Abstract
The receptor tyrosine kinase MET is overexpressed in human colorectal adenomas and carcinomas, suggesting an instrumental role for MET signaling in the onset and progression of colorectal cancer. To corroborate this role, animal models are needed. To study the expression of Met in the normal and neoplastic mouse intestine, we generated an Armenian hamster monoclonal antibody against mouse Met. By using this antibody in immunohistochemical studies, we observed strong Met expression in fetal mouse intestinal epithelial cells. In contrast, in the intestines of adult mice, Met expression was very low whereas the protein was undetectable on the neoplastic epithelium of intestinal adenomas in Apc +/min mice. By immunoblotting, we were also unable to detect Met in intestinal adenomas, whereas Met mRNA levels in microdissected adenomas were very low. The absence of detectable Met protein expression in adenomas of Apc +/min mice contrasts sharply with the vast overexpression of the protein in adenomas of humans with familial adenomatous polyposis or sporadic colorectal carcinomas. Our results imply that deregulation of Wnt signaling in mouse − unlike in human − intestinal epithelium does not result in Met overexpression. Our findings thus reveal important interspecies differences in the regulation of Met expression during intestinal tumorigenesis. (Cancer Sci 2006; 97: 710–715)
Abbreviations:
- ACF
abberant crypt foci
- CRC
colorectal cancer
- DIG
digoxigenin
- E15
embryonic day 15
- HGF
hepatocyte growth factor
- IMDM
Iscove's Modified Dulbecco's Media
- mAb
monoclonal antibody
- nt
nucleotide
- PAGE
polyacrylamide gel electrophoresis
- PCR
polymerase chain reaction
- PFA
para formaldehyde
- RT
reverse transcription
- SDS
sodium dodecylsulfate
- TBP
TATA box binding protein.
Introduction
Proper functioning of the receptor tyrosine kinase MET is of critical importance during both embryogenesis and adult life.( 1 , 2 ) During embryogenesis, the tight spatiotemporal regulation of activation of MET by its ligand, HGF, results in cell type‐dependent mitogenesis, morphogenesis or motogenesis required for formation of the trophoblast, liver and subsets of skeletal muscles.( 3 , 4 , 5 ) During adult life, MET plays an important role in tissue homeostasis, remodeling and repair.( 2 , 6 )
In addition to its physiological roles during development and adult life, MET signaling has been implicated in the pathogenesis of a variety of neoplasms, including glioma, lymphoma, renal cell carcinoma and CRC.( 2 , 6 ) In these malignancies, MET is either mutated or overexpressed, resulting in uncontrolled activation of the MET signaling pathway. In CRC, MET is overexpressed at all stages of the adenoma–carcinoma sequence. At the early stages of disease, MET overexpression appears to be caused mainly by transcriptional activation through the WNT signaling pathway.( 7 , 8 ) However, a study by Di Renzo et al. demonstrated amplification of the MET gene in a small subset of primary colorectal carcinomas and in the majority of liver metastases studied,( 9 ) suggesting that the transcriptional overexpression present during the initial stages of the disease becomes ‘fixed’ during tumor progression. Taken together, these findings suggest that MET signaling is not only involved in the onset of CRC, but also in disease progression and in the formation of metastases.
Mouse models have been of great value in unraveling the pathogenesis of CRC,( 10 , 11 ) but the role of Met in intestinal tumorigenesis in mice has thus far not been explored. Based on mRNA in situ hybridization studies, Met expression in normal embryonic and adult mouse tissues appears to be confined largely to epithelial cells, including those of the intestine.( 12 , 13 ) However, due to a lack of antibodies suitable for immunohistochemistry, data on Met protein expression in the normal intestine and intestinal adenomas of mice are currently lacking. This prompted us to generate an Armenian hamster mAb against mouse Met. By using this antibody, we demonstrate that Met, while expressed at high levels in the embryonic mouse intestine, is hardly or not detectable in the normal adult mouse intestine and is not overexpressed in intestinal adenomas of Apc +/min mice.
