Abstract
The frequency of t(14;18) in follicular lymphoma (FL) in Japan has been reported to be low compared to North America and other European countries. Recently, it has also been reported that FL lacks t(14;18), mainly among histological grade 3b, and occasionally has a rearranged Bcl‐6 gene. It is not known whether a difference in histology or immunostaining pattern exists between FL with and without t(14;18). We performed interphase fluorescence in situ hybridization (FISH) analysis to detect Bcl‐2/IgH, Bcl‐6 gene rearrangement, Bcl‐2 gene amplification, and the cyclinD1/IgH gene in formalin‐fixed paraffin embedded specimens from our FL archives. The correlation between morphological features, histological grades, immunohistochemical findings, and cytogenetical aberrations was studied. In total, we found that 28 of 47 cases (59.6%) had t(14;18). Bcl‐6 gene rearrangement and extra Bcl‐2 gene signals were found in five and two cases, respectively. Only one had cyclinD1/IgH fusion. Ten of 12 grade 1, nine of 17 grade 2, and 0 of two grade 3 cases had fusion signals, respectively. None of the above abnormalities were detected in 12 of 47 cases (25.5%). Our data confirmed a high frequency of t(14;18) in FL in grade 1, but a lower incidence among grade 2, that could be attributed to the lower incidence of the translocation in FL in Japan. Immunostaining of both Bcl‐2 and CD10 was highly predictable for the presence of t(14;18); the positive predictive value was 75%, suggesting the usefulness of the staining. (Cancer Sci 2005; 96: 77–82)
Follicular lymphoma (FL) is derived from follicular center B‐cells and morphologically has a predominantly follicular pattern. 1 , 2 , 3 The neoplastic cells are commonly positive for CD10 and Bcl‐2 protein. The natural history of FL is characterized by occasional transformation to aggressive large B‐cell lymphoma after indolent behavior. The translocation (14;18)(q32;q21) has been recognized as a hallmark of FL, and was detected in approximately 80–100% of FL cases in North America and European countries. 4 , 5 However, in South‐east Asia, including Japan, the incidence of FL is low among non‐Hodgkin's lymphoma (NHL) cases, 6 , 7 , 8 , 9 and moreover, the typical cytogenetic abnormality has been considered relatively low, 5 , 10 , 11 , 12 suggesting the geographic heterogeneity of this type of lymphoma.
Recently, it has been reported that FL without t(14;18), including cases with Bcl‐6 gene rearrangements and other cytogenetic abnormalities such as trisomy 18, is detected mainly in histological grade 3b in North America and European countries. 13 , 14 , 15 , 16 However, the relationship between the chromosomal aberration and morphology, histological grade, and immunohistochemistry has not yet been clarified. We preformed interphase fluorescence in situ hybridization (FISH) analysis to detect Bcl‐2/IgH, Bcl‐6 gene rearrangement, Bcl‐2 gene amplification, and the cyclinD1/IgH gene in formalin‐fixed paraffin embedded specimens from our FL archives. We also characterized Japanese FL with and without t(14;18) in terms of morphological features, histological grades, and immunohistochemical findings.
Patients and Methods
Patient samples. Cases diagnosed as having FL between December 1998 and December 2003 were selected from the database of the National Cancer Center Hospital (NCCH), Japan. Only cases with complete records of CD10 and Bcl‐2 staining by immunohistochemistry were included in this study.
Samples for FISH analysis were obtained from lymph nodes, the gastrointestinal tract, nasopharyngeal area, gallbladder, and bone in 37, 4, 4, 1, and 1 case, respectively, and selected from archives of formalin‐fixed paraffin embedded specimens kept at the NCCH.
Histology. Each specimen was stained with hematoxylin and eosin. We categorized three groups by morphological findings: (i) typical types with a lymphoid cuff which indicated a mantle zone, and a diffuse area less than 50%; (ii) a reverse variant which lacked a lymphoid cuff; (17) and (iii) others. Others were defined as none of the above and included those samples in which the diffuse area was more than 50%. Histological grading was also determined according to the World Health Organization classification. (1) Cases with a diffuse large B‐cell lymphoma (DLBCL) component in addition to FL were also included. In this study, histological transformation to large B‐cell lymphoma was defined as follows: (i) cases with prior biopsy specimens confirmed as FL, or (ii) cases with multiple specimens showing a diagnosis of both FL and DLBCL.
Immunohistochemistry. Immunohistochemichal studies were performed on formalin‐fixed paraffin embedded specimens by the standard avidin–biotin methods. Monoclonal antibodies used were for: CD20 (Dako, Glostrup, Denmark), CD3 (Novocastra, Newcastle‐upon‐Tyne, UK), Bcl‐2 (Dako), and CD10 (Novocastra). CD5 (Novocastra), cyclin D1 (Dako), and CD21 (Dako) were added, if necessary.
