Abstract
We treated elderly patients with relapsed or refractory peripheral T‐cell lymphoma (PTCL) using a CMD (CPT‐11, mitoxantrone [MIT], dexamethasone [DEX]) regimen and studied its safety and efficacy. The subjects were 70–79‐year‐old patients with relapsed or refractory PTCL. CPT‐11 at 25 mg/m2 on days 1 and 2, MIT at 8 mg/m2 on day 3, and DEX at 40 mg/day on days 1–3 were administered once every 3 weeks, and this was performed for six cycles. Eleven (37%) of the 30 patients achieved complete remission and seven patients (23%) achieved partial remission. With a median follow‐up period of 32 months, the 3‐year survival rate was 28.2% and the 3‐year progression‐free survival rate was 17.5%. The main adverse drug reaction was hematological toxicity and there were no deaths related to the treatment. B‐type natriuretic peptide and troponin T levels did not increase after the treatment and none of the patients showed electrocardiogram or echocardiogram abnormalities. Our results indicate that the CMD regimen is safe in elderly patients and no cardiotoxicities developed as a result of this regimen. In addition, it was effective in patients who had previously been treated with doxorubicin and good treatment results were obtained in elderly patients with relapsed PTCL. (Cancer Sci 2007; 98: 109–112)
Mature T‐cell and natural killer‐cell lymphomas represent 5–25% of all non‐Hodgkin's lymphomas (NHL) in Western countries and Asia.( 1 , 2 ) The International Peripheral T‐cell Lymphoma (PTCL) Project includes 1162 patients who were diagnosed with de novo PTCL or natural killer/T‐cell lymphoma from 1990 to 2002 at 20 sites in North America, Europe and Asia. A formal classification and establishment of therapy will be performed in the future.( 3 ) Compared with diffuse large B‐cell lymphoma, PTCL has a significantly lower complete response (CR) rate and overall survival (OS) rate and many patients with PTCL relapse.( 4 , 5 ) An effective salvage therapy in elderly patients with relapsed PTCL has not been reported. In addition, they usually have previously been treated with an anthracycline such as doxorubicin (DXR), which can lead to cardiotoxicity. Therefore, the possibility of DXR‐induced cardiomyopathy must be taken into consideration when drugs for salvage therapy are selected.
Recently, irinotecan hydrochloride (CPT‐11), a potent inhibitor of topoisomerase I, has been used in the treatment of cancer of the lung, ovary, colon and stomach. The efficacy of CPT‐11 as a single therapy or in combination with other anticancer agents is being studied. The activity of CPT‐11 is not affected by P‐glycoprotein expression and there is minimum cross‐resistance with DXR or vincristine.( 6 ) It has been reported that administration of multiple small doses of CPT‐11 has a higher response rate than a single high‐dose administration in the treatment of NHL.( 7 ) In addition, there are many issues to evaluate, such as the kinds of drugs to combine with CPT‐11 and the order of administration of the drugs. In preclinical studies, the combination of topoisomerase I and topoisomerase II inhibitors showed an anticancer effect that was more than additive.( 8 , 9 ) The therapeutic activity of mitoxantrone (MIT), a topoisomerase II inhibitor, in NHL has been demonstrated in patients who had previously been treated with other regimens, with an objective response in 20–30% of patients with NHL.( 10 ) Therefore, we previously studied the efficacy of the combination of MIT and CPT‐11. The CMD (CPT‐11, MIT, dexamethasone) regimen was administered to 32 patients with relapsed or refractory NHL.( 11 ) CR was seen in 11 cases (34.4%) and partial remission (PR) in nine cases (28.1%). The pilot study included eight patients who were over 70 years of age; three of the eight patients achieved CR and three patients achieved PR.
In the present clinical phase II study, we administered the CMD regimen to elderly patients with relapsed or refractory PTCL, and its safety and efficacy were evaluated. As particularly severe toxicity was not observed in the pilot study,( 11 ) a higher dosage of MIT in the CMD regimen was administered in the present study. In addition, the dosage used in the pilot study was used in patients aged over 70 years because no particularly severe toxicity was observed. In order to evaluate whether MIT worsens cardiac function, the serum troponin T level, plasma B‐type natriuretic peptide (BNP) level and left ventricular ejection fraction (LVEF) as determined by echocardiogram were measured and cardiac function was evaluated.