Materials and Methods
Generation of a monoclonal antibody against mouse Met
The Armenian hamster fibroblast line ARHO12 (kindly provided by Dr Jannie Borst, NKI, Amsterdam, the Netherlands) was cultured as described previously.( 14 ) In brief, cells were grown in flasks (Greiner Bio‐One BV, Alphen a/d Rijn, the Netherlands) in IMDM medium supplemented with 10% fetal calf serum, 20 mM l‐glutamine (both from Gibco BRL/Life Technologies, Paisley, UK), 1 mg/mL gentamicin (Invitrogen, Paisley, UK) and 2‐mercaptoethanol (Sigma, St Louis, MO, USA). ARHO12 cells were transfected with pcDNA3.1/Neo(+) containing murine Met cDNA, using Lipofectamine Plus (Gibco BRL/Life Technologies) and were selected with geneticin (G418 sulfate; Invitrogen) at 0.1 mg/mL. Resulting clones were tested for Met expression by immunoblotting with the polyclonal anti‐Met antibody SP260 (Santa Cruz Biotechnology, Santa Cruz, CA, USA). An Armenian hamster (Cricetulus migratorius; Cytogen, West Roxbury, MA, USA) was injected intraperitoneally five times at weekly intervals with 107 4% PFA‐fixed ARHO12 cells expressing Met stably. Three weeks after the fifth injection, the hamster was boosted intraperitoneally with 107 cells. Three days later, hamster spleen cells were fused with mouse myeloma SP2/0 cells by standard hybridoma technology. Binding of hybridoma supernatants to the ARHO12 clone stably expressing Met was tested by flow cytometry as well as by immunohistochemistry. As a negative control, binding of hybridoma supernatants to non‐transfected ARHO12 cells was tested. Hybridomas binding specifically to the ARHO12‐Met cells were subcloned until they were monoclonal and stable. The hybridoma, designated We‐1, was grown in large amounts and immunoglobulin was purified using Protein A bound to Sepharose CL‐4B (Sigma). Purified antibody was conjugated with DIG (Roche Diagnostics, Almere, the Netherlands).
SDS‐PAGE
The isotype of We‐1 was determined by SDS‐PAGE. The antibody was run on both a reduced (10% SDS) and a non‐reduced (7% SDS) polyacrylamide gel. Immunoglobulin bands were detected using Coomassie brilliant blue staining.
Immunohistochemistry
Staining with the mAb We‐1‐DIG was carried out on ARHO12 cells (transfected with or without Met and fixed with 4% PFA) and on cytospins of mouse fibroblast cells (NIH/3T3 cells and NIH/3T3‐Met cells; a kind gift from Dr G. F. Vande Woude). Furthermore, different organs from adult C57BL6/J mice and Apc +/min mice (ages ranging from 9 to 20 weeks) (The Jackson Laboratory, Bar Harbor, ME, USA) were isolated and mounted into tissue‐tek (Miles Scientific, Naperville, IL, USA). Also, whole C57BL6/J embryos were embedded into tissue‐tek. Sections (5 µm) were prepared and stained with the primary antibodies, We‐1‐DIG, goat‐antimouse macrophage stimulating protein receptor (MSPR) (R&D Systems, Abingdon, UK), goat‐antihuman/mouse/rat‐β‐catenin (R&D Systems) and 9A4 (against mouse Cd44v6), followed by an avidinbiotinylated horse radish‐peroxidase complex (Dakopatts, Glostrup, Denmark). The peroxidase was visualized with 3‐amino‐9‐ethylcarbazole (Sigma).
Micro‐dissection and RT‐PCR
Using laser microdissection, tissue from normal mouse intestines and adenomas was isolated. cDNA was synthesized by a standard RT reaction, using Moloney leukemia virus reverse transcriptase (GibcoBRL/Life Technologies) and pd(N)6 random hexamer primers (Amersham Pharmacia Biotech, Aylesbury, UK). RT‐PCR was carried out using Taq DNA Polymerase (GibcoBRL/Life Technologies), 200 µM of each dNTPs (Amersham Pharmacia Biotech) and 1.5 mM MgCl2 in 1× PCR buffer (GibcoBRL/Life Technologies). Primers used were: Met forward, GTC CAC AAC AAG ACG GGT GC (nt 3703); Met reverse, GGA GAC CAG TTC GGA AAA GG (nt 3999); Ron forward, GTG TCC TAC AGG TGG AGA TA (nt 1670); Ron reverse, TGC TCC TCA TTG ACC TGT TC (nt 2450); and as a loading control, TBP forward, CAG GAG CCA AGA GTG AAG AAC (nt 794); and TBP reverse, AAT TCT GGC TCA TAG CTA CT (nt 995). PCR was started with a 5 min denaturation step at 95°C and followed by amplification in 39 cycles of denaturation at 95°C for 30 s, annealing at the appropriate melting temperature for 20 s and elongation at 72°C for 30 s. After a final elongation step at 72°C for 7 min, samples were cooled on ice and analyzed by electrophoresis.
