Abstract
Hepatocyte growth factor (HGF) promotes malignant development of cancer cells by enhancing invasion and metastasis. NK4, a competitive antagonist for HGF, is a bifunctional molecule that acts as a HGF antagonist and angiogenesis inhibitor. Although successful tumor inhibition by NK4 gene expression in tumor models has been demonstrated, the effects of systemic NK4 gene introduction are yet to be addressed. Here we show that systemic administration of a replication‐defective adenovirus expressing NK4 (Ad.NK4) inhibits tumor growth and lung metastasis of B16F10 melanoma and Lewis lung carcinoma in syngeneic mice. Single tail‐vein injection of Ad.NK4 achieved therapeutic levels of NK4 in the circulation and in multiple organs. Despite NK4 expression that was highest in the liver, toxicity in the liver was minimal. Ad.NK4‐mediated growth inhibition was associated with decreased blood vessel density and increased apoptosis in tumor tissues, which suggests that NK4 suppressed tumor growth as an angiogenesis inhibitor. Metastasis of B16F10 melanoma and Lewis lung carcinoma cells to the lung was potently inhibited by systemic Ad.NK4‐administration. Our results demonstrated that the adenovirus‐mediated induction of high levels of circulating NK4 significantly inhibited in vivo tumor growth and distant metastasis without obvious side effects. NK4 gene therapy is thus a safe and promising strategy for the treatment of cancer patients, and further validation in clinical trials is needed. (Cancer Sci 2009; 100: 1351–1358)
Hepatocyte growth factor (HGF), originally identified and cloned as a potent mitogen for hepatocytes,( 1 , 2 ) plays biological and physiological roles in both embryonic development and adult tissue regeneration.( 3 , 4 ) In malignant tumors, HGF enhances dissociation of cell–cell adhesiveness, cell motility, and extracellular matrix breakdown through the Met receptor tyrosine kinase, thereby leading to tumor invasion and metastasis.( 4 , 5 , 6 ) Blockade of HGF–Met signaling is thought to be a strategy for cancer therapy, and various approaches for inhibiting the HGF–Met pathway are currently being tested, including Met tyrosine kinase inhibitors,( 7 ) neutralizing antibodies,( 8 , 9 ) siRNA knockdown,( 10 ) and selected domains of Met or HGF such as decoy Met, Sema domain of Met, and NK4.( 4 , 5 , 6 )
We earlier prepared NK4 as a competitive antagonist for HGF–Met association.( 11 ) NK4 is composed of the NH2‐terminal and four‐kringle domains in the α‐chain of HGF.( 11 ) NK4 binds to Met and competitively antagonizes HGF‐induced activation of Met.( 12 , 13 , 14 , 15 , 16 ) Furthermore, in addition to its activity as a HGF antagonist, NK4 inhibits angiogenesis driven by basic fibroblast growth factor (bFGF), vascular endothelial growth factor, and HGF.( 17 , 18 ) The inhibition of Met activation by NK4 is not involved in the inhibitory effect of NK4 on bFGF‐induced and vascular endothelial growth factor‐induced angiogenic responses, suggesting a mechanism independent of its HGF‐antagonist action.( 18 ) Although the mechanism by which NK4 inhibits angiogenesis is yet to be defined, the bifunctional properties of NK4 (angiogenesis inhibitor and HGF antagonist) have led to a therapeutic approach for the treatment of invasive and metastatic tumors.( 15 )
Gene therapy is considered as another approach for antiangiogenesis therapy by virtue of its ability to locally and/or systemically deliver polypeptide angiogenesis inhibitors for a certain period.( 19 ) In multiple human cancer xenograft mouse models, NK4 gene delivery has been shown to inhibit tumor growth, invasion, and metastasis,( 15 , 16 ) and thus is now under development for clinical trials of cancer gene therapy. On the other hand, the safety and efficacy of systemic induction of NK4 genes must be carefully examined in experimental tumor models, as systemic delivery of viral vectors is currently one of the most effective means of gene therapy( 20 ) and antitumor activity of NK4 would be anticipated for any toxicity or side effects in real patients. In addition, previous studies have been done in immunodeficient nude mice with human cancer cells,( 21 , 22 , 23 ) thus NK4 gene delivery to immunocompetent animals needs to be addressed. In the present study we developed a highly efficient systemic NK4 delivery system in mice by taking advantage of the intravenous injection of NK4‐expressing adenoviral vector. We show here that in syngeneic immunocompetent mice systemic NK4 gene therapy inhibited tumor growth and lung metastasis of Lewis lung carcinoma (LLC) and B16F10 melanoma.
