Abstract
Previous studies suggest that some S100 proteins are involved in the progression of certain types of cancer. However, no comprehensive data is currently available on the expression of S100 family genes in lung adenocarcinomas. Oligonucleotide array, quantitative reverse transcription–polymerase chain reaction and western blot analyses of lung adenocarcinoma cell lines and bronchiolar epithelial cells (SAEC and NHBE) revealed that S100A2 and S100A4 were the most strikingly downregulated and upregulated members of the S100 family, respectively. Immunohistochemical analyses of 94 primary lung adenocarcinomas showed that positive S100A2 expression (33/94, 35.1%) was significantly associated with lymphatic invasion (P = 0.0233) and positive S100A4 expression (19/94, 20.2%) with vascular invasion (P = 0.0454). Interestingly, a strong inverse relationship was found between S100A4 and p53 expression (P = 0.0008). Survival analyses showed that S100A4 positivity was associated with poor patient prognosis (P = 0.042). S100A2 positivity was not associated with patient survival when the whole patient group was analyzed; however, S100A2 positivity was a favorable prognostic indicator in patients with p53‐negative tumors (P = 0.0448). Finally, we used oligonucleotide array analyses and identified potential S100A2 and S100A4 target genes involved in cancer progression: S100A2 induced RUNX3 and REPRIMO; S100A4 induced EZRIN, RUNX1 and WISP1; S100A2 repressed EGFR, NFKB2 and RELA2; and S100A4 repressed ANXA10 and IL1RN. Thus, the present study demonstrates involvement of S100A2 and S100A4 in the progression of lung adenocarcinomas and an inverse association between S100A4 and p53 expression, and provides a list of targets regulated by S100A2 and S100A4. (Cancer Sci 2005; 96: 844–857)
Lung cancer is the leading cause of cancer mortality in the USA, Japan, and other developed countries.( 1 ) Moreover, the incidence of lung adenocarcinoma is widely recognized to be increasing among industrialized countries.( 2 ) The prognosis of lung carcinoma patients is poor: even if diagnosed successfully, patients with stage I lung carcinoma have a 5‐year survival rate of only 70% after surgical resection.( 3 ) Also, patients with small‐sized adenocarcinoma (maximum dimension 2 cm or less) have a 5‐year survival rate of 77.2%,( 4 ) indicating that small tumor size is not necessarily a predictor of good patient prognosis. Without question, better molecular prognostic markers and therapies are urgently needed to improve the survival of patients with lung carcinoma, especially adenocarcinoma. This is why the molecular prognostic markers and targets for lung cancer therapy have been investigated intensively in recent years.( 5 )
The differential expression of the S100 proteins in neoplastic tissues has generated major interest in the S100 family over the last several years.( 6 , 7 ) The S100 proteins are Ca2+‐binding proteins characterized by the EF‐hand motif.( 6 , 7 ) To date, 20 different proteins have been assigned to the S100 protein family, and the genes encoding most of them are located in a cluster on human chromosome 1q21.( 6 , 7 ) The physiological and structural properties of S100 proteins suggest that they are trigger or activator proteins. The S100 proteins are implicated in diverse cellular functions, including cell proliferation, differentiation, metabolism, motility and signal transduction.( 6 , 7 ) Several S100 proteins, including S100A2, S100A4, S100A6, S100A7, S100A11, S100P and S100B, are postulated to play a role in the progression of human cancer.( 6 , 7 )
In spite of the potential importance of the S100 proteins in various types of cancer, there has been no comprehensive analysis to date concerning the expression of the S100 family of proteins in lung adenocarcinomas. We began our study by analyzing lung adenocarcinoma cell lines and normal counterparts using oligonucleotide microarrays, real‐time reverse transcription–polymerase chain reaction (RT‐PCR) and western blot analyses to identify the S100 family members whose expressions were altered in the former. Through these analyses, we found that the expressions of S100A2 and S100A4 were conspicuously downregulated and upregulated, respectively, in lung adenocarcinoma cell lines. We also studied their expression in surgically resected primary lung carcinomas of small size (maximum dimension 3 cm or less) and determined their clinical significance. Finally, we used oligonucleotide microarray analyses to identify potential target genes that were either induced or suppressed by S100A2 and S100A4.
Materials and Methods
Cell lines and medium
Normal human bronchial epithelial cell (NHBE) lines and SAEC (small airway epithelial cells) were purchased from BioWhittaker (Walkerville, MD, USA) and cultured in medium provided by the manufacturer. Lung adenocarcinoma cell lines were obtained from several sources: H23, H460, H522, H1299, H1395, H1648, H2009 and H2347 from ATCC (American Type Culture Collection, Manassas, VA, USA); RERF‐LC‐MS, RERF‐LC‐KJ, A549, PC3, VMRC‐LCD and ABC‐1 from the Japanese Cancer Research Resources Bank (Osaka, Japan); and HLC‐1 and LC‐2/ad from the RIKEN Cell Bank (Tsukuba, Japan). All cell lines were maintained in the culture mediums recommended by the suppliers (Dulbecco's Modified Eagle's Medium [DMEM], RPMI 1640, HAMF 12 + DMEM) supplemented with 10% fetal calf serum, glutamine and antibiotics in a humidified atmosphere with 5% CO2 and 95% air.
RNA extraction
Total RNA was isolated by the acid guanidium/phenol/chloroform method( 8 ) using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA). All samples were treated with RNase‐free DNase (Qiagen, Valencia, CA, USA) during the isolation according to the manufacturer's protocol. The purity and concentration of RNA were determined by spectrometry at 260 nm and 280 nm.
Oligonucleotide microarray analysis One normal bronchial epithelial cell line (SAEC) and seven lung adenocarcinoma cell lines (A549, H23, H522, H1395, H1648, H2009 and H2347) were analyzed by oligonucleotide microarray (GeneChip Human Genome U133A array; Affymetrix, Santa Clara, CA, USA). This array contained probe sets interrogating approximately 14 000 clusters from the UniGene database (Build 133). Analysis was carried out essentially as described previously,( 9 ) according to the instructions from the Affymetrix GeneChip Expression Analysis Technical Manual. Briefly, double‐stranded cDNA was synthesized from 10 µg of total RNA with oligo(dT)24 T7 primer using the SuperScript II System (Invitrogen). In vitro transcription was carried out to produce biotin‐labeled cRNA using a BioArray High Yield RNA Transcript Labeling Kit (Affymetrix). The biotinylated RNA was cleaned with an RNAeasy Mini Kit (Qiagen), fragmented to 50–200 nucleotides and hybridized to the oligonucleotide microarrays. After washing, the arrays were stained with streptavidin–phycoerythrin (Molecular Probes, Eugene, OR, USA), amplified by biotinylated streptavidin (Vector Laboratories, Burlingame, CA, USA), and analyzed on an Affymetrix GeneChip Scanner 2500 to collect the image data. GeneChip Analysis Suite software 5.0 was used to calculate the signal intensity for each gene probe set on the array (expressed as an intensity value of the gene expression). Signals on each chip were scaled to a mean intensity of 100. Annotations of all filtered transcripts were updated using Affymetrix′ Netaffx (http://www.netaffx.com), based on the October 2003 annotation update.
Quantitative reverse transcription–polymerase chain reaction.
Total RNA (1 µg) was reverse transcribed in a reaction volume of 33 µL using pd(N)6 Random Hexamer (Amersham, Piscataway, NJ, USA) and Ready‐To‐Go You‐Prime First‐Strand Beads (Amersham). The PCR were carried out using the QuantiTect TM SYBR Green PCR KIT (Qiagen). The PCR amplification was carried out using a 96‐well optical tray and caps in a final reaction volume of 25 µL containing 3 mM MgCl2, 12.5 pmol of each PCR primer, 12.5 µL of the QuantiTect TM SYBR Green PCR MIX, and 5 µL of cDNA at a dilution of either 1/10 (for S100A2 and S100A4) or 1/500 (for 18S rRNA). PCR was carried out for 45 cycles (30 s denaturation at 94°C, 30 s annealing at 60°C, and 30 s extension at 72°C) for S100A2 and S100A4, and 45 cycles (30 s denaturation at 94°C, 30 s annealing at 58°C, and 30 s extension at 72°C) for 18S rRNA. Real‐time detection of the amplified cDNA was carried out using the iCycler iQTM Multi‐Color Real Time PCR Detection System (Bio‐Rad, Hercules, CA, USA). The following oligonucleotides were used for PCR: S100A2 primer (forward), 5′‐CTGGCTGTGCTGGTCACTAC‐3′; S100A2 primer (reverse), 5′‐TGGGCAGCTCCTTGTGCAGA‐3′; S100A4 primer (forward), 5′‐TGTGTCTTCCTGTCCTGCAT‐3′; S100A4 primer (reverse), 5′‐CCCAACCACATCAGAGGAGT‐3′; 18sR primer (forward), 5′‐CGGCTACCACATCCAAGGAA‐3′; and 18sR primer (reverse), 5′‐GCTGGAATTACCGCGGCT‐3′. These primers were designed using the computer program Primer 3 available at http://www‐genome.wi.mit.edu/cgi‐bin/primer/primer3_www.cgi
Several precautions were taken when designing these primers to obtain specific amplification of the targets. As the sequences of the S100 protein family are highly homologous, we first aligned the amino acid and nucleotide sequences of S100A2 and S100A4 using Genetyx computer software. Next, in designing the primers, we took the 3′ ends from areas that showed sequence differences between S100A2 and S100A4. The sequence specificity of the primers was also confirmed by homology searches through databases at NCBI using the computer program BLASTN. As the S100A2 and S100A4 genes consist of only three exons, it proved to be extremely difficult to design primer sets from sequences of different exons. Thus, the S100A2 and S100A4 primers were chosen from sequences of the same exons. To ensure that none of the PCR products were from residual genomic DNA, we confirmed that no PCR products could be generated in the samples without prior cDNA synthesis. The primers were purchased from Invitrogen. The data was normalized for 18S rRNA quantitated by real time RT‐PCR. After normalization, the results were expressed in arbitrary units. Negative controls lacking template RNA were always included in each experiment.
