Abstract
In mammalian cells, four types of sialidase have been described and found to behave in different ways during carcinogenesis. We previously demonstrated that a human sialidase associated with plasma membranes (NEU3) is up‐regulated in human colon cancer and is involved in suppression of apoptosis. Here we document altered expression of another human sialidase, the recently identified NEU4, and evidence of its influence on the malignant phenotype in colon cancers. Human colon mucosa was relatively rich in NEU4, which has been observed to possess short and long isoforms, but hardly contained the latter form. In clear contrast to the NEU3 case, the levels of mRNA for this sialidase were found by quantitative RT‐PCR to be markedly decreased in colon cancers. In cultured human colon cancer cells, the enzyme was up‐regulated in the early stage of apoptosis induced by either the death ligand TRAIL or serum‐depletion, and transfection of NEU4 resulted in acceleration of apoptosis and in decreased invasion and motility. The siRNA‐mediated NEU4 targeting, on the other hand, caused a significant inhibition of apoptosis and promotion of invasion and motility. Lectin blot analyses revealed that desialylated forms of nearly 100 kDa glycoproteins were prominently increased with PNA in NEU4 transfectants, whereas only slight changes in glycolipids were detected as assessed by thin layer chromatography. These results suggest that NEU4 plays important roles for maintenance of normal mucosa mostly through desialylation of glycoproteins and that down‐regulation may contribute to invasive properties of colon cancers. (Cancer Sci 2007; 98: 299–307)
Abbreviations
- 4MU‐NeuAc
4‐methylumbelliferyl‐neuraminic acid
- DMEM
Dulbecco's modified Eagle's medium; ECL, enhanced chemiluminescence
- EDTA
ethylene diamine tetra‐acetic acid
- GAPDH
glyceraldehyde‐3‐phosphate dehydrogenase
- HPTLC
high‐performance thin layer chromatography
- Lac‐cer
lactosylceramide
- MAM
maackia amurens mutagen
- NaBT
sodium butyrate
- PBS
phosphate‐buffered saline
- PMSF
phenylmethanesulfonylfluoride
- PNA
peanut agglutinin
- pTNM
pathological tumor–node–metastasis; PVDF, polyvinylidene difluoride
- RCA
ricinus communis agglutinin
- RPMI
Roswell Park Memorial Institute 1640 medium
- RT‐PCR
reverse transcription‐polymerase chain reaction
- SDS‐PAGE
sodium dodecylsulfate–polyacrylamide gel electrophoresis
- SSA
sambucus siebaldiana agglutinin
- TLC
thin layer chromatography
- TRAIL
tumor necrosis factor (TNF)‐related apoptosis‐inducing ligand.
Alterations in sialic acids, generally found at the non‐reducing termini of most glycoproteins and glycolipids, have been observed to be closely associated with the malignant phenotype in terms of metastatic potential and invasiveness.( 1 , 2 , 3 , 4 , 5 , 6 ) However, the underlying molecular mechanisms are not fully understood. To elucidate the causes of aberrant sialylation and the consequences of these changes, we have focused on mammalian sialidases, which cleave sialic acid residues from glycoproteins and glycolipids to control their cellular content in collaboration with sialyltransferases. Sialidase expression levels indeed change in response to various cellular phenomena and especially in relation to the cancer phenotype.( 7 )
Endogenous sialidases of mammalian cells have been classified into four types (abbreviated to Neu1, Neu2, Neu3, and Neu4), differing in subcellular localization and enzymatic properties, and this multiple nature suggests that each form might play a unique role depending on its properties. Our observations of human sialidase revealed that NEU1, NEU3, and NEU4 seemed to be actually expressed in human tissues.( 8 ) The recently identified NEU4,( 8 , 9 , 10 ) is unique in that it possesses two forms, long and short, and can hydrolyze gangliosides as well as glycoproteins and oligosaccharides.( 8 ) The two forms differ in the presence and absence of 12 N‐terminal amino acid residues, which act as a mitochondrial targeting sequence and their localizations are actually mitochondria and intracellular membranes, respectively. The isoforms are also differentially expressed in a tissue‐specific manner; brain, muscle and kidney containing both, and the liver and colon possessing predominantly the short form, as assessed by RT‐PCR.
We have previously described murine sialidases Neu1 and Neu2 to exhibit altered expression, playing important roles in cancer development.( 11 , 12 , 13 ) Furthermore, we have documented increased expression of human plasma membrane‐associated sialidase NEU3 in colon,( 14 ) and renal( 15 ) cancers and presented evidence of their roles in suppression of apoptosis. However, human NEU1 and NEU4 have not been investigated extensively. In this report, we describe our findings for expression of NEU4 in colon cancer and its contribution to apoptosis, invasion and motility in vitro.
Materials and Methods
Patient samples. Surgical specimens were obtained from 41 colon cancer patients (aged 41–83 years, mean 63.9 ± 10.2 years), 22 males and 19 females who underwent resection of their tumors at Miyagi Cancer Center. The histological differentiation of the tumors was well (13 patients), moderate (27 patients) and mucinous (one patient), and pTNM pathological stages were: stage 1 for two patients, stage 2 for 16, stage 3 for 16 and stage 4 for seven. Samples were taken from non‐necrotic areas of tumors and from adjacent non‐tumor tissues, and immediately frozen on dry ice. Informed consent was obtained from each patient to allow the use of portions of tissue for research purposes, and the study was approved by the Committee on Human Rights in Research at Miyagi Cancer Center.