Patients and Methods
Study design. This was an open‐label, single‐arm phase II study of the CMD regimen for the treatment of relapsed or refractory PTCL in elderly patients. We evaluated the response rate, cardiotoxicity (plasma BNP level, serum troponin T level, LVEF), survival and progression‐free survival (PFS). LVEF was measured before the start of treatment and 3 months after the end of treatment. In addition, the safety of this regimen was investigated. UCT was performed by a single cardiologist using the GE Yokokawa Medical System echocardiogram (UCT) apparatus. This study was approved by the Ethics Committee of Kitasato University School of Medicine and was carried out in accordance with the guidelines of the Declaration of Helsinki.
Eligibility criteria. Patients with relapsed or refractory PTCL who were being treated at Kitasato University School of Medicine were enrolled between April 2001 and March 2005. Eligible patients had histologically documented relapse or refractory PTCL, including PTCL, unspecified PTCL (PTCL‐U), angioimmunoblastic T‐cell lymphoma (AILT) and anaplastic large‐cell lymphoma (ALCL), as defined by the World Health Organization lymphoma classification.( 12 ) Patients who were between the ages of 70 and 79 years with expected survival of greater than 4 months and a performance status of 0–2 on the Eastern Co‐operative Oncology Group scale were included. Patients with stage II, III or IV disease, as assessed by the Ann Arbor classification, were included.( 13 ) We included patients who had previously received a total dose of DXR of 300 mg/m2 or less. Pretreatment laboratory examination was carried out within 2 weeks of study entry, and patients with the following range of laboratory results were included: absolute neutrophil count (ANC), >0.5 × 109/L; platelets, >75 × 109/L; creatinine, <1.5 × upper limit of normal (ULN); bilirubin, <2.0 × ULN; and aspartate transaminase (AST), <5 × ULN. Patients with uncontrolled infection (one patient), concomitant malignancy (eight patients), unstable angina pectoris (one patient), symptomatic cardiac arrhythmia (two patients), clinical heart failure (two patients) or symptomatic pleural effusions (one patient) were excluded. All patients gave informed consent for both treatment and sample collection in accordance with institutional policy.
Treatment. Patients were treated with the CMD regimen. The CMD regimen consisted of CPT‐11 (25 mg/m2, i.v.) on days 1 and 2, MIT (8 mg/m2, i.v.) on day 3, and dexamethasone (40 mg/day, i.v.) on days 1–3. Each patient was administered six cycles of CMD, once every 3 weeks. The schedule of granulocyte colony‐stimulating factor (G‐CSF) administration was designed so as to maintain the dose intensity of chemo‐therapeutic agents. If the nadir ANC was less than 5 × 109/L on one or two measurements, the same doses of drugs were administered in the present cycle as in the previous cycle. If the nadir ANC was less than 0.5 × 109/L on at least three measurements, the doses of CPT‐11 and MIT in the present cycle were reduced by 20% compared with the respective dose in the previous cycle. If the nadir platelet count was less than 25 × 109/L, the doses of CPT‐11 and MIT in the present cycle were reduced by 20% compared with the respective dose in the previous cycle.
Response criteria. Tumor response was assessed after the six cycles of treatment or at the end of chemotherapy treatment. Tumor response was classified as CR, unconfirmed complete response (CRu), PR, stable disease or progressive disease according to the International Workshop for NHL response criteria.( 14 )
Troponin T and BNP assays. For determination of the serum troponin T level, the blood samples were centrifuged at 1000 g at 4°C for 15 min and stored at −70°C until assayed. The third‐generation Enzymun Test Troponin T assay was used, which was developed based on a prototype of the new electrochemiluminescence‐based Elecsys system (Roche Diagnostics, Tokyo, Japan). The lower limit of detection of troponin T was 0.01 µg/mL. For determination of the plasma BNP level, blood samples were collected in chilled tubes containing ethylenediaminetetracetic acid, disodium salt and aprotinin (500 IU/mL). The plasma was separated by centrifugation at 1000 g at 4°C for 15 min and then stored at −70°C until analysis. The BNP concentration was measured by a commercial radioimmunoassay kit for human BNP (Shiono RIA BNP assay; Shionogi Co., Tokyo, Japan). The lower limit of detection and the upper limit of the reference interval of the BNP assay were 0.2 and 20 pg/mL, respectively.( 15 ) The blood samples for determination of the serum troponin T and plasma BNP levels, were obtained immediately after the fourth cycle of treatment was completed. Blood samples were also obtained before the treatment and 4 weeks after completion of treatment. If the troponin T level became greater than 1 µg/mL or the BNP level became greater than 200 pg/mL, MIT administration was stopped.
