Abstract
Thioredoxin (Trx) is a multifunctional redox protein that has growth‐promoting and anti‐apoptotic effects on cells and protects cells from endogenous and exogenous free radicals. Recently, altered expression of Trx has been reported in various cancers. In the present study, we investigated altered expression of Trx at the precancerous and carcinogenic phases during cholangiocarcinogenesis in a hamster cholangiocarcinoma (ChC) model, using semiquantitative immunohistochemical and Western blot analyses. Moreover, to determine if the results correlated well with those in human ChCs, we carried out a comparative immunohistochemical study for Trx in tissue‐arrayed human ChCs with different grades of tumor cell differentiation. Trx was found highly expressed in the cytoplasm of dysplastic bile ducts with highly abnormal growth patterns and ChCs irrespective of tumor type or tumor cell differentiation. Overexpression of Trx at the precancerous and carcinogenic phases was further supported by significant elevation of Trx protein in Western blotting. The results from the hamster ChCs were in good agreement with those from human ChCs. Our results strongly suggested that the redox regulatory function of Trx plays an important role in bile duct cell transformation and tumor progression during cholangiocarcinogenesis. (Cancer Sci 2009)
Cholangiocarcinoma (ChC) is a highly malignant epithelial cancer of the biliary tract.( 1 , 2 ) Although recent studies have indicated that alteration of selected growth factor pathways are likely to be closely associated with cholangiocarcinogenesis in rodent ChC models and in humans,( 2 ) the molecular pathogenesis is still largely unknown. In human, primary sclerosing cholangitis, hepatolithiasis, fibropolycystic diseases of the biliary tract, Caroli’s disease, and liver fluke infection have been considered as the risk conditions for development of ChC.( 1 , 3 ) These risk conditions share, as a common feature, long‐standing inflammation, resulting in chronic injury to the biliary tract in the form of DNA damage due to reactive oxygen species and chronic stimulation of biliary cell proliferation.( 2 , 4 ) Therefore, dysfunction of the intracellular redox regulatory system might be involved in cholangiocarcinogenesis.
Thioredoxin (Trx), first described in Escherichia coli by Laurent et al.,( 5 ) is a multifunctional redox protein that has a redox‐active disulfide/dithiol within the conserved active site sequence, Cys‐Gly‐Pro‐Cys. Trx is identical to adult T‐cell leukemia‐derived factor, which has a growth‐promoting effect on human T‐cell lymphotrophic virus‐1‐transformed lymphocytes.( 6 ) As a redox regulating system, Trx plays an important role in cell proliferation by regulating DNA synthesis and sensitivity of cells to various growth factors.( 7 , 8 ) Moreover, Trx enhances cell survival by inhibiting apoptosis and protecting cells from both endogenous and exogenous free radicals.( 8 , 9 ) Recent studies have indicated that Trx regulates transcription factors such as nuclear factor‐κB (NF‐κB), p53, and activator protein‐1 (AP‐1) that have important functions in cellular physiology.( 8 , 10 ) Because of these properties of Trx, there have been in vitro and in vivo studies to investigate its role in various human primary tumors, particularly in association with cell proliferation and apoptosis. Recently, altered expression of Trx has been reported in various human epithelial tumors such as gastric and colorectal cancer, breast cancer, and non‐small cell lung carcinoma( 11 , 12 , 13 , 14 ) and malignant mesothelioma.( 15 ) However, a possible role of Trx in cholangiocarcinogenesis has not been explored.
A hamster ChC model composed of liver fluke infestation and carcinogen treatment has been shown to be a useful animal model to study human cholangiocarcinogenesis.( 4 , 16 ) The hamster ChC model is characterized by severe chronic inflammation and bile duct injury, resulting in atypical bile duct hyperplasia advancing to ChC development, comparable to the histopathogenesis of human ChC, especially those cases associated with liver fluke infection.( 4 , 16 )
In the present study, to evaluate the potential contribution of Trx in cholangiocarcinogenesis, we investigated altered expression patterns of Trx protein during hamster cholangiocarcinogenesis using immunohistochemical and Western blot analyses. Moreover, we carried out a comparative immunohistochemical study for Trx in tissue‐arrayed human ChC cases.
Materials and Methods
Chemicals. Dimethylnitrosamine (DMN) was purchased from Tokyo Kasei Kogyo (Tokyo, Japan). Goat antihuman Trx IgG, purified using human Trx–Sepharose immunoaffinity column chromatography, was purchased from American Diagnostica (Greenwich, CT, USA). The Vectastain Elite avidin–biotin complex (ABC) immunostaining kit and avidin–biotin blocking kit were purchased from Vector Laboratories (Burlingame, CA, USA). Target retrieval solution for antigen unmasking and 3,3‐diaminobenzidine tetrahydrochloride were obtained from DakoCytomation (Glostrup, Denmark). The horseradish‐conjugated secondary antibody and the detection reagent for Western blotting were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Animals. Syrian golden hamsters, each 130–150 g in body weight, were purchased from Japan SLC (Shizuoka, Japan). They were housed five per polycarbonate cage in a clean rack maintained at room temperature (22–26°C) under a 12‐h light/dark illumination cycle. Animals were given standard pelleted chow (Samyang, Wonju, Korea) and tap water ad libitum throughout the experimental period, except for 4 weeks when carcinogen was added to the drinking water. All animal research procedures were approved by the Kangwon National University Animal Care and Use Committee.