Materials and Methods
Materials. Recombinant HGF and NK4 were purified from the conditioned medium of CHO cells transfected with cDNA for human HGF and NK4 respectively.( 14 , 24 , 25 ) A polyclonal antibody against human HGF and ELISA kits for detection of human HGF and mouse HGF were obtained from B‐Bridge International (Mountian View, CA, USA). Animal experiments were carried out in accordance with the institutional guidelines of the animal committee of Osaka University Medical School.
Cell culture and invasion assay. LLC, B16F10, and HEK293 cells were obtained from the American Type Culture Collection (Manassas, VA, USA). SUIT2 cells were generously provided by Dr Iguchi (Fukuoka, Japan). LLC and SUIT2 cells were cultured in RPMI‐1640 medium containing 10% FBS, and HEK293 and B16F10 cells were maintained in DMEM containing 10% FBS.( 14 ) For the invasion assay, cells were seeded on Matrigel invasion chamber plates (Becton Dickinson Labware, Franklin Lakes, NJ, USA) at a density of 1.5 × 104 cells/cm2 and cultured in the medium containing 2% FBS. HGF and/or NK4 were added to the lower chamber, cells were cultured for 36 h, and invasive cells were stained with hematoxylin–eosin.
Adenovirus‐mediated NK4 expression. Replication‐defective adenovirus type 5‐based vectors lacking E1 and E3 regions were used to generate Ad.NK4 and Ad.LacZ expressing the NK4 and LacZ genes respectively under the cytomegarovirus immediate early promoter/enhancer (CMV) promoter.( 22 ) The adenoviral vectors were propagated in HEK293 cells. For detection of NK4, the conditioned medium of HEK293 cells infected with either Ad.NK4 or Ad.LacZ were subjected to immunoprecipitation and western blotting. The amount of NK4 in the medium was determined using an ELISA kit for human HGF. For in vivo systemic gene delivery, adenovirus (1 × 109 pfu in 100 µL of 3% glycerol in PBS) was administered intravenously into mice via the tail vein. Tissues were homogenized in buffer composed of 20 mM Tris‐HCl (pH 7.5), 2 M NaCl, 0.1% Tween‐80, 2 mM EDTA, and 1 mM PMSF, and centrifuged at 12 000g for 30 min. The supernatant was used as the tissue extract. Concentrations of mouse HGF in tumor tissues and NK4 in tissues and plasma were determined using ELISA kits for mouse HGF and human HGF respectively. The human HGF ELISA kit detects both human NK4 and human HGF, but does not detect mouse HGF. The mouse HGF ELISA kit does not detect NK4.
Tumor models. Male C57BL/6 mice (6 weeks old) (SLC, Hamamatsu, Japan) were implanted subcutaneously with either syngeneic LLC or B16F10 cells (106 cells in 100 µL saline), both of which were established from C57BL/6 mice. Twenty‐four hours after tumor implantation, the mice were injected intravenously in the tail vein with 100 µL of either the vehicle (3% glycerol in PBS), Ad.LacZ, or Ad.NK4 (MOI of 1 × 109 pfu for each). The tumor volume was calculated using the following formula: tumor volume (mm3) = 0.52 × (width [mm])2 × (length [mm]). All mice were killed 3 weeks after tumor implantation when control mice began to die. For the lung metastasis model, 106 cells in 100 µL saline were injected into the tail vein and the mice were injected intravenously in the tail vein with either the vehicle, Ad.LacZ, or Ad.NK4 (1 × 109 pfu for each) at 24 h after tumor inoculation. Mice were killed when control mice began to die on day 21 after tumor implantation. The number of metastatic nodules was counted microscopically.
Histopathological analysis. Apoptotic cells were determined by terminal deoxynucleotidyl transferase‐mediated dUTP nick‐end labeling (TUNEL) assay (Promega, Madison, WI, USA).( 12 , 17 ) For detection of proliferating cells and blood vessels, tissue sections were subjected to immunostaining for proliferating cell nuclear antigen (PCNA)( 12 , 17 ) and von Willebrand factor (vWF).( 17 ) The number of TUNEL‐ or PCNA‐positive cells and vWF‐positive vessels was counted under a microscope at 100× magnification using at least 20 randomly selected fields per section.
Statistical analyses. Data are presented as mean values ± SEM. Normally distributed data were analyzed using an unpaired t‐test. Data not normally distributed were analyzed using the Mann–Whitney test. P < 0.05 was considered significant.