Antibodies
Rabbit polyclonal anti‐S100A2 (1/400 dilution for immunohistochemistry and 1/800 dilution for western blotting) and goat polyclonal anti‐S100A4 (1/800 dilution for immunohistochemistry and 1/800 dilution for western blotting) were used for the analyses. Rabbit antihuman S100A2 and goat antihuman S100A4 antibodies are described elsewhere.( 10 ) The specificity of these antibodies had been tested by western blotting of tissue and cell lysates and bacterially expressed recombinant S100 family proteins.( 10 ) Mouse monoclonal anti‐p53 antibody (clone DO‐7) was used to detect p53 (dilution 1/100). As secondary antibodies, we used a prediluted mixture of antimouse and antirabbit IgG biotin conjugate (DakoCytomation, Glostrup, Denmark, 1/1 dilution for immunohistochemistry), antigoat IgG biotin conjugate (Sigma, Tokyo, Japan, 1/800 dilution for immunohistochemistry), antirabbit IgG peroxidase conjugate (Amersham; 1/1000 dilution for Western blotting) and antigoat IgG peroxidase conjugate (Sigma; 1/5000 dilution for western blotting).
Western blot analysis
Cells were lysed in a lysis buffer consisting of 50 mM Tris‐HCl (pH 6.8) and 2% sodium dodecyl sulfate with a cocktail of proteinase inhibitors. After sonication, lysates were boiled at 95°C for 5 min and cleared by centrifugation. Protein concentrations were determined using the DC Protein Assay kit (Bio‐Rad). For western blotting, equal amounts of protein samples were size‐separated on 12.5% polyacrylamide gels and electroblotted onto polyvinylidene difluoride (PVDF) membrane. Non‐specific binding was blocked by immersion of the membranes for 1 h in 5% skim milk in Tris‐buffered saline (TBS) at 4°C. The membranes were washed with TBS buffer containing 0.1% Tween, incubated for 1 h at room temperature with primary antibodies, washed again and incubated for 1 h with secondary antibodies. The antigen was detected using ECL Western Blotting Detection Reagents (Amersham) according to the manufacturer's instructions.
Lung adenocarcinoma patients and tissues
We examined a consecutive series of 94 primary small lung adenocarcinomas (maximum diameter 3 cm or less) resected at Tokyo Metropolitan Komagome Hospital, Tokyo, Japan, between 1977 and 1990. The patients consisted of 56 men and 38 women ranging in age from 34 to 89 years (average 60.3 years). The observation periods ranged from 1 month to 162 months, with a median follow‐up period of 65.9 months. The cases were staged according to the tumor‐node‐metastasis system adopted by the American Joint Committee on Cancer and the International Union Against Cancer.( 11 ) The cases consisted of 47 stage I (27 stage IA, 20 stage IB), seven stage II (one stage IIA, six stage IIB), 39 stage III (24 stage IIIA, 15 stage IIIB), and one stage IV adenocarcinomas. The tumors were also histologically evaluated for lymph node metastasis, pleural infiltration, blood vessel invasion and lymphatic vessel invasion. Pleural infiltration and blood vessel invasion were evaluated by routinely staining the sections with elastica van Gieson to identify the elastic fibers of pleura and blood vessels.
Immunohistochemical staining for carcinoma specimens
Immunohistochemical staining was carried out on formalin‐fixed, paraffin‐embedded tissue sections of consecutive lung adenocarcinoma specimens. Sections (5‐µm thick) were deparaffinized in xylene and rehydrated in decreasing concentrations of ethanol. Antigen retrieval was carried out by heating for 20 min at 95°C in 0.01 M citrate buffer (pH 6.0) in a waterbath, followed by cooling for 20 min. After blocking endogenous peroxidase activity with a 3% aqueous H2O2 solution for 5 min, the sections were reacted with primary antibodies for 1 h at room temperature. After washing with TBS buffer, appropriate secondary antibodies (see above) were applied for 1 h at room temperature. Subsequently, the sections were allowed to react for 30 min with avidin–biotin–peroxidase complex (ABC) using a Vectastain ABC kit (Vector Laboratories). The DAB (3‐,3′‐diaminobenzidine tetrahydrochloride) Liquid System (DakoCytomation) was used to detect the immunostaining. Immunoreactivity was evaluated by two investigators (TN and DM), and immunoreactivity was categorized as either positive or negative using a cut‐off value of 5% for S100A2 and 30% for S100A4 following criteria used in previous studies.( 12 , 13 , 14 ) For the evaluation of p53, a cut‐off value of 20% was used.( 15 )
Plasmid vector construction and gene transfection
Human S100A2 and S100A4 cDNAs were purchased from Invitrogen. The S100A2 and S100A4 cDNAs were amplified with primers containing restriction enzyme recognition sites at the 5′ ends. After enzymatic digestion, the resultant PCR products were inserted into expression vector pIRES2‐EGFP (BD Biosciences Clontech, SanJose, CA, USA) at either of two positions; that is, the position between the BglII and EcoRI sites for S100A2 (designated pS100A2‐IRES2‐EGFP), and the position between the BglII and PstI sites for S100A4 (designated pS100A4‐IRES2‐EGFP). Correct construction of the designed vectors was confirmed by DNA sequencing. The genes were transfected into cells by lipofection. Expression of the desired genes was confirmed by green fluorescence under fluorescence microscopy or immunohistochemical staining for S100A2, S100A4 or green fluorescent protein (GFP).
Flow cytometry and fluorescence‐activated cell sorter
The pS100A2‐IRES2‐EGFP, pS100A4‐IRES2‐EGFP or empty vectors were transfected into A549 cells plated on a 10‐cm dish (GIBCO, Invitrogen Japan K.K., Tokyo, Japan). pEGFP‐C1 (BD Biosciences Clontech) was used as an empty vector expressing only GFP. The cells were trypsinized and sorted after 48 h using EPICS ALTRA (Coulter Electronics, Luton, UK), and the total RNA was extracted immediately after cell sorting.
Statistical analysis
Associations were determined using the χ2‐test. Survival was calculated from the day of the last follow up. The Kaplan–Meier method was used to analyze patient survival, and the Mantle–Cox method was used to evaluate the statistical significance of the results. A P‐value less than 0.05 was considered to indicate statistical significance.
Results
Expression analysis of the S100 family of genes by oligonucleotide microarray analysis
We began our experiments by investigating the expressions of 16 authentic members of the S100 family (S100A1 through S100A14, S100B and S100P) in SAEC and seven human lung adenocarcinoma cell lines (A549, H23, H522, H1395, H1648, H2009 and H2347) using oligonucleotide microarray analysis. The results are shown in Table 1. Overall, the SAEC and seven lung adenocarcinoma cell lines expressed the S100 family of genes at various levels. In a close comparison between SAEC and seven lung adenocarcinoma cell lines, the expression of three of the 16 genes analyzed, that is, S100A2, S100A4 and S100P, were strikingly altered between the former and the latter. Whereas S100A2 expression was high in SAEC, it was low in six of the seven lung adenocarcinoma cell lines and modest in the seventh (H1648). In contrast, the levels of S100A4 mRNA were low in SAEC, but more than 10‐fold higher in two (A549 and H2347) of the seven adenocarcinoma cell lines. The levels of S100P transcripts in three (A549, H1395 and H1648) of the seven cell lines were more than 10‐fold higher than the levels measured in SAEC. With regard to other S100 family members, S100A1, S100A3, S100A8, S100A10, S100A11 and S100A14 appeared be downregulated to some extent in adenocarcinoma cell lines.
Table 1.
