Abstract
The aim of this study was to clarify the clinical implications of a unique carbohydrate determinant, MECA‐79, in gastric cancer specimens and cells. Immunohistochemical analysis showed that 62 of 225 (27.6%) cases were defined as positive for MECA‐79. MECA‐79 expression was correlated with depth of invasion, venous invasion, TNM stage, and distant metastasis. In survival analyses, patients with MECA‐79 expression had worse prognosis by the log–rank test. Multivariate analysis of the Cox proportional hazard model showed that MECA‐79 expression was an independent factor of a worse cancer‐specific survival. Among 11 gastric cancer cells, MECA‐79 was observed in only MKN7 cells, which also expressed GlcNAc6ST‐2 transcript. A knockdown of GlcNAc6ST‐2 in MKN7 cells showed a markedly reduced expression of MECA‐79, suggesting that GlcNAc‐sulfation of MECA‐79 is mainly synthesized by GlcNAc6ST‐2. Furthermore, real‐time RT‐PCR analysis revealed that GlcNAc6ST‐2 was significantly increased in cancer tissues compared with paired normal mucosa. In conclusion, the expression of MECA‐79 could be a useful marker for the prognosis of gastric cancer. Our results might also provide novel perspectives on the biology of MECA‐79 and GlcNAc6ST‐2 in cancer progression and metastasis. (Cancer Sci 2011; 102: 1088–1094)
Despite a decreasing trend in its incidence, gastric cancer remains a common disease and the second leading cause of cancer‐related death worldwide, affecting approximately one million people per year.( 1 ) The discovery of additional prognostic indicators, including tumor markers, would improve the poor survival outcome and permit earlier diagnosis.
It has been known that malignant transformation is associated with abnormal glycosylation induced by several molecular mechanisms, resulting in an expression of altered carbohydrate determinants.( 2 , 3 , 4 , 5 ) Sialyl Lewis a (sLea, also known as CA19‐9) as well as sialyl Lewis x (sLex, also known as SLX) were shown to play a principal role in the metastasis of cancer cells derived from the colorectum, breast, ovary, lung, pancreas, and biliary tract.( 2 , 6 ) SLex has also been widely investigated in patients with gastric cancer, suggesting that increased expression of sLex may serve as a prognostic indicator.( 7 , 8 , 9 , 10 ) SLea/x are ligands for E‐,P‐selectins, whereas 6‐sulfo sLex has been found as a ligand for L‐selectin, which overlaps with MECA‐79 epitope. It is known that the selectin family of adhesion molecules, E‐,L‐,P‐selectins, plays a pivotal role in cancer progression and metastasis by mediating interactions between selectin‐expressing host cells and their respective selectin ligands on tumor cells.( 3 , 11 ) Although sLex/a‐mediated cancer progression has been well documented, the role of L‐selectin and its ligand in cancer progression is still not fully understood.
Lymphocyte recirculation has been studied during the past few decades, which resulted in the discovery of L‐selectin as the homing receptor involved in the interaction of lymphocytes with high endothelial venules (HEVs) of lymph nodes.( 12 ) L‐selectin expressed on naive lymphocytes interacts with HEVs to participate in lymphocyte homing to secondary lymphoid organs. The ability of L‐selectin ligands to function entirely depends on their specific carbohydrate structures decorated with 6‐sulfo sLex (sialic acid‐α2→3Galβ1→4[Fucα1→3(sulfo→6)]GlcNAc), which contains fucose, sialic acid, and sulfate.( 12 ) A unique mAb, MECA‐79, specifically stains HEV in peripheral lymph node and inhibits lymphocyte attachment to HEV and lymphocyte homing in vitro and in vivo. The critical structure of MECA‐79 epitope is 6‐sulfated N‐acetyllactosamine (6‐sulfo LacNAc) on the extended core‐1 O‐glycans, Galβ1→4(sulfo→6)GlcNAcβ1 3Galβ1→3GalNAc→O‐R.( 13 ) MECA‐79 has been used as a powerful tool for studying the HEVs in lymph nodes or HEV‐like vessels at the site of chronic inflammation, including rheumatoid arthritis, inflammatory bowel diseases, thyroiditis, and most recently gastritis induced by Helicobacter pylori infection.( 14 )
To our knowledge, MECA‐79 expression has not been examined in any malignancies. We investigated the clinical significance of MECA‐79 expression in gastric cancer, as well as the biosynthesis of MECA‐79 determinant in gastric cancer cells.
Materials and Methods
Patient population. This study included a total of 250 Japanese patients with primary gastric cancer, who had undergone gastric resection. The study was approved by the institutional review committee of Fukushima Medical University (Fukushima, Japan). None received chemotherapy or radiotherapy before surgery.
For immunohistochemistry, paraffin‐embedded gastric cancer tissue samples were randomly selected from the database and obtained from 121 patients recruited from Fukushima Medical University from 1991 to 2004, and from 104 patients recruited from Ohta‐Nishinouchi Hospital from 2001 to 2003. Among these cases, samples of adjacent non‐malignant mucosa were also available in 94 and 75 cases, respectively. All 225 specimens were used for MECA‐79 staining, and 121 specimens recruited from Fukushima Medical University were also used for sLex staining. Clinicopathological findings were determined according to the TNM classification set out by the International Union Against Cancer. There were 114 tubular adenocarcinomas, seven papillary adenocarcinomas, 71 poorly differentiated adenocarcinomas, six mucinous adenocarcinomas, and 27 signet ring cell carcinomas. Histological types were further divided into differentiated and undifferentiated types, as described previously.( 15 ) In survival analyses, the end point of interest was cancer‐specific survival. The end point of follow‐up was the date of the last contact or the date of death through March 2009. For analyses of gastric cancer‐specific survival, deaths as a result of other causes were censored. The median follow‐up time in the present study was 2491 days (range, 50–6407 days).
