucleotide complementary (antisense) or anticomplementary (sense, negative control) probe to a portion of the EBER‐1 gene. The sequence of the antisense probe was 5′‐AGACACCGTCCTCACCACCCGGGACTTGTA‐3′. The in situ hybridization was carried out on routinely processed sections of the paraffin‐embedded PGDL samples using the DAKO in situ hybridization kit (DakoCytomation, Kyoto, Japan), according to the manufacturer's instructions, and visualized with peroxidase‐diaminobenzidine‐4HCI (DAB). Patients in which over half of the lymphoma cells were positive were evaluated as EBER‐1 in situ positive (Fig. 1b).
Paraffin section immunohistochemistry for EBV‐related antigens was carried out in EBER‐1‐positive cases, using anti‐latent membrane protein (LMP)1 (DakoCytomation) and anti‐Epstein Barr virus nuclear antigen (EBNA)2 (DakoCytomation) antibodies as primary antibodies.
Statistical analysis
The correlation between EBV and the patients’ clinical outcomes was analyzed using the χ2‐test.
Results
Patient population
The median age of the patients was 62 years (range 20–73 years). Twenty‐six patients were male and 24 were female.
Response, survival and clinicopathology of unfavorable patients
Complete response was achieved in 45 (90%) of the 50 patients, PR was achieved in one patient and PD was achieved in four patients. Three patients underwent salvage gastrectomy due to disease persistence or recurrence after completion of radiotherapy. One CR patient developed recurrence in the cerebellum 3 months after the completion of treatment and was alive without disease for 9 months following whole brain radiotherapy and salvage chemotherapy. Two patients showed local recurrence 6 months after CR. In total, eight patients experienced disease progression, partial response or recurrence. The clinicopathological features of these eight patients are summarized in Table 1. Four of these patients died of disease progression.
Table 1.
Summary of the clinical records of patients with unfavorable prognoses for primary gastric diffuse large B‐cell lymphoma
| Patient no. | Age (years) | Sex | CS | IPI | Depth | Response | Recurrence (organ, time after CR) | Prognosis | Survival time (months) | Surgery | EBV |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 13 | 61 | Female | II1 | 1 | MP | PD | DOD | 7 | – | + | |
| 24 | 64 | Female | I | 1 | SS | PD | DOD | 5 | – | + | |
| 38 | 60 | Male | II1 | 0 | MP | PR | A | 29 | + | + | |
| 44 | 66 | Male | I | 1 | SS | CR | + (CNS; 3 months) | A | 28 | – | + |
| 3 | 66 | Male | I | 1 | SS | CR | + (Local; 6 months) | A | 51 | + | – |
| 19 | 61 | Male | II1 | 1 | SS | CR | + (Local; 6 months) | A | 44 | – | – |
| 43 | 57 | Male | I | 0 | SS | PD | DOD | 11 | + | – | |
| 48 | 64 | Female | I | 2 | SS | PD | DOD | 10 | – | – |
A, alive; CNS, central nervous system; CR, complete remission; CS, clinical stage; DOD, died of disease; EBV, Epstein–Barr virus; IPI, international prognostic index; MP, proper muscle layer; PD, progressive disease; PR, partial response; SS, subserosal layer.
For these eight patients who experienced disease progression, partial response or recurrence, their ages ranged 57–66 years. Five patients stage I and three had stage II1 diseases, two had elevated lactate dehydrgenase (LDH) before the start of treatment, and seven were of international prognostic index (IPI) low risk and one was of IPI low–intermediate risk. The lesions were found in the upper, middle and lower gastric parts of three, four and one patient, respectively. The macroscopic features of the lesions were as follows: five ulcerative, two superficial and one protruding. Comparing these patients with the other patients who showed favorable clinical courses, there was no difference in age, sex, clinical stage, IPI score or other pathological features, such as depth, localization and features of the lesions (P > 0.05).
Of the eight patients who showed unfavorable courses, four were associated with EBV infection; over half of their lymphoma cells were positive for EBV by in situ hybridization. Of the patients who showed favorable clinical courses (i.e. achieved CR without recurrence), none were EBV positive. The occurrence of EBV association was statistically higher in the unfavorable group than in the favorable group (P < 0.001). Three patients in the unfavorable group were LMP1 positive and EBNA2 negative, and the other was LMP1 positive and EBNA2 positive. EBV‐positive patients consisted of two PD, one PR and one CR with distant recurrences, and two patients died of disease.
Discussion
There are several lymphoproliferative disorders that are associated with EBV: Burkitt's lymphoma of endemic type, NK natural killer/T‐cell lymphoma of nasal type, post‐transplant lymphoproliferative disorder, human immunodeficiency virus (HIV)‐related lymphoma, pyothorax‐associated lymphoma, senile (age‐related) EBV‐positive B‐cell lymphoproliferative disorder, methotrexate‐associated lymphoma and Hodgkin's lymphoma. According to the list above, some EBV‐positive lymphoproliferative disorders are related to immunodeficiency. In the present study, four patients were associated with EBV, with three in latency II and the other patient in latency III. Latency II and III are often found in Hodgkin's and nasal type NK/T‐cell lymphomas, and lymphomas in immunocompromised host or pyothorax‐associated lymphomas, respectively. However, as far as we know, four EBV‐positive patients in the present study did not show any clinical evidence of immunodeficiency.