mRNA levels for the S100 family in small airway epithelial cells (SAEC) and seven lung adenocarcinoma cell lines
| SAEC | A549 | H23 | H522 | H1395 | H1648 | H2009 | H2347 | |
|---|---|---|---|---|---|---|---|---|
| S100A1 | 45.4 | 37.3 | 16.7 | 28.3 | 60.9 | 29.3 | 3.1 | 10.3 |
| S100A2 | 7249 | 42.9 | 71.1 | 24.1 | 100 | 1693 | 113 | 443 |
| S100A3 | 107 | 71.9 | 49.6 | 3.9 | 24.3 | 26 | 27.2 | 37.8 |
| S100A4 | 117 | 1182 | 18.2 | 12.4 | 9.4 | 201 | 133 | 2832 |
| S100A5 | 5 | 6.5 | 26.4 | 17.3 | 2.6 | 11.2 | 7.5 | 80 |
| S100A6 | 4287 | 2954 | 2464 | 12.6 | 4645 | 2611 | 3788 | 6582 |
| S100A7 | 4.2 | 1.5 | 4.4 | 3.3 | 4.3 | 41.2 | 1.2 | 1.8 |
| S100A8 | 400 | 12.8 | 14 | 17.8 | 18.7 | 997 | 27 | 11.2 |
| S100A9 | 208 | 7.1 | 5.1 | 24.8 | 22.9 | 1074 | 261 | 3.1 |
| S100A10 | 4378 | 2704 | 2268 | 1416 | 1983 | 1867 | 2823 | 3645 |
| S100A11 | 3074 | 1196 | 2437 | 2.4 | 3799 | 1760 | 1723 | 1908 |
| S100A12 | 26.9 | 25.3 | 32.5 | 33.2 | 47.8 | 32.5 | 33.5 | 31.1 |
| S100A13 | 599 | 207 | 99 | 93.7 | 550 | 259 | 602 | 916 |
| S100A14 | 798 | 31.9 | 37.3 | 36.3 | 1038 | 447 | 359 | 261 |
| S100B | 3.1 | 6.0 | 1.7 | 1.9 | 4.3 | 3.4 | 1.4 | 3.8 |
| S100P | 55.7 | 683 | 3.2 | 3.1 | 4659 | 2317 | 17.2 | 3.9 |
Expression analysis of the S100 family of genes by real‐time reverse transcription–polymerase chain reaction and western blot analysis
We sought to confirm the results obtained from our microarray analysis by further examining other normal bronchial cells and lung adenocarcinoma cell lines. We examined the expression of S100A2 and S100A4 in SAEC, NHBE and nine lung adenocarcinoma cell lines. The latter included A549, a line analyzed earlier by oligonucleotide microarray analysis, and eight new lines (LC‐2/ad, ABC1, H460, HLC1, H1299, PC3, VMRC‐LCD and RERF‐LC‐KJ). Total RNA was extracted from these cells and subjected to quantitative RT‐PCR and western blot analysis. The results are shown in Fig. 1. While S100A2 expression was high in both NHBE and SAEC, S100A2 mRNA and protein were only present at detectable levels in one (HLC‐1) of the nine lung adenocarcinoma cell lines (Fig. 1a,c). The level of S100A2 in HLC‐1 was still less than one‐third of that in NHBE cells. The levels of S100A4 mRNA and protein were low in both NHBE and SAEC, but elevated in four (LC‐2/ad, A549, H460 and HLC1) of the nine lung adenocarcinoma cell lines (Fig. 1b,d). The results of the RT‐PCR and the western blot analyses were generally in good agreement.
Figure 1.
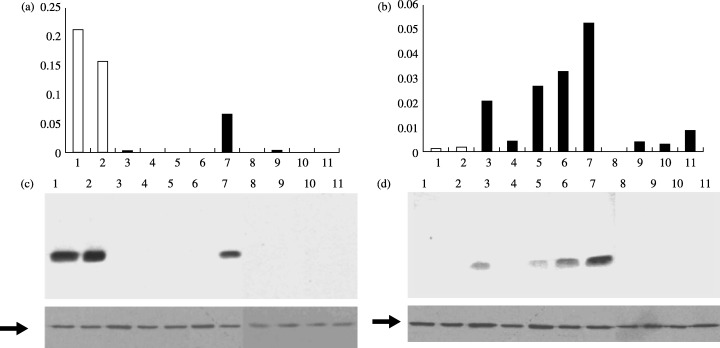
Expression analyses of S100A2 and S100A4 in bronchial epithelial cells (NHBE and SAEC) and nine lung adenocarcinoma cell lines. Levels of S100A2 (a) and S100A4 (b) mRNA were determined by real‐time reverse transcription–polymerase chain reaction analyses. Protein levels of S100A2 (c) and S100A4 (d) were examined by western blot analysis. The lower panels in (c) and (d) represent β‐actin expression (arrows) serving as an internal control. Lanes: 1, NHBE; 2, SAEC; 3, LC‐2/ad; 4, ABC‐1; 5, A549; 6, H460; 7, HLC1; 8, H1299; 9, PC3; 10, VMRC‐LCD; 11, RERF‐LC‐KJ.
Immunohistochemical analysis of S100A2 and S100A4 in human lung adenocarcinoma specimens
Next, we sought to determine whether the expression levels of S100A2 and S100A4 were altered in surgically resected specimens of primary lung adenocarcinomas. We examined a consecutive series of 94 primary small lung adenocarcinomas (maximum diameter 3 cm or less) by immunohistochemistry to determine the levels of S100A2 and S100A4 proteins.
S100A2 immunoreactivity was absent in normal lung tissue, but in some cases it was observed in basal cells of the bronchial epithelium, typically in inflamed areas near invading tumor cells (Fig. 2a). High levels of S100A2 immunoreactivity (> 30% tumor cells positive for S100A2) were observed in nine cases (9.6%) and low levels (5–30% tumor cells positive for S100A2) were observed in 24 cases (25.5%). S100A2 immunoreactivity was found in both the cytoplasm and nuclei of the cancer cells. The S100A2 staining tended to be localized in the invasive areas and negative in the tumor cells showing a lepidic growth pattern.
Figure 2.
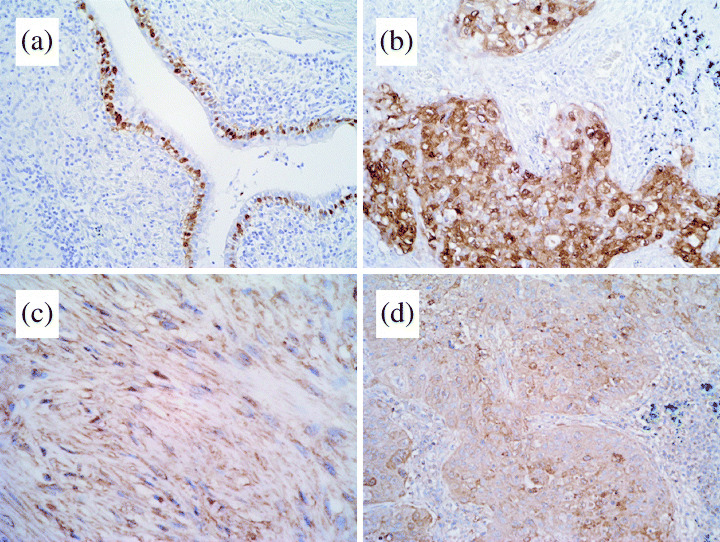
Immunohistochemical analyses of S100A2 and S100A4 expression in primary lung adenocarcinoma specimens. (a) S100A2 immunoreactivity was absent in normal lung tissue, but in some cases it was observed in basal cells of the bronchial epithelium, typically in inflamed areas near invading tumor cells. (b) S100A2‐positive tumor. S100A2 immunoreactivity was found in both the cytoplasm and nuclei of cancer cells. (c) S100A4 staining was found in both lymphocytes and fibroblasts. (d) S100A4‐positive tumor. S100A4 immunoreactivity was found in both the cytoplasm and nuclear membranes of cancer cells.
Positive staining for S100A4 was observed in both the lymphocytes and fibroblasts (Fig. 2c) used as internal controls. S100A4 positivity (> 30% tumor cells positive for S100A4) was found in 19 lung adenocarcinomas (20.2%). Although low levels of S100A4 expression were detected in 38 (40.4%) cases, these were categorized as negative following the criteria of Kimura et al.( 14 ) S100A4 staining was apparent in the cytoplasms and nuclear membranes. Again, the S100A4 staining tended to be more intense in the invading fronts than in the areas showing a lepidic growth pattern.
S100A2 and S100A4 expression and clinicopathological correlations
Next, we examined whether the expression of S100A2 and S100A4 was correlated with any clinicopathological parameters. The results are shown in Table 2. S100A2 expression showed a significant correlation with lymphatic invasion (P = 0.0233), but not with the pathological stage, nodal status, pleural or vascular invasion. In contrast, S100A4 expression correlated with vascular invasion (P = 0.0454), whereas it showed no correlations with pathological stage, nodal status, or pleural or lymphatic invasion. Because recent studies indicate close genetic and functional relationships between p53 and S100A4( 16 , 17 , 18 ) we investigated the relationship between nuclear accumulation of p53 and the expression of S100A2 and S100A4. Interestingly, there was a strong inverse relationship between p53 and S100A4 expression (P = 0.0008), whereas no association was found between p53 and S100A2 expression (P = 0.3269).
Table 2.
Expression of the S100A2 and S100A4 proteins and clinicopathological correlations
| Clinical feature | No. cases | S100A2 expression | S100A4 expression | ||||
|---|---|---|---|---|---|---|---|
| Positive | Negative | P‐value | Positive | Negative | P‐value | ||
| Pathological stage | |||||||
| I | 47 | 17 | 30 | 0.8289 | 8 | 39 | 0.4410 |
| II + III + IV | 47 | 16 | 31 | 11 | 36 | ||
| Nodal involvement (pN) | |||||||
| Negative (pN0) | 56 | 20 | 36 | 0.8808 | 8 | 48 | 0.0824 |
| Positive (pN1,2) | 38 | 13 | 25 | 11 | 27 | ||
| Pleural invasion | |||||||
| Negative | 39 | 13 | 26 | 0.7617 | 5 | 34 | 0.1329 |
| Positive | 55 | 20 | 35 | 14 | 41 | ||
| Vascular invasion | |||||||
| Negative | 21 | 6 | 15 | 0.4765 | 1 | 20 | 0.0454 |
| Positive | 73 | 27 | 46 | 18 | 55 | ||
| Lymphatic invasion | |||||||
| Negative | 21 | 3 | 18 | 0.0233 | 4 | 17 | 0.8801 |
| Positive | 73 | 30 | 43 | 15 | 58 | ||
| p53 | |||||||
| Negative | 52 | 16 | 36 | 0.3269 | 17 | 35 | 0.0008 |
| Positive | 42 | 17 | 25 | 2 | 40 | ||
Relationship between S100A2 and S100A4 expression and patient survival
To ascertain the significance of the expression of S100A2 and S100A4 in lung adenocarcinoma patients, we undertook survival analyses using the Kaplan–Meier method. The survival curves of 94 lung adenocarcinoma cases indicated that S100A4 expression was significantly associated with poor prognosis (P = 0.0269, Mantle–Cox Method) (Fig. 3b). Patients with S100A2‐positive tumors tended to have better prognoses than those with S100A2‐negative tumors (Fig. 3a), but the difference was not statistically significant.
