Abstract
Because autonomous proliferating cancer cells are often exposed to hypoxic conditions, there must be an alternative metabolic pathway, such as autophagy, that allows them to obtain energy when both oxygen and glucose are depleted. We previously reported finding that autophagy actually contributes to cancer cell survival in colorectal cancers both in vitro and in vivo. Pancreatic cancer remains a devastating and poorly understood malignancy, and hypoxia in pancreatic cancers is known to increase their malignant potential. In the present study archival pancreatic cancer tissue was retrieved from 71 cases treated by curative pancreaticoduodenectomy. Autophagy was evaluated by immunohistochemical staining with anti‐LC3 antibody, as LC3 is a key component of autophagy and has been used as a marker of autophagy. The results showed that strong LC3 expression in the peripheral area of pancreatic cancer tissue was correlated with a poor outcome (P = 0.0170) and short disease‐free period (P = 0.0118). Two of the most significant correlations among the clinicopathological factors tested were found between the peripheral intensity level of LC3 expression and tumor size (P = 0.0098) or tumor necrosis (P = 0.0127). Activated autophagy is associated with pancreatic cancer cells, and autophagy is thought to be a response to factors in the cancer microenvironment, such as hypoxia and poor nutrient supply. This is the first study to report the clinicopathological significance of autophagy in pancreatic cancer. (Cancer Sci 2008; 99: 1813–1819)
Cancers are abnormal tissue masses whose growth exceeds and is uncoordinated with that of adjacent normal tissues, and which persist in the same excessive manner after cessation of the stimulus that evoked them.( 1 ) All cancers ultimately depend on the host for their nutrition and blood supply, but as the preexisting vasculature is obviously insufficient to support the cancers’ unlimited requirements for energy and nutrition as a result of their unregulated growth, angiogenesis has been considered pivotal to providing proliferating cancer cells with an adequate source of oxygen, energy, and nutrients. However, recent studies have revealed that even after new blood vessels have formed, both the oxygen and glucose supply is insufficient for the aggressively proliferating cancer cells in locally advanced cancers.( 2 , 3 , 4 ) Tumor hypoxia has been used as a marker of poor prognosis;( 5 , 6 ) however, how cancer cells become more malignant or survive with an extremely poor blood supply, as for example in pancreatic cancer, is poorly understood.( 7 ) When cancer cells are exposed to hypoxia, anaerobic glycolysis increases and provides energy for cell survival, but as the glucose supply is also insufficient because of the poor blood supply, there must be an alternative metabolic pathway that provides energy when both oxygen and glucose are depleted.( 8 , 9 ) We have reported that several cancer cell lines, including pancreatic cancer‐ and colorectal cancer‐derived cell lines, are resistant to nutrient‐deprived conditions. We have named this starvation‐resistant phenotype ‘austerity’ and speculated that austerity may contribute to cancer cell survival in a nutrient‐deficient microenvironment.( 8 , 9 )
Autophagy has long been known to be a non‐specific self‐degradation mechanism that is triggered by nutrient deprivation, but recently it has been shown to play an important role in removing redundant or faulty cell components, such as damaged mitochondria and other organelles that are targeted for lysosomal degradation.( 10 ) The autophagic process occurs in three steps: (1) autophagosome formation; (2) lysosomal fusion with the autophagosome; and (3) lysosomal degradation. In step 1, a cup‐shaped lipid bilayer called the isolation membrane is formed and engulfs cytosolic components, including organelles. In step 2, the isolation membrane closes, forming an autophagosome. Cytosolic LC3 (microtubule‐associated protein 1 light chain 3), a mammalian homolog of yeast ATG8, is converted to LC3‐I (soluble unlipidated form of LC3) during this step and LC3‐I is then modified to form LC‐3‐II (a membrane‐bound form) and becomes localized on autophagosomes. Autophagosomes fuse with lysosomes to form autolysosomes. In step 3, the contents of the autolysosomes are degraded rapidly by lysosomal hydrolases, including cathepsins B, D, and L. Intra‐autophagosomal LC3‐II is also degraded at the same time.( 11 ) Thus, LC3 is a key component of autophagy, and it has been used as a marker of autophagy.