Cholangiocarcinoma and bile duct ligation models. The experimental protocol for hamster ChC and bile duct ligation models are summarized in Fig. 1. As previously described,( 17 ) 16 hamsters were infected with 15 metacercariae of liver fluke, Clonorchis sinensis (Cs), followed by the addition of 15 ppm DMN in their drinking water for 4 weeks. In order to collect liver tissues with dysplastic bile ducts, six hamsters were killed at 8 weeks after initiation of the experiment; based on previous studies, 8 weeks after hamster ChC model initiation was the precancerous phase prior to cancer development.( 16 , 17 ) Advanced and invasive ChCs were collected from the 10 hamsters killed at 27 weeks. As controls, five hamsters were used for the Cs infected group without DMN treatment, or the negative control group without Cs infection or DMN treatment.
Figure 1.
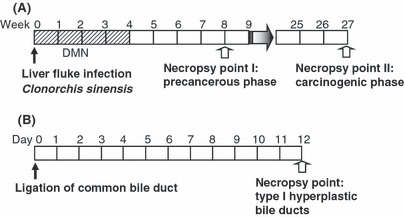
Experimental protocol for (A) hamster cholangiocarcinoma and (B) bile duct ligation models. DMN, dimethylnitrosamine.
In order to obtain carcinogen‐unaffected liver tissues with non‐tumorigenic hyperplastic bile ducts, five hamsters were subjected to ligation of the common bile duct. The hamsters were provided with tap water and standard pellet chow, then killed 12 days after the surgery.
Histology and immunohistochemistry of Trx. Hamster liver tissues were fixed in 10% buffered neutral formalin for 48 h. After routine tissue processing, tissues were embedded in a low‐melting‐point paraffin. Tissue sections were cut at 3 μm, and stained with H&E for histological examination. The histological findings were classified according to the scheme of Shimonish T( 18 ) for precancerous lesions and Endo K( 19 ) for ChCs.
The ABC method was used for immunohistochemical detection of Trx. After deparaffinization and hydration, liver tissues were immersed for 30 min in methanol solution containing 0.3% hydrogen peroxide in order to block endogenous peroxidase activity. After washing in distilled water, the sections were microwaved in preheated Dako antigen retrieval solution (pH 6.0; Dakocytomation) for 15 min at high power, followed by treatment with 0.05% Tween‐20 in PBS (pH 7.2) for 30 min at room temperature. After washing in PBS, the tissue sections were incubated in normal blocking serum provided in the Vectastain Elite ABC immunostaining kits. The sections were then incubated overnight at 4°C with the diluted primary antibody for Trx (1:300). For negative controls, PBS was applied to the sections instead of primary antibody. The tissue sections were then incubated with biotinylated secondary antibody for 40 min at room temperature, followed by incubation with ABC reagent for 30 min at room temperature. The specific bindings of antibodies within the tissue sections were visualized with the 3,3‐diaminobenzidine tetrahydrochloride solution, followed by counterstaining with Mayer’s hematoxylin.
Semiquantitative analysis of Trx immunoreactivity. For semiquantitative evaluation of Trx immunoreactivity in the biliary cell populations during cholangiocarcinogenesis, we counted the hyperplastic and dysplastic bile ducts in the six hamster livers at 8 weeks (total 555 bile ducts) and in the 10 hamster livers at 27 weeks (total 2502 bile ducts). More than 1000 individual neoplastic cells per case were counted in the 10 ChC cases. The immunoreactivity of Trx in the biliary cell populations, including biliary cancer cells, was represented as the percentage of strong (3+), moderate (2+), or weak (1+) positive bile ducts or neoplastic cells for Trx to the total counted bile ducts or neoplastic cell numbers. The criteria of grading is illustrated in Fig. 2. Based on the criteria, two different veterinary pathologists separately and blindly evaluated the immunoreactivity for Trx. The statistically significant differences between the biliary cell populations were detected using Student’s t‐test. Statistical significance was determined at P < 0.05 or P < 0.01.
Figure 2.
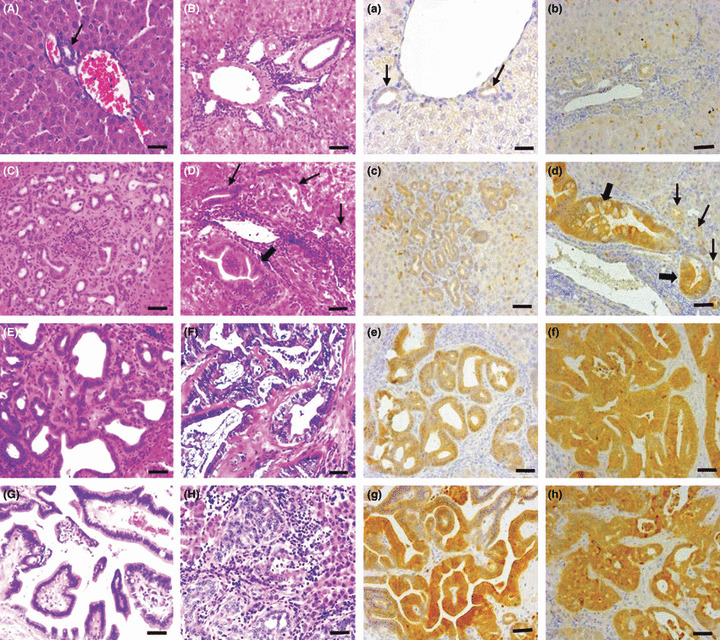
Histology (A–H, using H&E stain) and immunohistochemistry for thioredoxin (Trx) (a–h, using the avidin–biotin complex method) in a hamster cholangiocarcinoma (ChC) model. Normal bile ducts (arrows A and a), hyperplastic small bile ducts (B and b, C and c), and dysplastic bile ducts (D and d, E and e) are shown, as well as different types of ChCs (F and f, tubular type; G and g, tubulopapillary type; H and h, undifferentiated type). The Trx immunoreactivity was markedly increased in the cytoplasm of dysplastic bile ducts (d and e), depending on the grade of dysplastic changes. Note the highly increased expression of Trx in the dysplastic bile ducts with high abnormality of growth (thick arrows) and comparatively weak expression in those with mild morphological changes (thin arrows). The neoplastic cells of ChC consistently indicated high expression of Trx irrespective of tumor type or cell differentiation grade (f–h). In these figures, (b) and (c) scored 1+, (e) scored 2+, and (d, f–h) scored 3+. Bar = 25 μm.