Preparation of glass slides for FISH analysis. The established tissue FISH procedure was performed with minor modifications of parameters as follows. Each specimen was cut at a thickness of approximately 4 µm. The sample was treated in a solution of 0.3% pepsin/0.01 N HCL for 14 min at 37°C, denatured in 70% formamide/20 × SSC for 2 min at 73°C. A microwave treatment procedure was then carried out to intensify the signals. The microwave (MI‐77, Azumaya Company, Tokyo, Japan) was set to beam irradiation at intervals of 3 s on and 2 s off, at a frequency of 2.45 GHz at 250 W output power, with the temperature sensor set to 37°C for 60 min. (18) Incubation was performed overnight at 37°C. Slides were covered with an antifade solution and viewed under a BX60 fluorescence microscope (Olympus, Tokyo, Japan) using a ×100 oil immersion lens and appropriate filters.
We used the reactive lymph node as a negative control and the threshold was determined. The lymph nodes were obtained from surgically resected specimens of solid cancer and had been confirmed to be negative for the cancer cells. If the frequency was above this level, the sample was judged to be positive for the aberration. Each specimen was overviewed with low magnification and only cells located on the center of the follicle were evaluated. Each specimen was examined independently by three investigators (Y K, Y Y, N S), and when the judgment was the same, the result was counted as successful staining and included in this study.
Probes applied to FISH analysis. All the probes were purchased from the manufacturer (Vysis, Downers Grove, IL, USA). To detect t(14;18), we used the LSI IGH/BCL2 Dual Color, Dual Fusion Translocation Probe. To detect Bcl‐6 gene rearrangement, the LSI BCL6 Dual Color, Break Apart Rearrangement Probe was used. To detect amplification or deletion of chromosome 18, the CEP 18 Spectrum Orange probe, which hybridizes to the centromeric region of chromosome 18, was used. To detect t(11;14), we used the LSI IGH/CCND1 Dual Color, Dual Fusion Translocation Probe.
First, all the specimens were analyzed by both probes to detect t(14;18) and Bcl‐6 gene rearrangements (Fig. 1a,b). If extra Bcl‐2 signals were detected (Fig. 1c), the CEP18 Spectrum Orange probe was applied to confirm if the extra signals were due to either gaining chromosome 18 or amplification of Bcl‐2 gene/Bcl‐2 gene rearrangement with other genes. Finally, samples without abnormalities following these procedures underwent FISH using LSI IGH/CCND1 Dual Color, Dual Fusion Translocation Probe to detect t(11;14).
Figure 1.
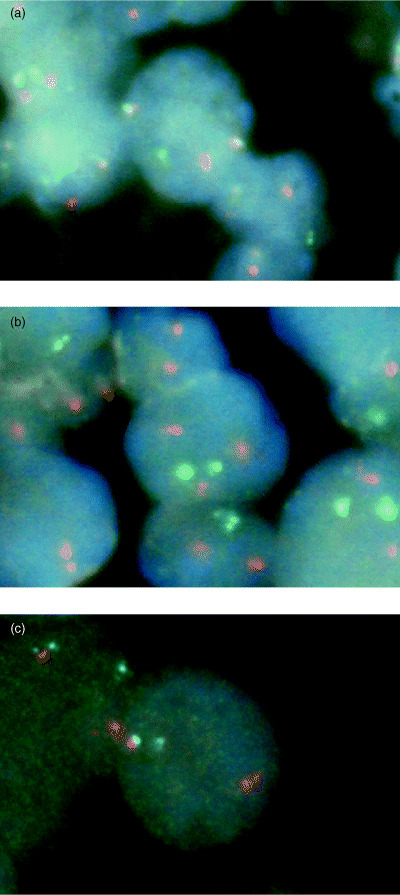
Representative result of fluorescence in situ hybridization analysis. (a) IgH and Bcl‐2 gene fusion pattern with the LSI IGH/BCL2 Dual Color, Dual Fusion Translocation Probe (Vysis, Downers Grove, IL, USA). One orange (1O) Bcl‐2 signal, one green (1G) IgH signal, and two fusion (2F) IgH/Bcl‐2 signals are present. (b) Bcl‐2 gene amplification or rearrangement with other genes with LSI IGH/BCL2 Dual Color, Dual Fusion Translocation Probe. 3O 2G signals are seen. (c) Bcl‐6 gene rearrangement with LSI BCL6 Dual Color, Break Apart Rearrangement Probe. 1O 1G 1F signals are shown.
Statistical analysis. To compare incidence of t(14;18) among each histologic grade group, we performed chi‐squared independence test using a 4 × 2 contingency table. Because almost all cases with FL + DLBCL had grade 3 FL, we analyzed grade 3a, grade 3b, and FL + DLBCL together as a grade 3 group in the analysis. After the analysis, we performed Fisher's exact probability test to compare the incidence of t(14;18) in each histologic grade.