Tissue culture. Human colon cancer cell lines used in this study were HCT‐15 (Cancer Cell Repository, Tohoku University, Sendai, Japan), DLD‐1 (HSRRB, Osaka, Japan) and HCT‐116 (ATCC, Manassas, VA, USA). HCT‐15 cells were maintained in RPMI containing 10% fetal bovine serum and antibiotics and DMEM was used for the latter two.
NEU4 transfection. A NEU4 expression plasmid was constructed by subcloning the open reading frame of human NEU4 cDNA,( 8 ) into the pCAGGS expression vector with the chicken β‐actin promoter,( 16 ) generously provided by Dr Jun‐ichi Miyazaki, Osaka University School of Medicine. The plasmid was transiently introduced into HCT‐15, HCT‐116 and DLD‐1 cells using the Effectene (QIAGEN) reagent, and harvested after 24–48 h. To obtain NEU4 stable transfectants, an expression plasmid (pCEP‐hNeu4) was constructed by inserting the gene into the pCEP4 expression vector (Invitrogen), and hygromycin B (Roche Applied Science)‐resistant clones of DLD‐1 cells were isolated at a concentration of 250 µg/mL.
SiRNA Transfection. siRNAs targeting NEU4 and NEU3 and their control scrambled siRNAs were obtained from Dharmacon RNA Technologies, Inc. (La‐fayette, CO, USA). The sequence for targeting NEU4 was NEU4 siRNA (CCTTCTACAGCGATGACCA) beginning at nucleotides 632 of the NEU4 open reading frame sequence, and the scrambled siRNA was the control (TAACGCCGCTTATACCGAC). The sequence for NEU3 was NEU3 siRNA (AAGGGAGTGTGGTAAGTTT) beginning at nucleotides 839 of the open reading frame sequence, and the scrambled siRNA was (GCGATTAATGTAGGTTCGA). Transfection of siRNA into HCT‐15 cells was carried out in 60‐mm dishes by nucleofection with the Nucleofector System (Amaxa Biosystems). At 24 h after transfection, cells were used for experimentation.
Quantification of the mRNA levels of NEU4, NEU1 and NEU3 sialidases. Human NEU4, NEU1 and NEU3 mRNA levels were evaluated by quantitative real time RT‐PCR using a LightCycler rapid thermal cycler system (Roche) as described previously,( 8 ). Total RNA was isolated from primary tumors and adjacent tissues and also from colon cancer cells with an RNeasy kit (QIAGEN) as recommended by the manufacturer. First strand cDNAs were synthesized by reverse transcription and used as templates for PCR. Human NEU4 primers were 5′‐CCGTCTTCCTCTTCTTCATCGC‐3′ (forward), and 5′‐CATTGCAGTAGAGGAAGCTGCC‐3′ (reverse), which yielded a 411‐bp fragment. The primers for NEU1 were 5′‐TGAGAACGACTTCGGTCTGGTG‐3′ (forward), and CCAGGAAACACCATCATCCTTG‐3′ (reverse), yielding a 403‐bp fragment, those for NEU3 were 5′‐AGGTCAGTCTCCAGTACCTTC‐3′ (forward), and 5′‐ACATCCAGCATCCTGACTGTAG‐3′ (reverse), which yielded a 401‐bp fragment. To normalize for sample variation, expression of GAPDH was also assessed as an internal control. Standard curves for NEU4, NEU1 and NEU3 cDNAs were generated by serial dilution of the Bluescript vector containing the respective genes encoding the entire open reading frame. For the long form of NEU4 (NEU4L), the primers used were 5′‐CCACCCATGATGAGCTCTGCAG‐3′ (forward), and 5′‐GCGATGAAGAAGAGGAAGACGG‐3′ (reverse). The level of the short form was obtained by subtracting the value for the long form from the whole NEU4 level. The PCR reaction was carried out in glass capillary reaction vessels (Roche) in a 20‐µL volume of a reaction mixture containing 0.5 µM primers, cDNA, and QuantiTect SYBR Green PCR master mix (QIAGEN). Amplification of the cDNAs involved a 15‐min denaturation step, followed by 45 cycles at 94°C for 15 s, 60°C for 30 s and 72°C for 30 s. Fluorescence from SYBR Green bound to the PCR product was detected, and specificity of the reactions was confirmed by melting curve analysis and subsequently, by agarose gel electrophoresis.
Sialidase activity assays. Cells were sonicated in nine volumes of PBS containing 1 mM EDTA, 0.5 mM PMSF, leupeptin and pepstatin, and centrifuged at 1000 g for 10 min. The supernatant (crude extract) was then used for measurement of sialidase activity at pH 4.6 with the synthetic substrate 4MU‐NeuAc or with mixed gangliosides. The 4‐methylumbellyferone released from the former substrate was determined fluorometrically, and the modified thiobarbituric acid method was used for the assay with the latter as described elsewhere.( 17 ) Protein concentrations were determined by dye‐binding assay (Bio‐Rad Laboratories, Hercules, CA, USA). One unit was defined as the amount of enzyme cleaving 1 nmol of sialic acid/h.