Pancreatic cancer remains a devastating and poorly understood malignancy. Its poor prognosis has been attributed to the inability to make a diagnosis while the tumor is still resectable and a propensity toward early vascular dissemination and spread to regional lymph nodes. Up to 60% of patients have advanced pancreatic cancer at the time of diagnosis, and their median survival time is a dismal 3–6 months.( 12 ) Several pathological features, including tumor size, tumor grade, nodal metastasis, lymphatic or vascular infiltration, and perineural invasion, have been reported to be prognostic pathological parameters for patients with invasive ductal carcinoma (IDC) of the pancreas.( 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 ) However, their prognostic value has been a matter of controversy because the studies were based on relatively small numbers of IDC patients. Hypoxia in pancreatic cancer has been reported to increase its malignant potential.( 5 , 6 ) Studies investigating associations between tumor necrosis in IDC and expression of hypoxic markers, such as hypoxia‐inducible factor‐1α or carbonic anhydrase IX (CA IX), are expected to provide useful information concerning hypoxia‐driven angiogenesis in IDC of the pancreas.( 21 , 22 , 23 ) However, most tissue samples of IDC of the pancreas have been found to be relatively hypovascular compared with the surrounding pancreatic tissue.( 7 ) Proliferating cancer cells require more nutrients than surrounding non‐cancerous cells do, though nutrition is supplied via functionally structurally immature neovessels. In other words, because autonomous proliferating cancer cells are often exposed to hypoxic conditions, autophagy is a marker of malignant transformation under hypoxic stress. We speculate that the important process for cancer cells might be autophagy as hypoxia in pancreatic cancer has been reported to be a marker of poor prognosis.( 5 , 6 )
Experimental evidence supports a role for autophagy in both cancer development and suppression. Because autophagy‐specific genes promote the survival of normal cells during nutrient starvation in all eukaryotic organisms, autophagy may support the survival of rapidly growing cancer cells that have outgrown their vascular supply and are exposed to an inadequate oxygen supply or metabolic stress. By contrast, excessive levels of autophagy promote cell death, presumably via self‐cannibalization. Antitumor effects are observed at all levels of autophagy, in the form of either cell death (when the autophagy level is very low or very high) or cell death‐independent tumor‐suppressor effects (when the autophagy level is intermediate).( 24 ) There might be a balance between the autophagy level and survival of rapidly growing cancer cells if autophagy were turned on or off, according to the nutritional status of cancer cells during the processes of growth, invasion, and metastasis. In a previous study, we showed that autophagosomes are produced actively and consumed promptly in colorectal cancer cells during amino acid starvation, and autophagosome formation was seen only in the tumor cells, never in the adjacent non‐cancerous cells. In other words, we found that active autophagy contributes to cancer cell survival in colorectal cancers both in vitro and in vivo.( 25 )
It is easy to speculate that the pathophysiological role of autophagy in proliferating cancer cells varies with the type of cancer cell. Autophagy is thought to react to the cancer microenvironment, such as hypoxia and low nutrient supply. There have been no reports of studies on how autophagy functions in cancer tissue, comprising cancer cells and their microenvironment. In the present study we extended our investigation to an in vivo study of pancreatic cancer tissue and to elucidating the significance of autophagy in cancer tissue.
Materials and Methods
Patients. The subjects of this study were 71 patients who underwent curative pancreaticoduodenectomy at the National Cancer Center Hospital East, Chiba, Japan between December 1992 and February 2004. The pathological diagnosis in every case was IDC of the pancreas. The median age of the patients was 65 years (mean age 64.3 years), and 31 patients were women. None of the patients received neo‐adjuvant therapy before the operation. Regional lymph node dissection was carried out in all patients. None of the 71 patients received adjuvant therapy. All patients agreed to enrollment in the study and each gave informed consent. The institutional review board of the National Cancer Center approved all protocols on the patients’ agreements.
Pathological examination. The resected specimens were fixed in 10% formalin at room temperature, and the size and gross appearance of the tumors were recorded. The entire tumor was sectioned at intervals of approximately 0.5 cm, and all tumor‐containing sections were processed routinely and embedded in paraffin. Serial sections of each tumor were cut and stained with hematoxylin–eosin, and then examined to confirm the pathological diagnosis. Elastica staining was used to examine them for blood vessel infiltration.
Clinicopathological parameters. The prognostic value of the following histological parameters was assessed in the present study: (1) age (≥65 vs <65 years); (2) sex; (3) tumor size (≥3 vs <3 cm); (4) predominant differentiation of the tumor (well, moderately, or poorly differentiated); (5) lowest degree of tumor differentiation (well, moderately, or poorly differentiated); (6) lymphatic vessel infiltration (≥2 [score 2 or 3] vs <2 [score 0 or 1]); (7) blood vessel infiltration (≥2 [score 2 or 3] vs <2 [score 0 or 1]); (8) intrapancreatic neural invasion (≥2 [score 2 or 3] vs <2 [score 0 or 1]); (9) retroperitoneal invasion (absent vs present); (10) International Union Against Cancer (UICC) pathological T (pT) category (pT1, pT2, or pT3); (11) UICC pathological N (pN) category (pN0 vs pN1); (12) UICC pathological stage (pStage) (≥pStageIIB vs ≤pStageIIA); (13) tumor necrosis (absent vs present); and (14) nerve plexus invasion (absent vs present).( 26 ) Fourteen histological parameters were evaluated in this study according to the UICC,( 26 ) World Health Organization,( 27 ) and the Japan Pancreas Society.( 28 ) Predominant and lowest differentiation was evaluated according to the World Health Organization classification.( 27 ) Tumor necrosis was defined as confluent cell death in invasive areas of primary cancers, visible at an objective lens magnification of ×4.( 20 )
Outcome. All 71 patients were followed for survival, and the follow‐up period was measured from the date of surgery to 29 November 2004. The median follow‐up period was 371 days. Overall, 34 patients were diagnosed with local recurrence, 31 patients with liver metastasis, and nine patients with peritoneal metastasis during the follow‐up period. Fifty‐eight patients died of their disease. Recurrence was defined as initial tumor recurrence. Metastasis or local recurrence was considered as evidence of tumor relapse, and only deaths from pancreatic cancer were considered for the purposes of this study.
Immunohistochemical staining. The method of production of rabbit polyclonal LC3 antibody and of immunohistochemical staining for LC3 have been described previously.( 25 , 29 ) Formalin‐fixed, paraffin‐embedded tissue sections containing the maximal cancer tissue area were processed for immunohistochemical staining to enable evaluation of the several clinicopathological factors described below. Some representative cases were used for immunohistochemical staining using rabbit polyclonal CA IX antibody (1 : 50 dilution) (H‐120; sc‐25599; Santa Cruz Biotechnology) as a hypoxia marker,( 21 ) to examine whether the tumor cells with enhanced LC3 expression were under hypoxic stress.