To test whether or not immunohistochemical staining could predict the presence of t(14;18), we performed sensitivity and specificity tests. A true positive (TP) was defined as being positive for immunohistochemical results in t(14;18)‐positive patients, whereas a false‐positive (FP) was defined as being negative for immunohistochemical results in t(14;18)‐positive patients. A true negative (TN) was defined as being negative for immunohistochemical results in t(14;18)‐negative patients, whereas a false‐negative (FN) was defined as being positive for immunohistochemical results in t(14;18)‐negative patients.
The sensitivity was calculated by TP/(TP + FN), whereas the specificity was calculated by TN/(FP + TN). The positive predictive value was found by TP/(TP + FP) and the efficacy was found by (TP + TN)/total.
Results
Determination of the threshold. Reactive lymph nodes (five samples) were used as negative controls. The thresholds of fused signal frequencies were determined as the mean plus 3 standard deviations (SD) of the number of overlapping or touching signals from 100 to 200 nuclei on each slide (Table 1). ‘One fusion signal pattern’ was defined by observation of one Orange (O), one Green (G), and ‘one Orange/Green (yellow) fusion (F) signal’ (1O1G1F pattern) was commonly observed in paraffin‐embedded tissue specimens in addition to the theoretical fusion pattern (1O1G2F pattern). We evaluated the frequency of one fusion signal pattern and the threshold was also determined. The calculated thresholds of the IGH/BCL2 2 fusion pattern and 1 fusion pattern were 2% and 7%, respectively (Table 1). If the fusion signals were greater than these thresholds, the samples were judged to be positive for fusion. In the same manner, the cases were judged to have an extra number of Bcl‐2 signals if the frequency was more than 1.5%. The thresholds to 2F and 1F were over 1% and 6%, respectively, when IgH/CCND1 dual color, dual fusion translocation probes were used. The breaking apart of the signal threshold was 1.5% and was again calculated as the mean plus 3 SD of the frequency of break apart signals for the analysis using BCL6 dual color, break apart rearrangement probes.
Table 1.
Control of the IGH/BCL2 Dual Color, Dual Fusion Translocation Probe (Vysis, Downers Grove, IL, USA) for reactive lymph nodes
| Pattern | Mean (%) | SD | Mean + 3SD (%) | Threshold (%) |
|---|---|---|---|---|
| 1O 1G 2F | 0.2 | 0.48 | 1.54 | 2 |
| 1O 1G 1F | 3.6 | 0.9 | 6.28 | 7 |
| 3O 2G or 2O 3G | 0 | 0 | 0 | 1.5 |
| 1O 2G | 17.2 | 5.7 | NA | NA |
| 2O 1G | 15.2 | 4.7 | NA | NA |
| 2O 2G | 62 | 12.8 | NA | NA |
SD, standard deviation; O, orange; G, green; F, fusion; NA, not applicable.
Results of FISH analysis and immunohistochemistry. In total, we studied samples from 47 cases of FL (Table 2). Among them, two cases were not applicable for CD10 because of insufficient staining for evaluation. Excluding these cases, 41 were positive for CD10 (91.1%) with immunohistochemistry.
Table 2.
Results of clinical data, morphological feature, histologic grade, and fluorescense in situ hybridization
| Case no. | Sex | Age | Biopsy sites | Morphological feature | Histologic grade | CD10 | Bcl‐2 | t(14;18) | Bcl‐6 B.A. | Extra Bcl‐2 | t(11;14) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 60 | Mesentric LN, ileum | Transformed FL | transformed FL | – | + | – | – | – | – |
| 2 | F | 72 | Cervical LN | Typical | G2 | + | – | – | – | – | + |
| 3 | F | 61 | Cervical LN, stomach | Transformed FL | transformed FL | + | + | + | – | – | N.D. |
| 4 | F | 66 | Inguinal LN | Others | G2 | + | – | – | – | – | N.D. |
| 5 | M | 59 | Inguinal LN | Others | G3a + DLBCL | + | + | – | + | – | N.D. |
| 6 | F | 30 | Inguinal LN | Typical | G3a | + | + | – | – | – | – |