Western blot analysis
Protein extracts were prepared from different organs by mincing into lysis buffer (10 mM Tris pH 8.0, 15 mM NaCl, 1% NP‐40, 10% glycerol, 0.4 mg/mL sodium orthovanadate). Protein extracts (50 µg) were separated by SDS‐PAGE and blotted onto immobilon‐P transfer membranes (Millipore, Bedford, MA, USA). Membranes were blocked in Tris‐buffered saline (100 mM Tris‐HCl pH 7.5, 150 mM NaCl) containing 0.1% Tween (Sigma) and 5% non‐fat dry milk, and probed with mAb AC‐15 against β‐actin (Sigma) or polyclonal antibody SP260 against Met (Santa Cruz Biotechnology). Proteins were detected with horseradish peroxidase‐conjugated secondary antibodies (Dakopatts) and a standard chemiluminescence immunoblotting protocol (ECL, Amersham Pharmacia Biotech). All signals were normalized for loading by comparison with the appropriate β‐actin signal.
Results
Generation of a monoclonal antibody against mouse Met
To study the expression of Met in the normal mouse intestine and in intestinal adenomas, we generated an antimouse Met mAb. To this end, the complete coding sequence of mouse Met was cloned into pcDNA3.1 and transfected stably into ARHO12 cells. These Met transfectants were used to immunize Armenian hamsters, and hybridomas were generated. By using this approach, a mAb specific for Met was obtained. This antibody, designated We‐1, strongly stained the plasma membranes of ARHO12 and NIH/3T3 cells transfected with mouse Met, but not that of non‐transfected control cells (Fig. 1A).
Figure 1.
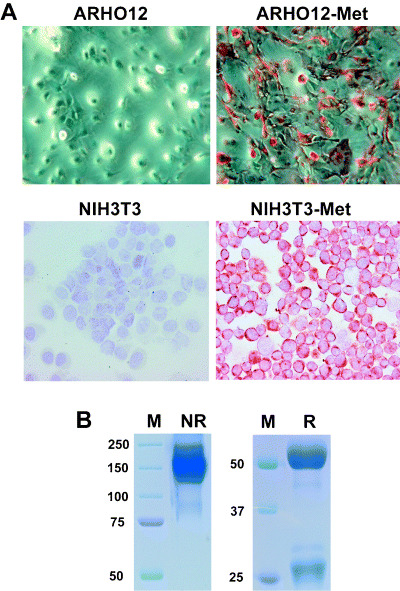
Characterization of a hamster monoclonal antibody, We‐1, against mouse Met. (A) We‐1 antibody was used to stain parental ARHO12 and NIH/3T3cells (left panel) or ARHO12 and NIH/3T3 cells transfected with Met (right panel). (B) The isotype of the antibody We‐1 was determined by sodium dodecylsulfate–polyacrylamide gel electrophoresis gel on reduced (R) and non‐reduced (NR) gels and staining with Coomassie brilliant blue. The immunoglobulin bands are visible next to the marker (M).
To determine the isotype of We‐1, the antibody was run on a SDS‐PAGE gel (Fig. 1B), which was stained with Coomassie Brilliant blue. On a non‐reduced gel this yielded a 150 kDa band, indicating that We‐1 is of immunoglobulin G isotype (Fig. 1B, left panel). Under reducing conditions, bands of 50 kDa and 25 kDa were present, representing the immunoglobulin heavy and light chains of the mAb, respectively (Fig. 1B, right panel).
Expression of Met in normal intestinal epithelium and in adenomas
We subsequently used We‐1 to assess the expression of Met protein in the normal murine intestinal mucosa and in intestinal adenomas. In the crypt and villus epithelium of the normal small and large intestine, we detected hardly any Met expression. In adenomas of the small and large intestine of Apc +/min mice, Met expression was low or absent (Fig. 2A, upper panel). In contrast, in the mouse kidney (Fig. 2A, lower right panel) and liver (data not shown), strong Met expression was present. In the kidney, tubular epithelial cells, glomerula and vessels stained positive for Met. In the liver, Met expression was detected on hepatoytes, bile ducts and vessels (data not shown). Our observation that mouse intestinal adenomas lack Met expression was unexpected as human intestinal tumors overexpress MET from the earliest stage (i.e. the ACF) onwards.( 8 ) This overexpression has been linked to deregulation of the WNT signaling pathway,( 8 ), which also underlies adenoma formation in Apc +/min mice. As has been shown by others,( 15 ) nuclear β‐catenin expression was detected in intestinal polyps of Apc ± /Min mice, indicating that activated Wnt signaling was present (Fig. 2A, lower left panel). Activated Wnt signaling in these polyps, however, did not result in Met overexpression.