Western blot analysis. The antibody of Trx used for immunohistochemistry originated from human Trx, therefore, the reactivity and specificity of the primary antibody against hamster Trx was tested by Western blot assay. In addition, Trx protein levels were respectively evaluated in three of the precancerous hamster livers and biliary tumor mass as well as the normal livers.
For protein extraction, the frozen hamster liver tissues were finely ground and dissolved by repeatedly passing through a 26 G syringe in protein extraction buffer (iNtRON, Chinju, Korea). After incubation for 15 min at −20°C the suspension was centrifuged at 17 900g for 5 min at 4°C. The protein concentration was measured using bicinchoninic acid solution (Sigma, St Louis, MO, USA) and spectrophotometer (Beckman Instruments, Fullerton, CA, USA). The protein extracts (15 μg) were subjected to 12% (w/v) SDS‐PAGE, then transferred to PVDF membranes (Bio‐Rad, Hercules, CA, USA). After blocking non‐specific binding sites by incubating the membranes with 5% non‐fat dried milk and 0.1% Tween‐20 in TBS (pH 7.4) for 1 h at room temperature, the membranes were incubated overnight at 4°C with diluted primary antihuman Trx antibody at 1:300 dilution, followed by incubation with secondary antibody for 50 min at room temperature. To visualize the bands, the membranes were treated with a detection reagent for 1 min, exposed to film, then developed using a SEER medical film processor (SEER Technologies, Kiheung, Korea). The statistically significant differences between the groups were detected using Student’s t‐test. Statistical significance was determined at P < 0.05 or P < 0.01.
Comparative study in tissue‐arrayed human ChCs. To compare the results of the hamster ChC model to those in human ChCs in Trx expression, we also carried out immunohistochemical staining for Trx in tissue‐arrayed human biliary cancers with different degrees of cell differentiation and normal bile ducts. The tissue array was made from the archival paraffin blocks in the Department of Pathology, Seoul National University College of Medicine (Seoul, Korea). The human biliary cancers were classified based on tumor cell differentiation grade according to the scheme of Endo K.( 19 ) The tissue array included 15 cases of well‐differentiated, eight moderately differentiated, and 24 poorly differentiated ChCs and three normal livers. The grade of Trx expression was semiquantitatively scored as negative (−), weak (1+), moderate (2+), or strong (3+) according to the criteria used for evaluating immunoreactivity of the hamster ChCs. Significant differences between the groups were determined at P < 0.05 by χ2 ‐test of independence.
Results
Histopathology and classification of hepatic biliary lesions. Gross and microscopic findings from the hamster livers at the precancerous and carcinogenic phases of the ChC model were consistent with those described in our previous study.( 17 ) The hamster livers at the precancerous phase of cholangiocarcinogenesis were characterized by proliferation of intrahepatic bile ducts with or without dysplastic changes and concurrent chronic inflammation in the periportal areas (Fig. 2C–E). Dysplastic bile ducts were determined by atypical irregular hyperplastic ducts lined by multi‐layered epithelial cells with piled‐up nuclei, sometimes forming papillary growths into the lumina (Fig. 2D,E). Moderate anisocytosis and anisokaryosis were observed in the cells lining dysplastic ducts. The dysplastic changes of bile ducts were more prominent and their frequency was also much higher in the livers at the carcinogenic phase than in those at the precancerous phase. Large hepatic ducts with dysplastic changes were also evident, severely dilated by the adult parasites (Fig. 3A).
Figure 3.
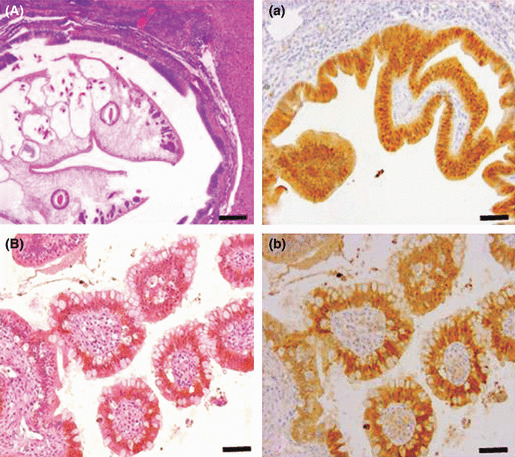
Histology (A,B, using H&E stain) and immunohistochemistry for thioredoxin (Trx) (a,b, using the avidin–biotin complex method) in a hamster cholangiocarcinoma (ChC) model. Hyperplastic large hepatic bile duct (A and a) and intestinal type ChC‐like lesion (B and b) are shown. Strong expression of Trx was noted in both cytoplasm and nucleus in these biliary cell populations. In contrast, Trx expression in the small dysplastic bile ducts and the tubular type of ChC, was restricted to cytoplasm. Bar = 100 μm (A,a) and 25 μm (B,b).
