Abstract
Ashwagandha (Withania somnifera) is widely used in the Indian traditional system of medicine, Ayurveda. Although it is claimed to have a large variety of health‐promoting effects, including therapeutic effects on stress and disease, the mechanisms of action have not yet been determined. In the present study, we aimed to investigate the growth inhibition and differentiation potential of the alcoholic extract of Ashwagandha leaves (i‐Extract), its different constituents (Withaferin A, Withanone, Withanolide A) and their combinations on glioma (C6 and YKG1) cell lines. Withaferin A, Withanone, Withanolide A and i‐Extract markedly inhibited the proliferation of glioma cells in a dose‐dependent manner and changed their morphology toward the astrocytic type. Molecular analysis revealed that the i‐Extract and some of its components caused enhanced expression of glial fibrillary acidic protein, change in the immunostaining pattern of mortalin from perinuclear to pancytoplasmic, delay in cell migration, and increased expression of neuronal cell adhesion molecules. The data suggest that the i‐Extract and its components have the potential to induce senescence‐like growth arrest and differentiation in glioma cells. These assays led us to formulate a unique combination formula of i‐Extract components that caused enhanced differentiation of glial cells. (Cancer Sci 2009; 100: 1740–1747)
Ashwagandha (Withania somnifera), an evergreen shrub belonging to the solanaceae family, is widely used in the Indian traditional system of medicine, Ayurveda. It is considered to be a rasayana herb, an adaptogen, and is commonly referred to as ‘Indian ginseng’. It is an evergreen shrub that grows to 1 m in height, remains leafy throughout the year and requires fertile, moist and well‐drained soil. The plant was first mentioned in English language text by Van Rheede in 1683, who described the use of its leaves in home ointments. Similar use of its roots was described later by Waring in 1868 (cited from Chopra( 1 )). In addition to leaves and roots, other parts of the Ashwagandha plant, including shoots, seeds and berries, have also been used in daily tonics and various home remedy recipes to increase health and longevity. They are a source of unique alkaloids and Withanolides that have been shown to act as steroidal hormones and antioxidants with favorable impacts on human health. Many recent studies have provided evidence for its antistress, antioxidant, analgesic, anti‐inflammatory, cardioprotective adaptogenic, antispasmodic, immunomodulatory and immunostimulant activities.( 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 ) The main active constituents of Ashwagandha leaves are alkaloids and steroidal lactones (commonly known as Withanolides). Recently, selective killing of human cancer cells by the leaf extract of Ashwagandha (i‐Extract) was reported.( 11 , 12 ) It effectively killed a large variety of tumor‐derived cells (bone, breast, lung, cervical, and brain tumors). The selective cancer cell killing activity was assigned to one of its components, Withanone, also called tumor inhibitory factor (i‐Factor).( 11 , 12 ) Ashwagandha extract was also reported to cause improvements in scopolamine‐induced memory deficits in mice( 13 ) and neurite extension, particularly in SH‐SY5Y cells.( 5 ) Withanolide A was shown to be a major active constituent that induced axonal and dendritic regeneration and synaptic reconstruction in the damaged mouse brain as well as in damaged cultured neurons.( 7 , 14 ) In view of these reports and the fact that gliomas constitute the largest group of primary brain tumors that escape existing therapies and remain incurable, we investigated whether Ashwagandha and its constituents could be used for redifferentiation and possibly leading to the treatment of gliomas.
Materials and Methods
Preparation of active constituents of Ashwagandha. The leaf extract and active constituents of Ashwagandha were prepared as described earlier.( 11 , 15 , 16 ) The chemical composition of the alcoholic extract is shown in Figure 1. It contained a high proportion of Withanone (i‐Factor), a moderate amount of Withaferin A and a low amount of Withanolide A.
Figure 1.
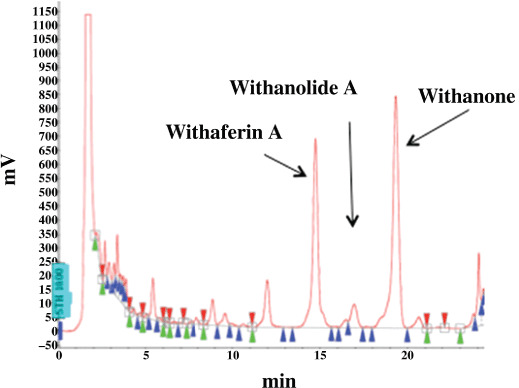
Chemical composition of i‐Extract.
Cell culture. Glioma cell lines C6 (rat) and YKG1 (human) were used in the present study. C6 was obtained from the Cell Resource Center for Biomedical Research (Tohoku University, Japan). YKG1 was obtained from the Health Science Research Resources Bank (Osaka, Japan). The cells were maintained in DMEM (Invitrogen, Grand Island, NY, USA) supplemented with 10% fetal bovine serum in a humidified incubator (37°C and 5% CO2). Cells (40–60% confluency) were treated with i‐Extract (0.8–5.0 µg/mL), Withaferin A (0.1–0.5 µM), Withanone (i‐Factor) (5–10 µg/mL), Withanolide A (5–10 µg/mL), and their combinations for 48–72 h as indicated.
Cytotoxicity and growth inhibition assay. The cytotoxicity of different constituents of i‐Extract was tested using the MTT test. Cell viability was quantified by the conversion of yellow MTT by mitochondrial dehydrogenases of living cells to purple formazan.( 17 ) MTT (0.5 mg/mL) was added to the cell culture medium for 4 h following the treatment of cells as indicated. MTT‐containing medium was then removed and 100 µL DMSO was added to each well for complete dissolution of formazan crystals. Absorbance was measured at 550 nm using a spectrophotometer (Wallac, ArvoSX, Tokyo, Japan).