Figure 2.
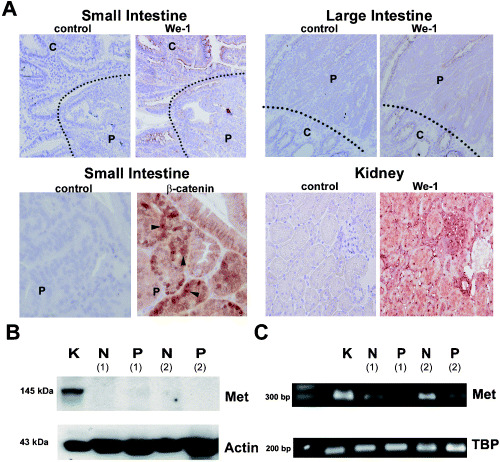
Expression of Met in normal intestinal epithelium and in adenomas of Apc +/min mice. (A) We‐1 antibody was used to stain normal intestinal tissue (C, crypts) and polyps (P) in the small and large intestine (upper panels). Dashed line depicts the boundary between normal intestinal tissue and adenomatous tissue. In addition, kidneys were stained with We‐1 (lower right panels). Also, intestinal tissue of the small intestine was stained for β‐catenin expression (lower left panel). Arrowheads indicate nuclear β‐catenin expression. (B) Met expression was detected by western blotting in lysates of kidney (K), normal intestinal tissue (N) and polyps (P) (n = 3). β‐Actin was used as a loading control. (C) Using laser microdissection we isolated normal intestinal tissue and tissue from polyps and determined Met mRNA levels using reverse transcription–polymerase chain reaction. Met mRNA levels were detected in the kidney, normal intestine or polyps (n = 3). As a loading control TATA box binding protein (TBP) levels were determined. In (B) and (C) the results of two different mice are shown (1 + 2).
To confirm that Met is indeed not expressed in mouse intestinal tumors, Met expression was also analyzed by immunoblotting using a polyclonal antibody against mouse Met (SP260).( 16 , 17 , 18 ) Using this approach, the Met protein was also not detected in the neoplastic (and normal) mouse intestine, although it was detected readily in kidney lysates (Fig. 2B). By using laser microdissection, we isolated adenomatous and adjacent normal intestinal tissue from Apc +/min mice and determined the Met mRNA levels by semiquantitative RT‐PCR. Although we did detect Met mRNA in both the normal intestine and in intestinal polyps, the expression levels were low compared to that in the kidney (Fig. 2C).
Met protein is expressed strongly by embryonic intestinal epithelial cells
Using in situ hybridization and Northern blot analysis, Sonnenberg and colleagues( 12 ) demonstrated that Met mRNA is expressed in the developing intestine, peaking at embryonic day 15 (E15). To explore whether Met protein expression can also be detected by We‐1 at this stage of development, we carried out immunohistochemical studies. Indeed, we observed that the intestinal epithelium of E15 mouse embryos stained strongly with We‐1 (Fig. 3A). Also, immunoblotting of E15 intestinal tissue demonstrated expression of Met protein. Taken together, these data demonstrate that Met is expressed in intestinal epithelial cells during development but hardly or not at all in the normal adult mouse intestine or in intestinal tumors of Apc +/min mice (Fig. 3B).
Figure 3.
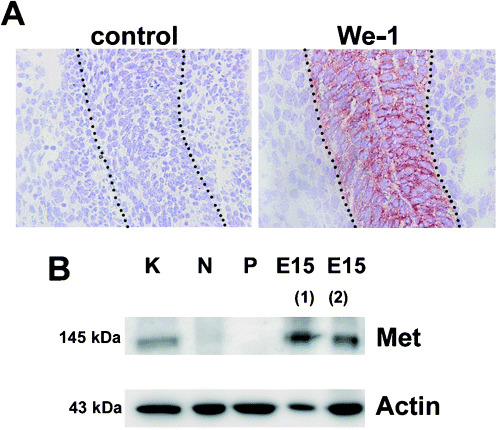
Met expression in the intestinal tract of mice at embryonic day 15 (E15). (A) The intestinal tract of embryos was stained with We‐1. Dashed line depicts the boundary between epithelial cells and surrounding cells. (B) Using immunoblotting Met protein expression was determined in lysates of kidney (K), adult intestinal tissue (N, normal; P, polyp) and intestinal tissue of two different E15 embryos (1 + 2). β‐Actin was used as a loading control.