For RT‐PCR analysis, another set of gastric specimens was used, obtained from 25 gastric cancer patients who underwent surgical resection at Fukushima Medical University between January 2008 and June 2009. Paired tumor and corresponding normal mucosa were obtained from each patient. The samples were immediately frozen in liquid nitrogen and stored at −80°C until RNA extraction.
Immunohistochemistry. Serial sections (4 μm) were deparaffinized in xylene and hydrated in ethanol. After the sections were rinsed in PBS, endogenous peroxidase was blocked with 0.3% H2O2 in methanol for 30 min. For sLex, antigens were retrieved by autoclaving sections on slides in 0.01 M pH 6.0 citrate buffer for 10 min. The sections were rinsed and incubated with each primary antibody overnight at 4°C. The primary antibodies were anti‐sLex (mouse mAb, KM93; Calbiochem, Darmstadt, Germany; 1:40) and anti‐MECA‐79 (rat mAb; Santa Cruz Biotechnology, Santa Cruz, CA, USA; 1:500). A further wash was followed by treatment with peroxidase‐labeled polymer conjugated to goat anti‐mouse immunogloblins (EnVision Kit; Dako, Glostrup, Denmark) for sLex for 30 min. For MECA‐79, sections were incubated with biotinylated rabbit anti‐rat immunogloblins as the secondary antibody for 20 min, then covered with the streptavidin–biotin peroxidase complex (LSAB; Dako) for 20 min. The staining was visualized with diaminobenzidine, followed by counterstaining with hematoxylin. Human tonsil tissue was used as a positive control for MECA‐79 and sLex. Colon cancer specimens known to carry sLex antigens were also included as a positive control.
Sections were considered positive for sLex when more than 5% of tumor cells were stained in the cytoplasm or cell membrane, based on the procedure reported previously.( 16 ) MECA‐79 positive staining was also evaluated using the same criteria. Assessment of the staining was evaluated by two independent pathologists without knowledge of the clinical status.
Cell culture. Eleven gastric cancer cell lines, including AGS, AZ‐521, KATO‐III, MKN‐1, MKN‐7, MKN‐28, MKN‐45, MKN‐74, NUGC‐3, NUGC‐4, and Okajima, were originally obtained from the American Type Culture Collection (Manassas, VA, USA) or kindly provided by Dr. Jun Yokota (National Cancer Research Institute, Tokyo, Japan). AZ521 was maintained with DMEM; others were with RPMI‐1640, supplemented with 10% FBS at 37°C in a humidified atmosphere of 5% CO2. The cells were detached using 0.25% trypsin/EDTA for 3 minutes at 37°C.
Reverse transcription–PCR analyses. Total RNA was isolated from cultured cell lines or tissue specimens using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions, and the amount of RNA was quantified by NanoDrop (Thermo Scientific, Waltham, MA, USA). The first‐strand cDNA was synthesized by 5 μg extracted total RNA using the SuperScript‐III First Strand cDNA Synthesis kit (Invitrogen), following the manufacturer’s instructions. Conventional PCR was carried out using TaKaRa ExTaq DNA polymerase (Takara, Shiga, Japan) and the following primers: β3GlcNAcT‐3 (B3GNT3), 5′‐TTCTTCAACCTCACGCTCAAGCAG‐3′ and 5′‐AGCATCTCATAAGGTAGGAAGCGG‐3′; Core2GlcNAcT‐1 (GCNT1), 5′‐CTCGAAACACCTCTCTTTTCTGGC‐3′ and 5′‐GGTCAGTGTTTTAATGTCTCCAAAG‐3′; GlcNAc6ST‐1 (CHST2), 5′‐CTCAAGGTCATCCACTTGGTGCG‐3′ and 5′‐GGGTCTTGCTGAGGTCTTTGACC‐3′; GlcNAc6ST‐2 (CHST4), 5′‐GCAGCATGAGCAGAAACTCAAG‐3′ and 5′‐TCCAGGTAGACAGAAGATCCAG‐3′; GlcNAc6ST‐3 (CHST5), 5′‐CAAGACAGTGACAGTGCTCC‐3′ and 5′‐TACGTCCTGCTTGCTGATGG‐3′; and β‐actin (ACTB) as an internal control, 5′‐GCTCGTCGTCGACAACGGCTC‐3′ and 5′‐CAAACATGATCTGGGTCATCTTCTC‐3′. The amplification was carried out at 94°C for 5 min with 30 cycles (25 cycles for β‐actin) of 94°C for 1 min, 55°C for 30 s, and 72°C for 1 min.
Quantitative real‐time RT‐PCR was carried out in triplicate using an ABI Prism 7500 (Applied Biosystems, Carlsbad, CA, USA) with TaqMan probes provided by the manufacturer. The TaqMan probes used were CHST4 (Id Hs00428480_m1) and ACTB (Id Hs99999903_m1). Threshold cycle numbers (Ct) were transformed by the using ΔCt method as described by the manufacturer, with ACTB used as the calibrator gene.