Results
NK4 inhibits invasion by B16F10 melanoma and LLC cells. We investigated the pro‐invasive properties of HGF and the anti‐invasive effect of NK4 on B16F10 melanoma and LLC cells using a Matrigel invasion chamber (Fig. 1). In control culture, the cells did not invade but remained above the filter membrane. The addition of HGF increased invasion by cancer cells in a dose‐dependent manner (Fig. 1), and maximal stimulation was achieved at 110 pM (10 ng/mL) HGF. Addition of NK4 in the presence of 110 pM HGF inhibited the invasion by cancer cells and nearly complete inhibition to the basal level (without HGF) was achieved by addition of 110 nM NK4. NK4 alone did not significantly affect invasion by cancer cells (not shown). These results indicate that HGF potently enhances invasion by B16F10 and LLC cells and that NK4 inhibits the invasion by those cells that is stimulated by HGF.
Figure 1.

Inhibitory effects of NK4 on invasion of B16F10 and Lewis lung carcinoma (LLC) cells. Typical appearances of (a) B16F10 and (c) LLC cells that invaded through the Matrigel and a membrane. Change in the number of invasive cells: (b) B16F10 or (d) LLC cells were cultured for 36 h. Each value represents the means ± SEM (n = 3 in each group). HGF, hepatocyte growth factor.
Expression and biological activity of virally generated NK4. The expression of NK4 in Ad.NK4‐infected 293 cells was checked by ELISA and western blotting. ELISA detected 808.9 ng/mL NK4 in the culture supernatants of 293 cells infected with Ad.NK4, whereas NK4 was not detectable in Ad.LacZ‐infected cells. Likewise, NK4 was specifically detected in the conditioned medium of Ad.NK4‐infected cells by western blotting with anti‐HGF antibody (Fig. 2a). The molecular size of the NK4 protein was 67 kDa, whereas the α‐chain of recombinant HGF was 69 kDa (Fig. 2a). Thus, 293 cells infected with the prepared Ad.NK4 successfully produced and secreted NK4 protein.
Figure 2.
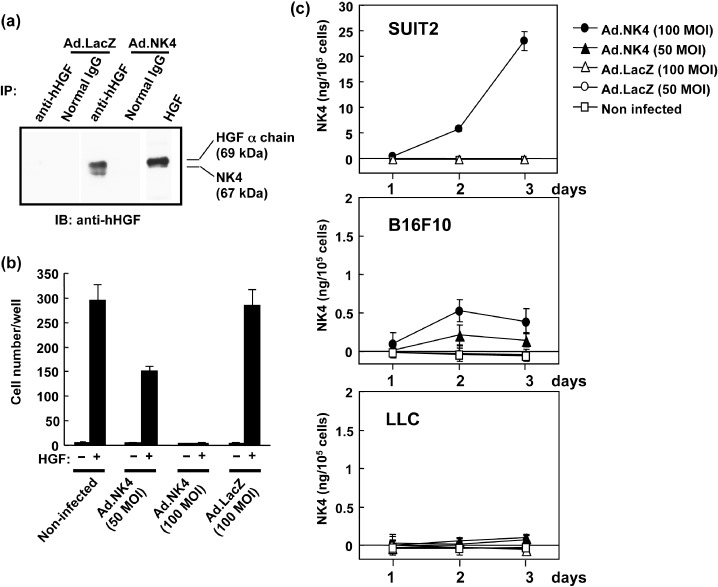
Adenovirus‐mediated NK4 gene expression in vitro. (a) Expression of NK4 protein. Cell lysates from HEK293 cells were analyzed by immunoprecipitation and western blotting using antihuman hepatocyte growth factor (HGF) antibody to detect NK4 (67 kDa). The α‐chain of HGF (69 kDa), also detected by the same antibody, was used as a control. (b) Inhibitory effect of adenovirally expressed NK4 on invasion of cancer cells. SUIT2 cells were infected with Ad.NK4 or Ad.LacZ and subjected to Matrigel invasion assay either with or without HGF (110 pM). (c) Inefficient adenovirus‐mediated NK4 induction in B16F10 and Lewis lung carcinoma (LLC) cells. SUIT2, B16F10, and LLC cells were infected with either Ad.NK4 or Ad.LacZ, and culture media were harvested for measurement of NK4. Each value represents the means ± SEM (n = 3 in each group).
Next, we determined the biological activities of virally produced NK4 in the invasion assay of SUIT2 human pancreatic cancer cells. The cells were infected with Ad.NK4, harvested after 3 days of culture, and subjected to invasion assay. NK4 protein levels reached ~23 ng/105 cells in the culture medium of SUIT2 cells infected with 100 MOI of Ad.NK4 (Fig. 2c). The addition of 10 ng/mL HGF strongly stimulated the invasion of uninfected SUIT2 cells through the Matrigel membrane, and control Ad.LacZ infection had no apparent effect on invasion (Fig. 2b). However, Ad.NK4 infection dose‐dependently blocked the HGF‐induced invasion, and the invasion was completely reversed to the baseline level by 100 MOI of Ad.NK4 (Fig. 2b). Therefore, NK4 produced by virally mediated gene expression is fully active in blocking tumor invasion as a HGF antagonist.