Figure 3.
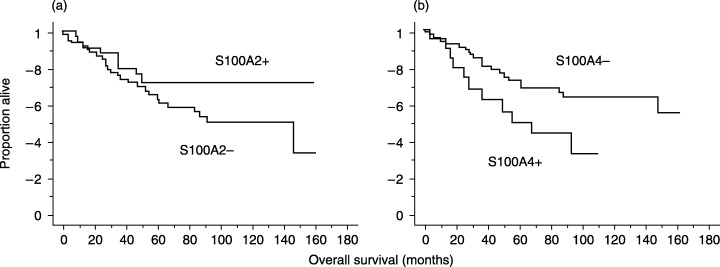
Patient survival according to the expression of S100A2 and S100A4. (a) Patients with S100A2‐positive tumors tended to have better prognoses than those with S100A2‐negative tumors, but the difference was not statistically significant (P = 0.1142). (b) S100A4 expression was significantly associated with poor prognosis (P = 0.0269).
S100A2 has been shown to increase the transcriptional activity of p53,( 19 ) indicating that the action of S100A2 may be dependent on the presence of wild‐type p53. This points to the possibility that the significance of S100A2 positivity may be lost in tumors where the p53 gene is mutated. Therefore, tumors were subdivided according to p53 positivity, and survival analysis was carried out in each subset. Interestingly, this subset analysis showed that S100A2 positivity was significantly associated with favorable outcome in patients with p53‐negative tumors but not in those with p53‐positive tumors (Fig. 4a,b).
Figure 4.
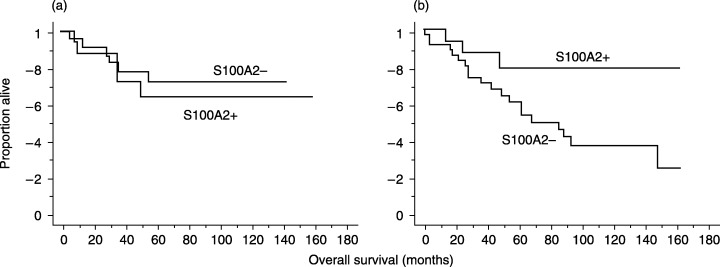
Patient survival according to the expression of S100A2 in patients with p53‐positive or p53‐negative tumors. (a) S100A2 positivity was not associated with prognosis of patients with p53‐positive tumors (P = 0.6517). (b) S100A2 positivity was significantly associated with favorable outcome in patients with p53‐negative tumors (P = 0.0448).
S100A2 has been shown to modulate the function of p53, thereby increasing the transcriptional activity of p53. This indicates that the action of S100A2 may be dependent on the presence of wild‐type p53. In other words, the significance of S100A2 positivity may be lost in tumors where the p53 gene is mutated.
Identification of genes regulated by S100A2 and S100A4 by oligonucleotide microarray
Numerous binding partners have been identified for the S100 proteins.( 6 , 7 ) However, little is known about the downstream genes whose expressions are altered when individual S100 proteins are overexpressed. Thus, we sought to identify the downstream target genes altered by overexpression of the S100A2 and S100A4 genes. After introducing the expression vector pS100A2‐IRES2‐EGFP, the expression vector pS100A4‐IRES2‐EGFP, or control vectors into A549 cells, we separated the cells positive for GFP and examined their gene expression profiles by oligonucleotide microarray analyses.
3, 4 list the genes whose expression levels were altered more than five‐fold by S100A2 or S100A4 over the control levels. An overview of 3, 4 revealed that both S100A2 and S100A4 modulated the expression of numerous genes encoding membrane proteins, notably channels and receptors. It was also interesting to note the appearance of numerous signaling molecules and transcription factors in the tables. Whereas S100A2 and S100A4 share many of the same target genes, they were found to differentially regulate several sets of genes. For example, cytoskeletal proteins such as ankyrin 1, kinesin‐associated protein 3 and myosin binding protein H were induced by S100A2, but not by S100A4. Further, several genes were found to be induced more potently by S100A2 than by S100A4, including REPRIMO (a candidate mediator of the p53‐mediated cell arrest) and Runx3 (runt‐related transcription factor 3, a mediator of transforming growth factor‐beta signaling).( 20 ) It was also interesting to note that S100A2 suppressed the expression of epidermal growth factor receptor (EGFR)( 21 ) and the signaling molecules of NF‐κB pathways,( 22 ) including NFKB2 and RELA2. S100A4 also preferentially induced numerous genes, including several of potential relevance to cancer progression; namely, ezrin,( 23 ) RUNX1 ( 24 ) and WISP1 (WNT1‐inducible signaling pathway protein 1).( 25 ) Lastly, S100A4 suppressed the expression of annexin A10 (ANXA10)( 26 ) and interleukin receptor antagonist (IL1RN).( 27 )
Table 3.
Summary of the genes upregulated or downregulated more than five‐fold by S100A2
| Gene upregulated | Fold change | Gene downregulated | Fold change | ||||
|---|---|---|---|---|---|---|---|
| S100A2 | S100A4 | S100A2 | S100A4 | ||||
| Adehesion | Melanoma cell adhesion molecule | 6.7 | 2.3 | Adhesion | Sperm adhesion molecule 1 | 0.23 | 0.48 |
| Junctional adhesion molecule 2 | 0.16 | 1.3 | |||||
| Desmocollin 2 | 0.099 | 0.059 | |||||
| Cell cycle | Cyclin‐dependent kinase 6 | 7.7 | 7.4 | ||||
| Mediator of the p53‐dependent | 6.4 | 3.3 | Apoptosis | Caspase 10 | 0.2 | 0.75 | |
| G2 arrest (REPRIMO) | 5.3 | 2.4 | |||||
| Cell division cycle 25C (CDC25C) | Cell cycle | Cyclin F | 0.19 | 0.55 | |||
| Channel | Solute carrier family 11, member 1 | 9 | 5.7 | CDC14 cell division cycle 14 homolog A | 0.18 | 0.7 | |
| Solute carrier family 5, member 2 | 6.6 | 4.8 | Cyclin D1 | 0.14 | 0.14 | ||
| ATPase, Ca2+ transporting, type 2C, member 1 | 6 | 1.6 | |||||
| Solute carrier family 16, member 10 | 5.9 | 5.6 | Channel | Solute carrier family 19, member 1 | 0.24 | 0.38 | |
| Mucolipin 3 | 5.6 | 5.8 | Solute carrier family 34, member 1 | 0.21 | 0.19 | ||
| Calcium channel, voltage‐ | 5.2 | 1.2 | Potassium voltage‐gated channel, | 0.21 | 0.62 | ||
| dependent, gamma subunit 2 | beta member 1 Solute carrier family 27, member 3 | 0.21 | 0.21 | ||||
| Cytoskeleton | Ankyrin 1, erythrocytic (ANK1) | 8.6 | 1.9 | Chloride channel 2 | 0.16 | 0.92 | |
| Kinesin‐associated protein 3 (KIFAP3) | 8.4 | 1.5 | Solute carrier family 1, member 3 | 0.15 | 0.18 | ||
| Microtubule‐associated protein 1A | 8 | 7.7 | |||||
| Myosin binding protein H | 5.7 | 0.81 | Cytokine | Eosinophil chemotactic cytokine | 0.12 | 0.16 | |
| Enzyme | Serine palmitoyltransferase, | 6.7 | 5.5 | ||||
| Long chain base subunit 2 | |||||||
| Guanidinoacetate N‐methyltransferase | 6.4 | 2.3 | Enzyme | Deiodinase, iodothyronine, type II | 0.25 | 1.1 | |
| Ribonuclease, RNase A family, 4 | 5.6 | 5.6 | Arginase, liver | 0.24 | 0.56 | ||
| Abhydrolase domain containing 6 | 5.2 | 5.7 | Hexosaminidase A | 0.22 | 0.23 | ||
| Heparan sulfate 6‐O‐sulfotransferase 1 | 0.21 | 0.9 | |||||
| Metabolism | Aldehyde dehydrogenase 4 family, | 6.9 | 6.4 | Carbonic anhydrase XI | 0.2 | 1.2 | |
| member A1 | Asparaginase like 1 | 0.16 | 0.58 | ||||
| Proteinase | Legumain | 8.7 | 7.4 | Choline acetyltransferase | 0.16 | 0.18 | |
| Meprin A, beta | 6.7 | 2.6 | Uridine monophosphate synthetase | 0.12 | 0.31 | ||
| Sorting nexin 11 | 6.4 | 4.9 | Dopachrome tautomerase | 0.08 | 0.4 | ||
| Receptor | Formyl peptide receptor‐like 1 | 10 | 7.8 | Receptor | Interleukin 7 receptor | 0.24 | 0.64 |
| Calcitonin receptor‐like | 6.9 | 3.8 | Benzodiazapine receptor associated protein 1 | 0.23 | 0.25 | ||
| Parathyroid hormone receptor 2 | 6.7 | 4.7 | Leukotriene B4 receptor | 0.23 | 0.24 | ||
| Thy‐1 cell surface antigen | 6.4 | 6.3 | Cholinergic receptor, nicotinic, beta polypeptide 2 | 0.23 | 0.19 | ||
| Cholinergic receptor, nicotinic, Alpha polypeptide 3 | 6.2 | 1.1 | Epidermal growth factor receptor (EGFR) | 0.22 | 1 | ||
| Rhodopsin (opsin 2, rod pigment) | 5.9 | 2.6 | Prostaglandin I2 receptor (IP) | 0.19 | 0.55 | ||
| Ephrin‐A1 | 0.17 | 0.39 | |||||
| Signal | Peptidyl‐prolyl cis/trans | 19 | 9.6 | Activin A receptor, type IB | 0.17 | 1 | |
| isomerase (PIN1) | (ACVR1B) | ||||||