Flow cytometry. The mAbs used in the study were as follows: MECA‐79 (rat mAb; Santa Cruz Biotechnology), anti‐sLex (mouse mAb, 2H5; Seikagaku, Tokyo, Japan), and anti‐Lewis x (mouse mAb, 73‐30; Seikagaku). The cancer cells were harvested at a subconfluent stage, and the cell suspensions (1 × 106 cells/mL) were incubated with each mAb, followed by staining with FITC‐conjugated sheep anti‐mouse (Chemicon, Billerica, MA, USA) or rabbit anti‐rat (Beckman Coulter, Brea, CA, USA) IgG, and analyzed on a FACScan (FACScalibur; Becton Dickinson, Franklin Lakes, NJ, USA).
Small interfering RNA experiments. The siRNA oligonucleotides of the human GlcNAc6ST‐2 (NM_005769, 5′‐GAUCUGAUACGGGCCGUCUUCUUGU‐3′ and 5′‐ACAAGAAGACGGCCCGUAUCAGAUC‐3′) and the non‐targeting control siRNA were purchased from Invitrogen. Each siRNA oligonucleotide was transfected into MKN7 cells using the Lipofectamine RNAiMAX reagent (Invitrogen), following the manufacturer’s instructions. Briefly, the cells were seeded onto a 6‐well plate at 1.5 × 105 cells per well and transfected with 40 nM respective siRNA. The cells were incubated for 72 h until they were harvested for FACS and RT‐PCR or used for further analysis.
Statistical analysis. Associations between categorical variables and the expression of MECA‐79 or sLex were evaluated with the χ2‐test, Fisher’s exact test, or a Mann–Whitney U‐test. Cumulative survival was estimated by the Kaplan–Meier method, and the differences between curves were analyzed by the log–rank test. The influence of each variable on survival was analyzed by the univariate and subsequent multivariate analysis of the Cox proportional hazard model. Multivariate analysis was adjusted for age, gender, histological type, lymph node metastasis, distant metastasis, and TNM stage. Final multivariate models were based on stepwise addition and removal of clinical covariates found to be associated with poor survival in univariate models (P < 0.10).
The differences at P‐values <0.05 were considered significant, and all P‐values reported were two‐sided. All statistical analyses were carried out using spss 11.0 software (SPSS, Chicago, IL, USA).
Results
Immunohistochemical analysis of MECA‐79 and sLex expression. In tonsil tissue and regional lymph node as positive controls of HEV, MECA‐79 staining showed a highly selective distribution occurring in the endothelial cells of venules (Fig. 1A,B). In the normal tissue, MECA‐79 staining was not completely detected in gastric epithelial cells (Fig. 1C), but it was in plump cuboidal endothelial cells restricted to venules, in which there was a high degree of leukocyte infiltration (Fig. 1D). That is, MECA‐79 expressing HEV‐like vessels were found in tertiary lymphoid organs that might arise in chronic inflammation. Additionally, HEV‐like vessels found in cancerous tissue were counted, and cases were subdivided into a high‐ or low‐expression groups, although there was no difference between the two groups and clinicopathological findings (data not shown).
Figure 1.
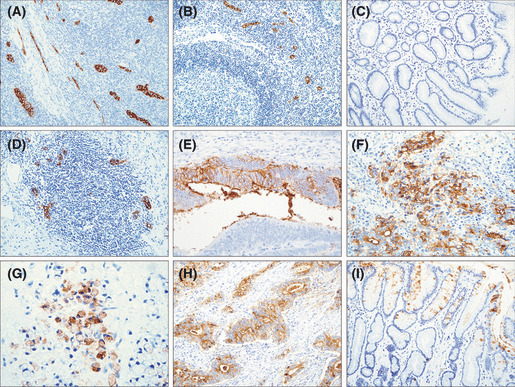
Representative images of immunohistochemistry for MECA‐79 and sLex. (A,B) MECA‐79 staining of high endothelial venule (HEV) endothelial cells in human tonsil (A) and regional lymph node (B) as a positive control (original magnification, ×20). (C) Normal gastric mucosa showing an entirely negative expression of MECA‐79 (×20). (D) High endothelial venule‐like vessels in gastric mucosal lymphatic follicle detected by MECA‐79 (×20). (E–G) MECA‐79 expressed prominently in the membrane and cytoplasm of gastric cancer cells (×40). (H) sLex observed diffusely in the cytoplasm and membrane of tumor cells (×20). (I) sLex staining of normal gastric mucosa detected heterogeneously in endothelial cells (×20).
MECA‐79 immunoreactivity was also detected in tumor cells (Fig. 1E–G). The expression of MECA‐79 was intense predominantly in membrane and also in the cytoplasm of tumor cells, and the expression pattern was heterogeneous in cancerous tissue. Sixty‐two (27.6%) of 225 cases were defined positive for MECA‐79. Table 1 shows the correlation between MECA‐79 expression and clinicopathological factors. MECA‐79 positive expression was significantly more frequent in cases with a deeper depth of invasion (P < 0.001) and presence of venous invasion (P < 0.001), advanced stage (P = 0.014), and presence of distant metastasis (P = 0.007). There was no significant difference between MECA‐79 expression and other variables, including age, gender, histological type, lymph node metastasis, and lymphatic invasion. In addition, we evaluated the relationships between MECA‐79 expression and World Health Organization histological types, however, there was no significant difference (data not shown).