Evaluation of LC3 expression level by immunohistochemical staining using LC3 antibody. Because nerve cells stain positive for LC3 immunohistochemically,( 25 ) we used the immunohistochemical staining of nerve cells in the tissue as an internal positive control to validate the immunohistochemical staining in each case. Cancer cells whose staining intensity was equal to or stronger than that of nerve cells were judged to be strongly positive, whereas those whose staining was clearly weaker than that of the nerve cells were judged to be weakly positive. Cancer cells that did not stain positively for LC3 immunohistochemically despite a positive internal control were judged to be negative. We selected sections containing the maximal cancer tissue area that included the center of the cancer tissue and the periphery and invasive border between the cancer tissue and non‐cancerous tissue. The midpoint between the margin and the center of the cancer tissue was defined as the border between the ‘peripheral area’ or ‘central area’. In other words, the area outside the border and the area inside the border were defined as the peripheral area or the central area, respectively. Thus, the peripheral area contained the invasive border of cancer tissue. Figure 1 shows a schema of how the central area and the peripheral area were defined for each tumor.
Figure 1.
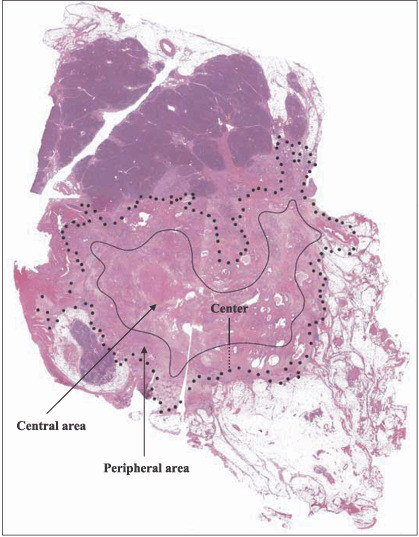
The central area and the peripheral area of the pancreatic cancer tissue. We mapped the margin of the tumor tissue area of each case and drew a bold dotted line surrounding the tumor tissue area using some slides covering the entire tumor tissue. The margin of tumor tissue is shown as the bold dotted line. The center is decided from the overview. The midpoint between the margin and the center of the cancer tissue was defined as the border between the ‘peripheral area’ or ‘central area’. As shown, the diameter (a small‐dotted straight black line) from the marginal bold dotted line of the tumor tissue to the border line and the diameter (a black straight line) from the border line to the center of the tumor tissue were the same. The border line (a black curved line) was drawn so that the diameters were almost the same inside the tumor tissue area. In other words, the area outside the border and the area inside the border were defined as the ‘peripheral area’ and the ‘central area’, respectively. Thus, the peripheral area contained the invasive border of cancer tissue.
The dominant intensity level of LC3‐positive cells was evaluated in each central area and peripheral area as follows. The level of intensity of LC3 staining in each area was determined by the percentage of cells that stained negative, weakly positive, and strongly positive. When more than 50% of the LC3‐positive cancer cells were strongly positive for LC3 in each area (peripheral area and central area), the area was evaluated as strongly positive, and when more than 50% of the LC3‐positive cancer cells were weakly positive for LC3, the area was designated weakly positive.
The cases were classified into three groups according to the dominant overall intensity of the cancer tissue: negative, weakly positive, or strongly positive. The dominant overall intensity in each case was determined according to the predominant intensity of LC3 positivity. When 30% of the cancer cells were weakly positive and 40% were strongly positive, the predominant intensity was recorded as strongly positive. One pathologist (S.F.) evaluated all of the immunohistochemical slides in the present study, and other pathologists (M.Y. and A.O.) also evaluated them to validate the reproducibility of immunohistochemical analyses.
The LC3 immunohistochemical staining factors were analyzed statistically to examine the relationship between clinicopathological factors, overall survival, and disease‐free survival.
Statistical analysis. All methods of statistical analysis have been described in a previous report.( 20 ) Overall survival curves and disease‐free survival curves were drawn using the Kaplan–Meier method. During the overall survival and disease‐free survival periods, significant differences in the levels of LC3 expression that were classified into two or three categories were examined using the log‐rank test. In the present study, the clinicopathological factors of pancreatic cancer were classified into two groups similar to the previous study.( 20 ) The relationships between the level of LC3 expression and clinicopathological factors were examined using the χ2‐test. The clinicopathological factors that were significantly associated with the expression level of LC3 in the peripheral area were further analyzed together in multivariate analyses using the Cox proportional hazard regression model to identify the factors that were most significantly associated with the tumor progression of pancreatic cancer. The P‐values were two‐sided, and the significance level was set at P < 0.05. All analyses were carried out using the Statview‐J 5.0 package, Windows version (SAS).