Met family member Ron is not expressed in intestinal adenomas of Apc +/min mice
Our observation that Met, although strongly overexpressed in human colorectal adenomas, is not expressed in adenomas of Apc +/min mice implies that Met is either not instrumental in intestinal tumor formation or that its role in intestinal tumorigenesis in the mouse is substituted for by a functional homolog. To explore the latter possibility, we studied the expression of Ron, which shows a high degree of homology to Met (75% amino acid identity) and represents the receptor for the only other member of the plasminogen‐like growth factor family (i.e. MSP). We observed that Ron is not expressed in intestinal adenomas, but only in the surrounding normal mucosa, specifically in the epithelial cells of the intestinal villi (Fig. 4A). The expression patterns of both Ron and Met are in sharp contrast to the expression of the Wnt target CD44v6,( 19 ) which is expressed in the epithelial cells of the crypts (Fig. 4A). Isolating adenomatous and normal intestinal tissue by laser microdissection corroborated the immunohistochemical data. In these tissues Ron mRNA was detected in the lysate of normal, but not neoplastic, intestinal cells (Fig. 4B).
Figure 4.
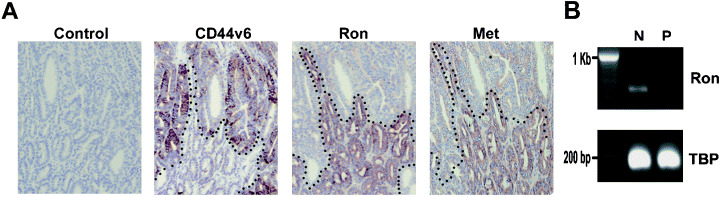
Expression of CD44v6, Ron and Met in normal intestinal epithelium and in polyps of Apc +/min mice. (A) Parallel sections of intestinal tissue were stained with antibodies against CD44v6, Ron and Met. Dashed line depicts the boundary between normal intestinal tissue and adenomatous tissue. (B) After microdissection Ron mRNA levels were determined in normal intestinal (N) or polyp (P) tissue using reverse transcription–polymerase chain reaction. As a loading control TATA box binding protein (TBP) levels were measured.
Discussion
To explore the role of Met in intestinal tumorigenesis in Apc +/min mice, we generated an Armenian hamster mAb against mouse Met. By using this antibody, designated We‐1, we detected strong expression of the Met protein in the intestinal epithelium of mouse embryos, a cell type that has previously been shown to express Met by mRNA in situ hybridization studies.( 12 ) In contrast, in the normal intestine of adult mice as well as in intestinal adenomas of Apc +/min mice, expression of the Met protein was very low. These observations suggest a role for the Hgf/Met signaling pathway in development of the mouse intestine, but also imply that Met signaling does not contribute to intestinal tumorigenesis in Apc +/min mice.
We demonstrated that the mAb We‐1 specifically stains ARHO12 and NIH/3T3 cells transfected with mouse Met, but not untransfected control cells (Fig. 1A). In addition, in immunohistochemical studies, We‐1 strongly stains kidney tubular and glomerular cells, which have previously been shown to express Met by mRNA in situ hybridization.( 12 ) We‐1 is of the immunoglobulin G isotype (Fig. 1B), and as the mAb did not detect Met by immunoblotting (data not shown), presumably it is directed against a conformational epitope on the Met protein.