Table 1.
MECA‐79 and sLex expression and clinicopathologic factors in patients with gastric cancer
| No. of patients (%) | MECA‐79 | P | No. of patients (%) | Sialyl Lewis x | P | |||
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | |||||
| n = 62 (27.6%) | n = 163 (72.4%) | n = 88 (72.7%) | n = 33 (28.3%) | |||||
| Age (years) | ||||||||
| ≥60 | 153 (68) | 46 | 107 | 0.219 | 74 (61) | 55 | 19 | 0.249 |
| <60 | 72 (32) | 16 | 56 | 47 (39) | 33 | 14 | ||
| Gender | ||||||||
| Male | 155 (69) | 46 | 109 | 0.289 | 82 (68) | 59 | 23 | 0.781 |
| Female | 70 (31) | 16 | 54 | 39 (32) | 29 | 10 | ||
| Histological type | ||||||||
| Differentiated | 120 (53) | 34 | 86 | 0.780 | 57 (47) | 43 | 14 | 0.527 |
| Undifferentiated | 105 (47) | 28 | 77 | 64 (53) | 45 | 19 | ||
| Depth of invasion | ||||||||
| T1 | 79 (35) | 10 | 69 | <0.001 | 40 (33) | 29 | 11 | 0.929 |
| T2 | 81 (36) | 25 | 56 | 46 (38) | 24 | 12 | ||
| T3 | 57 (25) | 24 | 33 | 35 (29) | 25 | 10 | ||
| T4 | 8 (4) | 3 | 5 | |||||
| Lymphatic invasion | ||||||||
| Present | 194 (86) | 55 | 136 | 0.324 | 99 (82) | 74 | 25 | 0.290 |
| Absent | 34 (14) | 7 | 27 | 22 (18) | 14 | 8 | ||
| Venous invasion | ||||||||
| Present | 168 (75) | 59 | 109 | <0.001 | 84 (69) | 58 | 26 | 0.171 |
| Absent | 57 (25) | 3 | 54 | 37 (31) | 30 | 7 | ||
| Lymph node metastasis | ||||||||
| Positive | 123 (55) | 39 | 84 | 0.126 | 67 (55) | 50 | 17 | 0.601 |
| Negative | 102 (45) | 23 | 79 | 54 (45) | 38 | 16 | ||
| Distant metastasis | ||||||||
| Present | 21 (9) | 11 | 10 | 0.007 | 4 (3) | 3 | 1 | 0.980 |
| Absent | 204 (91) | 51 | 153 | 117 (97) | 85 | 32 | ||
| Stage | ||||||||
| I | 110 (49) | 23 | 87 | 0.014 | 58 (48) | 43 | 15 | 0.712 |
| II | 37 (16) | 12 | 25 | 25 (21) | 18 | 7 | ||
| III | 48 (21) | 13 | 35 | 32 (26) | 23 | 9 | ||
| IV | 30 (13) | 14 | 16 | 6 (5) | 4 | 2 | ||
Regarding sLex, its staining was observed in most gastric cancer specimens. Of 121 cases used for sLex staining in this study, 88 cases (72.7%) were defined positive for sLex. The staining pattern was diffuse in cancerous tissues, and strongly in membrane and cytoplasm of cancer cells (Fig. 1H). Ninety‐four cases of normal gastric epithelium were evaluated; sLex expression was also found in 54 cases (40.0%) of normal mucosa (Fig. 1I). No significant correlation was found between sLex expression and any clinicopathological variables (Table 1). Moreover, there was no significant relationship between MECA‐79 and sLex expression.
Prognostic significance of MECA‐79 expression. Survival curves for patients with gastric cancer according to MECA‐79 expression are shown in Figure 2A. A positive expression of MECA‐79 was significantly associated with shorter cancer‐specific survival (P < 0.0001). The 5‐year survival rate was 30.6% in patients with MECA‐79 positive expression and 69.3% in those with MECA‐79 negative expression. In contrast, sLex status had no impact on prognosis (Fig. 2B). To evaluate the potential use of MECA‐79 expression, we carried out a stratified analysis by stage of disease (Fig. 3). When stratified by TNM staging, MECA‐79 expression was significantly associated with cancer‐specific mortality in stage I cases and stage III cases (P = 0.0010 and 0.0050, respectively).
Figure 2.
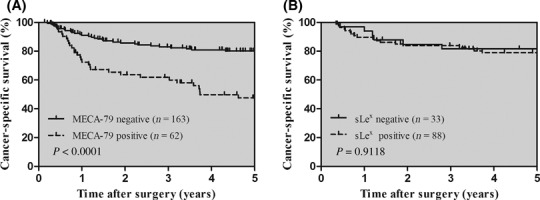
Kaplan–Meier curves in patients with gastric cancer. (A,B) Cancer‐specific survival for gastric cancer patients dependent on MECA‐79 (A) and sLex (B) expression. The P‐values were determined by the log–rank test.
Figure 3.