Adenovirus‐mediated NK4 gene transduction in mice. To evaluate the efficiency of adenoviral NK4 gene transduction in syngeneic tumor models, we infected tumor cells in vitro and host cells in vivo with Ad.NK4. When B16F10 cells were infected with Ad.NK4, they expressed NK4 and secreted ~0.6 ng/105 cells of NK4 in culture medium (Fig. 2c). Similarly, when LLC cells were infected with Ad.NK4 they produced NK4: 0.15 ng/105 cells in culture medium (Fig. 2c) and 1.5 ng/105 cells in cell lysates (not shown). Both tumor cells expressed NK4 when infected with Ad.NK4, but the expression levels of NK4 were much lower than that of SUIT‐2 cells: approximately 50 times lower in B16F10 cells and 100 times lower in LLC cells (Fig. 2c).
As NK4 treatment is advantageous in blocking tumor invasion and distant metastasis, NK4 gene transduction in host tumor‐surrounding tissues or in distant organs where tumor cells invade, or metastasize to, is significantly important. C57BL/6 mice were intravenously injected with Ad.NK4, and NK4 levels in the plasma and tissues were measured. Surprisingly, one shot of intravenous Ad.NK4 delivery achieved high expression levels of NK4 in the blood as well as in major organs, and expression was maintained for 28 days (Fig. 3). NK4 protein levels peaked at day 7 for lung (527.0 ± 40.0 ng/g tissue) and plasma (157.6 ± 27.0 ng/mL), and peaked at day 14 for kidney (800.0 ± 277.7 ng/g tissue) and liver (18.82 ± 2.69 µg/g tissue) (Fig. 3), whereas NK4 was not detectable in mice infected with Ad.LacZ. Those levels of NK4 induction are more than 80 times higher than the levels previously achieved by intraperitoneal injection.( 21 ) Although alanine transaminase (ALT), a marker for liver toxicity, was elevated to 155.7 ± 17.4 IU/L at 14 days after Ad.NK4 injection, it recovered to the normal level of 46.4 ± 27.8 IU/L at 4 weeks. No histological abnormalities of major organs were seen in the Ad.NK4 treatment group. Therefore, despite the inefficiency of adenoviral gene transduction in tumor cells per se, the intravenous injection of Ad.NK4 exerts an efficient systemic production of NK4 in the host cells of mice, and thus is suitable for examining the in vivo efficacy and safety of vigorous NK4 gene induction.
Figure 3.

In vivo adenovirus‐mediated NK4 gene induction in mice. Concentrations of NK4 in the (a) kidney, (b) liver, (c) lung, or (d) plasma after systemic administration of Ad.NK4. Mice were injected intravenously with Ad.NK4 or control Ad.LacZ via the tail vein. Each value represents the mean ± SEM (n = 5 in each group).
Inhibition of primary tumor growth. We next examined whether systemic expression of NK4 would suppress tumor growth in vivo. Either B16F10 cells or LLC cells were subcutaneously implanted into C57BL/6 mice, and either Ad.NK4, Ad.LacZ, or saline was intravenously introduced in the tail vein 1 day after tumor cell implantation (Fig. 4). Ad.LacZ treatment did not affect tumor growth of either tumor cell types as compared to vehicle treatment, indicating that there is no non‐specific tumor inhibition by adenoviral gene expression. In contrast, the volume of subcutaneous primary tumors in Ad.NK4‐treated mice was inhibited to 25% for LLC (P < 0.005, Fig. 4a) and to 24% for B16F10 melanoma (P < 0.005, Fig. 4b) on day 19 as compared to control mice. NK4 was mostly undetectable in tumor tissues of Ad.LacZ‐treated mice, whereas similar NK4 levels (13.2 ± 2.7 and 13.3 ± 6.0 ng/g tissue in LLC and B16F10 respectively) were detected in LCC and B16F10 tissues of Ad.NK4‐treated mice (Fig. 4c). In LLC tumors, HGF levels in Ad.LacZ‐ and Ad.NK4‐treated mice were 9.2 ± 0.6 and 14.6 ± 2.1 ng/g tissue, respectively, although we could not explain the reason for this change. In B16F10 tumors, similar levels of HGF (4.7–5.2 ng/g tissue) were detected Ad.LacZ‐ and Ad.NK4‐treated mice.
Figure 4.

Inhibitory effects of intravenous Ad.NK4 administration on primary tumor growth. Growth curves of subcutaneous tumor growth of (a) Lewis lung carcinoma (LLC) or (b) B16F10, and (c) NK4 and (d) hepatocyte growth factor (HGF) levels in tumor tissues on day 19. LLC or B16F10 cells were subcutaneously implanted in the right flank of mice on day 0. Vehicle, Ad.NK4, or Ad.LacZ was administered intravenously once on day 1. Each value represents the mean ± SEM (n = 8 in each group).