In the present study, we found that all four EBV‐associated PGDL patients took unfavorable clinical courses compared with the EBV‐negative patients. All EBV‐associated cases were classified as DLBCL, and it is rather difficult to predict the presence of EBV using histology only. If PGDL is positive for EBV, clinicians should undertake careful follow up, and if chemoradiotherapy is not effective, they should change the patient's therapy.
Several previous reports have described EBV association with primary gastric lymphomas: 11 out of 61 patients (Hui et al. Hong Kong),( 10 ) four out of 49 patients (Liu et al. Japan),( 11 ) seven out of 64 patients (Lee et al. Korea),( 12 ) two out of 33 patients (Yang et al. Korea),( 13 ) and 10 out of 46 patients (Chan et al. Hong Kong).( 14 ) Most reported cases were large B‐cell lymphomas, and EBV was not associated with low‐grade lymphomas, except for two cases in the study by Liu et al. Chan et al. reported EBV‐associated lymphomas showing much higher p53 gene mutations than EBV‐negative ones. To our knowledge, EBV association has not been well examined in Western countries except for in MALT lymphomas or immunocompromised patients.
The Southwest Oncology Group described a favorable 5‐year survival of 82% following three cycles of CHOP plus RT in patients with stage I or IE and non‐bulky stage II or IIE localized nodal and extranodal aggressive non‐Hodgkin's lymphoma.( 6 ) The German Multicenter Study Group reported an overall PGDL survival of 5 years for 78% of a non‐surgical group.( 15 ) These clinical outcomes are similar to those of our group, although the observation period in our study is too short to obtain a definite conclusion. Therefore, it may be worth checking for EBV infection in unfavorable cases of PGDL in Western countries though, the association with EBV is different between Western and Asian counties; nasal‐type NK lymphomas and pyothorax‐associated lymphomas are very rare in Western countries.
To date, the effects of EBV infection on the prognosis of lymphomas have not been well clarified. For example, the clinical outcome of Hodgkin's lymphoma in relation to EBV status has been controversial. However, Jarrett et al. have reported recently that overall survival (OS) is significantly better for EBV‐negative compared to EBV‐positive patients (P < 0.0001) in a population‐based study. They claimed that the impact of EBV status varied with age at diagnosis. In patients of younger age (16–34 years), there was no statistical significance between EBV status and OS. Among patients aged >50 years, EBV positivity was associated with a significantly poorer outcome.( 16 ) Oyama et al. have lately reported senile (age‐related) B‐cell lymphomas. Some of their patients were resistant to combination chemotherapy, and they assumed that EBV association may result in poorer clinical outcome.( 17 ) Their study comprised all Japanese patients, as did ours. Interestingly, all EBV‐postive PGDL cases in our study were over 50 years of age.
In the present study, we found that half of unfavorable PGDL cases treated non‐surgically were EBV positive. This finding strongly indicated that EBV‐associated PGDL was frequently resistant to this treatment strategy, although some other adverse factors remain unclear.
Acknowledgment
The authors are grateful to the pathologists affiliated with participating institutions for providing pathology materials for review. This study was supported by a Grant‐in‐Aid for Cancer Research (14‐9) from the Ministry of Health, Labour and Welfare, and a Grant‐in‐Aid for scientific research (B) (16390106) from the Japan Society for the Promotion of Science and Monbu Kagakusho, Japan.
Appendix I
Study participants
Hokkaido University (Toshiro Sugiyama, Tomoo Itoh), Sapporo; Sapporo Kosei Hospital (Tomonori Anbo, Shunji Muraoka), Sapporo; Asahi Central Hospital (Akira Nakamura), Asahi; Yamagata Prefectural Central Hospital (Hiroshi Saito, Shunichi Sasao), Yamagata; Tochigi Cancer Center (Hirofumi Fujii, Seiji Igarashi), Utsunomiya; Chiba Cancer Center (Toshiyuki Takagi, Makiko Itami) Chiba; National Cancer Center Hospital (Yukio Kobayashi, Ichiro Oda), Tokyo; Aichi Cancer Center (Tsuneya Nakamura, Michinori Ogura), Nagoya; Shiga Medical Center for Adults (Kazuhiko Mizuta, Eiji Takeuchi, Kazuo Ono), Moriyama; Kyoto Prefectural University of Medicine (Masafumi Taniwaki, Akio Yanagisawa), Kyoto; Osaka Medical College (Hiroya Takiuchi), Takatsuki; Okayama University (Hiroyuki Okada), Okayama; Hiroshima University (Shinji Tanaka, Hirofumi Nakayama, Koji Arihiro), Hiroshima; Shikoku Cancer Center (Tomohiro Nishina, Rieko Nishimura, Norihiro Teramoto), Matsuyama; Fukuoka University (Kazuo Tamura, Koichi Oshima), Fukuoka.