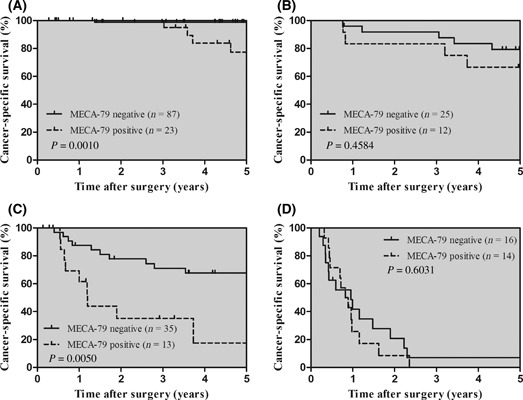
Kaplan–Meier curves in patients with gastric cancer, stratified by TNM stage. Cancer‐specific survival dependent on MECA‐79 expression, stage I (A), stage II (B), stage III (C), and stage IV (D).
The prognostic relevance of MECA‐79 expression was assessed using a univariate and multivariate Cox proportional hazard model adjusted for the established clinical prognostic factors (Table 2). MECA‐79 expression, lymph node metastasis, depth of invasion, TNM stage, and distant metastasis were recognized as independent prognostic factors for cancer‐specific survival. The expression of MECA‐79 increased the risk of cancer‐related death 2.334‐fold (95% CI, 1.094–4.982).
Table 2.
Univariate and multivariate Cox regression analyses of gastric cancer‐specific survival in 225 gastric cancer patients
| Comparison/referent | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Age | ≥60/<60 | 1.745 | 0.978–3.115 | 0.060 | NS | ||
| Gender | Female/male | 1.282 | 0.679–2.136 | 0.341 | |||
| Histological type | Undiff./diff. | 0.961 | 0.587–1.572 | 0.837 | |||
| Depth of invasion | T3–4/T1–2 | 2.019 | 1.697–2.404 | <0.001 | 1.307 | 1.039–1.644 | 0.022 |
| Lymph node metastasis | Present/absent | 10.836 | 4.670–25.145 | <0.001 | 3.551 | 1.300–9.703 | 0.013 |
| Distant metastasis | Present/absent | 14.014 | 7.674–25.595 | <0.001 | 3.555 | 1.876–6.736 | <0.001 |
| TNM stage | III–IV/I–II | 2.226 | 1.832–2.705 | <0.001 | 1.392 | 1.037–1.868 | 0.028 |
| MECA‐79 expression | Positive/negative | 2.856 | 1.744–4.678 | <0.001 | 2.034 | 1.202–3.444 | 0.008 |
CI, confidence interval; diff., differentiated; HR, hazard ratio; NS, not significant; undiff., undifferentiated.
Expression of MECA‐79 in cultured gastric cancer cells. To examine the expression of MECA‐79 in gastric cancer cell lines, we initially carried out a FACS analysis. Among 11 cell lines, only MKN7 cells expressed MECA‐79 significantly (Fig. 4A and Fig. S1). MKN7 cells also expressed Lex and sLex determinant, indicating that they possess the activities of enzymes, including sialyltransferases and fucosyltransferases responsible for sLex synthesis. Therefore, we focused especially on the significance of C‐6 sulfation and extended core‐1 structure, subsequently using RT‐PCR analysis.
Figure 4.
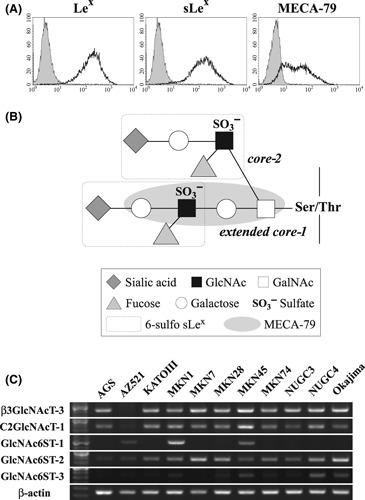
(A) Analyses of Lex, sLex, and MECA‐79 determinants on MKN7 cells using FACS. Among 11 cells tested, only MKN7 cells expressed MECA‐79, as well as Lex and sLex. (B) The L‐selectin ligand containing 6‐sulfo sLex on core‐2 branch and extended core‐1 structure. The epitopes for 6‐sulfo sLex and MECA‐79 are shown in the dash‐outlined boxes. (C) Reverse transcription–PCR analyses of sulfotransferase and glycotransferase genes involved in the synthesis of MECA‐79 determinant in 11 gastric cancer cells. GlcNAc6ST‐2 mRNA is expressed more highly than GlcNAc6ST‐1/3 in MECA‐79 expressing MKN7 cells.
Reverse transcription–PCR analysis of sulfotransferase and glycotransferase genes. To understand the contribution of sulfotransferases and glycotransferases toward the generation of MECA‐79, we examined gastric cancer cells by conventional RT‐PCR analyses of key glycogenes involved in the synthesis of MECA‐79. Because MECA‐79 determinant requires C‐6 sulfation of N‐acetylglucosamine (GlcNAc) and core‐1 extension, as well as sialylation and fucosylation (Fig. 4B), we analyzed the expression of mRNA, including β3GlcNAcT‐3 (for extended core‐1 structure), C2GlcNAcT‐1 (for core‐2 branching structure), and GlcNAc6STs (for sulfation of GlcNAc), which might play a role in MECA‐79 synthesis. β3GlcNAcT‐3 and C2GlcNAcT‐1 transcripts were constitutively expressed in almost all cells (Fig. 4C). In contrast, a minority of cells expressed GlcNAc6ST‐1 and/or ‐3. MECA‐79 expressing MKN7 cells showed a prominently higher expression of GlcNAc6ST‐2 transcript as compared to GlcNAc6ST‐1,‐3, suggesting the possibility that GlcNAc6ST‐2 is implicated in the generation of MECA‐79 epitope on cancer cells.