Using We‐1, we observed strong Met expression in the intestinal epithelium of E15 mouse embryos, an observation consistent with previous mRNA in situ hybridization studies.( 12 ) In contrast, Met was hardly detectable or not detectable in the adult mouse intestine and in intestinal adenomas of Apc +/min mice. In particular, the latter observation was unexpected as it is in striking contrast to the situation in human colorectal tumors, where strong overexpression of Met is present from the earliest stage of the adenoma–carcinoma sequence (i.e. ACF) onwards.( 7 , 8 ) Like familial adenomatous polyposis patients, Apc +/min mice lack one functional Apc allele; loss of the second allele leads to activation of the Wnt signaling pathway resulting in uncontrolled Tcf/β‐catenin‐mediated transcription. Activation of the WNT pathway, either by loss of APC or by mutation of β‐catenin, also presents the primary defect in the vast majority of sporadic human colorectal carcinomas. We have previously identified MET as a target gene of the WNT signaling pathway in human CRC.( 8 ) Furthermore, previous studies have shown that MET can also activate the Wnt signaling pathway.( 16 , 20 ) The absence of Met overexpression in intestinal adenomas of Apc +/min mice thus implies interspecies differences in the regulation of Met and WNT signaling targets between humans and mice.
The Met promoter region of mice and humans is approximately 70% identical at the nucleotide level. A search for TCF binding sites( 21 ) revealed no consensus TCF binding sites in both promoter regions. In human CRC, regulation of MET expression through WNT signaling is therefore probably an indirect effect. Enhancers and other binding sites may also influence Met expression. The SP1‐binding sites in the Met promoter are highly conserved between mice and man, implicating that transforming growth factor β (TGFβ) signaling may be important in regulating Met expression.
Our current finding that Met is not overexpressed in intestinal adenomas in Apc +/min mice is corroborated by a mRNA microarray study showing no regulation of Met in polyps of Apc +/min mice compared to normal intestinal epithelium (R. Smits, personal communication). In view of the compelling evidence for a key role of MET in tumor invasion and metastasis, it is tempting to speculate that the absence of Met overexpression in mouse intestinal adenomas could be a factor in their inability to progress to invasive and metastatic cancer.
Our observation that the Met protein, unlike in human adenomas, is not expressed in intestinal adenomas of Apc +/min mice implies that Met is not instrumental in their development. Ron, the receptor for MSP/hepatocyte growth factor line (HGFL),( 22 , 23 ) which is closely related to Met, is also not expressed by mouse intestinal adenomas and hence does not act as a functional substitute for Met. The absence of Ron expression in mouse intestinal tumors contrasts the reported overexpression of RON in human colorectal cancer.( 24 , 25 ) In the mouse intestine, we detected Ron expression on differentiated villus cells.
In conclusion, our findings demonstrate important interspecies differences in the regulation of Met expression during intestinal tumorigenesis. Our current study demonstrates that intestinal adenoma formation in Apc +/min mice is not accompanied by Met overexpression, implying that Wnt signaling deregulation in mouse, unlike in human intestinal epithelium, does not result in Met overexpression.
Acknowledgment
We would like to thank A. B. van der Wardt and K. Brandsma for technical assistance and animal care. Furthermore, we would like to thank N. Claessen and C.M. van der Loos for their advice on IHC staining and B. Westhoek and D. J. J. de Gorter for technical support.
References
- 1. Birchmeier C, Gherardi E. Developmental roles of HGF/SF and its receptor, the c‐Met tyrosine kinase. Trends Cell Biol 1998; 8: 404–10. [DOI] [PubMed] [Google Scholar]
- 2. Van Der Voort R, Taher TE, Derksen PW, Spaargaren M, Van Der Neut R, Pals ST. The hepatocyte growth factor/Met pathway in development, tumorigenesis, and B‐cell differentiation. Adv Cancer Res 2000; 79: 39–90. [DOI] [PubMed] [Google Scholar]
- 3. Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c‐met receptor in the migration of myogenic precursor cells into the limb bud. Nature 1995; 376: 768–71. [DOI] [PubMed] [Google Scholar]
- 4. Schmidt C, Bladt F, Goedecke S et al. Scatter factor/hepatocyte growth factor is essential for liver development. Nature 1995; 373: 699–702. [DOI] [PubMed] [Google Scholar]