| 7 | M | 67 | Mediastinal LN | Others | G1 | + | + | + | – | – | N.D. |
| 8 | F | 54 | Stomach, ileum | Transformed FL | transformed FL | + | + | + | – | – | N.D. |
| 9 | F | 78 | Tonsil, stomach | Reverse | G1 | + | + | + | – | – | N.D. |
| 10 | M | 44 | Cervical LN, iliac LN | Transformed FL | transformed FL | + | + | + | + | – | N.D. |
| 11 | F | 65 | Inguinal LN | Others | G3b + DLBCL | – | + | – | – | – | – |
| 12 | M | 19 | Inguinal LN | Typical | G3a + DLBCL | + | – | – | – | – | – |
| 13 | F | 50 | Cervical LN, inguinal LN | Others | G2 | + | + | + | – | – | N.D. |
| 14 | M | 61 | Inguinal LN | Reverse | G1 | + | + | + | – | – | N.D. |
| 15 | M | 63 | Iilum | Typical | G2 + DLBCL | + | + | + | – | – | N.D. |
| 16 | M | 74 | Tongue, gingiva | Transformed FL | transformed FL | + | + | + | – | – | N.D. |
| 17 | M | 48 | Inguinal LN | Reverse | G1 | + | + | + | – | – | N.D. |
| 18 | F | 44 | Mesentric LN, stomach | Others | G1 | + | + | + | – | – | N.D. |
| 19 | F | 62 | Cervical LN, stomach | Typical | G2 | + | + | + | – | – | N.D. |
| 20 | F | 55 | Axillary LN, stomach | Typical | G1 | + | + | + | – | – | N.D. |
| 21 | F | 38 | Inguinal LN | Others | G2 | + | + | – | + | – | N.D. |
| 22 | M | 59 | Cervical LN | Reverse | G2 | + | + | + | – | – | N.D. |
| 23 | F | 60 | Axillary LN | Others | G2 | + | + | – | – | – | – |
| 24 | F | 72 | Inguinal LN | Typical | G2 | + | + | – | – | – | – |
| 25 | F | 54 | Mesetric LN | Typical | G1 | + | + | + | – | – | N.D. |
| 26 | F | 45 | Inguinal LN, mesentric LN | Others | G2 | + | + | + | – | – | N.D. |
| 27 | M | 70 | Inguinal LN | Typical | G2 | + | + | – | – | + | N.D. |
| 28 | F | 32 | Mesentric LN | Typical | G1 | + | + | + | – | – | N.D. |
| 29 | F | 67 | Cervical LN | Others | G2 | + | – | – | – | – | – |
| 30 | M | 68 | Axillary LN | Typical | G3a + DLBCL | N.A. | + | – | + | – | N.D. |
| 31 | F | 52 | Mesentric LN | Others | G1 | + | + | + | – | – | N.D. |
| 32 | F | 42 | Axillary LN | Others | G3a + DLBCL | + | + | + | – | – | N.D. |
| 33 | M | 55 | Inguinal LN | Typical | G1 | + | + | + | – | – | – |
| 34 | M | 71 | Subcutaneous LN | Others | G3a + DLBCL | – | + | – | – | – | – |
| 35 | M | 52 | Cervical LN, stomach | Others | G2 | N.A. | + | + | – | – | N.D. |
| 36 | M | 49 | Inguinal LN | Others | G2 | + | + | – | – | – | – |
| 37 | M | 58 | Inguinal LN | Others | G1 | – | + | – | – | + | – |
| 38 | M | 54 | Supraclavicular LN | Others | G3a | + | – | – | – | – | – |
| 39 | M | 54 | Cervical LN | Others | G2 | + | + | + | – | – | N.D. |
| 40 | F | 46 | Inguinal LN, bladder | Transformed FL | transformed FL | + | + | + | – | – | N.D. |
| 41 | F | 69 | Inguinal LN | Typical | G2 | + | + | + | – | – | N.D. |
| 42 | F | 51 | Inguinal LN | Others | G2 | + | + | + | – | – | N.D. |
| 43 | M | 54 | Inguinal LN | Others | G3b + DLBCL | + | + | + | – | – | N.D. |
| 44 | F | 57 | Inguinal LN | Others | G1 | + | + | – | + | – | N.D. |
| 45 | M | 56 | Pharynx | Others | G3b + DLBCL | + | + | – | – | – | – |
| 46 | F | 62 | Duodenum | Typical | G3b + DLBCL | + | + | + | – | – | N.D. |
| 47 | F | 55 | Cervical LN | Typical | G2 | + | + | + | – | – | N.D. |
Bcl‐6 B.A., Bcl‐6 break apart; DLBCL, diffuse large B‐cell lymphoma; Extra Bcl‐2, Bcl‐2 gene amplification or rearrangement with other genes; F, female; FL, follicular lymphoma; G, grade; LN, lymphnode; M, male; N.A., not applicabable; N.D., not done; others, none of the above; reverse, reverse variant; typical, typical histology.
Forty‐two of 47 cases (89.4%) were positive for Bcl‐2 by immunostaining. Twenty‐eight of 47 cases (59.6%) were positive for t(14;18) by FISH analysis; one of these 28 patients was also positive for Bcl‐6 gene rearrangement by FISH analysis.