Determination of cellular sialic acids. Cells harvested at subconfluency were washed with PBS and lyophilized. The glycolipids were extracted in sequence with chloroform/methanol (C/M, 1:1, v/v), C/M (2:1,v/v) and C/M(1:2, v/v), followed by evaporation to dryness. The glycolipid extracts (lipid‐bound sialic acid) and the residues after extraction (protein‐bound sialic acid) were treated in 0.1 N H2SO4 at 80°C for 1 h and assayed for sialic acids by fluorometric high‐performance liquid chromatography with malononitrile.( 18 )
Lectin blotting. The lectins used were SSA, MAM, PNA and RCA (Honen, Tokyo, Japan). Lectin blot analysis was conducted as described previously.( 12 ) Briefly, cell homogenates were resolved on a 10% SDS‐PAGE gel and transferred to PVDF membranes. Blots were washed in 10 mM Tris‐HCl (pH 7.5) containing 100 mM NaCl, and 0.1% Tween 20 and incubated with biotinylated lectins in the buffer. After washing, lectin‐binding glycoproteins were visualized with horseradish peroxidase‐streptavidin (Vector, Burlingame, CA, USA).
Thin layer chromatography. Glycolipids were extracted from cells as described elsewhere,( 15 ) fractionated by thin layer chromatography on HPTLC plates (Baker, Phillipsburg, NJ, USA) in C/M/0.5% CaCl2 (60:40:9, v/v/v) and visualized with orcinol‐H2SO4.
Apoptosis assays. Apoptosis was induced by treatment of cells (5 × 104/mL) with 20 ng/mL TRAIL plus 1 µg/mL antipoly histidine (R & D Systems, Inc. MN, USA) for 10–24 h,( 19 ) or by culture under serum‐deleted conditions for 24–72 h. To observe the effects of NEU4 overexpression on apoptosis, the cells were treated at 24 h after NEU4 transfection, The cells were then fixed and stained with Hoechst 33258 and apoptotic bodies were counted. Alternatively, cells labeled with propidium iodide were analyzed with CellFix by flow cytometry. To evaluate the caspase‐3 activity level, cell homogenates were electrophoresed on a 12% SDS‐PAGE gel, and the proteins transferred to PVDF membranes were Western blotted with antibody for cleaved caspase‐3 (Asp‐175, Cell Signaling Technology) using an ECL system (Amersham Pharmacia Biotech).
Invasion and motility assays. Invasion assays were carried out using Matrigel‐coated culture inserts (Falcon). At 24 h after transfection, cells were seeded at 2.5 × 105/well onto the upper surface membranes of cell culture inserts and the lower chambers were filled with medium containing 10 µg/mL fibronectin (Asahi Techno Glass, Tokyo). After 24 h cells were fixed and stained with Wright‐Giemsa solution and all those present on the lower surface of the membranes were counted under a microscope. For cell motility, chemokinesis assays (undirected migration) were carried out using non‐coated cell culture inserts as above.
Results
Decreased expression of NEU4 in human colon cancers. To assess NEU4 expression levels, we carried out quantitative real time RT‐PCR assays with common primers between the long and short forms of NEU4. As reported previously,( 8 , 9 ) NEU4 was expressed at the highest level in human liver and at relatively high levels in the brain and colon mucosa, whereas other human tissues contained very low levels of NEU4, as determined by real time RT‐PCR and by Northern blotting. The level of the long form was similar to that of the short form in brain, and extremely low in liver and colon. Figure 1 shows the results for relative NEU4 mRNA levels in tumor and adjacent non‐tumor mucosa. A marked decrease was detected in tumors as compared with non‐tumor mucosa, although a wide variety of values were obtained especially for the levels with non‐tumor. The average reduction was 2.8‐fold (Fig. 1a). The expression ratio of tumor to non‐tumor (T/NT) in each case was shown in Fig. 1(b), and most cases gave the values below 1. The average T/NT value was 0.162, indicating over 80% of the level in non‐tumor was decreased in tumor. Levels did not significantly correlate with the histological differentiation or pathological stages, but the T/NT value remained significant at P = 0.025 with venous invasion between v 0 (n = 28) and v 1–3 (n = 13). It is now under investigation whether there is a correlation between the level and clinical prognosis. When relative expression levels of the two forms of NEU4 were quantified, consistent with our previous results,( 8 ) the apparent level of the long form was hardly detectable in either tumor or non‐tumor mucosa (less than 0.04% of the whole NEU4 level), while the two forms were nearly equally present in brain under the same assay conditions, indicating that the reduction of NEU4 in tumor is likely to be due to changes in the short form. To compare with expression of other sialidases, using these tissues we evaluated NEU1 and NEU3 mRNA, which are actually expressed in human tissues (Fig. 1c). NEU1 mRNA levels showed a tendency of decrease in tumors compared to non‐tumors, but it was statistically insignificant. On the other hand, NEU3 levels were found to be markedly up‐regulated in all cases of tumors, which was in line with our previous observation.( 14 ) Thus, the clear opposite changes between NEU3 and NEU4 expression seemed to give reverse effects on colon cancer malignancy.
Figure 1.
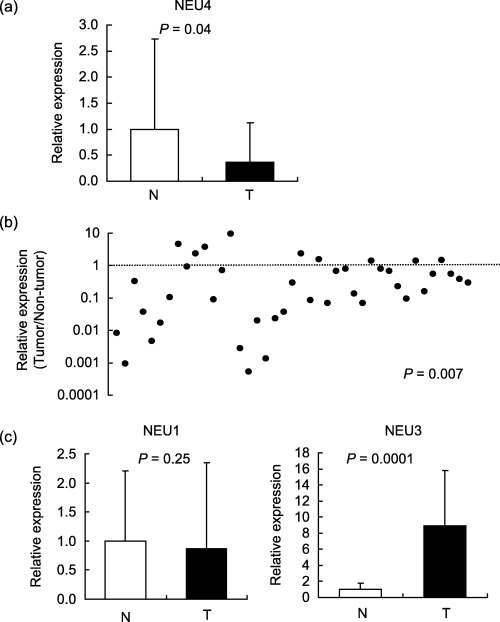
NEU4 expression in colon cancers and non‐cancerous mucosa. (a) Relative NEU4 mRNA levels in 41 tumor and adjacent non‐tumorous tissues were evaluated by quantitative real time reverse transcription‐polymerase chain reaction (RT‐PCR) and normalized for sample variation by glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) expression. (b) The expression ratio of tumor to non‐tumor (T/NT) in each case was shown and most cases gave the values below 1. (c) NEU1 and NEU3 mRNA levels were measured in the samples used for NEU4 assays. P‐values were evaluated by the Student's t‐test.