The ChCs developed were invasive primary biliary cancers of tubular or tubulopapillary type. The tubular types were composed of variably shaped tubules lined by single to multi‐layered tall columnar to cuboidal neoplastic cells and intermittent mucus‐producing goblet cells (Fig. 2F,G). These were usually well‐differentiated carcinomas, but solid foci of undifferentiated neoplastic cells were often noted in the boundary areas where neoplastic cells were infiltrating into adjacent hepatic parenchyma (Fig. 2H). Vigorous desmoplasia accompanied the proliferating neoplastic bile ducts as well.
The intestinal type ChC‐like lesion that frequently occurs in humans was evident in a large hepatic duct next to the hilus, characterized by villous projections of bile duct cells and interspersed goblet cells (Fig. 3B).
The Cs‐infected hamster livers without DMN treatment were characterized by hyperplastic small bile ducts with surrounding lymphoplasmacytic inflammation and mild fibrosis in portal areas (Supporting Information Fig. S1). Hyperplastic epithelia in the large hyperplastic bile ducts in which adult parasites were present were evident as well (Supporting Information Fig. S1).
In the hamsters with ligation of the common bile duct for 12 days, typical hyperplastic bile ductules were prominent in the portal areas, extending into the adjacent liver parenchyma (Fig. 2B). The hyperplastic bile ducts were lined by a single layer of well‐differentiated cuboidal epithelial cells (Fig. 2B). In addition, the hepatic bile ducts next to the hilus were severely dilated.
Immunohistochemistry for Trx and semiquantitative analysis. The specificity of Trx antibody in hamster liver tissues was verified by Western blot analysis. A clear single Trx protein band is shown at the molecular weight, approximately 12 kDa.
Trx was generally very weakly positive in the normal bile ducts (Fig. 2a). The immunoreactivity for Trx was slightly increased in the hyperplastic bile ducts with a well‐organized normal architecture in the ChC and bile duct ligation models (Fig. 2b,c) as well as in the hamster livers with Cs infection only (Supporting Information Fig. S1D). However, in general, the overall immunoreactivity for Trx was low.
Strong cytoplasmic staining of Trx was noted at the considerable number of bile ducts with dysplastic changes in the livers at the precancerous phase of the ChC model (Fig. 2d,e). Approximately 20% (102/555) of the total dysplastic bile ducts indicated high expression of Trx protein with >2+ grade (Fig. 4A). The strong expression of Trx was much more prominent in the dysplastic bile ducts of the non‐cancerous regions at the carcinogenic phase. Approximately 50% (1170/2502) of the dysplastic bile ducts were strongly positive for Trx (Fig. 4A). Note the significant increase in the number of dysplastic bile ducts indicating strong Trx immunoreactivity (>2+) in the livers at 27 weeks (Fig. 4B). Immunoreactivity for Trx in the dysplastic bile ducts corresponded well with the grade of dysplastic changes in the bile ducts (Fig. 2d).
Figure 4.
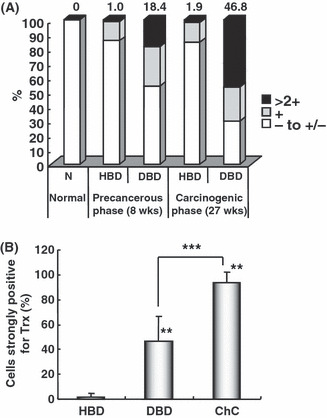
Semiquantitative analysis of thioredoxin (Trx) immunoreactivity in biliary cell populations at the precancerous and carcinogenic phases of cholangiocarcinogenesis in hamster. (A) Percentage of non‐cancerous biliary cell populations with different grades of Trx immunoreactivity during hamster cholangiocarcinogenesis. (B) Percentage of non‐cancerous biliary cell populations and neoplastic cells with strong Trx immunoreactivity (>2+) at the carcinogenic phase [27 weeks after cholangiocarcinoma (ChC) model initiation]. At the precancerous phase (8 weeks after ChC model initiation), high expression for Trx (>2+) was noted in a limited number of dyplastic bile ducts (DBD) (A). The number of DBD indicating strong Trx expression was significantly increased (A,B) at the carcinogenic phase (27 weeks after ChC model initiation), and most neoplastic cells were strongly positive for Trx (B). A total of 153, 555, and 2502 bile ducts were counted in the hamster livers at normal, precancerous, and carcinogenic phases, respectively. More than 1000 individual neoplastic cells per case were counted in 10 ChC cases. The numbers above the columns in (A) represent the percentages of cells indicated as strongly positive (>2+) for Trx. **Significant difference from hyperplastic bile ducts at P < 0.01; ***significant difference between the groups at P < 0.01. HBD, hyperplastic bile ducts.
The hyperplastic large hepatic bile ducts with dysplastic changes, which were severely dilated by the presence of adult parasites, also showed strong positivity for Trx (Fig. 3a). The expression pattern of Trx was, however, both cytoplasmic and nuclear in the hyperplastic large bile ducts as opposed to cytoplasmic in the dysplastic small ducts (Fig. 3a). The intestinal type ChC‐like lesion that occurred at the carcinogenic phase (27 weeks) also showed strong Trx immunoreactivity in both the cytoplasm and nucleus (Fig. 3b). Similarly, in the hyperplastic large bile ducts of Cs‐infected hamster livers without DMN treatment, Trx protein was overexpressed in both nuclei and cytoplasm (Supporting Information Fig. S1B,C). In the tubular or tubulopapillary types of ChC, nearly all of the neoplastic cells (>94%) were consistently strongly positive for Trx in their cytoplasm (2, 4), which is comparable to the dysplastic bile ducts of which Trx expression was heterogenous, usually depending on the abnormality of growth pattern. The small clusters of undifferentiated neoplastic cells also showed strong immunoreactivity for Trx in their cytoplasm to the same extent, as the well‐differentiated neoplastic cells (Fig. 2h).