Cell viability assay. The trypan blue dye exclusion assay was used to determine cell viability and membrane integrity. The cells (60% confluent) were cultured in 24‐well plates and treated with i‐Extract or its constituents. After 48–72 h, both floating and adherent cells were harvested and centrifuged at 100 g for 5 min. The cells were suspended in 100 µL PBS and stained with trypan blue at a final concentration of 10% (v/v). The non‐viable cells appeared as blue. The viable and non‐viable cells were determined by counting the cells in 10 µL of cell suspension using a hemocytometer. Furthermore, the cells were seeded in 48‐well plates, treated as indicated, and stained with crystal violet.
Morphological observations. The cells were cultured in 12‐well plates and treated with different concentrations of i‐Extract and its components at around 50% confluency. After 48–72 h, morphological changes were recorded with a phase contrast microscope.
Immunostaining. Cells were cultured and treated on glass coverslips placed in a 12‐well culture dish. At the end of the treatment, cells were washed with cold phosphate‐buffered saline (PBS) and fixed with pre‐chilled methanol : acetone (1:1 v/v) mixture for 5–10 min. Fixed cells were washed with PBS, permeabilized with 0.2% Triton X‐100 in PBS for 10 min, and blocked with 2% bovine serum albumin in PBS for 20 min. Cells were stained with anti‐glial fibrillary acidic protein (GFAP) (Sigma Aldrich, St. Louis, USA) and polyclonal anti‐mortalin( 18 ) antibodies. Immunostaining was visualized by secondary staining with Alexa‐488‐conjugated goat anti‐rabbit antibody (Molecular Probes, Eugene, OR, USA). After three to four washes with 0.2% Triton X‐100 in PBS (PBST), cells were overlaid with FA Mounting Fluid (VMRD, USA). The cells were examined under a microscope with epifluorescence optics.
Hoechst 33 258 staining. Glioma cells were cultured on coverslips placed in 12‐well plates and treated as indicated. At the end of treatment, cells were fixed with pre‐chilled methanol : acetone (1:1 v/v) and permeabilized with 0.2% PBST. Cells were exposed to the nuclear stain Hoechst 33 258 (0.5 µg/mL) for 5–10 min in the dark at room temperature. Cells were then washed with 0.1% PBST and finally with PBS, overlaid with Fluoromount mounting media, and observed under a microscope. Nuclear morphology and apoptotic cells in control and treated cultures were scored.
Western blotting. Cells were grown and treated in six‐well plates. After 48–72 h of treatments as indicated, the cells were lysed with NP‐40 lysis buffer. The protein (20 µg, estimated by the Bradford method) was separated on a SDS‐polyacrylamide gel and electroblotted onto a nitrocellulose membrane using a semidry transfer blotter. Immunoassays were done with anti‐GFAP, anti‐mortalin,( 18 ) anti‐Neural cell Adhesion Molecule (NCAM) (AbCys SA, Paris, France), and anti‐actin (Chemicon International, Temecula, CA, USA) antibodies. The immunocomplexes formed were visualized with horseradish peroxidase‐conjugated anti‐rabbit Ig (ECL; Amersham Pharmacia Biotech, Piscataway, NJ, USA).
Wound‐scratch assay. The cell migration capability of glioma cells in response to i‐Extract treatment was examined with a modified wound‐scratch assay. The cells were grown on 22 mm‐diameter glass coverslips. On approaching 100% confluency, the monolayer was wounded by uniformly scratching the surface with a needle (20 gauge). The initial wounding and the movement of both the control and the i‐Extract‐treated cells in the scratched area were monitored for 6 h under a phase contrast microscope. The cells were serially photographed. Four different fields from each sample were examined for quantitative estimation of the distance between the borderlines. The migration rate was expressed as percentage of the control and was calculated as the proportion of the mean distance between both the borderlines caused by scratching to the distance that remained cell free after regrowing (Image‐Pro Plus version 4.5.1.22, Media Cybernetics, Inc., MD, USA). To evaluate the correlation between NCAM protein expression and cell migration capability, at the end of the photographic monitoring, the cells were fixed and immunostained with anti‐NCAM antibody.
Cell cycle analysis. C6 glioma cells were treated with i‐Extract and its components for 48 h. After treatment the cells were harvested with trypsin, washed twice with PBS, and fixed with 70% ethanol at 4°C for 12 h. The fixed cells were centrifuged (400 g for 10 min), washed twice with cold PBS, and resuspended in 0.25 mL PBS. The cell suspension was stained with 10 µL of 1 mg/mL propidium iodide (PI) for 30 min in the dark. To avoid false DNA or PI staining, RNA was removed by adding 5 µL of 1 mg/mL RNAse A at 37°C for 1 h to the cell suspension before PI staining. After staining with PI, the cell cycle analysis was done using a BD FACS Aria flow cytometer (BD Biosciences, ON, Canada).