| Calmodulin‐like 3 | 9.2 | 14.9 | Angiotensin II receptor, type 2 (ANGTR2) | 0.16 | 1 | ||
| Suppressor of cytokine signaling 1 | 7.6 | 5.3 | G protein‐coupled receptor 20 | 0.1 | 0.41 | ||
| (SOCS1) | |||||||
| LIM protein | 6.9 | 5.2 | Pre T‐cell antigen receptor alpha | 0.07 | 0.12 | ||
| Dual‐specificity phosphorylation | 6.2 | 6.1 | |||||
| regulated kinase 2 | |||||||
| SH2 domain containing | 5.6 | 4.4 | Signal | Recoverin | 0.23 | 0.3 | |
| phosphatase anchor protein 1 | |||||||
| Kinase suppressor of ras | 5.6 | 4.7 | S100 calcium binding protein A12 | 0.22 | 0.61 | ||
| MAP/microtubule affinity‐regulating kinase 2 | 5.3 | 6.5 | Mitogen‐activated protein kinase 12 | 0.22 | 0.59 | ||
| Rho‐guanine nucleotide exchange factor | 5 | 2.7 | Myotubularin related protein 8 | 0.21 | 0.3 | ||
| Myotubularin related protein 1 | 0.21 | 0.66 | |||||
| Beta‐transducin repeat containing | 0.17 | 0.2 | |||||
| RAB40A, member RAS oncogene family | 0.08 | 0.18 | |||||
| Transcription | cbfa2T1 (ETO/MTG8) | 19 | 15 | ||||
| Kruppel‐like factor 12 | 7.4 | 4.3 | Transcription | cAMP responsive element binding protein‐like 1 | 0.23 | 0.42 | |
| Runt‐related transcription factor 3 (RUNX3) | 6 | 3.2 | Estrogen receptor 2 (ER beta) | 0.23 | 0.42 | ||
| MYC‐associated zinc finger protein | 5.9 | 4.8 | Paired‐like homeobox 2b | 0.19 | 0.57 | ||
| SRY (sex determining region Y)‐box 10 | 0.19 | 0.3 | |||||
| Others | Placenta‐specific 3 | 27 | 3.5 | Nuclear factor of kappa (NF‐kB2) | 0.17 | 0.86 | |
| Metallothionein 3 | 13 | 13 | Paired box gene 3 | 0.15 | 0.15 | ||
| Dihydropyrimidinase‐like 3 | 12 | 12 | Homeo box A10 | 0.12 | 0.1 | ||
| Zinc finger protein 42 | 11 | 5.2 | Rel A | 0.12 | 0.83 | ||
| Melanoma differentiation | 7.9 | 11 | Snf2‐related CBP activator protein | 0.12 | 0.12 | ||
| associated protein‐5 | |||||||
| Rhesus blood group, | 7.8 | 1.1 | Others | Sperm associated antigen 11 | 0.24 | 1 | |
| C glycoprotein | GM2 ganglioside activator protein | 0.23 | 0.34 | ||||
| Leucine zipper, putative tumor suppressor 1 | 0.22 | 0.26 | |||||
| Ewing sarcoma breakpoint region 1 | 0.22 | 0.2 | |||||
| F‐box only protein 22 | 0.21 | 0.38 | |||||
| Ras responsive element binding protein 1 | 0.21 | 1 | |||||
| Junctophilin 3 | 0.2 | 0.43 | |||||
| Cystatin SA | 0.17 | 0.49 | |||||
| Growth differentiation factor 3 | 0.16 | 0.69 | |||||
| Apolipoprotein A‐II | 0.15 | 0.17 | |||||
| Tripartite motif‐containing 2 | 0.14 | 0.24 | |||||
| Rng finger protein 19 | 0.14 | 0.54 | |||||
Table 4.
Summary of the genes upregulated or downregulated more than five‐fold by S100A4
| Gene upregulated | Fold change | Gene downregulated | Fold change | ||||
|---|---|---|---|---|---|---|---|
| S100A2 | S100A4 | S100A2 | S100A4 | ||||
| Adehesion | Integrin, beta 3 | 4.9 | 7.5 | Adhesion | Claudin 8 | 0.63 | 0.21 |
| Apoptosis | Endonuclease G | 4.8 | 8.5 | Desmocollin 2 | 0.1 | 0.06 | |
| Exonuclease NEF‐sp. | 4.3 | 7.2 | Apoptosis | Cylindromatosis(turban tumor syndrome) | 0.72 | 0.05 | |
| Cell cycle | Cyclin‐dependent kinase 6 | 7.7 | 7.4 | Cell cycle | CDC14 cell division cycle14 homolog A | 0.85 | 0.21 |
| Channel | ATP synthase, H + transporting, subunit c | 2.2 | 6.5 | Cyclin D2 | 0.45 | 0.19 | |
| Solute carrier family 11, member 1 | 9 | 5.7 | Cyclin D1 | 0.14 | 0.14 | ||
| Mucolipin 3 | 5.6 | 5.8 | Channel | Amiloride‐sensitive cation channel 2, neuronal | 0.58 | 0.25 | |
| Solute carrier family 7, member 8 | 2.9 | 5.4 | Solute carrier family 27, member 3 | 0.21 | 0.21 | ||
| Cytoskeleton | Microtubule‐associated protein 1A | 8 | 7.7 | Potassium inwardly rectifying Channel, member 6 | 0.29 | 0.2 | |
| Villin 2 (ezrin) | 3.9 | 7.3 | Solute carrier family 34, member 1 | 0.21 | 0.19 | ||
| Parvin, alpha | 3.7 | 6.5 | Solute carrier family 1, member 3 | 0.15 | 0.18 | ||
| Keratin, cuticle, ultrahigh sulfur 1 | 1.3 | 6.2 | ATP‐binding cassette, subfamily A, member 1 | 0.77 | 0.15 | ||
| ECM | Laminin, alpha 2 | 1.9 | 6.2 | Solute carrier family 25, member 14 | 1 | 0.07 | |
| Enzyme | Glutathione transferase zeta 1 | 2.9 | 9.7 | Cytokine | Eosinophil chemotactic cytokine | 0.12 | 0.16 |
| Peptidyl arginine deiminase, type IV | 1.1 | 8.1 | Cytoskeleton | Annexin A10 | 0.69 | 0.18 | |
| GalNAc‐T6 | 4.9 | 6.6 | Mucin | Mucin 3B | 1 | 0.21 | |
| MAP/microtubule affinity‐regulating kinase 2 | 5.3 | 6.5 | Enzyme | Adenosine deaminase, RNA‐specific, B2 | 0.31 | 0.23 | |
| Aldehyde dehydrogenase 4 family, member A1 | 6.9 | 6.4 | Histone deacetylase 9 | 0.72 | 0.23 | ||
| Hydroxyacid oxidase (glycolate oxidase) 1 | 3.7 | 5.8 | Hexosaminidase A (alpha polypeptide) | 0.22 | 0.23 | ||
| Pancreatic lipase‐related protein 1 | 4.2 | 5.7 | UDP‐Gal:betaGlcNAc beta1, 4‐galactosyltransferase | 0.67 | 0.22 | ||
| Ribonuclease, RNase A family, 4 | 5.6 | 5.6 | Formyltetrahydrofolate dehydrogenase | 0.98 | 0.2 | ||
| Serine palmitoyltransferase | 6.7 | 5.5 | Choline acetyltransferase | 0.16 | 0.18 | ||
| Heparanase | 2.5 | 5.4 | Proteinase | ADAMTS2 | 0.79 | 0.22 | |
| Tousled‐like kinase 1 | 4.5 | 5.3 | Receptor | Benzodiazapine receptor associated protein 1 | 0.23 | 0.25 | |
| Proteinase | Legumain | 8.7 | 7.4 | Leukotriene B4 receptor | 0.23 | 0.24 | |
| Receptor | G‐protein‐coupled receptor 6 | 2.2 | 10 | EphB4 | 0.93 | 0.22 | |
| Formyl peptide receptor‐like 1 | 1.3 | 6.5 | Adrenergic, alpha‐2C‐, receptor | 0.52 | 0.22 | ||
| Thy‐1 cell surface antigen | 6.4 | 6.3 | G protein‐coupled receptor 3 | 0.9 | 0.22 | ||
| CD1E antigen, e polypeptide | 2.4 | 5.8 | Cholinergic receptor, nicotinic, beta polypeptide 2 | 0.23 | 0.19 | ||
| EphB6 | 3.9 | 5.1 | Prostaglandin E receptor 3(subtype EP3) | 0.61 | 0.13 | ||
| Signal | Calmodulin‐like 3 | 9.21 | 15 | Pre T‐cell antigen receptor alpha | 0.07 | 0.12 | |
| Dual specificity phosphatase 7 (DUSP7) | 2 | 15 | Signal | PTK2B protein tyrosine kinase 2 beta | 0.35 | 0.22 | |
| Protein phosphatase 2 (PPP2R2B) | 2.7 | 13 | Guanine nucleotide binding protein, alpha 11 | 0.5 | 0.19 | ||
| Dihydropyrimidinase‐like 3 | 12 | 12 | TERF1 (TRF1)‐interacting nuclear factor 2 | 0.89 | 0.19 | ||
| BCR downstream signaling 1 | 0.63 | 8.9 | RAB40A, member RAS oncogene family | 0.08 | 0.18 | ||
| Mitogen‐activated protein kinase 4 (MAPK4) | 4.9 | 7.7 | G protein‐coupled receptor kinase‐interactor 2 | 0.27 | 0.15 | ||
| Protein phosphatase 1F (PPM1F) | 2.4 | 6.5 | Regulator of G‐protein signaling 3 | 0.82 | 0.13 | ||
| Dual‐specificity phosphorylation regulated kinase 2 | 6.2 | 6.1 | Interleukin 1 receptor accessory protein | 0.5 | 0.05 | ||
| Suppressor of cytokine signaling 1 (SOCS1) | 7.6 | 5.3 | Transcription | TAF6‐like RNA polymerase II | 0.73 | 0.25 | |
| Protein tyrosine phosphatase, receptor type, F | 4.4 | 5.2 | Nuclear factor of activated T‐cell 5 | 0.56 | 0.24 | ||
| TRAF interacting protein (TRIP) | 2.6 | 5.1 | MAX dimerization protein 1 | 0.49 | 0.23 | ||
| Transcription | cbfa2T1 (ETO/MTG8) | 19 | 15 | Snail homolog 1 (Drosophila) | 1.2 | 0.23 | |
| Zinc finger protein 42 | 1.3 | 6.6 | Interferon consensus sequence binding protein 1 | 0.72 | 0.19 | ||
| Runt‐related transcription factor 1 (RUNX1) | 1.1 | 6.3 | Zinc finger protein 36 (KOX 18) | 0.6 | 0.19 | ||
| SIN3 homolog B, transcriptional regulator | 3.4 | 5.7 | Paired box gene 3 | 0.15 | 0.15 | ||