Small interfering RNA‐mediated knockdown of GlcNAc6ST‐2. To confirm the role of GlcNAc6ST‐2 in the synthesis of MECA‐79 determinant, we carried out siRNA‐mediated knockdown of GlcNAc6ST‐2 on the MECA‐79 expressing cell line MKN7. Treatment with GlcNAc6ST‐2‐specific siRNA markedly decreased the GlcNAc6ST‐2 mRNA by real‐time RT‐PCR (Fig. 5A). Furthermore, MECA‐79 expression in GlcNAc6ST‐2 knockdown cells was also significantly reduced (Fig. 5B).
Figure 5.
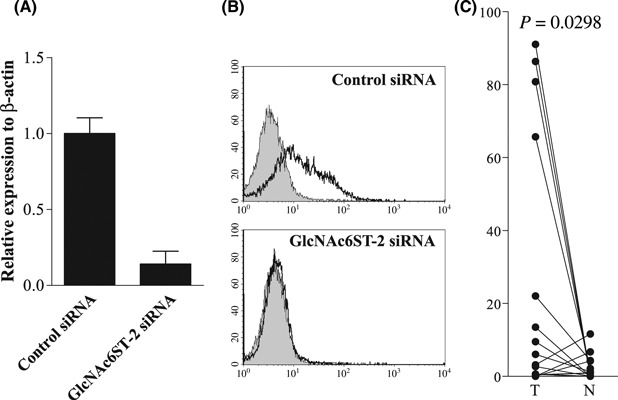
(A,B) Knockdown of GlcNAc6ST‐2 and reduced MECA‐79 expression by the siRNA technique. The real‐time PCR analysis showed that GlcNAc6ST‐2 mRNA was markedly decreased by siRNA in MKN7 cells (A). The MECA‐79 determinant was significantly reduced in GlcNAc6ST‐2 knockdown MKN7 cells (B). (C) A quantitative real‐time PCR analyses of GlcNAc6ST‐2 mRNA levels of 25 gastric cancer tissues (T) and corresponding normal mucosa (N) showed a marked increase in cancer samples. The P‐values were calculated by a paired t‐test.
Expression of GlcNAc6ST‐2 mRNA in gastric cancer specimens. To determine whether upregulated GlcNAc6ST‐2 in cell lines could correlate with that of clinical samples, we examined the GlcNAc6ST‐2 mRNA expression using paired 25 gastric clinical samples by quantitative real‐time RT‐PCR analyses (Fig. 5C). The level of GlcNAc6ST‐2 mRNA expression was increased significantly in cancer tissues compared with normal mucosa (P = 0.0298). Furthermore, cases were divided into high or low expression groups based on the fold change between tumor and normal mucosa (Table S1). High expression of GlcNAc6ST‐2 was significantly associated with advanced stage (P = 0.045).
Discussion
The present study has for the first time revealed that MECA‐79 is ectopically expressed in gastric cancer. Furthermore, we identified MECA‐79 determinant as a novel prognostic marker in primary gastric cancer. Our findings not only suggest the potentially promising usefulness of MECA‐79 as a prognostic indicator for poor survival, but also suggest that further studies are warranted into a possible link between the biological function of MECA‐79 and the progression and metastasis of gastric cancer.
In this study, we carried out immunohistochemical analyses of 225 gastric specimens, including normal mucosa. MECA‐79 positive expression was observed in 27.6% of gastric cancer specimens. The expression of MECA‐79 was significantly correlated with aggressive phenotypes, including deeper depth of invasion, presence of venous invasion, and advanced TNM stage. Moreover, there was a significant relationship between MECA‐79 expression and distant metastasis. In survival analyses, patients with tumors showing positive expressions of MECA‐79 had a significantly shorter survival time than those with negative expressions. Multivariate analyses also revealed that MECA‐79 expression was shown to be an independent prognostic factor for cancer‐specific survival. By contrast, sLex was found not only in most cancerous tissues, but also in normal mucosa. 72.7% of cancerous tissue was defined positive for sLex, although the expression of sLex was not related to any clinicopathological factors or to patient survival in our cohort. Our findings were consistent with previous studies.( 16 )
E‐,P‐selectin are typically inducible endothelial molecules, whereas L‐selectin expression is restricted to leukocytes and is highly expressed on peripheral blood lymphocytes.( 11 ) It is understood that E‐selectin expressed on the surface of activated endothelial cells mediates the tethering of cancer cells, thereby facilitating extravasation from the vasculature and the seeding of metastatic foci.( 2 ) Similarly, a direct role for P‐ and L‐selectin in the metastatic process has been revealed by the pronounced inhibition of metastasis in P‐ and/or L‐selectin‐deficient mice.( 17 , 18 ) It is believed that tumor cells can form the increased size of microemboli with platelets (through P‐selectin) and leukocytes (through L‐selectin) to facilitate mechanical trapping in the microvasculature of distant organs.( 19 ) Thus selectins can act cooperatively to promote tumor cell–host cell interactions and cancer metastasis. Our findings that tumors expressing MECA‐79 are associated with the presence of distant metastasis and worse patient survival are consistent with L‐selectin’s facilitating hematogenous metastasis.