Nineteen cases (40.4%) were negative for t(14;18). Among them, four cases (8.5% of total 47 cases) were positive for Bcl‐6 gene rearrangement, and two cases (4.3%) were accompanied by amplification of the Bcl‐2 gene or Bcl‐2 rearrangement with other genes. One case (2.1%) was shown to have t(11;14); the case was negative for CD5 and cyclin‐D1 by immunohistochemistry and re‐examination with a microscope showed again that the case had morphological features of FL without evidence of mantle cell lymphoma. Twelve patients (25.5%) had none of the above chromosomal aberrations as shown by FISH (Fig. 2).
Figure 2.
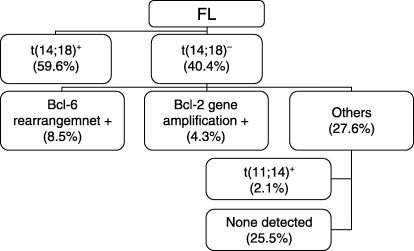
Results of follicular lymphoma subgrouping by fluorescence in situ hybridization analysis.
Relationship among morphological features, histological grades, and chromosomal aberrations by FISH analysis. The relationship between morphological features and presence of t(14;18), by FISH analysis, was analyzed in the 41 cases without transformation to DLBCL. A total 23 cases had t(14;18) typical histology and among them nine cases showed typical histology, while 18 cases did not have t(14;18), of which six cases showed typical histology. A reverse variant was recognized in four cases with t(14;18), but in no cases without t(14;18). The difference was not significant (Table 3).
Table 3.
Relationship between morphological features and follicular lymphoma with or without t(14;18)
| Morphological feature | Follicular lymphoma with t(14;18) | Follicular lymphoma without t(14;18) |
|---|---|---|
| Typical histology | 9 | 6 |
| Reverse variant | 4 | 0 |
| None of the above | 10 | 12 |
| Total | 23 | 18 |
The relationship between histological grades and chromosomal aberrations by FISH analysis is shown in Table 4. Ten of 12 cases (83.3%) were positive for t(14;18) in grade 1, and nine of 17 in grade 2 (52.9%), 0 of two in grade 3a (0%), and four of 10 in the FL + DLBCL (40%) groups. However, a relatively high incidence was observed in the histological transformation group at diagnosis (five of six cases, 83.3%).
Table 4.
Relationship between histologic grade or group, immunohistochemistry and karyotypic abberation by fluorescense in situ hybridization analysis
| Histologic grade | CD10(+) | Bcl‐2(+) | t(14;18)(+) | t(14;18)(+) Bcl‐6(–) | t(14;18)(+) Bcl‐6(–) | t(14;18)(+) Bcl‐6(+) | Extra Bcl‐2 | t(11;14)(+) | Total |
|---|---|---|---|---|---|---|---|---|---|
| G1 | 11 | 12 | 10 | 10 | 1 | 0 | 1 | 0 | 12 |
| G2 | 16 (N.A.1) | 14 | 9 | 9 | 1 | 0 | 1 | 1 | 17 |
| G3a | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| G3b | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| FL + DLBCL | 7 (N.A.1) | 9 | 4 | 4 | 2 | 0 | 0 | 0 | 10 |
| Transformed FL | 5 | 6 | 5 | 4 | 0 | 1 | 0 | 0 | 6 |
| Total | 41(N.A.2) | 42 | 28 | 27 | 4 | 1 | 2 | 1 | 47 |
DLBCL, diffuse large B‐cell lymphoma; Extra Bcl‐2, Bcl‐2 gene amplification or rearrangement with other genes; FL, follicular lymphoma G, grade; N.A., not applicabable.
The incidence of t(14;18) among each histologic grade group was statistically different (P = 0.047). The incidence of t(14;18) in grade 2 tended to be lower, and statistically significantly lower in the grade 3 group compared to that in grade 1; the P‐values were 0.096, and 0.018, respectively.
Extra numbers of Bcl‐2 gene signals were detected both in grades 1 and 2. Bcl‐6 gene rearrangement was recognized in the grade 1, 2, and FL + DLBCL groups; we found five cases had rearrangements. The case judged to have t(11; 14) was grade 1.
Relationship between immunohistochemistry and t(14;18) by FISH analysis. The relationship between immunohistochemistry and t(14;18) by FISH analysis is shown in Table 5. Sensitivity was 100% in all groups, although specificity was 22.2%, 26.3%, and 50.0% in the CD10, Bcl‐2, and CD10/Bcl‐2‐positive groups, respectively. In both the CD10 and Bcl‐2 positive groups, the positive predictive value and efficacy were 68.9% and 80.0%, respectively.
Table 5.