Results
Dose‐dependent effects of i‐Extract and its components on glioma cells. C6 (rat glioma) and YKG1 (human glioma), the established models to study undifferentiated, oligodendrocytic, astrocytic, and neuronal phenotypes,( 19 ) were treated with i‐Extract and its components (Withaferin‐A, Withanone and Withanolide‐A). We observed a strong growth arrest in both the cells lines in response to all the four treatments in a dose‐dependent manner with a maximum effect seen between 48 and 72 h. The antiproliferative or growth inhibition effect of these constituents was quantitated by MTT and trypan blue assays. As shown in Figure 2(a–c), all the four treatments were found to cause strong growth arrest of both C6 and YKG1 cells. The IC50 values were 5 µg/mL for i‐Extract, 0.2 µM for Withaferin A, 40 µg/mL for Withanone and 35 µg/mL for Withanolide A in C6 rat gliomas. Human gliomas (YKG‐1) were more sensitive than the rat gliomas: the IC50 values were 2.5 µg/mL for i‐Extract, 0.1 µM for Withaferin A, 30 µg/mL for Withanone and 20 µg/mL for Withanolide A. Interestingly, whereas treatment with high doses of i‐Extract and its components caused apoptosis (data not shown), moderate concentrations caused growth arrest (Fig. 3A), as also confirmed by cell cycle distribution. The absence of apoptosis at low doses was confirmed by Hoechst staining (Fig. 3B). Low doses caused changes in cell morphology that ranged from polygonal appearance with few cytoplasmic processes to spindle shape with long cytoplasmic processes, similar to the ones observed for differentiated astrocytic cells (Fig. 3C). The differentiation of glioma cells was further investigated by immunostaining and western blotting for GFAP, a 50‐kDa type III intermediate filament protein, an established and reliable marker of mature astrocytes. As shown in Figure 4(A–C) there was a significant increase in GFAP expression in treated cells as compared to the control. To investigate if the cells had acquired the normal phenotype, we examined the level and staining pattern of mortalin protein that exhibits perinuclear staining in cancer and pancytoplasmic staining in normal cells.( 18 , 20 , 21 , 22 , 23 , 24 , 25 ) There was no difference in the level of expression of mortalin in control and treated cells (Fig. 4A); however, the treated cells showed pancytoplasmic staining (Fig. 4B,D), suggesting an induction of the senescence phenotype. This was also consistent with the cell cycle data that showed arrest of cells in S and G2/M phase. We anticipated that the differentiated glioma cells may also be retarded in their motility and hence carried out a wound healing assay in control and i‐Extract‐treated cells. As shown in Figure 5(a), untreated C6 cells were able to invade the scratched area and fully colonize within 6 h. The i‐Extract‐treated cells did not move to the scratched area, suggesting that there was a strong reduction in the migration rate of C6 glioma cells. Furthermore, we examined the expression of adhesion molecule NCAM and found that the i‐Extract‐treated cells had increased levels of NCAM expression (Fig. 5b). The above data showed that the i‐Extract and its components caused multiple dose‐dependent changes, including: (i) apoptosis, (ii) growth arrest, (iii) morphological conversion to astrocytes, (iv) cell cycle arrest, (v) induction of senescence, (vi) induction of differentiation, (vii) decrease in motility and (viii) increase in adhesion.
Figure 2.
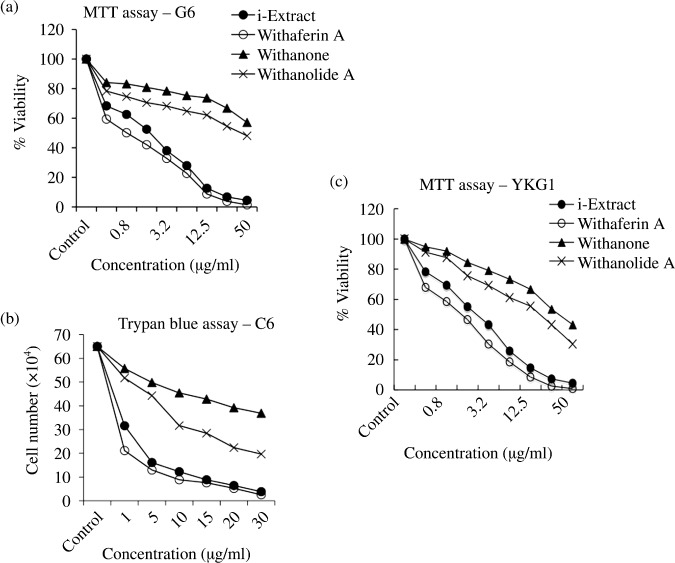
Growth curves showing the effect of i‐Extract and its constituents on proliferation of (a,b) C6 and (c) YKG‐1 glioma cells as assessed by (a,c) MTT assay and (b) trypan blue dye uptake assay (b). Cells were incubated with the indicated reagents for 72 h. Values are representative of three independent experiments and expressed as means. For Withaferin A, X µM = 10 X µg/mL.
Figure 3.
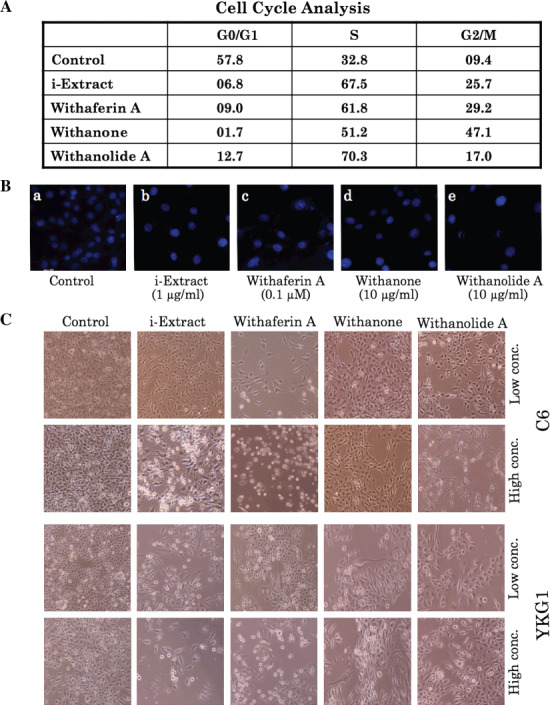
A, Effect of i‐Extract and its components on the cell cycle progression of C6 glioma cells. Cell cycle showed an arrest in S phase. B, Hoechst 33 258 staining of (a) control cells, and cells treated with (b) 1 µg/mL i‐Extract, (c) 0.1 µM Withaferin A, (d) 10 µg/mL Withanone, and (e) 10 µg/mL Withanolide A (magnification, 400×). C, Effects of Ashwagandha constituents on the morphological changes in C6 (72 h treatment) and YKG1 (48 h treatment) cells. Cells were treated with low and high concentrations of i‐Extract (0.8 and 5 µg/mL), Withaferin A (0.1 and 0.5 µM), Withanone (10 and 25 µg/mL), and Withanolide A (10 and 25 µg/mL). Low concentrations of treated cells showed multiple processes and differentiated morphology. High concentrations of i‐Extract, Withaferin A, and Withanolide A showed cytotoxicity.
