Abstract
Increased cancer risk occurs in inflammatory bowel disease (IBD) undergoing long‐term chronic inflammation. To evaluate whether inducible nitric oxide synthase (iNOS)‐dependent DNA damage plays a role in the carcinogenic process triggered by IBD, we prepared a mouse model of IBD induced by transfer of CD45RBhighCD4+ T cells lacking regulatory T cells to female severe combined immunodeficiency (SCID) mice. CD45RBhighCD4+ T cells were isolated from mouse spleen after staining with fluorescein isothiocyanate (FITC)‐conjugated anti‐CD45RB monoclonal antibody, followed by anti‐FITC‐conjugated microbeads. This IBD mouse model showed that the bodyweight increased with aging to a lesser extent than non‐treated controls, and that the intestine was shortened. Pathological findings of this mouse model, which showed severe inflammation in colon tissues, were similar to IBD patients. Double immunofluorescence technique revealed that both 8‐nitroguanine and 8‐oxo‐7,8‐dihydro‐2′‐deoxyguanosine (8‐oxodG) were formed mainly in epithelial cells of the IBD mouse model. 8‐Nitroguanine was formed in most of 8‐oxodG‐immunoreactive nuclei of epithelial cells. iNOS, proliferating cell nuclear antigen and p53 protein were also expressed in the colon epithelium. These results indicate that nitrative DNA damage, as well as oxidative DNA damage, is induced in colon epithelial cells of the IBD mouse model followed by proliferation of these cells, which may contribute to colon carcinogenesis. (Cancer Sci 2005; 96: 157–163)
Ulcerative colitis and Crohn's disease are well known as chronic inflammatory diseases in the lower bowel, and share many clinical and pathological characteristics. These diseases are referred to as inflammatory bowel disease (IBD), which leads to long‐term impairment of intestinal structure and function. (1) A large number of immunological abnormalities have been noted in patients with IBD. (2) It is well established that an increased cancer risk occurs in tissues undergoing chronic inflammation. Many cancers arise from sites of infection, chronic irritation and inflammation. (3) Epidemiological studies have suggested that the incidence of colorectal cancer in IBD is greater than the expected incidence in the general population. 4 , 5 , 6 The histological and molecular signatures suggest an inflammation‐driven carcinogenesis process in IBD patients. This detailed mechanism is unclear, although a recent report has shown that NF‐κB functions as a tumor promoter in inflammation‐associated cancer. (7)
NF‐κB can regulate genes like inducible nitric oxide synthase (iNOS). (8) Nitric oxide (NO) and reactive oxygen species (ROS) are considered to play the key role in inflammation‐mediated carcinogenesis. ROS can induce the formation of 8‐oxo‐7,8‐dihydro‐2′‐deoxyguanosine (8‐oxodG), which is an indicator of oxidative DNA damage. 9 , 10 , 11 , 12 The level of 8‐oxodG has been found to significantly increase in IBD patients (13) and animal models. (14) NO can mediate the formation of 8‐nitroguanine, a marker of nitrative DNA damage. 15 , 16 Akaike et al. reported that 8‐nitroguanine is formed in ribonucleic acid by viral infection as a biomarker of inflammation. (17) In addition, 8‐nitroguanine formed in DNA has been proposed to account for inflammation‐associated carcinogenesis. 9 , 18 , 19 , 20 Therefore, the accumulation of 8‐nitroguanine, as well as 8‐oxodG formation, may play a key role in the initiation and/or promotion of IBD‐mediated carcinogenesis. However, whether 8‐nitroguanine is formed in the colonic epithelium in IBD has not yet been determined.
To evaluate whether nitrative DNA damage plays a role in the carcinogenic process triggered by IBD, we prepared a mouse model of IBD induced by transfer of CD45RBhighCD4+ T cells lacking regulatory T cells to severe combined immunodeficiency (SCID) mice, 21 , 22 since mouse models of IBD can result from either excessive effector T cell function or deficient regulatory T cell function. (2) We performed a double immunofluorescent staining procedure to examine the formation of 8‐nitroguanine and 8‐oxodG in the colon tissues. We also examined the expression of iNOS by immunohistochemistry. To evaluate the proliferating activity of colonic epithelial cells and their response to DNA damage, we examined the expression of proliferating cell nuclear antigen (PCNA) and p53 in the colon tissues.