Up‐regulation of NEU4 in apoptosis‐induced human colon cancer cells. To understand the biological significance of the decreased NEU4 expression in colon cancer, we examined NEU4 changes in colon cancer cell lines during apoptosis induced by a death ligand TRAIL plus antipoly histidine,( 20 ) or by serum‐depletion in the human colon carcinoma HCT‐15, DLD‐1 and HCT‐116 cell lines. When expression levels of endogenous sialidases were measured by real time RT‐PCR in these cells, HCT‐15 cells were found to possess significantly higher NEU4 and NEU1 but lower NEU3 than the other two cells (Fig. 2a). In the HCT‐15 case, apoptotic cells accounted for 17.8% of the total population at 10 h after TRAIL treatment and 12.5% after 48 h under serum‐depleted conditions. Under these conditions, the endogenous NEU4 mRNA level was increased at the early phase of apoptosis, induced by either TRAIL or serum depletion as shown in Fig. 2(b). DLD‐1 and HCT‐116 cells also showed increased NEU4 expression; even their NEU4 levels were low. These data indicate that NEU4 expression is actually affected by cell apoptosis.
Figure 2.
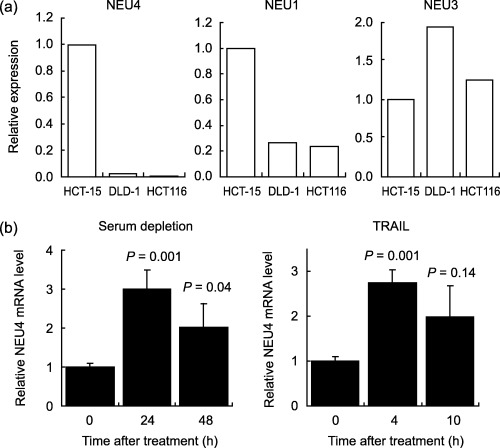
Alteration of NEU4 expression during apoptosis of human colon cancer cells. (a) The mRNA levels of endogenous sialidases, NEU1, NEU3, and NEU4 were compared by quantitative real time reverse transcription‐polymerase chain reaction (RT‐PCR) in the cells used in this study. (b) HCT‐15 cells were cultured under serum‐deleted conditions for 48 h (a) or treated with 20 ng/mL TRAIL plus 1 µg/mL antipoly histidine for 10 h (b). During the apoptosis wave, cells were collected and measured for endogenous NEU4 mRNA. The values were the means for three independent experiments in each case.
Promotion of apoptosis by NEU4 gene transfection. To observe the effects of NEU4 overexpression on apoptosis, the NEU4 gene was introduced into HCT‐15 human colon cancer cells. For overexpression of the gene, the short form was used, because normal colon mucosa contained mostly the short form as described above, and both forms showed similar substrate specificity.( 8 ) Transient transfection increased the sialidase activity to 10–15‐fold that of the vector‐transfected cells (4.9 ± 0.6 units/mg protein). It should be noted here that the gene transfection did not give any influence on the expression of other human sialidases in the cell system. In these NEU4 transfectants, the number of the cells stained with Hoechst 33258 after exposure to TRAIL was increased (Fig. 3a), and the population in the subG1 phase (apoptotic cells), expressed as a percentage of the total population, was also elevated (Fig. 3b). The increase of caspase‐3 activity with TRAIL was promoted remarkably by the transfection, as assessed by Western blot (Fig. 3c). To check further specific involvement of NEU4 in apoptosis, the NEU4 effect was observed in NEU3 silencing cells where NEU3 apoptosis‐suppressing function,( 14 ) was eliminated. Treatment with siRNA for NEU3 resulting in a 79% decrease of the mRNA caused increased apoptosis as expected, and then NEU4 overexpression further promoted cell apoptosis (Fig. 3d), indicating that NEU4 itself functions as an apoptosis enhancing molecule. Similar results were obtained with transient and stable transfectants of DLD‐1 cells treated with TRAIL. As shown in Figure 4a, the percentage of apoptotic cells was higher in the transiently transfected DLD‐1 cells than the controls. Using the stable transfectants (NEU4‐1 and NEU4‐2), apoptotic cells were found to be 57% and 82% in the cells with sialidase activity of 28 and 49 units/mg protein, respectively, when the vector transfected cells (4.5 unit/mg protein) showed 35% of apoptotic cells, suggesting that the effects were dependent on the NEU4 level. Consistent with these results, treatment of control HCT‐15 cells with TRAIL yielded a slightly higher percentage of apoptotic cells than the case of HCT‐116 cells having extremely low endogenous NEU4 level (30%vs 18% of apoptotic cells). Apoptotic cells were also more numerous after serum depletion in NEU4‐transfected DLD‐1 and HCT‐116 cells as compared with their controls as shown in Figure 4a (right panel) and 4b, respectively.
Figure 3.