- 5. Uehara Y, Minowa O, Mori C et al. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature 1995; 373: 702–5. [DOI] [PubMed] [Google Scholar]
- 6. Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nature Rev Mol Cell Biol 2003; 4: 915–25. [DOI] [PubMed] [Google Scholar]
- 7. Wielenga VJ, Van Der Voort R, Taher TE et al. Expression of c‐Met and heparan‐sulfate proteoglycan forms of CD44 in colorectal cancer. Am J Pathol 2000; 157: 1563–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boon EM, Van Der Neut R, Van de Wetering M, Clevers H, Pals ST. Wnt signaling regulates expression of the receptor tyrosine kinase met in colorectal cancer. Cancer Res 2002; 62: 5126–8. [PubMed] [Google Scholar]
- 9. Di Renzo MF, Olivero M, Giacomini A et al. Overexpression and amplification of the met/HGF receptor gene during the progression of colorectal cancer. Clin Cancer Res 1995; 1: 147–54. [PubMed] [Google Scholar]
- 10. Boivin GP, Washington K, Yang K et al. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterol 2003; 124: 762–77. [DOI] [PubMed] [Google Scholar]
- 11. Fodde R, Smits R. Disease model: familial adenomatous polyposis. Trends Mol Med 2001; 7: 369–73. [DOI] [PubMed] [Google Scholar]
- 12. Sonnenberg E, Meyer D, Weidner KM, Birchmeier C. Scatter factor/hepatocyte growth factor and its receptor, the c‐met tyrosine kinase, can mediate a signal exchange between mesenchyme and epithelia during mouse development. J Cell Biol 1993; 123: 223–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang XM, Park M. Expression of the hepatocyte growth factor/scatter factor receptor tyrosine kinase is localized to epithelia in the adult mouse. Lab Invest 1995; 73: 483–91. [PubMed] [Google Scholar]
- 14. Hamann J, Van Zeventer C, Bijl A, Molenaar C, Tesselaar K, Van Lier RA. Molecular cloning and characterization of mouse CD97. Int Immunol 2000; 12: 439–48. [DOI] [PubMed] [Google Scholar]
- 15. Sheng H, Shao J, Williams CS et al. Nuclear translocation of β‐catenin in hereditary and carcinogen induced intestinal adenomas. Carcinogenesis 1998; 19: 543–9. [DOI] [PubMed] [Google Scholar]
- 16. Danilkovitch‐Miagkova A, Miagkov A, Skeel A, Nakaigawa N, Zbar B, Leonard EJ. Oncogenic mutants of RON and MET receptor tyrosine kinases cause activation of the beta‐catenin pathway. Mol Cell Biol 2001; 21: 5857–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Taher TE, Tjin EP, Beuling EA, Borst J, Spaargaren M, Pals ST. c‐Cbl is involved in Met signaling in B cells and mediates hepatocyte growth factor‐induced receptor ubiquitination. J Immunol 2002; 169: 3793–800. [DOI] [PubMed] [Google Scholar]
- 18. Wright TG, Tsai J, Jia Z, Elliott BE. Inhibition by copper (II) binding of hepatocyte growth factor (HGF) interaction with its receptor Met and blockade of HGF/Met function. J Biol Chem 2004; 279: 32 499–506. [DOI] [PubMed] [Google Scholar]
- 19. Wielenga VJ, Smits R, Korinek V et al. Expression of CD44 in Apc and Tcf mutant mice implies regulation by the WNT pathway. Am J Pathol 1999; 154: 515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boon EMJ, Kovarikova M, Derksen PWB, Van Der Neut R. Met signalling in primary colon epithelial cells leads to increased transformation irrespective of aberrant WNT signaling. Br J Cancer 2005; 92: 1078–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. He TC, Sparks AB, Rago C et al. Identification of c‐MYC as a target of the APC pathway. Science 1998; 281: 1509–12. [DOI] [PubMed] [Google Scholar]
- 22. Huff JL, Jelinek MA, Borgman CA, Lansing TJ, Parsons JT. The protooncogene c‐sea encodes a transmembrane protein–tyrosine kinase related to the Met/hepatocyte growth factor/scatter factor receptor. Proc Natl Acad Sci USA 1993; 90: 6140–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ronsin C, Muscatelli F, Mattei MG, Breathnach R. A novel putative receptor protein tyrosine kinase of the met family. Oncogene 1993; 8: 1195–202. [PubMed] [Google Scholar]
- 24. Chen YQ, Zhou YQ, Angeloni D, Kurtz AL, Qiang XZ, Wang MH. Overexpression and activation of the RON receptor tyrosine kinase in a panel of human colorectal carcinoma cell lines. Exp Cell Res 2000; 261: 229–38. [DOI] [PubMed] [Google Scholar]
- 25. Zhou YQ, He C, Chen YQ, Wang D, Wang MH. Altered expression of the RON receptor tyrosine kinase in primary human colorectal adenocarcinomas: generation of different splicing RON variants and their oncogenic potential. Oncogene 2003; 22: 186–97. [DOI] [PubMed] [Google Scholar]