Results
LC3 is expressed in pancreatic cancer tissue. As imaging analyses of pancreatic cancer in vivo have shown that cancer tissue is more hypovascular than the surrounding non‐cancerous tissue,( 7 ) it is speculated that cancer cells use autophagy as a means of nutrition. We evaluated autophagy in surgically resected pancreatic cancer tissue by immunohistochemical staining with anti‐LC3 antibody. Formalin‐fixed and paraffin‐embedded tissue samples from 71 cases of IDC were processed for immunohistochemical analysis, and LC3 expression in the cancer tissue was assessed by comparing it with the staining intensity of nerve cells in intra‐ or peripancreatic tissue, which expresses LC3 consistently (2, 3). Most pancreatic cancer tissues stained positively for LC3. Weakly or strongly positive expression of LC3 was observed in 62 of the 71 cases of IDC of the pancreas, but there were no positive cells in the cancer tissue from the other nine cases. Table 1 compares the intensity of LC3 expression between the peripheral area and central area of the pancreatic cancer tissue. Intensity in the peripheral area was negative, weakly positive, and strongly positive in nine cases (12.7%), 23 cases (32.4%) and 39 cases (54.9%), respectively. The peripheral area of the pancreatic cancer tissue was strongly positive for LC3 expression in more than half of the cases examined in the present study. The intensity in the central area was negative, weakly positive, and strongly positive in 17 cases (23.9%), 33 cases (46.5%), and 21 cases (29.6%), respectively. The dominant overall intensity of LC3 expression (the most representative level of intensity in the cancer tissue as a whole) was negative, weakly positive, and strongly positive in nine cases (12.7%), 31 cases (43.65%), and 31 cases (43.65%), respectively (Table 1). Thus, the number of cases in which the individual cancer cells were weakly or strongly positive for LC3 was the same as the number of cases counted from the view of dominant overall intensity of LC3 expression.
Figure 2.
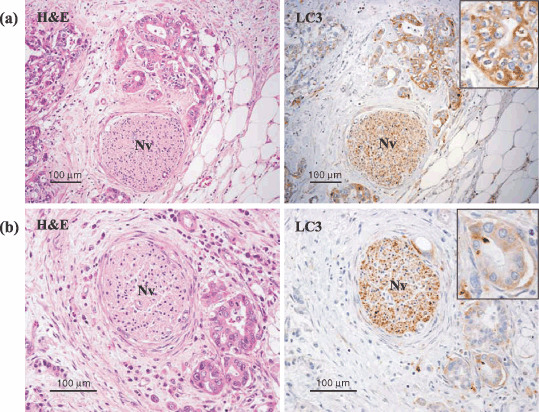
LC3 protein was detected in pancreatic cancer tissue by immunohistochemical staining with LC3 antibody. Cancer tissue stained immunohistochemically with LC3 antibody and corresponding hematoxylin–eosin (HE)‐stained sections are shown. A nerve cell (Nv) was used as an internal positive control to validate immunohistocheimical staining and evaluate the level of intensity of LC3 expression. The inserts are the photographs at higher magnification. (a) The cancer cells that stained as or more intensely for LC3 than the nerve cells were recorded as strongly positive. (b) The cancer cells that stained less intensely for LC3 than the nerve cells were recorded as weakly positive.
Figure 3.
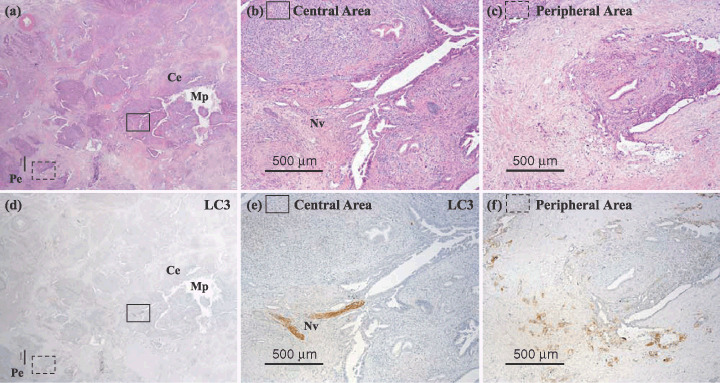
A representative pancreatic tumor is shown. Cancer tissue (d–f) stained immunohistochemically with LC3 antibody and (a–c) corresponding hematoxylin–eosin (HE)‐stained sections are shown. The peripheral area of the pancreatic cancer tissue (Pe) is more strongly positive for LC3 protein than the central area (Ce). Mp, main pancreatic duct; Nv, nerve cell.
Table 1.
Intensity level of LC3 expression between the peripheral area and central area of the pancreatic cancer tissue, and the dominant overall intensity of LC3 expression of the pancreatic cancer tissue
| Staining | Dominant intensity in the peripheral area (%) | Dominant intensity in the central area (%) | Dominant overall intensity (%) |
|---|---|---|---|
| Negative | 9 (12.7) | 17 (23.9) | 9 (12.7) |
| Weakly positive | 23 (32.4) | 33 (46.5) | 31 (43.65) |
| Strongly positve | 39 (54.9) | 21 (29.6) | 31 (43.65) |
| Total cases | 71 (100) | 71 (100) | 71 (100) |
We examined the difference in intensity level of LC3 expression in the peripheral area and central area of the pancreatic cancer tissue from all 71 cases (Table 2). We classified the pattern of intensity level into three groups according to whether the peripheral or central area contained more cells that stained immunohistochemically positive for LC3: (1) peripheral > central; (2) peripheral = central; and (3) peripheral < central. The peripheral intensity level of LC3 staining was stronger in 25 (40.3%) of the 62 cases that were positive for LC3 expression, and weaker in only two cases (3.2%), and LC3 expression in the peripheral area and central area was the same in 35 cases (56.5%). There was a trend toward a stronger level of intensity of LC3 expression in the peripheral area of the pancreatic cancer tissue, which included the invasive border.
Table 2.