| Paired box gene 8 | 4.6 | 5.7 | Snf2‐related CBP activator protein | 0.12 | 0.12 | ||
| Interferon regulatory factor 7 | 3.1 | 5.5 | Homeo box A10 | 0.12 | 0.1 | ||
| Homeo box B2 | 2.1 | 5.3 | Others | 5‐hydroxytryptamine (serotonin) receptor 1D | 0.46 | 0.24 | |
| Paired box gene 8 | 3.8 | 5.3 | Vacuolar protein sorting 33 A(yeast) | 0.91 | 0.24 | ||
| Estrogen receptor 1 | 1.1 | 5.2 | CD79A antigen | 0.57 | 0.24 | ||
| LIM protein | 6.9 | 5.2 | Tripartite motif‐containing 2 | 0.14 | 0.24 | ||
| Msh homeo box homolog 2 (Drosophila) | 4 | 5.1 | Neuron navigator 3 | 0.88 | 0.23 | ||
| Others | Metallothionein 3 | 13 | 13 | Gem (nuclear organelle) associated protein 4 | 0.82 | 0.23 | |
| Melanoma differentiation associated protein‐5 | 7.9 | 11 | Fibrinogen, A alpha polypeptide | 0.77 | 0.22 | ||
| Tissue inhibitor of metalloproteinase 3 | 0.86 | 8 | DEAD (Asp‐Glu‐Ala‐Asp) box polypeptide 17 | 0.5 | 0.2 | ||
| SP110 nuclear body protein | 0.51 | 7.8 | Semaphorin 3A | 0.75 | 0.2 | ||
| Apolipoprotein L, 6 | 4 | 7.7 | Beta‐transducin repeat containing | 0.17 | 0.2 | ||
| Hemoglobin, gamma G | 1.6 | 6.9 | Ewing sarcoma breakpoint region 1 | 0.22 | 0.2 | ||
| WNT1 inducible signaling pathway protein 1 (WISP1) | 1.1 | 6.9 | Lysosomal apyrase‐like protein 1 | 1.4 | 0.19 | ||
| Membrane‐spanning 4‐domains, member 6A | 3 | 6.1 | Apolipoprotein A‐II | 0.15 | 0.17 | ||
| Serine (or cysteine) proteinase inhibitor, member 9 | 2 | 5.7 | Interleukin 1 receptor antagonist | 0.7 | 0.17 | ||
| Abhydrolase domain containing 6 | 5.2 | 5.7 | Integral membrane protein 2C | 1.6 | 0.15 | ||
| Regenerating islet‐derived 1 alpha | 3.7 | 5.5 | Uromodulin | 0.74 | 0.15 | ||
| CD64 | 1.3 | 5.5 | Heat shock 70 kDa protein 4 | 0.95 | 0.13 | ||
| Complement component 1q receptor 1 | 4.6 | 5.1 | PR domain containing 2, with ZNF domain | 0.46 | 0.12 | ||
| Tissue inhibitor of metalloproteinase 2 | 4.4 | 5.1 | Fanconi anemia, complementation group F | 0.52 | 0.11 | ||
| XPA‐binding protein 2 | 0.9 | 0.11 | |||||
ECM, extracellular matrix.
Discussion
The S100 protein represents a family of acidic calcium‐binding proteins regulating diverse cellular functions such as metabolism, motility, proliferation and differentiation.( 5 , 6 ) Of the 20 members of this family thus far identified, studies indicate that some are involved in the progression of certain types of cancer.( 6 , 7 ) With regard to lung carcinomas, previous studies document the expression of some members of the S100 family;( 13 , 14 , 28 , 29 ) however, no investigations have been conducted so far to comprehensively analyze the expressions of S100 family members in lung adenocarcinomas.
Our group began this study by comparing the expression of the S100 family members in non‐transformed bronchial epithelial cells (SAEC and NHBE) with those in lung adenocarcinoma cell lines using a combination of oligonucleotide arrays, quantitative RT‐PCR and western blot analyses. Through these initial analyses we found that the expression of S100A4 was highly elevated in five of 15 lung adenocarcinoma cell lines. Immunohistochemical analyses of surgically resected primary lung adenocarcinomas showed that S100A4 expression was associated with the presence of vascular invasion. Survival analyses further showed that patients with S100A4‐positive tumors had a significantly shorter survival time than those with S100A4‐negative tumors, suggesting that S100A4 is involved in the progression of lung adenocarcinomas. Overall, these results are in keeping with those of the previous studies on colorectal,( 30 ) gastric,( 31 ) mammary,( 32 ) esophageal,( 33 ) pancreatic( 34 ) and prostate cancer.( 35 ) Kimura et al. previously investigated the expression of S100A4 in 135 cases of non‐small lung cancer including 101 lung adenocarcinomas.( 14 ) They found an association between S100A4 expression and poor patient survival. Our study not only confirms their findings but further demonstrates an inverse correlation between S100A4 expression and p53 nuclear accumulation. This inverse relationship is consistent with the previous in vitro study that demonstrated the induction of S100A4 by wild‐type p53;( 17 ) if p53 plays a critical role in S100A4 expression in vivo, functional abnormality of the p53 gene would result in reduced levels of S100A4 in tumor cells. An alternative explanation could be that high levels of S100A4 may somehow suppress the expression of p53, as it has been shown that S100B reduces the levels of p53 in melanoma cells.( 36 , 37 )
Experimental studies also support the role of S100A4 in cancer progression and metastasis. The metastatic potentials of murine mammary adenocarcinoma cell lines and B16 melanoma cells were found to be positively correlated with the level of S100A4 mRNA expression.( 38 ) Further, the expression of S100A4 in MMTV‐neu transgenic mice induced metastasis of mammary tumors( 39 ) and the expression of antisense RNA to the S100A4 gene in high‐metastatic cancer cells suppressed cell motility and in vitro invasiveness.( 40 ) It seems therefore that S100A4 may play a role in the progression of cancer, especially in the acquisition of the metastatic phenotype.
Interesting findings were also brought to light on the S100A2 protein. In keeping with the presumed role of S100A2 as a tumor suppressor( 6 , 7 ) our results clearly demonstrated downregulation of S100A2 mRNA in all the lung adenocarcinoma cell lines examined. S100A2 may act as a tumor‐suppressor in certain epithelial tissues by reducing cell migration.( 41 ) However, in contrast to the results obtained in the lung adenocarcinoma cell lines, S100A2 was expressed frequently in primary lung adenocarcinoma cells, and no associations were found between S100A2 expression and patient survival, stage or lymph node metastasis. Paradoxically, S100A2 expression was positively associated with the presence of lymphatic invasion. In fact, conflicting results are reported in previous studies concerning S100A2 expression in cancer. Overexpression of S100A2 has been reported in carcinomas of the stomach( 42 ) and ovary( 43 ) while other reports document downregulation of S100A2 in carcinomas of the prostate( 35 ) and breast.( 44 ) In laryngeal carcinomas, the expression of S100A2 was associated with better patient prognosis.( 12 ) Earlier findings on lung carcinomas have been discrepant: while an early study showed diminished expression of S100A2 through promoter methylation in non‐small cell lung carcinomas( 45 ) a subsequent study documented frequent expression of S100A2 in primary non‐small lung carcinomas.( 13 ) This paradox could be resolved if we assume that S100A2 expression is induced by inflammation, growth stimuli or oncogenic signals, and that S100A2 induction forms a negative feedback loop that suppresses progression of cancer cells in a certain context. Indeed, it has recently been shown that S100A2 increases the transcriptional activity of p53.( 19 ) If this is an important function of S100A2, the action of S100A2 may be dependent on the presence of wild‐type p53. This might explain why S100A2 positivity was significantly associated with favorable outcome only in patients with p53‐negative tumors (implying tumors with wild‐type p53).