Materials and Methods
Production of anti‐8‐nitroguanine antibody. As described previously, a polyclonal antibody against 8‐nitroguanine was produced. (23) Briefly, the 8‐nitroguanine‐aldehyde‐rabbit serum albumin conjugate was mixed with Freund's complete adjuvant and injected into a rabbit. We obtained the serum and purified the antibody by affinity chromatography. The specificity of this purified antibody was confirmed by a dot immunobinding assay and absorption test. (23)
Induction of IBD in SCID mice by adoptive transfer of CD45RBhighCD4+ T cells. Female BALB/c and SCID mice (C.B‐17) of the BALB/c background were obtained from CLEA JAPAN (Osaka, Japan), and used at >6 week of age. Mice were maintained at the Institute of Laboratory Animal of Life Science Center, Mie University. The animal experiments were conducted according to the guidelines of Mie University School of Medicine. Single cell suspensions of spleen from BALB/c mice were enriched for CD4+ T cells by negative selection using a CD4 isolation kit (Miltenyi Biotec, Bergisch, Germany). CD45RBhighCD4+ T cells were positively selected from the resultant CD4+ T cell preparation using AutoMACS (Miltenyi Biotec) after staining with FITC‐conjugated anti‐CD45RB monoclonal antibody (mAb) 16A (PharMingen, San Diego, CA, USA) followed by anti‐FITC‐conjugated microbeads (Miltenyi Biotec), according to the manufacturer's instruction. For induction of IBD, four C.B‐17 SCID mice were injected intraperitoneally with purified CD45RBhighCD4+ T cells (4 × 105 cells/mouse) in phosphate‐buffered saline as described previously. (21) Four non‐treated C.B‐17 SCID mice were used as the control. Mice were observed daily and weighed weekly. All mice were killed 12 weeks later. Under deep anesthesia, the mice were transcardially perfused with saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer. Colon tissues were embedded in paraffin and cut into 5 µm thick sections. The sections were routinely stained with hematoxylin and eosin (HE) for histopathological examination, or used for immunohistochemical analyses.
Light microscopic immunohistochemistry. As described previously, 8‐nitroguanine and 8‐oxodG immunoreactivity in the colon tissues was assessed by double immunofluorescence labeling study. (24) Sections were incubated with rabbit polyclonal anti‐8‐nitroguanine antibody (2 µg/mL) and mouse anti‐8‐oxodG mAb (4 µg/mL; Japan Institute for the Control of Aging, Fukuroi, Japan) overnight. The sections were then incubated for 3 h with Alexa 488‐labeled goat antibody against rabbit immunoglobulin G (IgG) and Alexa 594‐labeled goat antibody against mouse IgG (1:400; Molecular Probes, Oregon, USA). The stained sections were examined under an inverted Laser Scan Microscope (LSM 410; Zeiss, Gottingen, Germany). In a certain experiment, to demonstrate 8‐nitroguanine formation in DNA more clearly, tissue sections were pretreated with 10 µg/mL ribonuclease (RNase) at 37°C for 1 h according to the method described by Tsuruya et al. (25)
As described above, the expression of iNOS, PCNA and p53 were also assessed by double immunofluorescence labeling study with 8‐nitroguanine. Anti‐PCNA (1:200; Novocastra Laboratories Ltd, Newcastle‐upon‐Tyne, UK), anti‐iNOS (1:1000; Sigma, St Louis, MO, USA) and anti p53 (1:100; Novocastra Laboratories Ltd) mAbs were used as primary antibodies.
Electron microscopic immunohistochemistry. The colon tissues were postfixed in 2% glutaraldehyde in 0.1 M phosphate buffer for 3 h, and sectioned by a microslicer (D.S.K., Kyoto, Japan) in 100 µm thickness for electron microscopic immunohistochemistry. Staining by the postembedding immunogold method was performed as described previously. (26) The sections were embedded in Epon 812, and 75 nm thick ultra‐thin sections were cut. The ultra‐thin sections were incubated with 0.3% H2O2 in Tris‐buffered saline (TBS) for 7 min, rinsed in 0.1% solution of bovine serum albumin in TBS (BSA‐TBS) for 10 min, and incubated with 2% normal goat serum for 30 min. The sections were incubated with anti‐8‐nitroguanine antibody for 6 h at room temperature, rinsed twice in BSA‐TBS for 5 min, and incubated with goat antibody against rabbit IgG (diluted 1:40 in BSA‐TBS) and coupled to colloidal gold (15 nm; Janssen Life Sci. Prod., Beerse, Belgium), anddiluted 1:40 in BSA‐TBS for 3 h. The sections were counter‐stained with uranyl acetate for 15 min and 1% lead citrate for 2 min. Control staining was made by omission of 8‐nitroguanine antibody.