Figure 4.
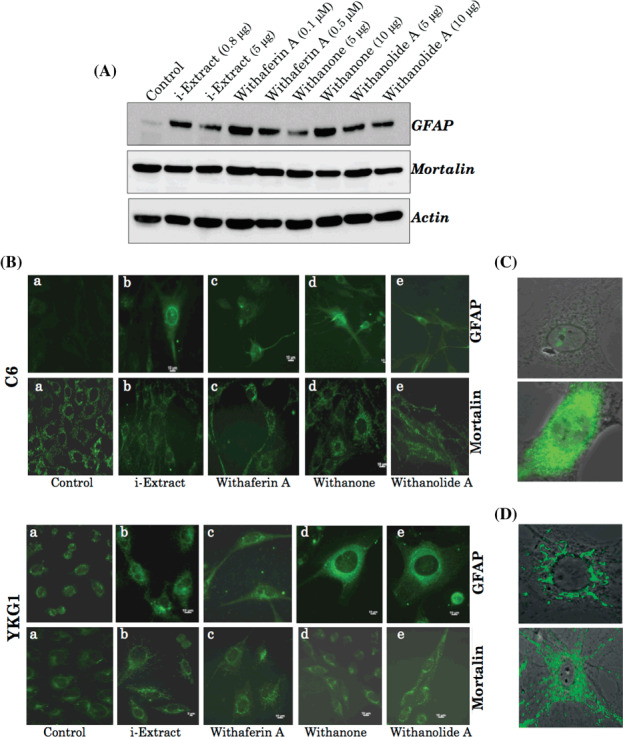
(A) Western blot analysis showing the expression levels of glial fibrillary acidic protein (GFAP), mortalin, and actin in YKG1 glioma cells treated with i‐Extract and its various components for 48 h. An increase in GFAP, but not mortalin, was observed in response to different treatments. (B) Immunofluorescence detection of GFAP and mortalin in C6 (after 72 h) and YKG1 (after 48 h) gliomas. High‐resolution images of treated cells showing the typical (C, lower panel) GFAP and (D) perinuclear mortalin stainings in control cells (upper panel) and pancytoplasmic staining in i‐Extract‐treated (lower panel) C6 cells.
Figure 5.
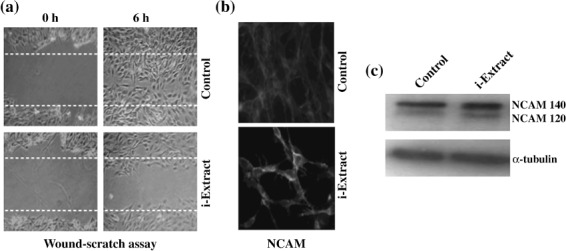
Effect of i‐Extract on cell motility and Neural Cell Adhesion Molecule (NCAM) expression. i‐Extract reduces C6 glioma cell motility. Representative phase contrast images of control and i‐Extract‐treated cells in the wound‐scratch assay are shown. In the control, the distance between the borderlines of the scratch became significantly narrow 6 h after wounding; it was still wide in i‐Extract‐treated samples. (a) Images shown are at the start point (0 h after scratch) and end point (6 h after scratch) of the assay. (b) NCAM staining in control and i‐Extract‐treated (6 h) cells is shown. There was a significant increase in the NCAM staining in the i‐Extract treatment groups as compared to the control. Western blot shows that the i‐Extract treatment results in increased expression of NCAM 140. (c) Tubulin was used as a loading control.
Combinational effects. The above data showed that low doses of i‐Extract and its components could be recruited for induction of differentiation in glioma cells. We anticipated that the low dose‐induced differentiation may serve as milder and effective therapy in contrast to the high dose‐induced apoptosis, usually limited by high toxicity. Furthermore, such differentiation of glioma cells may offer several advantages in the therapy and normal brain functions. Based on these data, we undertook a screen to achieve enhanced differentiation of glioma cells by a combination formula of the three purified factors: Withaferin A, Withanone (i‐Factor) and Withanolide A (Fig. 6). Eight combinations (A to H) were prepared and tested for their differentiation potential based on the morphological observations and expression level of GFAP. As shown in Figure 6(b,c), combination E (0.01 µM Withaferin A and 5 µg/mL Withanone) showed the highest induction of GFAP protein and the best‐differentiated phenotype (with longest cellular extensions) and is therefore proposed as an effective combination for milder and effective glioma therapy.
Figure 6.