Because tumor growth is regulated by the counterbalance of proliferation and death of cancer cells, proliferating and apoptotic cancer cells were investigated by immunohistochemistry for PCNA and TUNEL, respectively. The population of PCNA‐positive cells in LLC tumor tissues had no significant difference between values in Ad.LacZ and Ad.NK4 (59.8% in Ad.LacZ vs 59.0% in Ad.NK4, P = 0.748) (Fig. 5a,b). On the other hand, the population of TUNEL‐positive cancer cells was 1.08 ± 0.10% in control mice, whereas it significantly increased to 2.37 ± 0.40% in Ad.NK4‐treated mice (P < 0.01) (Fig. 5a,b). The blood vessel density in tumor tissues of control mice was 20.7 ± 2.6/field, whereas it decreased to 13.3 ± 1.6/field in Ad.NK4‐treated mice (P < 0.01). Similarly, in B16F10 tumors NK4 gene expression did not affect the number of PCNA‐positive tumor cells, but it increased the number of apoptotic cells (0.79% in Ad.LacZ vs 1.64% in Ad.NK4) and decreased the blood vessel density (24.0 ± 3.2 in Ad.LacZ vs 16.8 ± 2.4 in Ad.NK4, P < 0.05) (Fig. 5c,d).
Figure 5.

Enhanced tumor apoptosis by Ad.NK4‐treatment. Typical distribution of PCNA‐positive proliferating cells, TUNEL‐positive apoptotic cells, and von Willebrand factor (vWF)‐positive blood vessels in subcutaneous tumor tissues from (a) Lewis lung carcinoma (LLC) or (c) B16F10. Changes in PCNA‐positive cells, TUNEL‐positive cells, and vWF‐positive blood vessels in (b) the tumor tissues of LLC or in (d) B16F10. Tumors were excised on day 19. Each value represents the mean ± SEM (n = 8 in each group). **P < 0.01.
Inhibition of pulmonary metastasis. Because inhibition of tumor metastasis is still a major challenge for cancer treatment, we investigated whether experimental lung metastasis would be inhibited by systemic expression of NK4. Ad.NK4 or Ad.LacZ was intravenously administered at 24 h after injection of tumor cells from the tail vein, and the number of metastatic nodules on the lung surface and lung weights were measured 3 weeks after tumor implantation (Fig. 6). For LLC lung metastasis, control mice developed more than 100 metastatic nodules on their lung surface, whereas the number of metastatic nodules decreased to 14.6 nodules/lung in Ad.NK4‐treated mice (P < 0.005 compared to vehicle or Ad.LacZ) (Fig. 6a,b). Consistent with the decreased metastases, the lung weight of Ad.NK4‐treated mice was significantly lower than that of control mice, indicating that metastatic tumor burden was significantly attenuated in lungs of Ad.NK4‐treated mice (Fig. 6c). Similarly, lung metastasis of B16F10 was inhibited by Ad.NK4 (Fig. 6d), as determined by a decreased metastatic nodule number (Fig. 6e) and a reduced lung weight (Fig. 6f), compared to treatment with either the vehicle or Ad.LacZ (P < 0.005).
Figure 6.

Suppression of lung metastasis by systemic Ad.NK4‐treatment. Typical appearances of lungs metastasized with (a) Lewis lung carcinoma (LLC) or (d) B16F10. Numbers of metastatic nodules on the surface of lungs in the mice bearing either (b) LLC or (e) B16F10. Lung weight of the mice bearing (c) LLC or (f) B16F10. Lung tissues were harvested on day 21 after tumor inoculation. Each value (closed circle) represents the mean ± SEM.
Discussion
Both LLC cells and B16F10 cells were refractory to adenovirus‐mediated gene transduction compared with other Ad.NK4‐permissive cells in previous reports.( 15 , 16 , 21 , 22 ) Instead, when administered intravenously, Ad.NK4 induced strong expression of the NK4 transgene in host normal cells in vivo– likely the highest in liver cells. In addition, in contrast to the undetectable levels of plasma NK4 by intraperitoneal injection of Ad.NK4,( 22 ) intravenous injection achieved therapeutic levels of NK4 in circulation in this study, and thus is a good experimental model for systemic NK4 gene therapy. Intriguingly, ALT, a marker for liver toxicity, was slightly elevated in the mice receiving intravenous Ad.NK4 injection (155.7 ± 17.4 IU/L), but it was the same as, or even lower than, that in the intraperitoneal injections (185 ± 83 IU/L),( 22 ) suggesting that even high levels of NK4 protein do not induce overt side effects, at least in mice.