Statistical analyses. All statistical analyses were carried out using SAS software (version 9; SAS Institute, Cary, NC, USA). Data are expressed as mean ± SD unless otherwise indicated. The serum troponin T levels or plasma BNP levels at different time points were compared using the Wilcoxon signed rank test. The duration of the response and survival were assessed using the method of Kaplan and Meier.( 16 ) A P‐value < 0.05 was considered to indicate statistical significance.
Results
Patient characteristics. Thirty elderly patients with relapsed or refractory PTCL were enrolled in the present study. The clinical characteristics of the 30 patients are summarized in Table 1. There were 16 men and 14 women with a median age of 75 years (range, 70–79 years). The histological subtype was PTCL‐U in 27 patients and AILT in three patients. Seven patients had stage II disease, 13 patients had stage III disease and 10 patients had stage IV disease at the time of relapse. There were 25 relapsed patients and five patients who were resistant to the initial treatment. Twenty‐four patients had previously undergone one chemotherapy regimen and six patients had previously undergone two chemotherapy regimens. The total dose of DXR that had been administered in the previous chemotherapy regimens was 250 mg/m2 (median; range, 200–300 mg/m2).
Table 1.
Patient characteristics
| Characteristic | Total (n = 30) |
|---|---|
| Age (median years) | 75 (Range, 70–79) |
| Sex (Male/female) | 16/14 |
| Histology (PTCL‐U/AILT/ALCL) | 27/3/0 |
| Stage (II/III/IV) | 7/13/10 |
| LDH (normal/elevated) | 7/23 |
| Relapsed disease status | 25 |
| Primary refractory | 5 |
| No. prior chemotherapy regimens | |
| 1 | 24 |
| 2 | 6 |
| Total dose of doxorubicin (median) | 250 mg/m2 (Range, 200–300mg/m2) |
AILT, angioimmunoblastic T‐cell lymphoma; ALCL, anaplastic large‐cell lymphoma; LDH, lactate dehydrogenase; PTCL‐U, unspecified peripheral T‐cell lymphoma.
Response to the CMD regimen. The overall response rate to the CMD regimen was 60% (CR [including CRu], 11 patients, 37%; PR, seven patients, 23%) (Table 2). The CR rate of relapsed patients was 10/25 (40%), and the overall response rate was 74%. One (20%) of the five primary refractory patients achieved CR, and the overall response rate was 40%. However, the CR rate did not show any significant differences when the patients were divided into groups according to histology (PTCL‐U vs AILT), PS (0,1 vs 2), stage (I/II vs III/IV) or lactate dehydrogenase (LDH) level (normal vs abnormal). The 3‐year PFS rate was 17.5% and the 3‐year survival rate was 28.2% with a median follow‐up period of 32 months (Fig. 1). The 3‐year PFS rate was 0% among primary refractory patients, and 20.8% among relapsed patients. The 3‐year survival rate was 10.2% among primary refractory patients, and 35.8% among relapsed patients (data not shown).
Table 2.
Results of treatment
| Response | |
|---|---|
| CR (including CRu) | 11 (37%) |
| PR | 7 (23%) |
| Relapsed disease status | |
| CR | 10/25 (40%) |
| PR | 6/25 (34%) |
| Primary refractory | |
| CR | 1/5 (20%) |
| PR | 1/5 (20%) |
| Survival (median follow‐up 32 months) | |
| Overall survival (4‐years) | 28.2% |
| Progression‐free survival (4‐years) | 17.5% |
CR, complete response; CRu, complete response uncertain; PR, partial remission.
Figure 1.
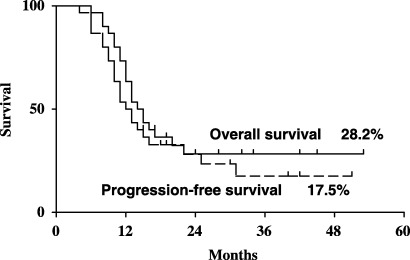
Overall and progression‐free survival curves of the 30 elderly patients with relapsed or refractory peripheral T‐cell lymphoma who were treated with the CMD (CPT‐11, mitoxantrone [MIT], dexamethasone [DEX]) regimen.