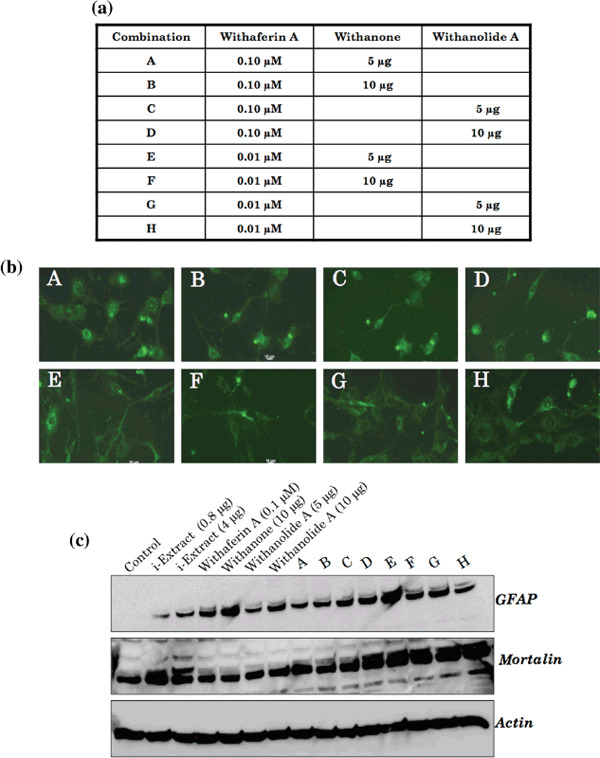
(a) Composition of combinations (A–H). (b) Induction of glial fibrillary acidic protein (GFAP) expression, as detected by immunostaining, in C6 cells after 72 h treatment with each of the combinations; dendritic extensions were most elongated with combination E. (c) Relative level of GFAP and mortalin expression in YKG1 cells in response to each of the combination treatments for 48 h.
Discussion
According to the American Brain Tumor Association, primary brain tumors occur at a rate of 12.8 per 100 000 people. The majority of these develop into malignant gliomas and remain incurable in spite of the therapies like external beam radiation, surgery and chemotherapy. These are further complicated by their heterogeneity, poor prognosis, blood brain barrier and unsuccessful chemotherapy and radiotherapy regimes. Hence, there is a strong demand for developing effective and alternate therapeutic strategies and reagents. In the present study, we used rat and human glioma cells as model systems to study the effect of active constituents (Withaferin‐A, Withanone and Withanolide A) of leaf extract (i‐Extract) of Ashwagandha. Whereas high doses of i‐Extract and Withaferin A caused apoptosis, low doses caused growth arrest that appeared to be associated with the induction of senescence. These data directly suggest the use of i‐Extract and its components in glioma treatment. Some other studies have reported anticancer activities in the leaf extract of Ashwagandha.( 26 , 27 , 28 , 29 , 30 , 31 , 32 ) We had previously reported that the treatment with i‐Extract and Withanone (i‐Factor) leads to selective killing of cancer cells by activation of the tumor suppressor p53 pathway.( 11 , 12 ) Aalinkeel et al. reported the role of Janus Kinase (JAK)–Signal Transducer and Activator of transdution (STAT) signaling in the antiproliferative effects of Ashwagandha extract in prostate cancer.( 28 ) Ashwagandha treatment significantly downregulated the expression of the proinflammatory cytokines Interleukin (IL)‐6, IL‐1β, chemokine IL‐8, Heat shock protein (HSP)70 and STAT‐2, whereas reciprocal upregulation was observed for p38MAPK, p13 K, caspase 6, cyclin D and c‐myc. Ashwagandha treatment significantly modulated the JAK–STAT pathway that regulates both the apoptosis process as well as MAP kinase signaling.
Our study showed an interesting and useful aspect of Ashwagandha‐based cancer therapy. The low dose and combination formula could be used for induction of differentiation in contrast to the apoptosis that is often complicated by in vivo toxicity. GFAP plays an important role in maintaining the normal astrocytic morphology and regulating aspects of astyrocytoma cell growth.( 33 , 34 , 35 ) Increased expression of GFAP coupled with morphological changes induced by i‐Extract and its components suggested the occurrence of differentiation. The staining pattern of mortalin that distinguishes the normal and cancer phenotype of cells( 11 , 18 , 20 , 21 , 22 , 36 , 37 , 38 ) suggested that the i‐Extract and its components induced senescence in glioma cells.
The antiproliferative effect of i‐Extract in C6 glioma cells was also accompanied by enhanced expression of NCAM, a cell surface Ig superfamily protein that has been implicated in cell–cell interaction throughout the nervous system and is expressed by many tumors of neuroectodermal origin, including astrocytic tumors.( 39 , 40 , 41 ) Through cell–cell adhesion, homophilic interaction of NCAM is known to induce signal transduction resulting in neuronal differentiation( 39 , 42 ) and inhibition of cell proliferation.( 43 , 44 ) Increase in expression of the NCAM 140 isoform was consistent with the reduction in cellular motility as also reported by Prag et al.( 45 ) Rao et al. have reported the inhibition of cell invasion in an invasive NCAM‐negative variant of the glioma BT4C by overexpression of the transmembrane isoform NCAM 140.( 46 ) The result was also accompanied by downregulation of the expression of MMP9, an important determinant of infiltrative behavior.( 47 , 48 ) Overexpression of the glycerophospholipid‐anchored isoform NCAM 120 was shown not to affect MMP expression, implying that the downstream signaling involving an intracellular domain of NCAM 140 isoform was critical. Consistent with these reports, we found that the expression level of NCAM 140, but not NCAM 120, increased in response to the treatment with i‐Extract that reduced their motility.
In our combinational approach, we found that the combinations of Withaferin A (0.01 and 0.1 µM) and Withanone (5 and 10 µg), and Withaferin A (0.01 and 0.1 µM) and Withanolide A (5 and 10 µg) were effective to induce differentiation. As compared to each individual reagent, these combinations caused stronger growth inhibition and differentiation, as seen by cell morphology and enhanced GFAP levels. The strongest differentiation potential was seen in combination E (0.01 µM Withaferin A and 5 µg/mL Withanone) as determined by GFAP expression and morphological changes. Furthermore, combinations D to H resulted in an increased level of mortalin expression (Fig. 6). Upregulation of mortalin was closely related with the differentiation of neuroblastoma cells both in vitro and in vivo.( 49 ) Hence, further studies are warranted to unravel the molecular mechanism of the role of induced mortalin expression in glial differentiation.