NK4 levels in subcutaneous LLC and B16F10 tissues reached 13.2 and 13.3 ng/g tissue, respectively, and NK4 levels in tumor tissues might be predominantly derived from circulating NK4 in the blood. On the other hand, endogenous HGF levels were 14.6 and 4.7 ng/g tissue in LLC and B16F10, respectively, and the main source of HGF in tumor tissues might be the host cells. A previous report demonstrated that intraperitoneal administration of Ad.NK4 inhibited HGF‐ and bFGF‐induced angiogenesis in subcutaneous Matrigel in mice.( 22 ) In this case, the NK4 level in subcutaneous tumor tissue reached 1.2 ng/g tissue by intraperitoneal administration of Ad.NK4 and this was associated with inhibition of tumor angiogenesis and growth. Taken together, although the precise concentration of NK4 to which subcutaneous tumor cells were exposed was unknown, it is highly likely that NK4 derived from blood circulation could exert biological activities as a HGF‐antagonist and angiogenesis inhibitor in subcutaneous tumor tissues.
Hepatocyte growth factor and NK4 had no direct effect on proliferation and apoptosis in LLC and B16F10 cells (not shown). Systemic gene expression of NK4 increased apoptotic cancer cells and decreased blood vessel density without a change in the number of mitotic cancer cells, suggesting that the growth suppression might be due to the increased apoptosis. However, as the increases in apoptotic tumor cells were 2.1–2.3‐fold compared to the control values, it is arguable whether apoptosis could entirely explain the mechanism of growth suppression by Ad.NK4‐treatment. The involvement of necrotic death in cancer cells, which also occurs by angiogenesis inhibition,( 26 , 27 ) is not excluded. Thus, angiogenesis inhibition by NK4 might have played a role in suppressing the growth of primary tumors, at least in part. On the other hand, it is highly likely that both activities as HGF antagonist and angiogenesis inhibitor are involved in antimetastasis, because NK4 suppressed invasion by cancer cells, and the inhibition of tumor angiogenesis was associated with a decrease in tumor metastasis.( 28 )
Intratumoral or intraperitoneal administration of Ad.NK4 suppressed metastasis and growth of human cancer xenografts.( 21 , 22 ) In intraperitoneal administration, the antitumor effects of Ad.NK4 were described for tumors in local abdominal organs, such as peritoneal dissemination, liver metastasis, and orthotopic pancreatic tumors.( 29 , 30 ) In the present study, we first demonstrated that intravenous injection of Ad.NK4 provides systemic inhibitory effects on tumor growth and metastasis. Although the intravenous delivery of adenoviral vector may be arguable or should be modified for gene therapy, it seems to have advantages in highly efficient transgene expression. The HGF–Met pathway participates in anchorage‐independent survival of tumor cells,( 31 , 32 ) and blood HGF level increases in patients with tumors.( 3 ) Thus activation of the HGF–Met pathway in circulating tumor cells may increase the incidence of metastatic colonization. Likewise, as malignant tumors frequently metastasize to distant organs and have already formed undetectable micrometastasis when primary tumors are found, a systemic increase in blood NK4 level may have significance to inhibit metastatic colonization, enlargement of metastatic tumors, and their further spreading to other organs. It is noteworthy that the clinical trial of intravenous infusion of adenovirus demonstrated that the replication‐selective adenoviruses were well tolerated at doses of up to 2 × 1013 particles in cancer patients.( 33 , 34 )
Inhibition of tumor invasion and metastasis awaits the development of new strategies in cancer treatment and prevention. The inhibition of tumor angiogenesis is also a key therapeutic strategy that holds promise for the advancement of cancer treatment. Based on the notion that the HGF–Met pathway confers invasive and metastatic characteristics in tumor cells, systemic NK4 gene therapy by adenoviral vector, plasmid‐based gene delivery,( 35 ) and other appropriate gene transduction methods( 36 ) could offer a new therapeutic option for the inhibition of cancer metastasis and growth, thereby offering better outcomes for cancer patients.
Acknowledgments
This work was supported by grants from the Ministry of Education, Culture, Science, Sports, and Technology of Japan (no. 18013031 to T.N., 18015031 to K.M., 19890062 to K.K., and the 21st Century global COE program to T.N.), and by the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation. K.K. is supported by the Takeda Science Foundation.