Differences in the intensity level of LC3 expression in the peripheral area and central area of the pancreatic cancer tissue
| No. cases | Pattern of intensity level | ||
|---|---|---|---|
| Peripheral > central (%) | Peripheral = central (%) | Peripheral < central (%) | |
| 62 | 25 (40.3) | 35 (56.5) | 2 (3.2) |
To address the question of whether peripheral tumor cells with enhanced LC3 expression are under hypoxic stress, we carried out immunohistochemical staining using CA IX as a hypoxia marker on some representative cases. The results showed that in some cases the tumor cells with strong LC3 expression at the peripheral area concomitantly showed enhanced expression of CA IX (Fig. 4).
Figure 4.
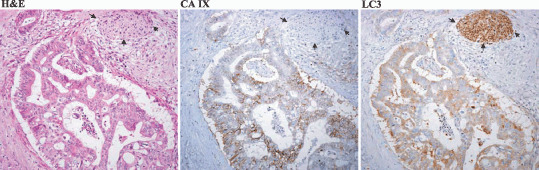
Tumor cells with enhanced LC3 expression at the peripheral area concomitantly expressed carbonic anhydrase IX (CA IX) as a hypoxia marker. Three photographs of a representative case are shown (hematoxylin–eosin and CA IX and LC3 expression by immununohistochemical staining). The black arrows show the peripheral nerve at the peripheral area of the tumor tissue and which served as a positive internal control of LC3 expression.
Strong expression of LC3 in the peripheral area of the cancer tissue correlates with poor outcome and a short disease‐free period. We analyzed the relationship between the intensity of LC3 expression (negative, weakly positive, strongly positive) in the peripheral area of pancreatic cancer tissue and overall survival. There was a trend toward the group with a strongly positive peripheral area to have a poor outcome (P = 0.0579) (Fig. 5a). We speculated that the cells strongly positive for LC3 in the peripheral area of the cancer tissue have a significant role in the characteristics of aggressive pancreas cancers and we divided the cases into two groups, a negative or weakly positive group and a strongly positive group, according to the results for staining in the peripheral area. Interestingly, there was a significant correlation between the level of intensity of LC3 expression in the peripheral area of the cancer tissue and poor outcome (P = 0.0170) (Fig. 5b). Moreover, cases in which the dominant overall intensity of LC3 expression was strongly positive had a poorer outcome than the group in which the dominant overall intensity was weakly positive or negative (P = 0.0301) (Fig. 5c). Cases with a strongly positive intensity level in the peripheral area of the cancer tissue had a significantly shorter disease‐free period than the cases with a negative or weakly positive intensity level (P = 0.0118) (Fig. 5d).
Figure 5.
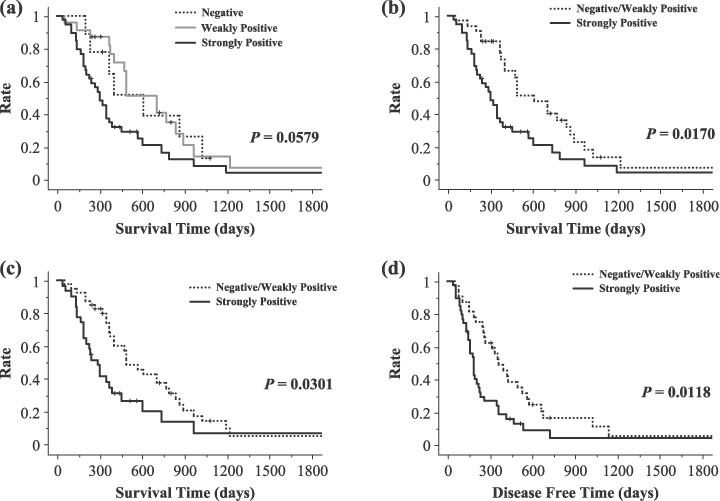
Overall survival curves and disease‐free curve according to the level of intensity of LC3 protein expression in the peripheral area and the dominant overall intensity of LC3 expression. (a) Overall survival curves according to the level of intensity of LC3 expression in the peripheral area of the cancer tissue in a negative group, weakly positive group, and strongly positive group. There was a trend for patients with strongly positive expression of LC3 protein to have a poor outcome (P = 0.0579). (b) Overall survival curves according to the level of intensity of LC3 protein expression in the peripheral area of the cancer tissue in the negative and weakly positive group and the strongly positive group. The group with strongly positive expression of LC3 protein had a significantly shorter survival time (P = 0.0170). (c) Overall survival curves according to the dominant overall intensity of LC3 protein expression in the negative and weakly positive group and the strongly positive group. The group with strongly positive expression of LC3 protein had a significantly shorter survival time (P = 0.0301). (d) Disease‐free curves according to the level of intensity of LC3 protein expression in the peripheral area of the cancer tissue in the negative and weakly positive group and the strongly positive group. The group with strongly positive expression of LC3 protein in the peripheral area of the cancer tissue had a significantly shorter disease‐free period (P = 0.0118).
LC3 expression is correlated with clinicopathological factors, including tumor necrosis, differentiation, blood vessel infiltration, and tumor necrosis. In our study, there were significant correlations between a strong intensity of LC3 expression in the peripheral area, which included the invasive border, and a poor outcome and short disease‐free survival time. In the next step, we investigated the relationship between LC3 expression and clinicopathological factors, including age, sex, tumor size, predominant differentiation, lowest differentiation, lymphatic vessel infiltration, blood vessel infiltration, intrapancreatic neural invasion, retroperitoneal invasion, UICC pT, UICC pN, UICC pStage, tumor necrosis, and nerve plexus invasion. The results showed significant correlations between the intensity level of LC3 expression in the peripheral area of the tumor and tumor size, predominant differentiation, lowest degree of differentiation, blood vessel infiltration, and tumor necrosis (P < 0.05) (Table 3), and two of the most significant correlations were with tumor size (P = 0.0098) or tumor necrosis (P = 0.0127) (Table 3).