In addition to S100A2 and S100A4, other members of the S100 family appear to be involved in the progression of certain types of cancer as well. Recent studies show that S100P is frequently overexpressed in carcinomas of the pancreas( 46 ) and lung.( 47 ) S100P in culture stimulated cellular proliferation and survival in NIH3T3 cells( 48 ) and these effects appeared to be mediated through extracellular‐regulated kinases and NF‐κB.( 48 ) In fact, our preliminary analysis showed that S100P was overexpressed in three of seven lung adenocarcinoma cell lines examined, suggesting that S100P may also be involved in the progression of lung adenocarcinoma. However, a lack of antibodies appropriate for immunohistochemical staining on paraffin sections prevented us from further analyzing the surgically resected tissues of primary lung adenocarcinoma. As such, the significance of S100P expression in lung adenocarcinoma requires further study in the future. Komatsu et al. reported that S100A6 was frequently overexpressed at the invading front of colorectal carcinomas.( 49 ) Arai et al. demonstrated the overexpression of S100A9 in a subset of lung adenocarcinomas and proved that this overexpression was associated with poorer differentiation.( 29 ) Using serial analysis of gene expression, El‐Rifai et al. demonstrated the overexpression of S100A7, S100A8, S100A9 and S100A10 in a subset of gastric cancer cases.( 42 ) In our analyses, however, the expression of S100A1, S100A3, S100A8, S100A10, S100A11 and S100A14 appeared to be downregulated in lung adenocarcinoma cell lines. In contrast to our findings on S100A4, additional experiments to immunohistochemically analyze the expression of S100A6 in primary lung adenocarcinomas revealed no clear relationships between S100A6 positivity and clinicopathological parameters (data not shown). The significance of these S100 family members (S100A1, S100A3, S100A6, S100A8, S100A9, S100A10, S100A11, and S100A14) in lung adenocarcinoma may need further investigations as well.
The S100 proteins perform various cellular functions by binding to target proteins and modulating their activities. Targets of the S100 proteins include signaling molecules and transcription factors. To identify the downstream genes whose expressions are regulated by S100A2 and S100A4, we constructed plasmid vectors that coexpress S100A2 (or S100A4) and green fluorescent protein by use of the internal ribosomal entry site (IRES). After the introduction of these plasmids, GFP‐positive cells were selected by a fluorescence‐activated cell sorter and subjected to comprehensive gene expression analyses by oligonucleotide array. This method allowed us to identify downstream genes without secondary genetic events that may occur during cell cloning. The results obtained identified several interesting genes relevant to cancer progression: S100A2 induced the expression of RUNX3, a regulator of the transforming growth factor (TGF)‐β pathway and a candidate tumor suppressor of gastric cancer( 20 ) while repressing the expressions of receptors such as EGFR and signaling molecules such as NFKB2 and RELA2. Recent studies indicate that the RUNX3 promoter is frequently methylated in various malignancies, including those of the head and neck, lung, liver and stomach.( 50 ) The roles of EGFR and NF‐κB in cancer are well documented in various types of malignancies as well.( 21 , 22 ) Given that S100A4 modulated the expression of molecules involved in cancer progression, such as EZRIN, RUNX1 and WISP1, the overexpression of S100A4 in cancer may alter the motility and growth of cancer cells through these molecules.
Although we have identified several interesting downstream target genes regulated by S100A2 and S100A4, we are yet to unravel the mechanisms underlying the overexpression of S100A4 and downregulation of S100A2. Previous studies have suggested that erbB2 is involved in the overexpression of S100A4 in medulloblastoma.( 51 ) To test the hypothesis that the activation of growth factor receptors and their downstream signal transduction pathways may be responsible for the overexpression of S100A4, we examined the effects of the growth factors TGF‐α, hepatocyte growth factor, TGF‐β and interleukin‐1, as well as four kinase inhibitors of signal transduction molecules, that is, ERK, JNK, p38 and PI3‐kinase. Our results revealed no significant changes in S100A4 mRNA levels following the addition of these molecules (our unpublished observations).
Other studies indicate that hypermethylation of the S100A2 promoter is responsible for the downregulation of S100A2 in cancer( 44 ) whereas the hypomethylation of S100A4 promoter may be responsible for overexpression of the gene in cancer.( 52 ) In fact, treatment with the demethylating agent 5‐aza‐2′‐deoxycytidine restored the expression of S100A2 mRNA in the lung adenocarcinoma cell line A549 (our unpublished observation), suggesting that promoter methylation is indeed involved in the downregulation of S100A2 in cancer. In any case, the mechanisms underlying the altered expression of S100A2 and S100A4 clearly warrant further study.
In summary, our study demonstrated the potential involvement of S100A2 and S100A4 in the progression of lung adenocarcinomas, and it provided a list of targets regulated by S100A2 and S100A4. Further studies will be required to clarify the mechanisms underlying the overexpression of S100A4 and downregulation of S100A2 in lung adenocarcinomas.
Acknowledgments
We thank Miyuki Saito for her valuable technical assistance with the immunohistochemistry. We also thank Dr Tetsuro Watabe, Department of Molecular Pathology, University of Tokyo, for technical advice on vector construction and for kindly reading the manuscript.
Present address of Toshiro Niki: Department of Integrative Pathology, Jichi Medical University, Yakushiji 3311‐1, Minamikawachi‐machi, Kawachi‐gun, Tochigi, 329‐0498 Japan.
Grant support: This study was supported in part by the Vehicle Racing Commemorative Foundation, the Ministry of Health, Labor and Welfare, a Grant‐in‐Aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology, and the Program for Promotion of Fundamental Studies in Health Sciences of the National Sciences of the National Institute of Biomedical Innovation.
References
- 1. Statistics and Information Department. Vital Statistics, 2000. Tokyo: Ministry of Health, Labor and Welfare, 2001. [Google Scholar]
- 2. Janssen‐Heijnen ML, Coebergh JW. Trends in incidence and prognosis of the histological subtypes of lung cancer in North America, Australia, New Zealand and Europe. Lung Cancer 2001; 31: 123–37. [DOI] [PubMed] [Google Scholar]
- 3. Naruke T, Tsuchiya R, Kondo H, Asamura H, Nakayama H. Implications of staging in lung cancer. Chest 1997; 112: 242S–8S. [DOI] [PubMed] [Google Scholar]
- 4. Moriya Y, Niki T, Yamada T, Matsuno Y, Kondo H, Hirohashi S. Increased expression of laminin‐5 and its prognostic significance in lung adenocarcinomas of small size. An immunohistochemical analysis of 102 cases. Cancer 2001; 91: 1129–41. [DOI] [PubMed] [Google Scholar]
- 5. Danesi R, De Braud F, Fogli S et al. Pharmacogenetics of anticancer drug sensitivity in non‐small cell lung cancer. Pharmacol Rev 2003; 55: 57–103. [DOI] [PubMed] [Google Scholar]
- 6. Marenholz I, Heizmann CW, Fritz G. S100 proteins in mouse and man: from evolution to function and pathology. Biochem Biophys Res Commun 2004; 322: 1111–22. [DOI] [PubMed] [Google Scholar]
- 7. Heizmann CW, Fritz G, Schafer BW. S100 proteins: structure, functions and pathology. Front Biosci 2002; 7: D1356–68. [DOI] [PubMed] [Google Scholar]
- 8. Chomczynski P, Sacchi N. Single‐step method of RNA isolation by acid guanidinium thiocyanate‐phenol‐chloroform extraction. Anal Biochem 1987; 162: 156–9. [DOI] [PubMed] [Google Scholar]
- 9. Ishii M, Hashimoto S, Tsutsumi S et al. Direct comparison of GeneChip and SAGE on the quantitative accuracy in transcript profiling analysis. Genomics 2000; 68: 136–43. [DOI] [PubMed] [Google Scholar]
- 10. Ilg EC, Schafer BW, Heizmann CW. Expression pattern of S100 calcium‐binding proteins in human tumors. Int J Cancer 1996; 68: 325–32. [DOI] [PubMed] [Google Scholar]
- 11. Sobin L, Wittekind L. TNM Classification of Malignant Tumours. New York: John Wiley & Sons, 1997. [Google Scholar]
- 12. Lauriola L, Michetti F, Maggiano N et al. Prognostic significance of the Ca (2+) binding protein S100A2 in laryngeal squamous‐cell carcinoma. Int J Cancer 2000; 89: 345–9. [DOI] [PubMed] [Google Scholar]