Relationship between immunohistochemistry and t(14;18) by fluorescense in situ hybridization analysis
| Sensitivity (%) † | Specificity (%) † | Positive predictive value (%) † | Efficacy (%) † | |
|---|---|---|---|---|
| CD10 | 100 | 22.2 | 65.9 | 68.9 |
| Bcl‐2 | 100 | 26.3 | 66.6 | 70.2 |
| CD10/Bcl‐2 | 100 | 50.0 | 75.0 | 80.0 |
Sensitivity, specificity, positive predictive value and efficacy were calculated as TP/(TP+FN), TN/(FP+TN), TP/(TP+FP), and (TP+TN)/total, where TP is true positive, FP is false positive, TN is true negative, and FN is false negative, respectively.
Discussion
We found 59.6% of FL cases had t(14;18), in the present study. The t(14;18) has been recognized as a hallmark of FL and it is detected in approximately 90% of FL cases in North America and European countries, whereas the incidence of t(14;18) in East Asia, including Japan, has been reported to be relatively lower than that of North America and European countries. 5 , 10 , 11 , 12 Amakawa et al. reported that only 10 of 30 cases (33.3%) had t(14;18), (10) while Yabumoto et al. also reported 45 of 79 cases (57.0%) had the abnormality by Southern blot hybridization. (11) Mitani et al. reported 13 of 41 cases (31.7%) had the typical aberration by polymerase chain reaction (PCR) analysis. (12) The higher incidence that we observed might be attributed to the methodology. Conventional cytogenetic analysis commonly fails to detect cytogetetic aberrations, especially in lymph node specimens with low grade lymphoma, including FL. PCR analysis still fails to detect translocation breakpoints outside major breakpoint and minor cluster regions. Therefore, FISH analysis can overcome these weak points and detects a greater number of positive cases. In the present study, using this method, however, the incidence of t(14;18) in Japan was still lower. This is consistent with the recent report by Matumoto et al., which showed 56% of Japanese FL had the translocation using the same procedure. (19)
It has also been reported that FL lacks t(14;18) mainly among histological grade 3b, and that it occasionally has a rearranged Bcl‐6 gene in North America and European countries. 13 , 14 Ott et al. reported that grade 1, 2, and 3a had typical t(14;18) in 61 of 73 cases (84%), whereas two of 16 cases (13%) had t(14;18), and seven of 16 cases (44%) had a chromosomal break at 3q27 in G3b ± DLBCL. (13) Bosga‐Bouwer et al. reported similar findings by using PCR, and FISH analysis, (14) they found that 10 of 32 cases of G3b without t(14;18) had a 3q27 aberration. In the present study, most grade 1 cases had t(14;18), whereas the incidence of the aberration was relatively low in grade 2, 3a, and FL + DLBCL. Especially in grade 2, only nine of 17 cases were positive for t(14;18), which would lower the total incidence of positive cases among Japanese FL.
We also checked the incidence of the Bcl‐6 gene rearrangement, anticipating a high incidence of this rearrangement among cases without t(14;18); however, that was not the case and the incidence was equal to that of North America and other European countries and was recognized regardless of histological grade. Bcl‐2 gene amplification was also detected in a limited numbers of cases. None of the abnormalities were detected in one‐quarter of the cases using our procedure.
We found that FL that transformed to aggressive large B‐cell lymphoma had a high frequency of t(14;18). We included these cases, because the clinical course was that of typical FL. The high frequency might reflect the selection bias that observations of a typical indolent clinical course per se reinforce the diagnosis of FL.
We also examined the relationship among morphological features, immunohistochemistry and t(14;18). In the present study, typical morphological features did not indicate the presence of t(14;18), while the reverse pattern did not exclude the absence of t(14;18), either. However, when immunostaining of both Bcl‐2 and CD10 were positive, the presence of t(14;18) was highly predictable. This is consistent with Godon et al., who reported that t(14;18) was detected in all 63 cases (100%) with CD10 and Bcl‐2‐positive FL using FISH analysis. (4) The importance of immunohistchemistry should be stressed when diagnosing FL.
In contrast, FL without t(14;18) tended to lack Bcl‐2 protein expression. Horseman et al. reported 50 cases of FL without t(14;18) by conventional cytogenetic analysis, and they found only 16 Bcl‐2 positive cases among them. (16) In their report, 11 of the 16 cases had a chromosome 18 aberration, including trisomy 18, and only one case had the 18q21 aberration of 34 cases without the Bcl‐2 protein. In the present study, we detected two cases with Bcl‐2 gene amplification or rearrangement with other genes and both cases were positive for Bcl‐2 protein, which is consistent with their report, suggesting that that Bcl‐2 over‐expression might be due to an amplified Bcl‐2 gene.