References
- 1. Nakamura T, Nawa K, Ichihara A. Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem Biophys Res Commun 1984; 122: 1450–9. [DOI] [PubMed] [Google Scholar]
- 2. Nakamura T, Nishizawa T, Hagiya M et al . Molecular cloning and expression of human hepatocyte growth factor. Nature 1989; 342: 440–3. [DOI] [PubMed] [Google Scholar]
- 3. Funakoshi H, Nakamura T. Hepatocyte growth factor: from diagnosis to clinical applications. Clin Chim Acta 2003; 327: 1–23. [DOI] [PubMed] [Google Scholar]
- 4. Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol 2003; 4: 915–25. [DOI] [PubMed] [Google Scholar]
- 5. Jiang WG, Martin TA, Parr C, Davies G, Matsumoto K, Nakamura T. Hepatocyte growth factor, its receptor, and their potential value in cancer therapies. Crit Rev Oncol Hematol 2005; 53: 35–69. [DOI] [PubMed] [Google Scholar]
- 6. Matsumoto K, Nakamura T. Hepatocyte growth factor and the Met system as a mediator of tumor–stromal interactions. Int J Cancer 2006; 119: 477–83. [DOI] [PubMed] [Google Scholar]
- 7. Christensen JG, Schreck R, Burrows J et al . A selective small molecule inhibitor of c‐Met kinase inhibits c‐Met‐dependent phenotypes in vitro and exhibits cytoreductive antitumor activity in vivo . Cancer Res 2003; 63: 7345–55. [PubMed] [Google Scholar]
- 8. Cao B, Su Y, Oskarsson M et al . Neutralizing monoclonal antibodies to hepatocyte growth factor/scatter factor (HGF/SF) display antitumor activity in animal models. Proc Natl Acad Sci USA 2001; 98: 7443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Michieli P, Mazzone M, Basilico C et al . Targeting the tumor and its microenvironment by a dual‐function decoy Met receptor. Cancer Cell 2004; 6: 61–73. [DOI] [PubMed] [Google Scholar]
- 10. Shinomiya N, Gao CF, Xie Q et al . RNA interference reveals that ligand‐independent met activity is required for tumor cell signaling and survival. Cancer Res 2004; 64: 7962–70. [DOI] [PubMed] [Google Scholar]
- 11. Date K, Matsumoto K, Shimura H, Tanaka M, Nakamura T. HGF/NK4 is a specific antagonist for pleiotrophic actions of hepatocyte growth factor. FEBS Lett 1997; 420: 1–6. [DOI] [PubMed] [Google Scholar]
- 12. Date K, Matsumoto K, Kuba K, Shimura H, Tanaka M, Nakamura T. Inhibition of tumor growth and invasion by a four‐kringle antagonist (HGF/NK4) for hepatocyte growth factor. Oncogene 1998; 17: 3045–54. [DOI] [PubMed] [Google Scholar]
- 13. Parr C, Hiscox S, Nakamura T, Matsumoto K, Jiang WG. NK4, a new HGF/SF variant, is an antagonist to the influence of HGF/SF on the motility and invasion of colon cancer cells. Int J Cancer 2000; 85: 563–70. [PubMed] [Google Scholar]
- 14. Tomioka D, Maehara N, Kuba K et al . Inhibition of growth, invasion, and metastasis of human pancreatic carcinoma cells by NK4 in an orthotopic mouse model. Cancer Res 2001; 61: 7518–24. [PubMed] [Google Scholar]
- 15. Matsumoto K, Nakamura T. Mechanisms and significance of bifunctional NK4 in cancer treatment. Biochem Biophys Res Commun 2005; 333: 316–27. [DOI] [PubMed] [Google Scholar]
- 16. Matsumoto K, Nakamura T. NK4 gene therapy targeting HGF‐Met and angiogenesis. Front Biosci 2008; 13: 1943–51. [DOI] [PubMed] [Google Scholar]
- 17. Kuba K, Matsumoto K, Date K, Shimura H, Tanaka M, Nakamura T. HGF/NK4, a four‐kringle antagonist of hepatocyte growth factor, is an angiogenesis inhibitor that suppresses tumor growth and metastasis in mice. Cancer Res 2000; 60: 6737–43. [PubMed] [Google Scholar]
- 18. Kuba K, Matsumoto K, Ohnishi K, Shiratsuchi T, Tanaka M, Nakamura T. Kringle 1‐4 of hepatocyte growth factor inhibits proliferation and migration of human microvascular endothelial cells. Biochem Biophys Res Commun 2000; 279: 846–52. [DOI] [PubMed] [Google Scholar]
- 19. Kuo CJ, Farnebo F, Yu EY et al . Comparative evaluation of the antitumor activity of antiangiogenic proteins delivered by gene transfer. Proc Natl Acad Sci USA 2001; 98: 4605–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Young LS, Searle PF, Onion D, Mautner V. Viral gene therapy strategies: from basic science to clinical application. J Pathol 2006; 208: 299–318. [DOI] [PubMed] [Google Scholar]