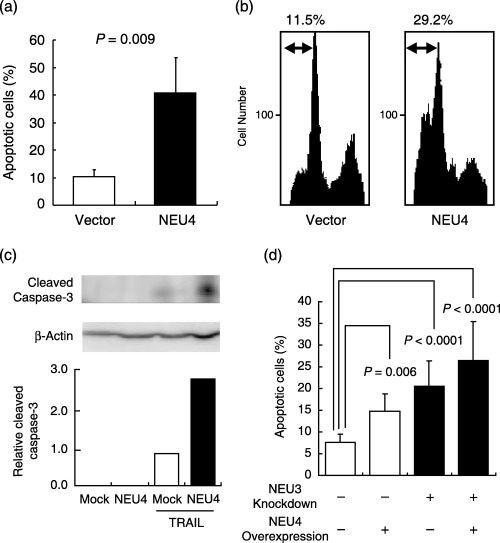
Effects of NEU4 overexpression on apoptosis induced by TRAIL treatment in human colon cancer cells. At 24 h after transient transfection, HCT‐15 cells were treated with TRAIL plus antipoly histidine for 12 h and apoptotic cells were detected by staining with Hoechst 33258, and counted to give percentages of the total cells (a). Bars indicate the standard deviations of the means for all experiments carried out in triplicate. P‐values were evaluated by the Student's t‐test. Apoptosis was also determined by analyzing cell populations in the subG1 phase fluorometrically (b), and by caspase‐3 activity assay by Western blot with an antibody for cleaved caspase‐3 (c). The NEU4 effect was also observed in the NEU3 silencing cells (d). The sialidase activities towards gangliosides were 4.1, 55.2, 3.4 and 60.8 units/mg protein for mock‐, NEU4‐transfected cells, NEU3 siRNA‐transfected cells, and NEU4‐cDNA and NEU3‐siRNA trans‐fected cells, respectively. The values were the means for two or three experiments in each case.
Figure 4.
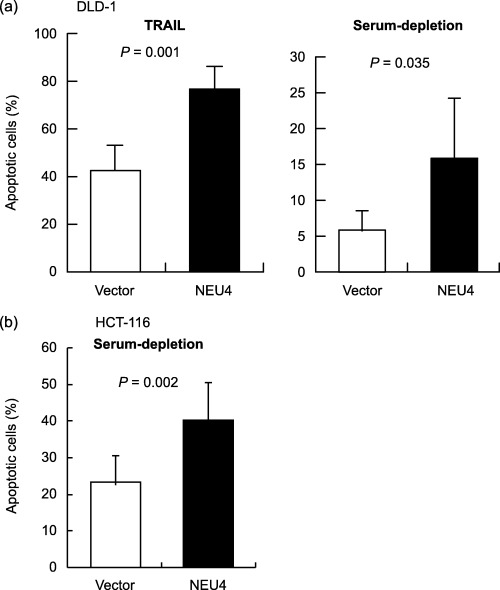
Effects of NEU4 overexpression on apoptosis induced by TRAIL or serum‐depletion in human colon cancer cells. After transient transfection, DLD‐1 cells were treated with TRAIL and apoptotic cells were detected with Hoechst 33258 (a, left panel). DLD‐1 (a, right panel) or HCT‐116 (b) cells were cultured under serum‐depleted conditions for 48 h and apoptotic cells were detected with Hoechst 33258. Bars indicate the standard deviations of the means for experiments carried out in triplicate. P‐values were evaluated by the Student's t‐test.
Inhibition of cell motility and invasion by up‐regulation of NEU4. The effects of NEU4 transfection on cell motility and invasion were observed using non‐coated and Matrigel‐coated culture inserts, respectively, in the presence of 10 µg/mL fibronectin. The chemokinesis assays (undirected migration) with non‐coated filters revealed that NEU4 overexpression decreased cell migration in HCT‐15 cells (Fig. 5a, left panel) and in DLD‐1 cells (Fig. 5b, left panel). In the latter case with stable transfectants of DLD‐1 cells having different levels of NEU4 expression as described above, reduction in cell motility might be dependent on the NEU4 level. Furthermore, the numbers of the HCT‐15 cells that penetrated Matrigel‐coated filters were also significantly decreased with transient expression of NEU4 (Fig. 5a, right panel). Similar results were obtained with stable transfectants of DLD‐1 cells (Fig. 5b, right panel).
Figure 5.
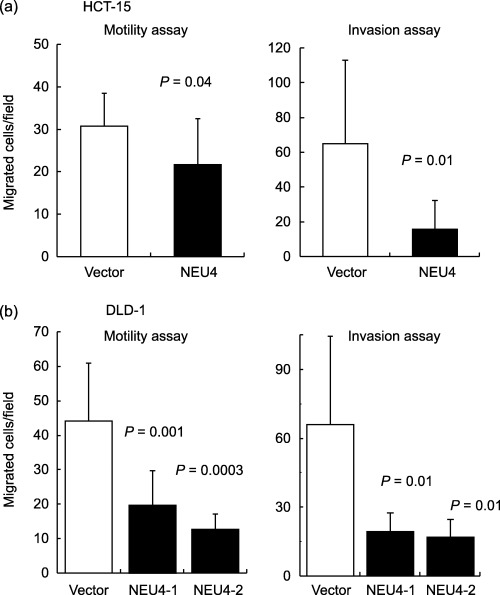
Effects of NEU4 overexpression on cell motility and invasion of human colon cancer cells. Twenty‐four hours after transfection, HCT‐15 or DLD‐1 cells were observed for cell motility and invasion using non‐coated and Matrigel‐coated culture inserts, respectively, in the presence of fibronectin in the lower chambers. The cells on the lower surface of the membranes were counted under a microscope. The stable transfectants of DLD‐1 cells, NEU4‐1 and NEU4‐2, showed sialidase activity of 28 and 49 units/mg protein, respectively. Bars indicate the standard deviations of the means for numbers of cells counted in 10 fields. P‐values were evaluated by the Student's t‐test.