Sinusoidal cells were immunoreactive for Trx, but hepatocytes were very weakly positive under our immunostaining protocol. In particular, the immunoreactivity of sinusoidal cells for Trx was consistent in all the liver samples examined. Therefore, the intensity of sinusoidal immunostaining in each liver sample was useful to validate the immunoreactivity of biliary cell populations.
Western blot analysis for Trx. Compared to that in the normal liver, Trx protein was prominently upregulated in the precancerous and neoplastic biliary tissues, in good agreement with the results of the immunohistochemistry, with 2.6‐fold and 4.9‐fold increases over the control value, respectively (Fig. 5). In the precancerous liver tissues, there were individual variances, which might be related to how many dysplastic bile ducts highly expressing Trx were present in the tissue (Fig. 5). Despite such individual variations, a notable difference in the Trx protein level was found between precancerous liver tissues and tumor samples; there was an approximate 2‐fold increase in the ChC tumor mass (Fig. 5).
Figure 5.
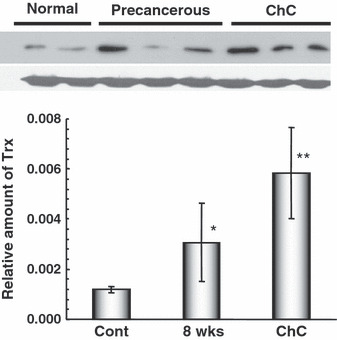
Western blot analysis for thioredoxin (Trx) protein in normal, precancerous, and neoplastic liver tissues in a hamster cholangiocarcinoma (ChC) model. Note the clear bands made by the same antibody used for immunohistochemistry. In addition, quantitative analysis indicated that Trx protein was significantly increased in the livers with frequently noted dysplastic bile ducts (precancerous, 8 weeks) and in ChCs. Compared to the control mean value (Cont), Trx protein was increased 2.6‐fold and 4.9‐fold in the precancerous (8 weeks) and ChC tissues, respectively. *P < 0.05 and **P < 0.01 versus the control.
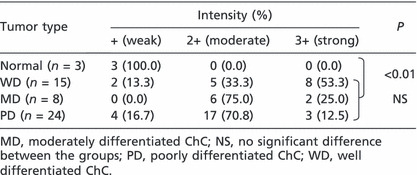
Expression of Trx in tissue‐arrayed human ChCs. The bile duct epithelial cells in the normal livers indicated relatively low expression of Trx (Fig. 6, Table 1). However, strongly positive staining of Trx was noted in the cytoplasm of most of the neoplastic epithelial cells in human ChCs. More than 85% of human ChCs were strongly positive for Trx (>2+) with significant differences from the normal at P < 0.01 (Fig. 6, Table 1). However, Trx expression was found irrespective of the differentiation grade of the tumor cells (Fig. 6, Table 1). The results from the human ChCs, therefore, closely correlated with those in the hamster ChC model.
Figure 6.
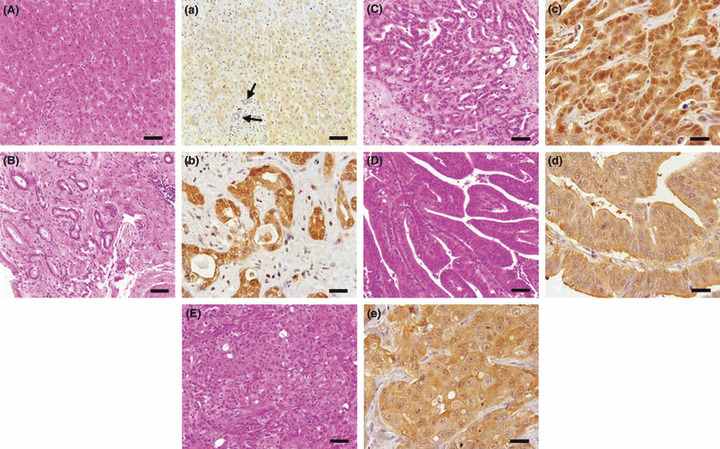
Histopathology (A–E, using H&E stain) and immunohistochemistry for thioredoxin (Trx) (a–e, using the avidin–biotin complex method) in tissue‐arrayed normal and cholangiocarcinoma (ChC) human liver tissues. Trx were reliably overexpressed in human ChCs, irrespective of tumor type or neoplastic cell differentiation grade. Compare the weak expression of Trx in normal bile ducts (arrows). A and a, normal liver; B and b, well‐differentiated ChC; C and c, moderately differentiated ChC; D and d, papillary type ChC; E and e, poorly differentiated ChC. Bars = 25 μm.
Table 1.
Immunoreactivity for thioredoxin in tissue‐arrayed archival human cholangiocarcinomas (ChCs) with different grades of neoplastic cell differentiation
Discussion
We have provided compelling evidence of altered expression of Trx in the precancerous and cancerous lesions obtained from a hamster ChC model, using immunohistochemical and Western blot analyses. The importance of Trx overexpression in cholangiocarcinogenesis was further indicated by the close immunohistochemical correlation in human ChCs.