- 13. Smith SL, Gugger M, Hoban P et al. S100A2 is strongly expressed in airway basal cells, preneoplastic bronchial lesions and primary non‐small cell lung carcinomas. Br J Cancer 2004; 91: 1515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kimura K, Endo Y, Yonemura Y et al. Clinical significance of S100A4 and E‐cadherin‐related adhesion molecules in non‐small cell lung cancer. Int J Oncol 2000; 16: 1125–31. [DOI] [PubMed] [Google Scholar]
- 15. Terasaki H, Niki T, Matsuno Y et al. Lung adenocarcinoma with mixed bronchioloalveolar and invasive components: clinicopathological features, subclassification by extent of invasive foci, and immunohistochemical characterization. Am J Surg Pathol 2003; 27: 937–51. [DOI] [PubMed] [Google Scholar]
- 16. Grigorian M, Andresen S, Tulchinsky E et al. Tumor suppressor p53 protein is a new target for the metastasis‐associated Mts1/S100A4 protein: functional consequences of their interaction. J Biol Chem 2001; 276: 22 699–708. [DOI] [PubMed] [Google Scholar]
- 17. Daoud SS, Munson PJ, Reinhold W et al. Impact of p53 knockout and topotecan treatment on gene expression profiles in human colon carcinoma cells: a pharmacogenomic study. Cancer Res 2003; 63: 2782–93. [PubMed] [Google Scholar]
- 18. EL Naaman C, Grum‐Schwensen B, Mansouri A et al. Cancer predisposition in mice deficient for the metastasis‐associated Mts1 (S100A4) gene. Oncogene 2004; 23: 3670–80. [DOI] [PubMed] [Google Scholar]
- 19. Mueller A, Schaefer BW, Ferrari S et al. The calcium binding protein S100A2 interacts with p53 and modulates its transcriptional activity. J Biol Chem 2005; 280: 29 186–93. [DOI] [PubMed] [Google Scholar]
- 20. Ito Y, Miyazono K. RUNX transcription factors as key targets of TGF‐beta superfamily signaling. Curr Opin Genet Dev 2003; 13: 43–7. [DOI] [PubMed] [Google Scholar]
- 21. Laskin JJ, Sandler AB. Epidermal growth factor receptor: a promising target in solid tumours. Cancer Treat Rev 2004; 30: 1–17. [DOI] [PubMed] [Google Scholar]
- 22. Rayet B, Gelinas C. Aberrant rel/nfkb genes and activity in human cancer. Oncogene 1999; 18: 6938–47. [DOI] [PubMed] [Google Scholar]
- 23. Yu Y, Khan J, Khanna C, Helman L, Meltzer PS, Merlino G. Expression profiling identifies the cytoskeletal organizer ezrin and the developmental homeoprotein Six‐1 as key metastatic regulators. Nat Med 2004; 10: 175–81. [DOI] [PubMed] [Google Scholar]
- 24. Planaguma J, Diaz‐Fuertes M, Gil‐Moreno A et al. A differential gene expression profile reveals overexpression of RUNX1/AML1 in invasive endometrioid carcinoma. Cancer Res 2004; 64: 8846–53. [DOI] [PubMed] [Google Scholar]
- 25. Margalit O, Eisenbach L, Amariglio N et al. Overexpression of a set of genes, including WISP‐1, common to pulmonary metastases of both mouse D122 Lewis lung carcinoma and B16–F10.9 melanoma cell lines. Br J Cancer 2003; 89: 314–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu SH, Lin CY, Peng SY et al. Down‐regulation of annexin A10 in hepatocellular carcinoma is associated with vascular invasion, early recurrence, and poor prognosis in synergy with p53 mutation. Am J Pathol 2002; 160: 1831–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pantschenko AG, Pushkar I, Anderson KH et al. The interleukin‐1 family of cytokines and receptors in human breast cancer: implications for tumor progression. Int J Oncol 2003; 23: 269–84. [PubMed] [Google Scholar]
- 28. Diederichs S, Bulk E, Steffen B et al. S100 family members and trypsinogens are predictors of distant metastasis and survival in early‐stage non‐small cell lung cancer. Cancer Res 2004; 64: 5564–9. [DOI] [PubMed] [Google Scholar]
- 29. Arai K, Teratani T, Nozawa R, Yamada T. Immunohistochemical investigation of S100A9 expression in pulmonary adenocarcinoma: S100A9 expression is associated with tumor differentiation. Oncol Rep 2001; 8: 591–6. [DOI] [PubMed] [Google Scholar]
- 30. Gongoll S, Peters G, Mengel M et al. Prognostic significance of calcium‐binding protein S100A4 in colorectal cancer. Gastroenterology 2002; 123: 1478–84. [DOI] [PubMed] [Google Scholar]
- 31. El‐Rifai W, Frierson HF Jr, Harper JC, Powell SM, Knuutila S. Expression profiling of gastric adenocarcinoma using cDNA array. Int J Cancer 2001; 92: 832–8. [DOI] [PubMed] [Google Scholar]
- 32. Rudland PS, Platt‐Higgins A, Renshaw C et al. Prognostic significance of the metastasis‐inducing protein S100A4 (p9Ka) in human breast cancer. Cancer Res 2000; 60: 1595–603. [PubMed] [Google Scholar]
- 33. Ninomiya I, Ohta T, Fushida S et al. Increased expression of S100A4 and its prognostic significance in esophageal squamous cell carcinoma. Int J Oncol 2001; 18: 715–20. [DOI] [PubMed] [Google Scholar]
- 34. Sato N, Fukushima N, Maitra A et al. Gene expression profiling identifies genes associated with invasive intraductal papillary mucinous neoplasms of the pancreas. Am J Pathol 2004; 164: 903–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gupta S, Hussain T, MacLennan GT, Fu P, Patel J, Mukhtar H. Differential expression of S100A2 and S100A4 during progression of human prostate adenocarcinoma. J Clin Oncol 2003; 21: 106–12. [DOI] [PubMed] [Google Scholar]
- 36. Lin J, Blake M, Tang C et al. Inhibition of p53 transcriptional activity by the S100B calcium‐binding protein. J Biol Chem 2001; 276: 35 037–41. [DOI] [PubMed] [Google Scholar]
- 37. Lin J, Yang Q, Yan Z et al. Inhibiting S100B restores p53 levels in primary malignant melanoma cancer cells. J Biol Chem 2004; 279: 34 071–7. [DOI] [PubMed] [Google Scholar]
- 38. Grigorian M, Ambartsumian N, Lykkesfeldt AE et al. Effect mts1(S100A4): expression on the progression of human breast cancer cells. Int J Cancer 1996; 67: 831–41. [DOI] [PubMed] [Google Scholar]
- 39. Davies MP, Rudland PS, Robertson L, Parry EW, Jolicoeur P, Barraclough R. Expression of the calcium‐binding protein S100A4 (p9Ka) in MMTV‐neu transgenic mice induces metastasis of mammary tumours. Oncogene 1996; 13: 1631–7. [PubMed] [Google Scholar]
- 40. Takenaga K, Nakamura Y, Sakiyama S. Expression of antisense RNA to S100A4 gene encoding an S100‐related calcium‐binding protein suppresses metastatic potential of high‐metastatic Lewis lung carcinoma cells. Oncogene 1997; 14: 331–7. [DOI] [PubMed] [Google Scholar]
- 41. Nagy N, Brenner C, Markadieu N et al. S100A2, a putative tumor suppressor gene, regulates in vitro squamous cell carcinoma migration. Laboratory Invest 2001; 81: 599–612. [DOI] [PubMed] [Google Scholar]
- 42. El‐Rifai W, Moskaluk CA, Abdrabbo MK et al. Gastric cancers overexpress S100A calcium‐binding proteins. Cancer Res 2002; 62: 6823–6. [PubMed] [Google Scholar]
- 43. Hough CD, Cho KR, Zonderman AB, Schwartz DR, Morin PJ. Coordinately up‐regulated genes in ovarian cancer. Cancer Res 2001; 61: 3869–76. [PubMed] [Google Scholar]
- 44. Liu D, Rudland PS, Sibson DR, Platt‐Higgins A, Barraclough R. Expression of calcium‐binding protein S100A2 in breast lesions. Br J Cancer 2000; 83: 1473–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Feng G, Xu X, Youssef EM, Lotan R. Diminished expression of S100A2, a putative tumor suppressor, at early stage of human lung carcinogenesis. Cancer Res 2001; 61: 7999–8004. [PubMed] [Google Scholar]
- 46. Crnogorac‐Jurcevic T, Missiaglia E, Blaveri E et al. Molecular alterations in pancreatic carcinoma: expression profiling shows that dysregulated expression of S100 genes is highly prevalent. J Pathol 2003; 201: 63–74. [DOI] [PubMed] [Google Scholar]
- 47. Beer DG, Kardia SL, Huang CC et al. Gene‐expression profiles predict survival of patients with lung adenocarcinoma. Nat Med 2002; 8: 816–24. [DOI] [PubMed] [Google Scholar]
- 48. Arumugam T, Simeone DM, Schmidt AM, Logsdon CD. S100P stimulates cell proliferation and survival via receptor for activated glycation end products (RAGE). J Biol Chem 2004; 279: 5059–65. [DOI] [PubMed] [Google Scholar]
- 49. Komatsu K, Andoh A, Ishiguro S et al. Increased expression of S100A6 (Calcyclin), a calcium‐binding protein of the S100 family, in human colorectal adenocarcinomas. Clin Cancer Res 2000; 6: 172–7. [PubMed] [Google Scholar]
- 50. Kim TY, Lee HJ, Hwang KS et al. Methylation of RUNX3 in various types of human cancers and premalignant stages of gastric carcinoma. Laboratory Invest 2004; 84: 479–84. [DOI] [PubMed] [Google Scholar]
- 51. Hernan R, Fasheh R, Calabrese C et al. ERBB2 up‐regulates S100A4 and several other prometastatic genes in medulloblastoma. Cancer Res 2003; 63: 140–8. [PubMed] [Google Scholar]
- 52. Sato N, Maitra A, Fukushima N et al. Frequent hypomethylation of multiple genes overexpressed in pancreatic ductal adenocarcinoma. Cancer Res 2003; 63: 4158–66. [PubMed] [Google Scholar]