Adverse drug reactions. Grade 3–4 hematological toxicities were observed in 18 (60%) of the 30 patients (Table 3). Eight patients (27%) developed grade 4 neutropenia, all of whom had been treated with G‐CSF; they were treated with antibiotics and recovered. Five patients developed grade 3/4 thrombocytopenia and they required platelet transfusion. Two patients developed grade 3 hemoglobin decrease and both received red blood cell transfusion. As for non‐hematological toxicities of grade 3 or higher, there was only febrile neutropenia, which developed in eight patients. There were no deaths associated with the treatment.
Table 3.
Adverse drug reactions
| Reaction | Grade 0/1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Hematological | ||||
| White blood cells | 1 | 11 | 10 | 8 |
| Neutrophils | 1 | 11 | 9 | 9 |
| Hemoglobin | 12 | 16 | 2 | 0 |
| Platelets | 15 | 10 | 4 | 1 |
| Non‐hematological | ||||
| Nausea or vomiting | 28 | 2 | 0 | 0 |
| Diarrhea | 29 | 1 | 0 | 0 |
| Mucositis | 28 | 2 | 0 | 0 |
| ALT/AST | 28 | 2 | 0 | 0 |
| Creatinine | 20 | 0 | 0 | 0 |
| Troponin T | 30 | 0 | 0 | 0 |
| Cardiac | 30 | 0 | 0 | 0 |
| Febrile neutropenia | 22 | 0 | 8 | 0 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Cardiotoxicities (serum troponin T and plasma BNP levels). We evaluated the cardiotoxicity of MIT with the serum troponin T and plasma BNP levels and the LVEF obtained by echocardiogram. The troponin T and BNP levels were measured before the start of treatment, after four cycles of treatment, and 1 month after completion of treatment. Serum troponin T was undetectable in all patients before the start of treatment, and it remained undetectable during and after the treatment in all patients. The BNP level was slightly elevated in three patients before the treatment (20.4, 22.6 and 26.8 pg/mL; normal, <20 pg/mL). After four cycles of treatment, the BNP levels in two of the three patients increased to 21.2 and 30.8 pg/mL, respectively. These two patients showed no abnormalities on electrocardiogram (ECG) and echocardiogram. The LVEF as measured on echocardiograms before the start of treatment was over 65% in the 30 patients (range, 66–78%). The LVEF measured after the end of treatment was over 60% in the patients (range, 62–77%). No patient had a reduction in LVEF of more than 5% at any time during or after the treatment. These findings suggest that the addition of MIT to the treatment regimen did not increase its cardiotoxicity.
Discussion
The treatment results of elderly patients with DLBCL have improved with advancements in chemotherapy combined with molecular targeted agents such as rituximab.( 17 ) However, there are few effective treatment regimens for relapsed PTCL. Younger patients with relapsed PTCL have been treated with autologous hematopoietic stem cell transplantation combined with high‐dose chemotherapy; however, a consensus opinion on its efficacy has not been reached. There have not been any reports regarding the treatment results in elderly patients with relapsed PTCL. In general, the prognosis is poor and few patients have long‐term survival.
CPT‐11 has been used in the treatment of relapsed or refractory NHL; however, the optimal administration schedule and synergistic combination of drugs have not been determined. In the treatment of colorectal cancer, CPT‐11 is generally administered at 350 mg/m2/day, once every 3 weeks.( 18 ) As to NHL, no patient has achieved remission by administration of CPT‐11 at 200 mg/m2/day every 3–4 weeks.( 7 ) However, administration of CPT‐11 at 40 mg/m2 for three consecutive days every week resulted in a CR rate of 23.2% and a PR rate of 11.1%. Administration of CPT‐11 at 40 mg/m2 for five consecutive days every 3–4 weeks resulted in a CR rate of 12.5% and a PR rate of 18.8%. These results from an early phase II study in Japan suggested that administration of multiple small doses of CPT‐11 is effective for NHL.( 7 ) Subsequently, it was reported in USA that upon administration of CPT‐11 at 300 mg/m2 every 21 days, nine (39%) of 23 cases of relapsed or refractory aggressive NHL showed remission (CR + PR). As for adverse drug reactions, the report listed diarrhea of grade 3 or higher in 15% of cases and nausea or vomiting in 14%.