Table 3.
Relationship between LC3 expression and clinicopathological factors of pancreatic cancer
| No. cases | LC3 expression | P‐value | |||
|---|---|---|---|---|---|
| N | W | S | |||
| Age | |||||
| <65 years | 37 | 3 | 10 | 24 | |
| ≥65 years | 34 | 6 | 13 | 15 | 0.1875 |
| Sex | |||||
| Male | 40 | 5 | 13 | 22 | |
| Female | 31 | 4 | 10 | 17 | 0.9987 |
| Tumor size | |||||
| <3.0 cm | 34 | 3 | 17 | 14 | |
| ≥3.0 cm | 37 | 6 | 6 | 25 | 0.0098 |
| Predominant differentiation | |||||
| Well | 25 | 5 | 13 | 7 | |
| Moderately | 37 | 3 | 8 | 26 | 0.0227 |
| Poorly | 9 | 1 | 2 | 6 | |
| Lowest differentiation | |||||
| Well | 8 | 2 | 5 | 1 | |
| Moderately | 27 | 2 | 11 | 14 | 0.0431 |
| Poorly | 36 | 5 | 7 | 24 | |
| Lymphatic vessel infiltration | |||||
| 0 or 1 | 50 | 6 | 19 | 25 | |
| 2 or 3 | 21 | 3 | 4 | 14 | 0.2940 |
| Blood vessel infiltration | |||||
| 0 or 1 | 7 | 1 | 5 | 1 | |
| 2 or 3 | 64 | 8 | 18 | 38 | 0.0497 |
| Intrapancreatic neural invasion | |||||
| 0 or 1 | 20 | 3 | 6 | 11 | |
| 2 or 3 | 51 | 6 | 17 | 28 | 0.1680 |
| Retroperitoneal invasion | |||||
| 0 or 1 | 27 | 4 | 12 | 11 | |
| 2 or 3 | 44 | 5 | 11 | 28 | 0.1567 |
| UICC pT | |||||
| pT1 or pT2 | 4 | 1 | 1 | 2 | |
| pT3 | 67 | 8 | 22 | 37 | 0.7415 |
| UICC pN | |||||
| pN0 | 11 | 2 | 5 | 4 | |
| pN1 | 60 | 7 | 18 | 35 | 0.4038 |
| UICC pStage | |||||
| IA/IB/IIA | 14 | 2 | 6 | 6 | |
| IIB/III/IV | 57 | 7 | 17 | 33 | 0.5805 |
| Tumor necrosis | |||||
| Absent | 50 | 7 | 21 | 22 | |
| Present | 21 | 2 | 2 | 17 | 0.0127 |
| Nerve plexus invasion | |||||
| Absent | 26 | 3 | 9 | 14 | |
| Present | 45 | 6 | 14 | 25 | 0.9450 |
N, negative; S, strongly positive; W, weakly positive.
Multivariate analyses of parameters significantly associated with overall survival. We carried out multivariate analyses to investigate the prognostic value of tumor size greater than 3.0 cm, predominantly poor differentiation, lowest degree of differentiation, 2 or 3 degrees of blood vessel infiltration, tumor necrosis and strongly positive intensity at the peripheral area, but only tumor size greater than 3.0 cm significantly increased the hazard ratio for overall survival (P = 0.0018, hazard ratio = 2.8, 95% confidence interval = 1.4–5.2). None of the other factors significantly increased the hazard ratio for overall survival in the multivariate analyses.
Discussion
The clinicopathological significance of autophagy in cancer progression has remained unclear, and the role of autophagy in cell fate decisions remains a matter of controversy. Autophagy has been regarded as playing a role in providing nutrients by degrading existing cellular components. It is referred to as a recycling of cell constituents and an adaptive response to various cell stresses, such as energy deficiency.( 10 ) Recently, the role of autophagy has also been shown to be an indispensable physiological reaction in caspase‐independent programmed cell death (autophagic death), and autophagic cell death has been found to eliminate damaged and harmful cells, such as cancer cells damaged by anticancer reagents and cells infected with pathogenic microorganisms.( 30 , 31 ) Paradoxically, autophagy has been proposed to be an indispensable physiological reaction for sustaining cell viability under nutrient‐starved conditions.( 10 ) In our previous study, we showed that colorectal cancer cells harbor functional autophagic machinery and that the autophagic machinery functions to prolong cell survival during shortages of nutrients.( 25 ) The membrane‐bound LC3‐II protein level is used as a marker of autophagosome formation. We detected LC3‐II by western blotting with the anti‐LC3 antibody used in the previous study, and LC3 protein has also been detected in colon cancer cells by immunohistochemical staining.( 25 ) We demonstrated that LC3 protein detected by immunohistochemical staining coincided with the localization of autophagosomes in colon cancer tissue specimens containing LC3 protein by electron microscopy and that autophagy contributes directly to cancer cell survival during nutrient starvation.( 25 ) In a previous experiment, both an autolysosomal protease inhibitor and 3‐methyladenine, an inhibitor of autophagosome formation, induced marked apoptotic death in all colorectal cancer cells examined.( 25 ) However, the significance of excess expression of autophagic machinery in cancer tissue samples remained unclear. In the present study we showed that LC3 positivity of pancreatic cancer tissue was correlated with poor overall survival and a shorter disease‐free period. Constitutive formation of autophagosomes in cancer cells may contribute to cell survival in the harsh cancer microenvironments in which cancers are known to progress.