Numerous glycosyltransferases and several sulfotransferases function in the biosynthesis of MECA‐79 as well as 6‐sulfo sLex epitope, including Core1‐β3GlcNAcT (which is identical to β3GlcNAcT‐3), C2GlcNAcT‐1, β4GalT‐IV, sialyltransferases, fucosyltransferases, and GlcNAc6STs.( 19 , 20 ) Because sulfation at C‐6 GlcNAc is essential for L‐selectin binding and for the MECA‐79 epitope,( 19 ) we focused on GlcNAc6STs that catalyze sulfate transfer to GlcNAc structures. Among five members of the GlcNAc6ST family cloned in humans, GlcNAc6ST‐1 shows broad distribution, including HEV, whereas GlcNAc6ST‐2 is very specific, restricted to HEV.( 20 ) GlcNAc6ST‐3 expression is also highly restricted to the intestine.( 21 ) Interestingly, several studies reported that a transcript of GlcNAc6ST‐2 gene was ectopically expressed in colonic adenocarcinoma but not in normal mucosa,( 22 , 23 ) suggesting that GlclcNAc6ST‐2 is the enzyme with cancer‐associated expression and is involved in the synthesis of a cancer‐associated carbohydrate determinant. However, its biological role in cancer still remains unclear. In our study, among 11 gastric cancer cell lines tested by FACS, we found that MECA‐79 was detected in only one cell line, MKN7, which expressed GlcNAc6ST‐2 mRNA much more highly than GlcNAc6ST‐1/‐3. We further showed that a disruption of GlcNAc6ST‐2 using the siRNA knockdown markedly decreased the expression of MECA‐79 in MKN7 cells, suggesting that GlcNAc‐6‐sulfation of MECA‐79 determinant was synthesized mainly by GlcNAc6ST‐2 in MKN7 cells. In addition, when we carried out real‐time RT‐PCR analysis, GlcNAc6ST‐2 transcript was expressed at a low level in normal mucosa, whereas it was found to be partially expressed in cancerous tissues. Our finding that GlcNAc6ST‐2 was partly upregulated in gastric cancer seems to be consistent with that in colonic cancer. One possibility is that upregulated GlcNAc6ST‐2 might play an important role in MECA‐79 synthesis as well as cancer progression in gastric cancer. However, as the mechanisms of glycosylation and post‐translational modifications of carbohydrate structures are extremely complicated, our experiments cannot clearly show that GlcNac6ST‐2 is directly responsible for MECA‐79 synthesis in cancer cells. Similarly, we could not show the significant functional relevance of MECA‐79 in MKN7 cells with or without knockdown of GlcNAc6ST‐2 (data not shown). Therefore, further studies are needed to clarify the functional and biological properties of MECA‐79 in gastric cancer.
Some antibodies detecting carbohydrate determinants, such as sLea, sLex, and NCC‐ST‐439, have been widely applied to serum diagnoses of cancers.( 2 ) In this study, we showed that MECA‐79 was more valuable than conventional sLex for indicating prognosis and metastasis. Therefore, in the future, MECA‐79 could be a potential serum marker for the detection of metastasis and for the prediction of the prognoses of patients with gastrointestinal cancer.
Because MECA‐79 blocks the L‐selectin‐mediated adhesion, this antibody could be clinically applicable for anti‐adhesive therapy. In fact, a recent study about inflammatory diseases reported that MECA‐79, given intravenously, has a significant therapeutic effect in a sheep model of asthma.( 24 ) Therefore, adhesion‐blocking agents, including the use of antibodies directed to MECA‐79, which involve the inhibition of L‐selectin‐mediated tumor cell–leukocytes adhesion, might be a potential strategy to suppress cancer metastasis.
In conclusion, our report provides the first description of MECA‐79 expression in gastric tumors and cells. Our study clearly shows that carbohydrate antigen MECA‐79 expression was significantly correlated with distant metastasis and poor outcome; furthermore, its prognostic impact was highly superior to that of conventional sLex expression. We also found that GlcNAc6ST‐2 was upregulated in MECA‐79 expressing cells and was associated with MECA‐79 synthesis. Further elucidation of the regulation and function of MECA‐79 as well as GlcNAc6ST‐2 will provide new insights that will contribute to the diagnosis and treatment of gastric cancer.
Disclosure Statement
The authors have no conflict of interest.
Supporting information
Fig. S1. Analyses of Lex, sLex, and MECA‐79 determinant on 11 gastric cancer cell lines using FACS.
Table S1. GlcNAc6ST‐2 expression and clinicopathologic factors in patients with gastric cancer.