( 19 ) In another report, upon administration of CPT‐11 350–500 mg/m2 on day 1 and every 3–4 weeks thereafter for up to six courses, CR was seen in one (3%) of 28 cases and PR in only one case. The duration of remission was short, and digestive and hematological toxicities were seen frequently.( 20 ) Therefore, it appears that a single high‐dose administration of CPT‐11 is less effective for NHL. We previously reported the safety and efficacy of the CMD regimen, which consisted of MIT (which is less cardiotoxic than DXR) combined with CPT‐11 and dexamethasone, in patients with relapsed NHL.( 11 ) In the present study, we treated elderly patients with relapsed or refractory PTCL with a CMD regimen and studied its efficacy and safety (especially cardiotoxicity). The response rate was 60% (18 of 30 cases) (CR 37%, PR 23%), the 3‐year PFS rate was 17.5%, and the 3‐year survival rate was 28.2%. As for adverse drug reactions, the majority were hematological toxicities and no serious cases were seen. Most DXR cardiotoxicities are irreversible and cause dilated cardiomyopathy and fatal chronic heart failure. In particular, elderly patients may already have arrhythmia or latent myocardial damage prior to the treatment. Cardiotoxicity is one reason to limit the use of anthracyclins. DXR‐induced cardiomyopathy can generally be detected by the LVEF, which is determined using echocardiography or radionuclide ventriculography; however, these methods are limited in their ability to detect cardiotoxicity. Recently, the usefulness of serum BNP as a biomarker of DXR‐induced cardiomyopathy has been reported.( 21 , 22 ) Koh et al. reported that there was a significant negative correlation between the percentage fractional shortening of the left ventricle and the serum BNP or plasma troponin T level in rats and that the troponin T level was highly sensitive for the detection of DXR‐induced cardiomyopathy.( 23 ) In the present study, the troponin T level was below the detection limit, and was normal before and after treatment with the CMD regimen in all patients; therefore, it was not helpful for the early detection of DXR‐induced cardiomyopathy. The BNP level was slightly elevated in three patients before the treatment; two of these three patients showed slightly elevated BNP levels 3 weeks after the treatment, but there was no clear significant difference before and after the treatment. There were no abnormalities in LVEF or percentage fractional shortening by echocardiogram before or after CMD treatment.
In our previous study on the R‐CMD regimen in elderly patients with diffuse large B‐cell lymphoma, hematological toxicity was seen.( 24 ) In the present study, five of 30 patients developed grade 3 thrombocytopenia and they required platelet transfusion. Four patients developed grade 3 hemoglobin decrease and two of these four patients received red blood cell transfusion. Therefore, the patients should be carefully monitored for thrombocytopenia and anemia.
In conclusion, our results suggest that the CMD regimen is safe in elderly patients with relapsed or refractory PTCL, with no cardiotoxicity developing from this regimen, although hematological toxicity was seen in some patients and transfusion of platelets or red blood cells was required. This regimen was effective in patients with relapsed or refractory PTCL who had previously been treated with DXR. However, there were some patients who relapsed soon after treatment, which is an issue to address in future studies.
Acknowledgments
We thank Dr S. Nakamura, Dr H. Nakamine, Dr T. Yoshino, Dr N. Nakamura, Dr Oshima and Dr J. Tamaru for performing pathological diagnoses.
References
- 1. The World Health Organization classification of malignant lymphomas in Japan: incidence of recently recognized entities. Lymphoma Study Group of Japanese Pathologists. Pathol Int 2000; 50: 696–702. [DOI] [PubMed] [Google Scholar]
- 2. Pinkus GS, Said JW. Periperal T‐cell lymphoma. In: Daniel MK, ed. Neoplastic Hematopathology. Baltimore: Williams & Wilkins, 2001: 1091–164. [Google Scholar]
- 3. Vose JM. The International PTCL Project International Peripheral T‐Cell Lymphoma (PTCL) Clinical and Pathologic Review Project: poor outcome by prognostic indices and lack of efficacy with anthracyclines. Blood 2005; 106: 811. [Google Scholar]