Glioblastoma is the most common and difficult malignant brain tumor to treat. Despite the use of different treatment strategies, including surgery, radiotherapy, and chemotherapy, most patients die within a year of diagnosis. Differentiation therapy is an attractive alternative therapeutic approach. The use of retinoids and adjuvant approaches using their catabolic inhibitors, interferon‐γ, taxol, paclitaxel and PKC inhibitors have been demonstrated to have considerably improved therapeutic potential.( 50 , 51 , 52 , 53 , 54 , 55 ) In most cases, induction of astrocytic differentiation, overexpression of GFAP, downregulation of telomerase expression, and increased sensitivity to chemical drugs have been reported.( 53 ) At the same time, it has been reported that some gliomas show strong resistance to the induction of differentiation or apoptosis by retinoids( 56 ) and hence the alternative drugs including the psychotropic agents phenothiazines, chlorpromazine and perphenazine, N‐(4‐hydroxyphenyl) retinamide, capsaicin (8‐methyl‐N‐vanillyl‐6‐nonenamide), panaxydol, cAMP and cholera toxin have been tested for their glioma therapeutic potential.( 57 , 58 , 59 , 60 ) Apparently, there is a considerable need to develop more effective antiglioma reagents. Based on our present data, we suggest the use of i‐Extract and its constituents (combinational formula) for differentiation‐based milder and effective glioma therapy.
Acknowledgments
The study was supported in part by grants from the National Institute of Advanced industrial Science & Technology (AIST) International Affairs Department under the AIST, Japan – Department of Biotechnology (DBT), India bilateral research collaboration. Navjot Shah was supported by an AIST Fellowship. Hardeep Kataria was supported by fellowship grant from the Council of Scientific and Industrial Research (CSIR), India.
References
- 1. Chopra RN. A review of work on Indian Medicinal plants. In: Pharmacopoeia of India. Indian Council of Medical Research. Cambridge Printing Works, Kashmere Gate, New Delhi, 1955. [Google Scholar]
- 2. Agarwal R, Diwanay S, Patki P, Patwardhan B. Studies on immunomodulatory activity of Withania somnifera (Ashwagandha) extracts in experimental immune inflammation. J Ethnopharmacol 1999; 67: 27–35. [DOI] [PubMed] [Google Scholar]
- 3. Mishra LC, Singh BB, Dagenais S. Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): a review. Altern Med Rev 2000; 5: 334–46. [PubMed] [Google Scholar]
- 4. Scartezzini P, Speroni E. Review on some plants of Indian traditional medicine with antioxidant activity. J Ethnopharmacol 2000; 71: 23–43. [DOI] [PubMed] [Google Scholar]
- 5. Tohda C, Kuboyama T, Komatsu K. Dendrite extension by methanol extract of Ashwagandha (roots of Withania somnifera) in SK‐N‐SH cells. Neuroreport 2000; 11: 1981–5. [DOI] [PubMed] [Google Scholar]
- 6. Davis L, Kuttan G. Effect of Withania somnifera on cell mediated immune responses in mice. J Exp Clin Cancer Res 2002; 21: 585–90. [PubMed] [Google Scholar]
- 7. Kuboyama T, Tohda C, Komatsu K. Neuritic regeneration and synaptic reconstruction induced by withanolide A. Br J Pharmacol 2005; 144: 961–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bhattacharya SK, Muruganandam AV. Adaptogenic activity of Withania somnifera: an experimental study using a rat model of chronic stress. Pharmacol Biochem Behav 2003; 75: 547–55. [DOI] [PubMed] [Google Scholar]
- 9. Gupta SK, Dua A, Vohra BP. Withania somnifera (Ashwagandha) attenuates antioxidant defense in aged spinal cord and inhibits copper induced lipid peroxidation and protein oxidative modifications. Drug Metabol Drug Interact 2003; 19: 211–22. [DOI] [PubMed] [Google Scholar]
- 10. Singh B, Chandan BK, Gupta DK. Adaptogenic activity of a novel withanolide‐free aqueous fraction from the roots of Withania somnifera Dun. (Part II). Phytother Res 2003; 17: 531–6. [DOI] [PubMed] [Google Scholar]
- 11. Widodo N, Kaur K, Shrestha BG et al . Selective killing of cancer cells by leaf extract of Ashwagandha: identification of a tumor‐inhibitory factor and the first molecular insights to its effect. Clin Cancer Res 2007; 13: 2298–306. [DOI] [PubMed] [Google Scholar]
- 12. Widodo N, Takagi Y, Shrestha BG, Ishii T, Kaul SC, Wadhwa R. Selective killing of cancer cells by leaf extract of Ashwagandha: Components, activity and pathway analyses. Cancer Lett 2008; 262: 37–47. [DOI] [PubMed] [Google Scholar]
- 13. Dhuley JN. Nootropic‐like effect of ashwagandha (Withania somnifera L.) in mice. Phytother Res 2001; 15: 524–8. [DOI] [PubMed] [Google Scholar]
- 14. Kuboyama T, Tohda C, Komatsu K. Withanoside IV and its active metabolite, sominone, attenuate Abeta (25–35)‐induced neurodegeneration. Eur J Neurosci 2006; 23: 1417–26. [DOI] [PubMed] [Google Scholar]
- 15. Rani G, Kaur K, Wadhwa R, Kaul SC, Nagpal A. Evaluation of the anti‐genotoxicity of leaf extract of Ashwagandha. Food Chem Toxicol 2005; 43: 95–8. [DOI] [PubMed] [Google Scholar]