Effects of siRNA‐mediated NEU4 silencing on cell apoptosis, motility and invasion. To confirm the NEU4 effect on apoptosis, motility and invasiveness, we examined whether NEU4 silencing affected these cell events in the opposite direction using the siRNA targeting both forms of the NEU4 gene. Introduction of NEU4 siRNA into HCT‐15 cells reduced mRNA levels by 64% and the scrambled control showed no significant change to the level (Fig. 6a), as estimated by real time RT‐PCR using GAPDH as the internal control. The sialidase activities were 4.8 and 2.8 units/mg protein in the cells with NEU4 siRNA and with the control, respectively. It was also confirmed by RT‐PCR that the siRNA did not exert any influence on the expression of other human sialidases. Opposite to the case of NEU4 overexpression, apoptotic cells at 12 h after TRAIL treatment were significantly decreased with the siRNA as compared with the scrambled control (Fig. 6b), which was confirmed by reduction of caspase‐3 activity in the siRNA‐treated cells as shown in Western blot (Fig. 6c). Furthermore, NEU4 silencing caused promotion of cell migration and invasion (Fig. 6d) under the same conditions as observed in NEU4‐transfected cells. These data strengthen the notion that NEU4 is essentially involved in expression of malignant properties of cancer cells including cell apoptosis, migration and invasion.
Figure 6.
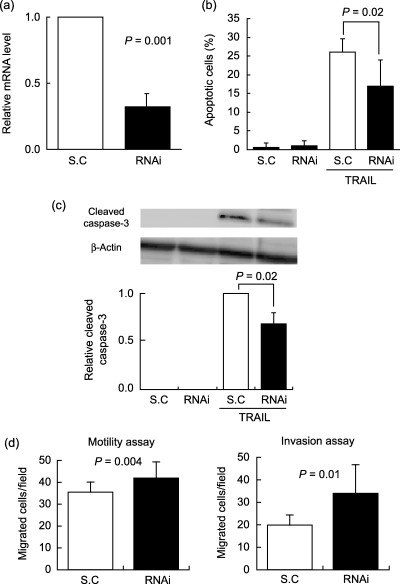
Effects of siRNA‐mediated NEU4 silencing on cell apoptosis, motility and invasion of human colon cancer cells. NEU4 mRNA levels were estimated by real time reverse transcription‐polymerase chain reaction (RT‐PCR) using glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) as the internal control after treatment with NEU4 siRNA or scrambled control (SC) (a). At 24 h after NEU4 siRNA introduction, HCT‐15 cells were treated with TRAIL for 12 h and estimated apoptotic cells (b) and caspase‐3 activity by Western blot (c) as described in Figure 3. Cell migration and invasion were observed using non‐coated and Matrigel‐coated culture inserts in the siRNA‐ or scrambled control‐treated cells (d).
Qualitative change in glycosylation in NEU4 transfected cells. To elucidate the molecular basis of the cell phenomena described above, we investigated how much sialic acid contents are removed as the result of sialidase reaction in the cells and whether specific ganglioside and glycoprotein molecules exist as targets for the sialidase. Transfectants with an activity of 45 units/mg protein showed decreased sialic acid contents (1.76 nmol/107 cells) as compared to those of the mock‐transfectants, and 58% of the whole amount of sialic acid reduced was protein‐bound and 42% for lipid‐bound cellular sialic acids, which was consistent with our previous data on substrate specificity that this sialidase hydrolyzes both glycolipids and glycoproteins. We then examined qualitative changes in cellular glycolipids and glycoproteins by thin layer chromatography and lectin blotting, respectively. When the glycolipids were subjected to thin layer chromatography, the levels of glycolipids corresponding to Lac‐Cer and globoside seemed to be slightly lower in NEU4 transfected cells than in control cells, although GM3 showed similar levels in both cells (Fig. 7a). NEU4 transfection might tend to cause acceleration of glycolipid degradation probably accompanied with compensation of reduced GM3. SSA, MAM, RCA and PNA lectins, which recognize preferentially α2‐6, α2‐3 sialyl linkages, Gal‐GlcNAc and Gal‐GalNAc, respectively, were then used for glycoprotein analysis. A significant difference in the binding affinity was not detectable between the transfected and control cells with the former three lectins, but the analysis with PNA revealed a prominent increase in the binding affinity to desialylated forms of about 100 kDa glycoproteins in the transfectants (Fig. 7b), indicating the removal of sialic acids from the molecules as a result of the sialidase reaction. We failed to detect any significant decrease in the affinity to either SSA or MAM lectin corresponding to increase to PNA, possibly being under cover of a large amount of sialo‐glycoproteins insensitive to the sialidase. These results indicate that some 100 kDa glycoproteins are likely targets for the expressing sialidase, and that their desialylation might be related to enhanced apoptosis and reduced cell invasion and motility, although the effects of slight changes in glycolipids can not be excluded.
Figure 7.