- 21. Ogura Y, Mizumoto K, Nagai E et al . Peritumoral injection of adenovirus vector expressing NK4 combined with gemcitabine treatment suppresses growth and metastasis of human pancreatic cancer cells implanted orthotopically in nude mice and prolongs survival. Cancer Gene Ther 2006; 13: 520–9. [DOI] [PubMed] [Google Scholar]
- 22. Maemondo M, Narumi K, Saijo Y et al . Targeting angiogenesis and HGF function using an adenoviral vector expressing the HGF antagonist NK4 for cancer therapy. Mol Ther 2002; 5: 177–85. [DOI] [PubMed] [Google Scholar]
- 23. Saimura M, Nagai E, Mizumoto K et al . Tumor suppression through angiogenesis inhibition by SUIT‐2 pancreatic cancer cells genetically engineered to secrete NK4. Clin Cancer Res 2002; 8: 3243–9. [PubMed] [Google Scholar]
- 24. Wen J, Matsumoto K, Taniura N, Tomioka D, Nakamura T. Inhibition of colon cancer growth and metastasis by NK4 gene repetitive delivery in mice. Biochem Biophys Res Commun 2007; 358: 117–23. [DOI] [PubMed] [Google Scholar]
- 25. Seki T, Ihara I, Sugimura A et al . Isolation and expression of cDNA for different forms of hepatocyte growth factor from human leukocyte. Biochem Biophys Res Commun 1990; 172: 321–7. [DOI] [PubMed] [Google Scholar]
- 26. Heidenreich R, Machein M, Nicolaus A et al . Inhibition of solid tumor growth by gene transfer of VEGF receptor‐1 mutants. Int J Cancer 2004; 111: 348–57. [DOI] [PubMed] [Google Scholar]
- 27. Chang YS, Adnane J, Trail PA et al . Sorafenib (BAY 43‐9006) inhibits tumor growth and vascularization and induces tumor apoptosis and hypoxia in RCC xenograft models. Cancer Chemother Pharmacol 2007; 59: 561–74. [DOI] [PubMed] [Google Scholar]
- 28. Gasparini G, Longo R, Toi M, Ferrara N. Angiogenic inhibitors: a new therapeutic strategy in oncology. Nat Clin Pract Oncol 2005; 2: 562–77. [DOI] [PubMed] [Google Scholar]
- 29. Saimura M, Nagai E, Mizumoto K et al . Intraperitoneal injection of adenovirus‐mediated NK4 gene suppresses peritoneal dissemination of pancreatic cancer cell line AsPC‐1 in nude mice. Cancer Gene Ther 2002; 9: 799–806. [DOI] [PubMed] [Google Scholar]
- 30. Murakami M, Nagai E, Mizumoto K et al . Suppression of metastasis of human pancreatic cancer to the liver by transportal injection of recombinant adenoviral NK4 in nude mice. Int J Cancer 2005; 117: 160–5. [DOI] [PubMed] [Google Scholar]
- 31. Qiao H, Hung W, Tremblay E et al . Constitutive activation of met kinase in non‐small‐cell lung carcinoma correlates with anchorage‐independent cell survival. J Cell Biochem 2002; 86: 665–77. [DOI] [PubMed] [Google Scholar]
- 32. Abounader R, Lal B, Luddy C et al . In vivo targeting of SF/HGF and c‐met expression via U1snRNA/ribozymes inhibits glioma growth and angiogenesis and promotes apoptosis. FASEB J 2002; 16: 108–10. [DOI] [PubMed] [Google Scholar]
- 33. Nemunaitis J, Cunningham C, Buchanan A et al . Intravenous infusion of a replication‐selective adenovirus (ONYX‐015) in cancer patients: safety, feasibility and biological activity. Gene Ther 2001; 8: 746–59. [DOI] [PubMed] [Google Scholar]
- 34. Reid T, Warren R, Kirn D. Intravascular adenoviral agents in cancer patients: lessons from clinical trials. Cancer Gene Ther 2002; 9: 979–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wen J, Matsumoto K, Taniura N, Tomioka D, Nakamura T. Hepatic gene expression of NK4, an HGF‐antagonist/angiogenesis inhibitor, suppresses liver metastasis and invasive growth of colon cancer in mice. Cancer Gene Ther 2004; 11: 419–30. [DOI] [PubMed] [Google Scholar]
- 36. Kim KS, Kim HS, Park JS, Kwon YG, Park YS. Inhibition of B16BL6 tumor progression by coadministration of recombinant angiostatin K1‐3 and endostatin genes with cationic liposomes. Cancer Gene Ther 2004; 11: 441–9. [DOI] [PubMed] [Google Scholar]