- 16. Kaur K, Rani G, Widodo N et al . Evaluation of the anti‐proliferative and anti‐oxidative activities of leaf extract from in vivo and in vitro raised Ashwagandha. Food Chem Toxicol 2004; 42: 2015–20. [DOI] [PubMed] [Google Scholar]
- 17. Hansen MB, Nielsen SE, Berg K. Re‐examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods 1989; 119: 203–10. [DOI] [PubMed] [Google Scholar]
- 18. Wadhwa R, Kaul SC, Mitsui Y, Sugimoto Y. Differential subcellular distribution of mortalin in mortal and immortal mouse and human fibroblasts. Exp Cell Res 1993; 207: 442–8. [DOI] [PubMed] [Google Scholar]
- 19. Kanno H, Shinonaga M, Kuwabara T, Umeda M. [Growth factors produced by rat glioma cells: activities of transforming growth factors]. No To Shinkei 1989; 41: 905–9. [PubMed] [Google Scholar]
- 20. Wadhwa R, Sugihara T, Yoshida A et al . Selective toxicity of MKT‐077 to cancer cells is mediated by its binding to the hsp70 family protein mot‐2 and reactivation of p53 function. Cancer Res 2000; 60: 6818–21. [PubMed] [Google Scholar]
- 21. Michishita E, Nakabayashi K, Suzuki T et al . 5‐Bromodeoxyuridine induces senescence‐like phenomena in mammalian cells regardless of cell type or species. J Biochem 1999; 126: 1052–9. [DOI] [PubMed] [Google Scholar]
- 22. Deocaris CC, Widodo N, Shrestha BG et al . Mortalin sensitizes human cancer cells to MKT‐077‐induced senescence. Cancer Lett 2007; 252: 259–69. [DOI] [PubMed] [Google Scholar]
- 23. Wadhwa R, Taira K, Kaul SC. Mortalin: a potential candidate for biotechnology and biomedicine. Histol Histopathol 2002; 17: 1173–7. [DOI] [PubMed] [Google Scholar]
- 24. Wadhwa R, Colgin L, Yaguchi T, Taira K, Reddel RR, Kaul SC. Rhodacyanine dye MKT‐077 inhibits in vitro telomerase assay but has no detectable effects on telomerase activity in vivo . Cancer Res 2002; 62: 4434–8. [PubMed] [Google Scholar]
- 25. Ohtsuka R, Abe Y, Fujii T et al . Mortalin is a novel mediator of erythropoietin signaling. Eur J Haematol 2007; 79: 114–25. [DOI] [PubMed] [Google Scholar]
- 26. Malik F, Kumar A, Bhushan S et al . Immune modulation and apoptosis induction: two sides of antitumoural activity of a standardised herbal formulation of Withania somnifera . Eur J Cancer 2009; 45: 1494–509. [DOI] [PubMed] [Google Scholar]
- 27. Malik F, Kumar A, Bhushan S et al . Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic cell death of human myeloid leukemia HL‐60 cells by a dietary compound withaferin A with concomitant protection by N‐acetyl cysteine. Apoptosis 2007; 12: 2115–33. [DOI] [PubMed] [Google Scholar]
- 28. Aalinkeel R, Hu Z, Nair BB et al . Genomic analysis highlights the role of the JAK–STAT signaling in the anti‐proliferative effects of dietary flavonoid ‘Ashwagandha’ in prostate cancer cells. Evid Based Complement Alternat Med 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Diwanay S, Chitre D, Patwardhan B. Immunoprotection by botanical drugs in cancer chemotherapy. J Ethnopharmacol 2004; 90: 49–55. [DOI] [PubMed] [Google Scholar]
- 30. Christina AJ, Joseph DG, Packialakshmi M et al . Anticarcinogenic activity of Withania somnifera Dunal against Dalton's ascitic lymphoma. J Ethnopharmacol 2004; 93: 359–61. [DOI] [PubMed] [Google Scholar]
- 31. Kuttan G. Use of Withania somnifera Dunal as an adjuvant during radiation therapy. Indian J Exp Biol 1996; 34: 854–6. [PubMed] [Google Scholar]
- 32. Leyon PV, Kuttan G. Effect of Withania somnifera on B16F‐10 melanoma induced metastasis in mice. Phytother Res 2004; 18: 118–22. [DOI] [PubMed] [Google Scholar]
- 33. Rutka JT, Hubbard SL, Fukuyama K, Matsuzawa K, Dirks PB, Becker LE. Effects of antisense glial fibrillary acidic protein complementary DNA on the growth, invasion, and adhesion of human astrocytoma cells. Cancer Res 1994; 54: 3267–72. [PubMed] [Google Scholar]
- 34. Kokunai T, Izawa I, Tamaki N. Overexpression of p21WAF1/CIP1 induces cell differentiation and growth inhibition in a human glioma cell line. Int J Cancer 1998; 75: 643–8. [DOI] [PubMed] [Google Scholar]
- 35. Das A, Banik NL, Ray SK. N‐(4‐Hydroxyphenyl) retinamide induced both differentiation and apoptosis in human glioblastoma T98G and U87MG cells. Brain Res 2008; 1227: 207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nakabayashi K, Ogata T, Fujii M et al . Decrease in amplified telomeric sequences and induction of senescence markers by introduction of human chromosome 7 or its segments in SUSM‐1. Exp Cell Res 1997; 235: 345–53. [DOI] [PubMed] [Google Scholar]
- 37. Bertram MJ, Berube NG, Hang‐Swanson X et al . Identification of a gene that reverses the immortal phenotype of a subset of cells and is a member of a novel family of transcription factor‐like genes. Mol Cell Biol 1999; 19: 1479–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fujii M, Ide T, Wadhwa R, Tahara H et al . Inhibitors of cGMP‐dependent protein kinase block senescence induced by inactivation of T antigen in SV40‐transformed immortal human fibroblasts. Oncogene 1995; 11: 627–34. [PubMed] [Google Scholar]