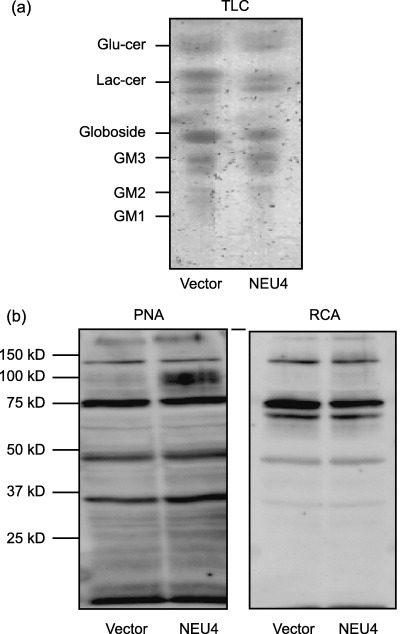
Thin layer chromatography and Lectin blotting of NEU4 transfected cells. Glycolipids were extracted from NEU4 transfected DLD‐1 cells, subjected to thin layer chromatography and visualized with orcinol‐H2SO4 (a). Glycoproteins of the DLD‐1 cells were analyzed by lectin blotting with ricinus communis agglutinin (RCA) or peanut agglutinin (PNA) lectins as described in ‘Materials and Methods’ and compared with those of the mock transfectants (b). Numbers indicate molecular size of the standard markers.
Discussion
The present study demonstrated that NEU4 mRNA level is decreased in colon cancers, in clear contrast to the case with NEU3.( 14 ) Up‐regulation was evident with the early stage of apoptosis induced by TRAIL treatment or serum depletion in the cancer cells, and NEU4 overexpression resulted in acceleration of apoptosis accompanied by caspase‐3 activation. As an essential involvement of NEU4 in apoptosis was confirmed by siRNA‐mediated NEU4 targeting, an early increase of endogenous NEU4 during apoptosis suggests that NEU4 itself seems to induce apoptosis for maintenance of normal mucosa and thus, the decrease of NEU4 in colon cancer might lead to protection against programmed cell death. The molecular mechanism of the NEU4 effect on apoptosis in response to TRAIL and serum depletion is uncertain at present, but it is possible that NEU4 might down‐regulate the MEK/ERK signaling pathway because both of the stimuli lead to apoptosis via decreasing ERK phosphorylation.( 21 ) The NEU4 reduction rate (T/NT) was only correlated statistically with venous invasion but not with histological differentiation or pathological stages, even the clear NEU4 effects were observed in the cells. It is unclear why the NEU4 effects do not always reflect on the clinical features, but one of the factors for this ambiguity is possibly due to the participation of NEU1 level influencing malignancy, as suggested in our previous work.( 11 ) NEU4 and NEU1 would collaborate together in the same direction toward reduced invasive properties of cancer cells.
Consistent with the data on cellular sialic acid contents, NEU4 possesses broad substrate specificity, capable of hydrolyzing most oligosaccharides, glycoproteins and gangliosides,( 8 ) whereas NEU1 acts effectively on oligosaccharides and glycopeptides and also glycoproteins to a lesser extent if highly activated, and NEU3 hydrolyzes preferentially gangliosides. Bearing this in mind, it is likely to be very difficult to distinguish the endogenous sialidase activity of NEU4 from the other two. However, we now understand the conundrum regarding our previous results on NEU3( 14 ) as to why the activity towards gangliosides was not as low as expected in non‐tumorous as compared to mucosa, despite the marked difference from tumor in NEU3 mRNA levels. This possibly occurred because ganglioside sialidase activity for NEU4 remained relatively high in the mucosa. The results on sialic acid analysis and lectin blotting suggest that effects of NEU4 on cell motility, invasiveness as well as cell apoptosis is mostly through desialylation of O‐glycosylated proteins, rather than hydrolysis of gangliosides, although a slight decrease in Lac‐cer and globoside by NEU4 may also affect these cell events. It would be most feasible that the desialylated O‐glycosylated proteins of about 100 kDa are possibly some types of mucins that are known to establish a selective molecular barrier at the epithelial cell surface and conduct signals in response to external stimuli as cell surface receptors.( 22 ) Since aberrant alteration in mucin expression or glycosylation has been implicated to accompany the development of cancer and influence various cellular phenomena including proliferation, differentiation, apoptosis, adhesion, invasion and immune surveillance,( 22 , 23 ) desialylation of mucins by NEU4 might cause changes in cell apoptosis, motility and invasion. For further understanding, it will be necessary to identify an endogenous substrate(s) for NEU4.
The present results on apoptosis induction provide an important clue to the biological significance of NEU4 expression in colon cancer. The opposing alteration expression patterns of NEU3 and NEU4 in colon cancer and during cell apoptosis suggest opposite influences on the malignant phenotype with reference to apoptosis, cell motility and invasion. In fact, our recent studies revealed that up‐regulation NEU3 plays roles in determining malignant properties of cancer cells.( 15 , 20 ) In contrast, NEU4 appears to be involved in maintaining the normal mucosa. The functional difference between the two sialidases might be due to the difference in their substrate specificities. NEU4 is likely a positive regulator for cell death, possibly through desialylation of O‐glycosylated proteins, whereas NEU3 could play a role as a negative regulator by modulation of gangliosides. Although molecular mechanisms underlying NEU4 effects on apoptosis, cell invasion and motility have yet to be fully elucidated, the present results point to a biological role of NEU4 in cell homeostasis, which is in accord with down‐regulation in human cancer.