According to our results, Trx was strongly expressed in cytoplasm of the dysplastic bile ducts at the precancerous phase and the ChCs in the hamster ChC model. In the dysplastic bile ducts, immunoreactivity for Trx was found positively related to the grade of dysplastic changes of the bile ducts. In contrast, it was negative to weakly positive in the hyperplastic bile ducts induced by bile duct ligation and Cs infection only. Overexpression of Trx in the precancerous and carcinogenic phases was further verified by significant elevation of protein at those phases in the Western blot analysis. These results strongly supported our postulation that Trx can play an important role in transformation of bile duct cells and progression of ChC. In good agreement with the results in hamsters, our comparative study using human tissues also provided compelling evidence of altered expression of Trx in ChC. Most of the human biliary cancers (more than 85%) showed high cytoplasmic expression of Trx as graded >2+. This differential expression pattern of Trx proposes a strong potential for Trx as a new molecular marker for diagnosis and as a novel therapeutic target protein against cholangiocarcinogenesis.
Trx expression did not correlate with the grade of malignancy of neoplastic cells in either hamster or human ChCs. The results in ChCs were at variance with those of other tumors. Soini et al. ( 14 ) reported stronger Trx expression in the lower‐graded non‐small cell lung carcinomas rather than in the higher‐graded tumors. In sharp contrast, Noda et al. reported higher expression of Trx in undifferentiated gastric cancers than in the differentiated type.( 12 ) Thus, the expression patterns of Trx might be unique depending on tumor type.
The subcellular localization of Trx in other cancers was also reported to be cell type‐specific, that is, either cytoplasmic or both nuclear and cytoplasmic.( 11 , 13 , 14 , 20 ) The dysplastic bile ducts and ChCs were cytoplasmic. It was of interest in the present study that the hyperplastic large hepatic bile ducts, unlike the intrahepatic small bile ducts, showed strong immunoreactivity for Trx in both cytoplasm and nuclei. High expression of Trx in both cytoplasm and nuclei was also evident in the papillary intestinal type ChC‐like lesion that developed in a hepatic duct at the carcinogenic phase.
Overexpression of Trx in the dysplastic and neoplastic bile duct cells could be associated with producing oxidative stress, promoting cell proliferation and/or inhibiting apoptosis in the hamster cholangiocarcinogenesis, as postulated in recent published reports.( 7 , 8 , 9 ) The long‐lasting inflammation in the parasite‐infected hamster ChC models have been shown to produce high levels of reactive oxygen species and nitric oxide, inducing bile duct cell DNA damage( 4 , 21 , 22 ) and influencing the redox status.( 23 ) In the present study, Cs infection enhanced Trx expression in the hamster livers independent of DMN treatment in the parasite‐containing large hyperplastic bile ducts with severe mechanical injury and surrounding inflammatory reaction (Supporting Information Fig. S1). However, Cs infection by itself did not affect Trx overexpression in the hyperplastic intrahepatic small bile ducts (Supporting Information Fig. S1). Therefore, we speculate that cytoplasmic overexpression of Trx in the intrahepatic hyperplastic bile ducts with dysplastic changes might be associated with transformation of the bile ducts for tumor development. Then, Trx overexpressing cells could resist oxidative stress and apoptosis signaling and be advantageous in cell proliferation by activating transcription factors such as AP‐1 and NF‐κB.( 8 , 23 ) Indeed, the expression patterns of Trx in the present study were strongly correlated with the proliferating activity of biliary cell populations.( 17 ) The labeling index of proliferating cell nuclear antigen significantly increased in the dysplastic and neoplastic biliary cell populations in the hamster ChC model, compared with that of normal and hyperplastic bile ducts.( 17 ) Likewise, a good positive correlation between Trx expression and proliferating activity was also indicated in breast and gastric carcinomas.( 20 , 24 ) The promoting effect of Trx on cell growth is associated with activation of AP‐1 transcription factor.( 10 ) According to Freemerman et al.’s study,( 25 ) AP‐1 dramatically increased up to 10‐fold by Trx overexpression in MCF‐7 breast cancer cells. In cholangiocarcinogenesis, Trx expression patterns in this study are strongly correlated with those of c‐Fos, an AP‐1 family protein,( 17 ) and evidenced to play a critical role in cell proliferation.( 10 , 26 , 27 , 28 , 29 )
Significant progress in understanding of the genetic and epigenetic mechanisms has been made in human and animal ChC, including mutations of specific oncogenes such as K‐ras and p53( 30 , 31 ) and aberrant expression and activation of growth factors and their receptor tyrosine kinases, such as epidermal growth factor and ErbB2, hepatocyte growth factor and Met, and interleukin‐6 signaling.( 32 ) Also, COX‐2, transmembrane mucins including MUC1 and MUC4, insulin‐like growth factor‐1 receptor signaling, serine/threonine Akt/PK (Akt) and FADD‐like interleukin‐1 β converting enzyme (Flice)‐like inhibitory protein, myeloid cell leukemia 1 (Mcl1), and Bcl‐2 have been postulated to be involved in ChC carcinogenesis.( 32 ) There have been reports showing direct or indirect links between Trx and those ChC‐associated molecules (p53, COX‐2, Bcl‐2, VEGF).( 33 , 34 , 35 , 36 ) Further studies are warranted to establish the interacting functions of Trx with other ChC‐implicated molecules.
In summary, our present study showed the overexpression of Trx in dysplastic bile ducts and ChC in hamster, further supported by a comparative human study. Our results suggest that the endogenous redox regulatory protein, Trx, might play an important role at the phases of bile duct cell transformation and tumor progression during cholangiocarcinogenesis and warrant further mechanistic studies to elucidate the precise role of this important protein.