- 39. Walsh FS, Meiri K, Doherty P. Cell signalling and CAM‐mediated neurite outgrowth. Soc Gen Physiol Ser 1997; 52: 221–6. [PubMed] [Google Scholar]
- 40. Ditlevsen DK, Povlsen GK, Berezin V, Bock E. NCAM‐induced intracellular signaling revisited. J Neurosci Res 2008; 86: 727–43. [DOI] [PubMed] [Google Scholar]
- 41. Suzuki T, Izumoto S, Fujimoto Y, Maruno M, Ito Y, Yoshimine T. Clinicopathological study of cellular proliferation and invasion in gliomatosis cerebri: important role of neural cell adhesion molecule L1 in tumour invasion. J Clin Pathol 2005; 58: 166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Amoureux MC, Cunningham BA, Edelman GM, Crossin KL. N‐CAM binding inhibits the proliferation of hippocampal progenitor cells and promotes their differentiation to a neuronal phenotype. J Neurosci 2000; 20: 3631–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Krushel LA, Tai MH, Cunningham BA, Edelman GM, Crossin KL. Neural cell adhesion molecule (N‐CAM) domains and intracellular signaling pathways involved in the inhibition of astrocyte proliferation. Proc Natl Acad Sci USA 1998; 95: 2592–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim JH, Lee JH, Park JY et al . Retrovirally transduced NCAM140 facilitates neuronal fate choice of hippocampal progenitor cells. J Neurochem 2005; 94: 417–24. [DOI] [PubMed] [Google Scholar]
- 45. Prag S, Lepekhin EA, Kolkova K et al . NCAM regulates cell motility. J Cell Sci 2002; 115: 283–92. [DOI] [PubMed] [Google Scholar]
- 46. Rao JS, Steck PA, Mohanam S, Stetler‐Stevenson WG, Liotta LA, Sawaya R. Elevated levels of Mr 92 000 type IV collagenase in human brain tumors. Cancer Res 1993; 53: 2208–11. [PubMed] [Google Scholar]
- 47. Edvardsen K, Brunne N, Spang‐Thomsen M, Walsh FS, Bock E. Migratory, invasive and metastatic capacity of NCAM transfected rat glioma cells. Int J Dev Neurosci 1993; 11: 681–90. [DOI] [PubMed] [Google Scholar]
- 48. Andersson AM, Moran N, Gaardsvoll H et al . Characterization of NCAM expression and function in BT4C and BT4Cn glioma cells. Int J Cancer 1991; 47: 124–9. [DOI] [PubMed] [Google Scholar]
- 49. Hsu WM, Lee H, Juan HF et al . Identification of GRP75 as an independent favorable prognostic marker of neuroblastoma by a proteomics analysis. Clin Cancer Res 2008; 14: 6237–45. [DOI] [PubMed] [Google Scholar]
- 50. Westarp ME, Westarp MP, Grundl W, Biesalski H, Kornhuber HH. Improving medical approaches to primary CNS malignancies: retinoid therapy and more. Med Hypotheses 1993; 41: 267–76. [DOI] [PubMed] [Google Scholar]
- 51. Haque A, Banik NL, Ray SK. Emerging role of combination of all‐trans retinoic acid and interferon‐gamma as chemoimmunotherapy in the management of human glioblastoma. Neurochem Res 2007; 32: 2203–9. [DOI] [PubMed] [Google Scholar]
- 52. Zhang R, Banik NL, Ray SK. Combination of all‐trans retinoic acid and interferon‐gamma upregulated p27 (kip1) and downregulated CDK2 to cause cell cycle arrest leading to differentiation and apoptosis in human glioblastoma LN18 (PTEN‐proficient) and U87MG (PTEN‐deficient) cells. Cancer Chemother Pharmacol 2008; 62: 407–16. [DOI] [PubMed] [Google Scholar]
- 53. Das A, Banik NL, Ray SK. Retinoids induced astrocytic differentiation with down regulation of telomerase activity and enhanced sensitivity to taxol for apoptosis in human glioblastoma T98G and U87MG cells. J Neurooncol 2008; 87: 9–22. [DOI] [PubMed] [Google Scholar]
- 54. Karmakar S, Banik NL, Ray SK. Combination of all‐trans retinoic acid and paclitaxel‐induced differentiation and apoptosis in human glioblastoma U87MG xenografts in nude mice. Cancer 2008; 112: 596–607. [DOI] [PubMed] [Google Scholar]
- 55. Sharif TR, Sharif MA. High throughput system for the evaluation of protein kinase C inhibitors based on Elk1 transcriptional activation in human astrocytoma cells. Int J Oncol 1999; 14: 327–35. [DOI] [PubMed] [Google Scholar]
- 56. Schmidt F, Groscurth P, Dichgans J, Weller M. Human malignant glioma cell lines are refractory to retinoic acid‐mediated differentiation and sensitization to apoptosis. Cell Physiol Biochem 2000; 10: 159–68. [DOI] [PubMed] [Google Scholar]
- 57. Nordenberg J, Fenig E, Landau M, Weizman R, Weizman A. Effects of psychotropic drugs on cell proliferation and differentiation. Biochem Pharmacol 1999; 58: 1229–36. [DOI] [PubMed] [Google Scholar]
- 58. Hai J, Lin Q, Lu Y, Yi J, Zhang H. Growth inhibition and induction of differentiation by panaxydol in rat C6 glioma cells. Neurol Res 2008; 30: 99–105. [DOI] [PubMed] [Google Scholar]
- 59. Li Y, Yin W, Wang X, Zhu W, Huang Y, Yan G. Cholera toxin induces malignant glioma cell differentiation via the PKA/CREB pathway. Proc Natl Acad Sci USA 2007; 104: 13 438–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hai J, Lin Q, Lu Y. Phosphatidylinositol 3‐kinase activity is required for the induction of differentiation in C6 glioma cells by panaxydol. J Clin Neurosci 2009; 16: 444–8. [DOI] [PubMed] [Google Scholar]