- 4. Armitage JO, Weisenburger DD. New approach to classifying non‐Hodgkin's lymphomas: clinical features of the major histologic subtypes. Non‐Hodgkin's Lymphoma Classification Project. J Clin Oncol 1998; 16: 2780–95. [DOI] [PubMed] [Google Scholar]
- 5. Gisselbrecht C, Gaulard P, Lepage E et al. Prognostic significance of T‐cell phenotype in aggressive non‐Hodgkin's lymphomas. Groupe d’Etudes des Lymphomes de l’Adulte (GELA). Blood 1998; 92: 76–82. [PubMed] [Google Scholar]
- 6. Pommier Y. DNA topoisomerase I and II in cancer chemotherapy: update and perspectives. Cancer Chemother Pharmacol 1993; 32: 103–8. [DOI] [PubMed] [Google Scholar]
- 7. Ohno R, Yoshida Y, Oguro M et al. An early phase II study of CPT‐11 (Irinotecan hydrochloride) in patients with hematological malignancies. Jpn J Cancer Chemother 1994; 21: 75–82. [PubMed] [Google Scholar]
- 8. Kano Y, Suzuki K, Akutsu M, Suda K, Inoue Y, Yoshida M. Effects of CPT‐11 in combination with other anti‐cancer agents in culture. Int J Cancer 1992; 50: 604–10. [DOI] [PubMed] [Google Scholar]
- 9. Kim R, Hirabayashi N, Nishiyama M, Jinushi K, Toge T, Okada K. Experimental studies on biochemical modulation targeting topoisomerase I and II in human tumor xenografts in nude mice. Int J Cancer 1992; 50: 760–6. [DOI] [PubMed] [Google Scholar]
- 10. Coltman CA Jr, Coltman TM, Balcerzak SP, Morrison FS, Von Hoff DD. Mitoxantrone in refractory non‐Hodgkin's lymphoma: A Southwest Oncology Group study. Semin Oncol 1984; 11: 50–3. [PubMed] [Google Scholar]
- 11. Niitsu N, Iijima K, Chizuka A. Combination therapy with irinotecan (CPT‐11), mitoxantrone, and dexamethasone in relapsed or refractory non‐Hodgkin's lymphoma: a pilot study. Ann Hematol 2001; 7: 411–16. [DOI] [PubMed] [Google Scholar]
- 12. Jaffe ES, Harris NL, Stein H, Vardiman JW, eds. World Health Organization Classification of Tumours. Pathology and Genetics. Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press, 2001. [Google Scholar]
- 13. Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Res 1971; 31: 1860–1. [PubMed] [Google Scholar]
- 14. Cheson BD, Horning SJ, Coiffier B et al. Report of an international workshop to standardize response criteria for non‐Hodgkin's lymphomas. NCI‐Sponsored International Working Group. J Clin Oncol 1999; 17: 1244–53. [DOI] [PubMed] [Google Scholar]
- 15. Ishii J, Cui W, Kitagawa F et al. Prognostic value of combination of cardiac troponin T and B‐type natriuretic peptide after initiation of treatment in patients with chronic heart failure. Clin Chem 2003; 49: 2020–6. [DOI] [PubMed] [Google Scholar]
- 16. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–81. [Google Scholar]
- 17. Feugier P, Van Hoof A, Sebban C et al. Long‐term results of the R‐CHOP study in the treatment of elderly patients with diffuse large B‐cell lymphoma: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol 2005; 23: 4117–26. [DOI] [PubMed] [Google Scholar]
- 18. Haller DG. An overview of adjuvant therapy for colorectal cancer. Eur J Cancer 1995; 31A: 1255–63. [DOI] [PubMed] [Google Scholar]
- 19. Sarris AH, Phan A, Goy A et al. Irinotecan in relapsed or refractory non‐Hodgkin's lymphomas: Indications of activity in a phase II trial. Oncology 2002; 16: 27–31. [PubMed] [Google Scholar]
- 20. Ribrag V, Koscielny S, Vantelon JM et al. Phase II trial of irinotecan (CPT‐11) in relapsed or refractory non‐Hodgkin's lymphomas. Leuk Lymphoma 2003; 44: 1529–33. [DOI] [PubMed] [Google Scholar]
- 21. Nousiainen T, Vanninen E, Jantunen E et al. Natriuretic peptides during the development of doxorubicin‐induced left ventricular diastolic dysfunction. J Intern Med 2002; 251: 228–34. [DOI] [PubMed] [Google Scholar]
- 22. Auner HW, Tinchon C, Linkesch W et al. Prolonged monitoring of troponin T for the detection of anthracycline cardiotoxicity in adults with hematological malignancies. Ann Hematol 2003; 82: 218–22. [DOI] [PubMed] [Google Scholar]
- 23. Koh E, Nakamura T, Takahashi H. Troponin‐T and brain natriuretic peptide as predictors for adriamycin‐induced cardiomyopathy in rats. Circ J 2004; 68: 163–7. [DOI] [PubMed] [Google Scholar]
- 24. Niitsu N, Kohuri M, Higashihara M, Bessho M. Phase II study of the CPT‐11, mitoxantrone and dexamethasone regimen in combination with rituximab in elderly patients with relapsed diffuse large B‐cell lymphoma. Cancer Sci 2006; 97: 933–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