Supporting information
Fig. S1. Immunohistochemistry for thioredoxin (Trx), using H&E stain, in hamster livers with Clonorchis sinensis (Cs) infection only for 8 weeks. Trx protein was evident in both nuclei and cytoplasm of the large normal hepatic bile ducts (A). Trx expression was highly enhanced in both nuclei and cytoplasm of the Cs infection‐induced large hyperplastic bile ducts (B,C, thick arrows), but not as enhanced in cytoplasm of the intrahepatic hyperplastic small bile ducts (B,D, thin arrows). Magnification, ×400 (A,C); ×100 (B); and ×200 (D).
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Acknowledgments
This work was supported by the Korea Research Foundation Grant (KRF‐2004‐041‐E00324) and the BK21 Program for Veterinary Science.
References
- 1. Sirica AE, Lai GH, Endo K, Zhang Z, Yoon BI. Cyclooxygenase‐2 and ERBB‐2 in cholangiocarcinoma: potential therapeutic targets. Semin Liver Dis 2002; 22: 303–13. [DOI] [PubMed] [Google Scholar]
- 2. Sirica AE. Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy. Hepatology 2005; 41: 5–15. [DOI] [PubMed] [Google Scholar]
- 3. Khan SA, Thomas HC, Davidson BR, Taylor‐Robinson SD. Cholangiocarcinoma. Lancet 2005; 366: 1303–14. [DOI] [PubMed] [Google Scholar]
- 4. Pinlaor S, Hiraku Y, Ma N et al. Mechanism of NO‐mediated oxidative and nitrative DNA damage in hamsters infected with Opisthorchis viverrini: a model of inflammation‐mediated carcinogenesis. Nitric Oxide 2004; 11: 175–83. [DOI] [PubMed] [Google Scholar]
- 5. Laurent TC, Moore EC, Reichard P. Enzymatic synthesis of deoxyribonucleotides. VI. Isolation and characterization of thioredoxin, the hydrogen donor from Escherichia coli. B J Biol Chem 1964; 239: 3436–44. [PubMed] [Google Scholar]
- 6. Wakasugi H, Rimsky L, Mahe Y et al. Epstein‐Barr virus‐containing B‐cell line produces an interleukin 1 that uses as a growth factor. Proc Natl Acad Sci U S A 1987; 84: 804–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Powis G, Mustacich D, Coon A. The role of the redox protein thioredoxin in cell growth and cancer. Free Radic Biol Med 2000; 29: 312–22. [DOI] [PubMed] [Google Scholar]
- 8. Powis G, Montfort WR. Properties and biological activities of thioredoxins. Annu Rev Biophys Biomol Struct 2001; 30: 421–55. [DOI] [PubMed] [Google Scholar]
- 9. Iwata S, Hori T, Sato N et al. Adult T cell leukemia (ATL)‐derived factor/human thioredoxin prevents apoptosis of lymphoid cells induced by L‐cystine and glutathione depletion: possible involvement of thiol‐mediated redox regulation in apoptosis caused by pro‐oxidant state. J Immunol 1997; 158: 3108–17. [PubMed] [Google Scholar]
- 10. Liu H, Colavitti R, Rovira II, Finkel T. Redox‐dependent transcriptional regulation. Circ Res 2005; 97: 967–74. [DOI] [PubMed] [Google Scholar]
- 11. Matsutani Y, Yamauchi A, Takahashi R et al. Inverse correlation of thioredoxin expression with estrogen receptor‐ and p53‐dependent tumor growth in breast cancer tissues. Clin Cancer Res 2001; 7: 3430–6. [PubMed] [Google Scholar]
- 12. Noda N, Ochiai A, Miyazaki K, Sugimura T, Terada M, Wakasugi H. Detection of thioredoxin in gastric cancer: association with histological type. Antioxid Redox Signal 2000; 2: 519–28. [DOI] [PubMed] [Google Scholar]
- 13. Raffel J, Bhattacharyya AK, Gallegos A et al. Increased expression of thioredoxin‐1 in human colorectal cancers is associated with decreased patient survival. J Lab Clin Med 2003; 142: 46–51. [DOI] [PubMed] [Google Scholar]
- 14. Soini Y, Kahlos K, Näpänkangas U et al. Widespread expression of thioredoxin and thioredoxin reductase in non‐small cell lung carcinoma. Clin Cancer Res 2001; 7: 1750–7. [PubMed] [Google Scholar]
- 15. Kahlos K, Soini Y, Saily M et al. Up‐regulation of thioredoxin and thioredoxin reductase in human malignant pleural mesothelioma. Int J Cancer 2001; 95: 198–204. [DOI] [PubMed] [Google Scholar]
- 16. Lee JH, Rim HJ, Sell S. Heterogeneity of the “oval‐cell” response in the hamster liver during cholangiocarcinogenesis following Clonorchis sinensis infection and dimethylnitrosamine treatment. J Hepatol 1997; 26: 1313–23. [DOI] [PubMed] [Google Scholar]
- 17. Yoon BI, Kim YB. Overexpression of c‐Fos in the dysplastic bile ducts and cholangiocarcinoma and its association with cell proliferating activity in the hamster cholangiocarcinoma model. Lab Anim Res 2008; 25: 41–6. [Google Scholar]
- 18. Shimonishi T, Sasaki M, Nakanuma Y. Precancerous lesions of intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Surg 2000; 7: 542–50. [DOI] [PubMed] [Google Scholar]
- 19. Endo K, Yoon BI, Pairojkul C, Demetris AJ, Sirica AE. ERBB‐2 overexpression and cyclooxygenase‐2 up‐regulation in human cholangiocarcinoma and risk conditions. Hepatology 2002; 36: 439–50. [DOI] [PubMed] [Google Scholar]