On the other hand, autophagy may not be a causal step in malignant transformation at the cellular level and may instead be an indicator of a poor blood supply in the cancer microenvironment.( 32 ) Interestingly, however, significant associations were found between the LC3 expression level in the peripheral area, which included the invasive margin, and several clinicopathological factors, including tumor size, predominant differentiation, lowest differentiation, blood vessel infiltration, and tumor necrosis. Multivariate analyses showed that the LC3 expression level in the peripheral area was not an independent prognostic factor, but that tumor size greater than 3.0 cm, which was the factor most significantly correlated with LC3 expression (P = 0.0098, Table 3), was significantly associated with poor overall survival. Moreover, there was a correlation between tumor necrosis and strong expression of LC3 in pancreatic cancer tissue (P = 0.0127; Table 3). Two cases showed decreased intensity of LC3 expression by immunohistochemical staining at the peripheral area (Table 2). Both of them showed an absence of tumor necrosis and the sizes of both tumors were less than 3 cm. Nine cases were totally negative for LC3 expression (Table 3). Only two cases (2.8%) were negative for LC3 expression and tumor necrosis, whereas 19 cases (26.8%) were positive for LC3 expression and tumor necrosis. Six cases (8.5%) were negative for LC3 expression and had a tumor size of 3.0 cm ≤ 0. Twenty‐five cases (35.2%) were positive for LC3 expression and had a tumor size of ≤3.0 cm. Negative cases showed lesser tumor size (<3.0 cm) and an absence of tumor necrosis with significant correlations. These results suggest the functional significance of autophagy in pancreatic cancer tissue. This observation indicates that autophagy may promote cell viability in hypovascular pancreatic cancer tissue, where only limited oxygen and nutrient supplies would be expected. To address the question of whether peripheral tumor cells with enhanced LC3 expression are under hypoxic stress, we carried out immunohistochemical staining using CA IX as a hypoxia marker on some representative cases. There were some cases whose tumor cells with strong LC3 expression at the peripheral area showed concomitant enhanced expression of CA IX (Fig. 4). However, further studies are needed to address the relationship between autophagy and hypoxia, angiogenesis, and nutrient starvation by investigating a large number of cases and many kinds of cancer tissues. No LC3 expression was detected in the non‐cancerous ductal epithelium of the pancreas in the present study (data not shown). We do not understand this discrepancy if autophagy is regarded as just a physiological response to poor blood supply. Moreover, as LC3 expression was significantly correlated with various clinicopathological factors, further study will be needed to determine its significance in relation to the malignant character of cancer cells.
Based on all of the above, taken together, we conclude that activated autophagy is associated with pancreatic cancer cells and that LC3 expression by pancreatic cancer cells is significantly correlated with a poor outcome. This is the first study to show the clinicopathological significance of autophagy in relation to a poor outcome and associations with clinicopathological parameters. It is speculated that autophagy may play a variety of pathophysiological roles in carcinogenesis, cancer progression, and metastasis, and that its role may vary with the cancer cell type due to differences in the characters of the cancer cells themselves and the microenvironment of the cancer tissue. To better understand the autophagic machinery for cancer cell survival related to carcinogenesis and cancer progression, we plan to extend our investigation to other cancer cell types by using cancer tissue samples.
Acknowledgments
This work was supported by a grant from the Ministry of Health, Labour, and Welfare for the Third‐Term Comprehensive 10‐year Strategy for Cancer Control. We wish to thank Miss Mai Okumoto for her excellent technical assistance.