References
- 1. Wotherspoon AC. A critical review of the effect of Helicobacter pylori eradication on gastric MALT lymphoma. Curr Gastroenterol Rep 2000; 2: 494–8. [DOI] [PubMed] [Google Scholar]
- 2. Bozzetti F, Audisio RA, Giardini R, Gennari L. Role of surgery in patients with primary non‐Hodgkin's lymphoma of the stomach: an old problem revisited. Br J Surg 1993; 80: 1101–6. [DOI] [PubMed] [Google Scholar]
- 3. Ruskone‐Fourmestraux A, Aegerter P, Delmer A et al. Primary digestive tract lymphoma: a prospective multicentric study of 91 patients. Groupe d’Etude des Lymphomes Digestifs. Gastroenterol 1993; 105: 1662–71. [DOI] [PubMed] [Google Scholar]
- 4. Bartlett DL, Karpeh MS Jr, Filippa DA, Brennan MF. Long‐term follow‐up after curative surgery for early gastric lymphoma. Ann Surg 1996; 223: 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vaillant JC, Ruskone‐Fourmestraux A, Aegerter P et al. Management and long‐term results of surgery for localized gastric lymphomas. Am J Surg 2000; 179: 216–22. [DOI] [PubMed] [Google Scholar]
- 6. Miller TP, Dahlberg S, Cassady JR et al. Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate‐ and high‐grade non‐Hodgkin's lymphoma. N Engl J Med 1998; 339: 21–6. [DOI] [PubMed] [Google Scholar]
- 7. Ishikura S, Tobinai K, Ohtsu A, Nakamura S et al. Japanese multicenter phase II study of CHOP followed by radiotherapy in stage I–II, diffuse large B‐cell lymphoma of the stomach. Cancer Sci 2005; 96: 349–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jaffe ES, Harris NL, Stein H et al. World Health Organization Classification of Tumors: Pathology and Genetics of Tumors of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press, 2001. [Google Scholar]
- 9. Rohatiner A, D’Amore F, Coiffier B et al. Report on a workshop convened to discuss the pathological and staging classifications of gastrointestinal tract lymphoma. Ann Oncol 1994; 5: 397–400. [DOI] [PubMed] [Google Scholar]
- 10. Hui PK, Tokunaga M, Chan WY, Ng CS, Chow J, Lee JC. Epstein–Barr virus‐associated gastric lymphoma in Hong Kong Chinese. Hum Pathol 1994; 25: 947–52. [DOI] [PubMed] [Google Scholar]
- 11. Liu Q, Ohshima K, Masuda Y, Kikuchi M. Detection of the Epstein–Barr virus in primary gastric lymphoma by in situ hybridization. Pathol Int 1995; 45: 131–6. [DOI] [PubMed] [Google Scholar]
- 12. Lee SS, Jang JJ, Cho KJ, Khang SK, Kim CW. Epstein–Barr virus‐associated primary gastrointestinal lymphoma in non‐immunocompromised patients in Korea. Histopathol 1997; 30: 234–42. [DOI] [PubMed] [Google Scholar]
- 13. Yang WI, Cho MS, Tomita Y, Ohsawa M, Aozasa K. Epstein–Barr virus and gastrointestinal lymphomas in Korea. Yonsei Med J 1998; 39: 268–76. [DOI] [PubMed] [Google Scholar]
- 14. Chan WY, Chan EK, Chow JH. Epstein–Barr virus‐associated gastric lymphomas are distinct from mucosa‐associated lymphoid tissue‐type lymphomas: genetic abnormalities of p53 gene. Diagn Mol Pathol 2001; 10: 153–60. [DOI] [PubMed] [Google Scholar]
- 15. Koch P, del Valle F, Berdel WE et al. Primary gastrointestinal non‐Hodgkin's lymphoma. II. Combined surgical and conservative or conservative management only in localized gastric lymphoma − results of the prospective German Multicenter Study GIT NHL 01/92. J Clin Oncol 2001; 15: 3874–83. [DOI] [PubMed] [Google Scholar]
- 16. Jarrett RF, Stark GL, White J et al. Impact of tumour Epstein–Barr virus status on presenting features and outcome in age‐defined subgroups of patients with classical Hodgkin lymphoma: a population‐based study. Blood 2005; 106: 2444–51: [Epub June 7 ahead of print]. [DOI] [PubMed] [Google Scholar]
- 17. Oyama T, Ichimura K, Suzuki R et al. Senile EBV+ B‐cell lymphoproliferative disorders: a clinicopathologic study of 22 patients. Am J Surg Pathol 2003; 27: 16–26. [DOI] [PubMed] [Google Scholar]