Acknowledgments
We appreciate the expert technical assistance of Ms. Setsuko Moriya. This study was supported in part by Grants‐in Aid from the Mitsubishi Foundation, and by Grants‐in Aid for Scientific Research on Priority Areas Cancer from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1. Warren L, Buck CA, Tuszynski GP. Glycopeptide changes and malignant transformation. A possible role for carbohydrate in malignant behaviour. Biochim Biophys Acta 1978; 516: 97–127. [DOI] [PubMed] [Google Scholar]
- 2. Yogeeswaran G, Salk PL. Metastatic potential is positively correlated with cell surface sialylation of cultured murine tumor cell lines. Science 1981; 212: 1514–16. [DOI] [PubMed] [Google Scholar]
- 3. Fogel M, Altevogt P, Schirrmacher V. Metastatic potential severely altered by changes in tumor cell adhesiveness and cell‐surface sialylation. J Exp Med. 1983; 157: 371–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Collard JG, Schijven JF, Bikker A, La Riviere G, Bolscher JG, Roos E. Cell surface sialic acid and the invasive and metastatic potential of T‐cell hybridomas. Cancer Res 1986; 46: 3521–7. [PubMed] [Google Scholar]
- 5. Dennis JW, Lafarté S, Waghorne C, Breitman ML, Kerbel RS. β1‐6 branching of Asn‐linked oligosaccharides is directly associated with metastasis. Science 1987; 236: 582–5. [DOI] [PubMed] [Google Scholar]
- 6. Passaniti A, Hart GW. Cell surface sialylation and tumor metastasis. Metastatic potential of B16 melanoma variants correlates with their relative numbers of specific penultimate oligosaccharide structures. J Biol Chem 1988; 263: 7591–603. [PubMed] [Google Scholar]
- 7. Miyagi T, Wada T, Yamaguchi K, Hata K. Sialidase and malignancy: A minireview. Glycoconjugate J 2004; 20: 189–98. [DOI] [PubMed] [Google Scholar]
- 8. Yamaguchi K, Hata K, Koseki K et al. Evidence for mitochondrial localization of a novel human sialidase (NEU4). Biochem J 2005; 390: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Monti E, Bassi MT, Bresciani R et al. Molecular cloning and characterization of NEU4, the fourth member of the human sialidase gene family. Genomics 2004; 83: 445–53. [DOI] [PubMed] [Google Scholar]
- 10. Seyrantepe V, Landry K, Trudel S, Hassan JA, Morales CR, Pshezhetsky AV. Neu4, a novel human lysosomal lumen sialidase, confers normal phenotype to sialidosis and galactosialidosis cells. J Biol Chem 2004; 279: 37021–9. [DOI] [PubMed] [Google Scholar]
- 11. Kato T, Wang Y, Yamaguchi K et al. Overexpression of lysosomal‐type sialidase leads to suppression of metastasis associated with reversion of malignant phenotype in murine B16 melanoma cells. Int J Cancer 2001; 92: 797–804. [DOI] [PubMed] [Google Scholar]
- 12. Tokuyama S, Moriya S, Taniguchi S et al. Suppression of pulmonary metastasis in murine B16 melanoma cells by transfection of a sialidase cDNA. Int J Cancer 1997; 73: 410–15. [DOI] [PubMed] [Google Scholar]
- 13. Sawada M, Moriya S, Saito S et al. Reduced sialidase expression in highly metastatic variants of mouse colon adenocarcinoma 26 and retardation of their metastatic ability by sialidase overexpression. Int J Cancer 2002; 97: 180–5. [DOI] [PubMed] [Google Scholar]
- 14. Kakugawa Y, Wada T, Yamaguchi K et al. Up‐regulation of plasma membrane‐associated ganglioside sialidase (Neu3) in human colon cancer and its involvement in apoptosis suppression. Proc Natl Acad Sci USA 2002; 99: 10718–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ueno S, Saito S, Wada T et al. Plasma membrane‐associated sialidase is up‐regulated in renal cell carcinoma and promotes interleukin‐6‐induced apoptosis suppression and cell motility. J Biol Chem 2006; 281: 7756–64. [DOI] [PubMed] [Google Scholar]
- 16. Niwa H, Yamamura K, Miyazaki J. Efficient selection for high‐expression transfectants with a novel eukaryotic vector. Gene 1991; 108: 193–9. [DOI] [PubMed] [Google Scholar]
- 17. Miyagi T, Tsuiki S. Purification and characterization of cytosolic sialidase from rat liver. J Biol Chem 1985; 260: 6710–16. [PubMed] [Google Scholar]
- 18. Li K. Determination of sialic acids in human serum by reversed‐phase liquid chromatography with fluorimatric detection. J Chromatogr 1992; 579: 209–13. [DOI] [PubMed] [Google Scholar]
- 19. Kato K, Shiga K, Yamaguchi K et al. Plasma membrane‐associated sialidase (NEU3) differentially regulates integrin‐mediated cell proliferation through laminin‐ and fibronectin‐derived signaling. Biochem J 2006; 394: 647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Holmstrom TH, Tran SE, Johnson VL, Ahn NG, Chow SC, Eriksson JE. Inhibition of mitogen‐activated kinase signaling sensitizes HeLa cells to Fas receptor‐mediated apoptosis. Mol Cell Biol 1999; 19: 5991–6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tran SE, Holmstrom TH, Ahonen M, Kahari VM, Eriksson JE. MAPK/ERK overrides the apoptotic signaling from Fas, TNF, and TRAIL receptors. J Biol Chem 2001; 276: 16484–90. [DOI] [PubMed] [Google Scholar]
- 22. Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nature Rev Cancer 2004; 4: 45–60. [DOI] [PubMed] [Google Scholar]
- 23. Wei X, Xu H, Kufe D. Human MUC1 oncoprotein regulates p53‐ responsive gene transcription in the genetoxic stress response. Cancer Cell 2005; 7: 167–78. [DOI] [PubMed] [Google Scholar]