- 20. Grogan TM, Fenoglio‐Prieser C, Zeheb R et al. Thioredoxin, a putative oncogene product, is overexpressed in gastric carcinoma and associated with increased proliferation and increased cell survival. Hum Pathol 2000; 31: 475–81. [DOI] [PubMed] [Google Scholar]
- 21. Kawanishi S, Hiraku Y, Pinlaor S, Ma N. Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation‐related carcinogenesis. Biol Chem 2006; 387: 365–72. [DOI] [PubMed] [Google Scholar]
- 22. Prawan A, Buranrat B, Kukongviriyapan U, Sripa B, Kukongviriyapan V. Inflammatory cytokines suppess NAD(P)H:quinine oxidoreductase‐1 and induce oxidative stress in cholangiocarcinoma cells. J Cancer Res Clin Oncol 2009; 135: 515–22. [DOI] [PubMed] [Google Scholar]
- 23. Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress‐induced cancer. Chem Biol Interact 2006; 160: 1–40. [DOI] [PubMed] [Google Scholar]
- 24. Turunen N, Karihtala P, Mantyniemi A et al. Thioredoxin is associated with proliferation, p53 expression and negative estrogen and progesterone receptor status in breast carcinoma. APMIS 2004; 112: 123–32. [DOI] [PubMed] [Google Scholar]
- 25. Freemerman AJ, Gallegos A, Powis G. Nuclear factor kappaB transactivation is increased but is not involved in the proliferative effects of thioredoxin overexpression in MCF‐7 breast cancer cells. Cancer Res 1999; 59: 4090–4. [PubMed] [Google Scholar]
- 26. Bamberger AM, Milde‐Langosch K, Rössing E, Goemann C, Löning T. Expression pattern of the AP‐1 family in endometrial cancer: correlations with cell cycle regulators. J Cancer Res Clin Oncol 2001; 127: 545–50. [DOI] [PubMed] [Google Scholar]
- 27. Holt JT, Gopal TV, Moulton AD, Nienhuis AW. Inducible production of c‐fos antisense RNA inhibits 3T3 cell proliferation. Proc Natl Acad Sci U S A 1986; 83: 4794–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nishikura K, Murray JM. Antisense RNA of proto‐oncogene c‐fos blocks renewed growth of quiescent 3T3 cells. Mol Cell Biol 1987; 7: 639–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sunters A, Thomas DP, Yeudall WA, Grigoriadis AE. Accelerated cell cycle progression in osteoblasts overexpressing the c‐fos proto‐oncogene: induction of cyclin A and enhanced CDK2 activity. J Biol Chem 2004; 279: 9882–91. [DOI] [PubMed] [Google Scholar]
- 30. Khan SA, Thomas HC, Toledano MB et al. p53 mutations in human cholangiocarcinoma: a review. Liver Int 2005; 25: 704–16. [DOI] [PubMed] [Google Scholar]
- 31. Tangkawattana S, Kaewkes S, Pairojkul C, Tangkawattana P, Sripa B. Mutations of KRAS and TP53 in a minor proportion of Opisthorchis viverrini‐associated cholangiocarcinomas in a hamster model. Asian Pac J Cancer Prev 2008; 9: 101–6. [PubMed] [Google Scholar]
- 32. Sirica AE, Nathanson MH, Gores GJ, LaRusso NF. Pathobiology of biliary epithelia and cholangiocarcinoma: proceedings of the Henry M. and Lillian Stratton basic research single‐topic conference. Hepatol 2008; 48: 2040–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Welsh SJ, Bellamy WT, Briehl MM, Powis G. The redox protein thioredoxin‐1 (trx‐1) increases hypoxia‐inducible factor 1α protein expression: Trx‐1 overexpression results in increased vascular endothelial growth factor production and enhanced tumor angiogenesis. Cancer Res 2002; 62: 5089–95. [PubMed] [Google Scholar]
- 34. Li Y, Lu Z, Chen F et al. Antisense Bcl‐2 transfection up‐regulates anti‐apoptotic and anti‐oxidant thioredoxin in neuroblastoma cells. J Neurooncol 2005; 72: 17–23. [DOI] [PubMed] [Google Scholar]
- 35. Csiki I, Yanagisawa K, Haruki N et al. Thioredoxin‐1 modulates transcription of cyclooxygenase‐2 via hypoxia‐inducible factor‐1α in non‐small cell lung cancer. Cancer Res 2006; 66: 143–50. [DOI] [PubMed] [Google Scholar]
- 36. Hadj Amor IY, Smaoui K, Chaabène I et al. Human p53 induces cell death and downregulates thioredoxin expression in Saccharomyces cerevisiae . FEMS Yeast Res 2008; 8: 1254–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Immunohistochemistry for thioredoxin (Trx), using H&E stain, in hamster livers with Clonorchis sinensis (Cs) infection only for 8 weeks. Trx protein was evident in both nuclei and cytoplasm of the large normal hepatic bile ducts (A). Trx expression was highly enhanced in both nuclei and cytoplasm of the Cs infection‐induced large hyperplastic bile ducts (B,C, thick arrows), but not as enhanced in cytoplasm of the intrahepatic hyperplastic small bile ducts (B,D, thin arrows). Magnification, ×400 (A,C); ×100 (B); and ×200 (D).
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item