One of the other purposes of the present study was to examine the impact of FISH analysis on the pathological diagnosis. The Non‐Hodgkin's Lymphoma Classification Project reported that information from immunophenotypic analyses and cytogenetics did not contribute to the accurate diagnosis of FL, although its importance was pointed out for mantle cell lymphoma (MCL). 2 , 3 We sometimes encounter difficulty distinguishing MCL from FL by pathological diagnosis only. Therefore, we added the LSI IGH/CCND1 Dual Color, Dual Fusion Translocation Probe to detect t(11;14) in patients without any abnormality following an earlier procedure. In the present study, t(11;14) was detected in one case; this patient was negative for CD5 and cyclin D1 by immunohistochemistry. Rosenwald et al. identified MCL without cyclin D1 over‐expression and they over‐expressed cyclin D2 or cyclin D3 by gene expression profiling of cDNA. (20) As the morphology and cytogenetics of these cases had not been reported, we cannot rule out the contamination of such cases. The mechanism causing the absence of cyclin D1 expression, despite cyclin D1 and immunoglobulin gene rearrangement in this case, is currently unknown. Alternatively, the possibility of a false‐negative result of CD5 and cyclin D1 should be considered; however, FL might include such cases. Further studies are required in a large series of patients.
In conclusion, the present data supported the recent idea that FL is a heterogeneous disease according to cytogenetics, histology and phenotype, although the following two points were different. First, the frequency of FL with t(14;18) in Japan was relatively high in grade 1 and transformed to aggressive large B‐cell lymphoma cases, whereas it was relatively low in grade 2, 3, and FL + DLBCL cases. Second, FL with positive staining of both CD10 and Bcl‐2 is more likely to have t(14;18). Molecular techniques, including FISH analysis, in daily clinical use for FL might contribute not only to an accurate diagnosis, but also novel findings regarding prognosis and therapeutic approaches.
Acknowledgments
This study was supported by a Grant‐in‐Aid for Cancer Research from the Ministry of Health, Labor and Welfare of Japan (15–11), and a Bristol Meyers Squib Unrestricted Grant to National Cancer Center Hospital (2003). This paper was partly published at the 45th General Meeting of American Society of Hematology. The authors wish to thank to Dr Masafumi Taniwaki and Dr Kenichi Nomura at Kyoto Prefectural University of Medicine for technical advice.
References
- 1. Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller‐Hermelink HK, Vardiman J, Lister TA, Bloomfield CD. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: Report of the Clinical Advisory Committee meeting Airlie House, Virginia, November 1997. J Clin Oncol 1999; 17: 3835–49. [DOI] [PubMed] [Google Scholar]
- 2. Armitage JO, Weisenburger DD. New approach to classifying non‐Hodgkin's lymphomas: Clinical feature of the major histologic subtypes. J Clin Oncol 1998; 16: 2780–95. [DOI] [PubMed] [Google Scholar]
- 3. The Non‐Hodgkin's Lymphoma Classification Project. A clinical evaluation of the international lymphoma Study Group classification of non‐Hodgkin's lymphoma. Blood 1997; 89: 3909–18. [PubMed] [Google Scholar]
- 4. Godon A, Moreau A, Talmant P, Baranger‐Papot L, Geneviève F, Milpied N, Zandecki M, Avet‐Loiseau H. Is t(14;18)(q32;q21) a constant findings in follicular lymphoma? An interphase FISH study on 63 patients. Leukemia 2003;. 17 : 255–9. [DOI] [PubMed] [Google Scholar]
- 5. Biagi JJ, Seymour JF. Insights into the molecular pathogenesis of follicular lymphoma arising from analysis of geographic variation. Blood 2002; 99: 4265–75. [DOI] [PubMed] [Google Scholar]
- 6. Anderson JR, Armitage JO, Weisenburger DD. Epidemiology of the non‐Hodgkin's lymphomas: Distributions of the major subtypes differ by geographic locations. Ann Oncol 1998; 9: 717–20. [DOI] [PubMed] [Google Scholar]
- 7. Lymhoma Study Group of Japanese Pathologists. The World Health Organization classification of malignant lymphomas in Japan: Incidence of recently recognized entities. Pathol Int 2000; 50: 696–702. [DOI] [PubMed] [Google Scholar]