References
- 1. Kumar V, Cotran RS, Robbins SL. Robbins Pathologic Basis of Disease, 5th edn. Philadelphia: W.B. Saunders, 1992. [Google Scholar]
- 2. Jain RK. Molecular regulation of vessel maturation. Nat Med 2003: 9: 685–93. [DOI] [PubMed] [Google Scholar]
- 3. Vaupel P, Thews O, Hoeckel M. Treatment resistance of solid tumors: role of hypoxia and anemia. Med Oncol 2001; 18: 243–59. [DOI] [PubMed] [Google Scholar]
- 4. Harris AL. Hypoxia – a key regulatory factor in tumour growth. Nat Rev Cancer 2002; 2: 38–47. [DOI] [PubMed] [Google Scholar]
- 5. Koong AC, Mehta VK, Le QT et al . Pancreatic tumors show high levels of hypoxia. Int J Radiat Oncol Biol Phys 2000; 48: 919–22. [DOI] [PubMed] [Google Scholar]
- 6. Buchler P, Reber HA, Lavey RS et al . Tumor hypoxia correlates with metastatic tumor growth of pancreatic cancer in an orthotopic murine model. J Surg Res 2004; 120: 295–303. [DOI] [PubMed] [Google Scholar]
- 7. Kitano M, Kudo M, Maekawa K et al . Dynamic imaging of pancreatic diseases by contrast enhanced coded phase inversion harmonic ultrasonography. Gut 2004; 53: 854–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Izuishi K, Kato K, Ogura T, Kinoshita T, Esumi H. Remarkable tolerance of tumor cells to nutrient deprivation: possible new biochemical target for cancer therapy. Cancer Res 2000; 60: 6201–7. [PubMed] [Google Scholar]
- 9. Esumi H, Izuishi K, Kato K et al . Hypoxia and nitric oxide treatment confer tolerance to glucose starvation in a 5′‐AMP‐activated protein kinase‐dependent manner. J Biol Chem 2002; 277: 32 791–8. [DOI] [PubMed] [Google Scholar]
- 10. Levine B, Klionsky DJ. Development by self‐digestion: molecular mechanisms and biological functions of autophagy. Dev Cell 2004; 6: 463–77. [DOI] [PubMed] [Google Scholar]
- 11. Tanida I, Minematsu‐Ikeguchi N, Ueno T, Kominami E. Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy 2005; 1: 84–91. [DOI] [PubMed] [Google Scholar]
- 12. Sener SF, Fremgen A, Menck HR, Winchester DP. Pancreatic cancer: a report of treatment and survival trends for 100 313 patients diagnosed from 1985 to 1995, using the National Cancer Database. J Am Coll Surg 1985; 189: 1–7. [DOI] [PubMed] [Google Scholar]
- 13. Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg 1993; 165: 68–72. [DOI] [PubMed] [Google Scholar]
- 14. Sohn TA, Yeo CJ, Cameron JL et al . Resected adenocarcinoma of the pancreas‐616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg 2000; 4: 567–79. [DOI] [PubMed] [Google Scholar]
- 15. Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population‐based, linked database analysis of 396 patients. Ann Surg 2003; 237: 74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuhlmann KF, De Castro SM, Wesseling JG et al . Surgical treatment of pancreatic adenocarcinoma: actual survival and prognostic factors in 343 patients. Eur J Cancer 2004; 40: 549–58. [DOI] [PubMed] [Google Scholar]
- 17. Luttges J, Schemm S, Vogel I, Hedderich J, Kremer B, Kloppel G. The grade of pancreatic ductal carcinoma is an independent prognostic factor and is superior to the immunohistochemical assessment of proliferation. J Pathol 2000; 191: 154–61. [DOI] [PubMed] [Google Scholar]
- 18. Takai S, Satoi S, Toyokawa H et al . Clinicopathologic evaluation after resection for ductal adenocarcinoma of the pancreas: a retrospective, single‐institution experience. Pancreas 2003; 26: 243–9. [DOI] [PubMed] [Google Scholar]
- 19. Takahashi S, Hasebe T, Oda T et al . Extra‐tumor perineural invasion predicts postoperative development of peritoneal dissemination in pancreatic ductal adenocarcinoma. Anticancer Res 2001; 21: 1407–12. [PubMed] [Google Scholar]
- 20. Mitsunaga S, Hasebe T, Iwasaki M, Kinoshita T, Ochiai A, Shimizu N. Important prognostic histological parameters for patients with invasive ductal carcinoma of the pancreas. Cancer Sci 2005; 96: 858–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kivelä AJ, Parkkila S, Saarnio J et al . Expression of transmembrane carbonic anhydrase isoenzymes IX and XII in normal human pancreas and pancreatic tumours. Histochem Cell Biol 2000; 114: 197–204. [DOI] [PubMed] [Google Scholar]
- 22. Shibaji T, Nagao M, Ikeda N et al . Prognostic significance of HIF‐1 alpha overexpression in human pancreatic cancer. Anticancer Res 2003; 23: 4721–7. [PubMed] [Google Scholar]
- 23. Sipos B, Weber D, Ungefroren H et al . Vascular endothelial growth factor mediated angiogenic potential of pancreatic ductal carcinomas enhanced by hypoxia: an in vitro and in vivo study. Int J Cancer 2002; 102: 592–600. [DOI] [PubMed] [Google Scholar]
- 24. Levine B. Cell biology: autophagy and cancer. Nature 2007; 446: 745–7. [DOI] [PubMed] [Google Scholar]
- 25. Sato K, Tsuchihara K, Fujii S et al . Autophagy is activated in colorectal cancer cells and contributes to the tolerance to nutrient deprivation. Cancer Res 2007; 67: 9677–84. [DOI] [PubMed] [Google Scholar]
- 26. Sobin HL, Wiitekind C, eds. TNM Classification of Malignant Tumors, 6th edn. New York: Wiley‐Liss, 2002. [Google Scholar]
- 27. Hamilton RS, Aaltonen AL, eds. World Health Organization Classification of Tumours. Pathology and Genetics of Tumors of the Digestive System. Lyon: IARC Press, 2000. [Google Scholar]
- 28. Japan Pancreas Society . The Classification of Pancreatic Carcinoma, 1st English edn. Tokyo: Kanehara, 1996. [Google Scholar]
- 29. Jäger S, Bucci C, Tanida I et al . Role for Rab7 in maturation of late autophagic vacuoles. J Cell Sci 2004; 117: 4837–48. [DOI] [PubMed] [Google Scholar]
- 30. Kirkegaard K, Taylor MP, Jackson WT. Cellular autophagy: surrender, avoidance and subversion by microorganisms. Nat Rev Microbiol 2004; 2: 301–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer 2005; 5: 726–34. [DOI] [PubMed] [Google Scholar]
- 32. Klionsky DJ, Abeliovich H, Agostinis P et al . Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryocytes. Autophagy 2008; 4: 151–75. [DOI] [PMC free article] [PubMed] [Google Scholar]