Supporting info item
Supporting info item
References
- 1. Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 2006; 24: 2137–50. [DOI] [PubMed] [Google Scholar]
- 2. Kannagi R, Izawa M, Koike T, Miyazaki K, Kimura N. Carbohydrate‐mediated cell adhesion in cancer metastasis and angiogenesis. Cancer Sci 2004; 95: 377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hakomori S. Glycosylation defining cancer malignancy: new wine in an old bottle. Proc Natl Acad Sci USA 2002; 99: 10231–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kumamoto K, Goto Y, Sekikawa K et al. Increased expression of UDP‐galactose transporter messenger RNA in human colon cancer tissues and its implication in synthesis of Thomsen‐Friedenreich antigen and sialyl Lewis A/X determinants. Cancer Res 2001; 61: 4620–7. [PubMed] [Google Scholar]
- 5. Koike T, Kimura N, Miyazaki K et al. Hypoxia induces adhesion molecules on cancer cells: a missing link between Warburg effect and induction of selectin‐ligand carbohydrates. Proc Natl Acad Sci USA 2004; 101: 8132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Takada A, Ohmori K, Yoneda T et al. Contribution of carbohydrate antigens sialyl Lewis A and sialyl Lewis X to adhesion of human cancer cells to vascular endothelium. Cancer Res 1993; 53: 354–61. [PubMed] [Google Scholar]
- 7. Ura H, Denno R, Hirata K, Yamaguchi K, Yasoshima T, Shishido T. Close correlation between increased sialyl‐Lewisx expression and metastasis in human gastric carcinoma. World J Surg 1997; 21: 773–6. [DOI] [PubMed] [Google Scholar]
- 8. Futamura N, Nakamura S, Tatematsu M, Yamamura Y, Kannagi R, Hirose H. Clinicopathologic significance of sialyl Le(x) expression in advanced gastric carcinoma. Br J Cancer 2000; 83: 1681–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Amado M, Carneiro F, Seixas M, Clausen H, Sobrinho‐Simoes M. Dimeric sialyl‐Le(x) expression in gastric carcinoma correlates with venous invasion and poor outcome. Gastroenterology 1998; 114: 462–70. [DOI] [PubMed] [Google Scholar]
- 10. Nakagoe T, Fukushima K, Sawai T et al. Increased expression of sialyl Lewis(x) antigen as a prognostic factor in patients with stage 0, I, and II gastric cancer. Cancer Lett 2002; 175: 213–21. [DOI] [PubMed] [Google Scholar]
- 11. Witz IP. The selectin‐selectin ligand axis in tumor progression. Cancer Metastasis Rev 2008; 27: 19–30. [DOI] [PubMed] [Google Scholar]
- 12. Rosen SD. Homing in on L‐selectin. J Immunol 2006; 177: 3–4. [DOI] [PubMed] [Google Scholar]
- 13. Yeh JC, Hiraoka N, Petryniak B et al. Novel sulfated lymphocyte homing receptors and their control by a Core1 extension beta 1,3‐N‐acetylglucosaminyltransferase. Cell 2001; 105: 957–69. [DOI] [PubMed] [Google Scholar]
- 14. Kobayashi M, Mitoma J, Nakamura N, Katsuyama T, Nakayama J, Fukuda M. Induction of peripheral lymph node addressin in human gastric mucosa infected by Helicobacter pylori . Proc Natl Acad Sci USA 2004; 101: 17807–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Okayama H, Kumamoto K, Saitou K et al. CD44v6, MMP‐7 and nuclear Cdx2 are significant biomarkers for prediction of lymph node metastasis in primary gastric cancer. Oncol Rep 2009; 22: 745–55. [DOI] [PubMed] [Google Scholar]
- 16. Nakamori S, Furukawa H, Hiratsuka M et al. Expression of carbohydrate antigen sialyl Le(a): a new functional prognostic factor in gastric cancer. J Clin Oncol 1997; 15: 816–25. [DOI] [PubMed] [Google Scholar]
- 17. Borsig L, Wong R, Feramisco J, Nadeau DR, Varki NM, Varki A. Heparin and cancer revisited: mechanistic connections involving platelets, P‐selectin, carcinoma mucins, and tumor metastasis. Proc Natl Acad Sci USA 2001; 98: 3352–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Borsig L, Wong R, Hynes RO, Varki NM, Varki A. Synergistic effects of L‐ and P‐selectin in facilitating tumor metastasis can involve non‐mucin ligands and implicate leukocytes as enhancers of metastasis. Proc Natl Acad Sci USA 2002; 99: 2193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rosen SD. Ligands for L‐selectin: homing, inflammation, and beyond. Annu Rev Immunol 2004; 22: 129–56. [DOI] [PubMed] [Google Scholar]
- 20. Uchimura K, Rosen SD. Sulfated L‐selectin ligands as a therapeutic target in chronic inflammation. Trends Immunol 2006; 27: 559–65. [DOI] [PubMed] [Google Scholar]
- 21. Lee JK, Bhakta S, Rosen SD, Hemmerich S. Cloning and characterization of a mammalian N‐acetylglucosamine‐6‐sulfotransferase that is highly restricted to intestinal tissue. Biochem Biophys Res Commun 1999; 263: 543–9. [DOI] [PubMed] [Google Scholar]
- 22. Seko A, Nagata K, Yonezawa S, Yamashita K. Ectopic expression of a GlcNAc 6‐O‐sulfotransferase, GlcNAc6ST‐2, in colonic mucinous adenocarcinoma. Glycobiology 2002; 12: 379–88. [DOI] [PubMed] [Google Scholar]
- 23. Uchimura K, El‐Fasakhany FM, Hori M et al. Specificities of N‐acetylglucosamine‐6‐O‐sulfotransferases in relation to L‐selectin ligand synthesis and tumor‐associated enzyme expression. J Biol Chem 2002; 277: 3979–84. [DOI] [PubMed] [Google Scholar]
- 24. Rosen SD, Tsay D, Singer MS, Hemmerich S, Abraham WM. Therapeutic targeting of endothelial ligands for L‐selectin (PNAd) in a sheep model of asthma. Am J Pathol 2005; 166: 935–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Analyses of Lex, sLex, and MECA‐79 determinant on 11 gastric cancer cell lines using FACS.
Table S1. GlcNAc6ST‐2 expression and clinicopathologic factors in patients with gastric cancer.
Supporting info item
Supporting info item