- 8. Miyazato H, Nakatsuka S, Miyanaga I, Hanamoto H, Tatsumi Y, Matsuda M, Maeda Y, Kanamaru A, Aozasa K. Follicular lymphoma in Osaka, Japan: Histological features and chronological change. Int J Hematol 2002; 76: 333–7. [DOI] [PubMed] [Google Scholar]
- 9. Katsumata N, Matsuno Y, Nakayama H, Takenaka T, Kobayashi Y, Takeyama K, Narabayashi M, Fukushima T, Yokozawa T, Nakata M, Tajima K, Ikeda H, Tobinai K. Prognostic factors and a predictive model of follicular lymphoma: a 25‐year study at a single institution in Japan. Jpn J Clin Oncol 1996; 26: 445–54. [DOI] [PubMed] [Google Scholar]
- 10. Amakawa R, Fukuhara S, Ohno H, Doi S, Oguma S, Tanabe S, Yamabe H, Edamura S, Tomono N, Nasu K, Konaka Y, Shiomura T, Abe M, Wakasa H, Uchino H. Involvement of bcl‐2 gene in Japanese follicular lymphoma. Blood 1989; 73: 787–91. [PubMed] [Google Scholar]
- 11. Yabumoto K, Akasaka T, Muramatsu M, Kadowaki N, Hayashi T, Ohno H, Fukuhara S, Okuma M. Rearrangement of the 5′‐cluster region of the BCL2 gene in lymphoid neoplasm: a summary of nine cases. Leukemia 1996; 10: 970–7. [PubMed] [Google Scholar]
- 12. Mitani S, Aoki N, Mizutani S, Fujiwara M, Kitagawa T, Uehara T, Mori S. bcl‐2 gene rearrangement analysis of Japanese follicular lymphomas by polymerase chain reaction in formalin‐fixed, paraffin‐embedded tissue specimens. Jpn J Cancer Res 1993; 84: 37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ott G, Katzenberger T, Lohr A, Kindelberger S, Rüdiger T, Wilhelm M, Kalla J, Rosenwald A, Müller JG, Ott MM, Müller‐Hermelink HK. Cytomorphologic, immunohistochemical, and cytogenetic profiles of follicular lymphoma: 2 types of follicular lymphoma grade 3. Blood 2002; 99: 3806–12. [DOI] [PubMed] [Google Scholar]
- 14. Bosga‐Bouwer AG, Imhoff GWV, Boonstra R, Veen AVD, Haralambieva E, Berg AVD, Jong BD, Krause V, Palmer MC, Coupland R, Kluin PM, Berg EVD, Poppema S. Follicular lymphoma grade 3B includes 3 cytogenetically defined subgroups with primary t(14;18), 3q27, or other translocation: t(14;18) and 3q27 are mutually exclusive. Blood 2003;. 101 : 1149–54. [DOI] [PubMed] [Google Scholar]
- 15. Jardin F, Gaulard P, Buchonnet G, Contentin N, Leprêtre S, Lenain P, Stamatoullas A, Picquenot JM, Duval C, Parmentier F, Tilly H, Bastard C. Follicular lymphoma without t(14;18) and with BCL‐6 rearrangement: a lymphoma subtype with distinct pathological, molecular and clinical characteristics. Leukemia 2002;. 16 : 2309–17. [DOI] [PubMed] [Google Scholar]
- 16. Horsman DE, Okamoto I, Ludkovski O, Le N, Harder L, Gesk S, Siebert R, Chhanabhai M, Sehn L, Connors JM, Gascoyne RD. Follicular lymphoma lacking the t(14;18)(q32;q21): identification of two disease subtypes. Brit J Haematol 2003;. 120 : 424–33. [DOI] [PubMed] [Google Scholar]
- 17. Chan JKC, Ng CS, Hui PK. An unusual morphological variant of follicular lymphoma. Report of two cases. Histopathology 1988; 12: 649–58. [DOI] [PubMed] [Google Scholar]
- 18. Kitayama Y, Igarashi H, Sugimura H. Amplification of FISH signals using intermittent microwave irradiation for analysis of chromosomal instability in gastric cancer. J Clin Pathol 1999; 52: 357–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matsumoto Y, Nomura K, Matsumoto S, Ueda K, Nakao M, Nishida K, Sakabe H, Yokota S, Horiike S, Nakamine H, Nakamura S, Taniwaki M. Detection of t(14;18) in follicular lymphoma by dual‐color fluorescence in situ hybridization on paraffin‐embedded tissue sections. Cancer Genet Cytogenet 2004;. 150 : 22–6. [DOI] [PubMed] [Google Scholar]
- 20. Rosenwald A, Wright G, Wiestner Chan WC, Connors JM, Campo E, Gascoyne RD, Grogan TM, Muller‐Hermelink HK, Smeland EB, Chiorazzi M, Giltanane J, Hurt EM, Zhao H, Averett L, Henrickson S, Yang L, Powell J, Wilson WH, Jaffe ES, Simon R, Klausner RD, Montserrat E, Bosch F, Greiner TC, Weisenburger DD, Sanger WG, Dave BJ, Lynch JC, Vose J, Armitage JO, Fisher RI, Miller TP, LeBlanc M, Ott G, Kvaloy S, Holte H, Delabie J, Staudt LM. The proliferation gene expression signature is a quantitative integrator of oncogenic events that predicts survival in mantle cell lymphoma. Cancer Cell 2003; 3: 185–97. [DOI] [PubMed] [Google Scholar]