Results
Immunologic IBD mouse model. We isolated CD45RBhighCD4+ T cells from CD4+ T cells, obtained from mouse spleen, using FITC‐conjugated anti‐CD45RB mAb and anti‐FITC‐conjugated microbeads. The fluorescence intensity of CD45RB on CD4+ T cells was widely distributed (Fig. 1a), whereas isolated CD45RBhighCD4+ T cells showed a single peak (Fig. 1b). This result suggests that CD45RBhighCD4+ T cells were successfully isolated from mouse spleen.
Figure 1.
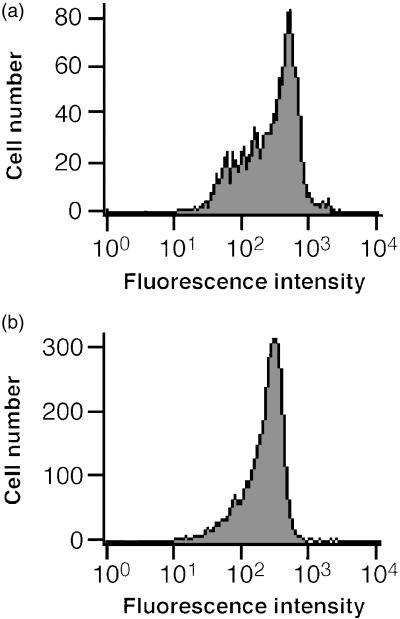
Flow cytometric analysis of CD45RB on total CD4+ T cells and isolated CD45RBhighCD4+ T cells. CD45RBhighCD4+ T cells were positively selected from CD4+ T cells obtained from BALB/c mice spleen after staining with fluorescein isothiocyanate (FITC)‐conjugated anti‐CD45RB monoclonal antibody, followed by anti‐FITC‐conjugated microbeads. (a) CD45RB on CD4+ T cells (before isolation), and (b) isolated CD45RBhighCD4+ T cells. The horizontal axis shows the fluorescence intensity, and the vertical axis shows the cell number.
Mice transferred with CD45RBhighCD4+ T cells showed an increase in bodyweight with aging to a lesser extent than non‐treated controls (Fig. 2). Bodyweight of the mice transferred with CD45RBhighCD4+ T cells was significantly lower than that of non‐treated controls at 12 weeks (P < 0.05). Seven weeks after CD45RBhighCD4+ T cell transfer, mice developed clinical signs of colitis, including diarrhea and weight loss. This IBD mouse model showed that the intestine was shortened compared with non‐treated controls.
Figure 2.
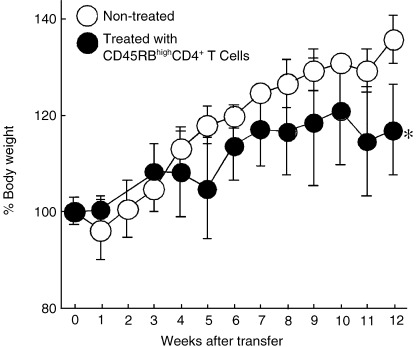
Change in bodyweight of the mice in the experiment duration. Bodyweight of the mice transferred with CD45RBhighCD4+ T cells (•) was significantly lower than that of non‐treated controls (○) at 12 weeks (n = 4, *P < 0.05).
Histopathological analysis of the IBD mouse model. The colon tissues of mice injected by CD45RBhighCD4+ T cells showed chronic inflammatory response (Fig. 3). Open ulcers, which were characterized by sloughing of epithelial cells and increased transmigration of leukocytes in the lamina propria, was observed in the colon tissues (Fig. 3a). Regenerated epithelial cells with mild dysplasia and goblet cell depression, besides remarkable subepithelial fibrosis, were observed in the epithelium (Fig. 3b). Some inflammatory cells infiltrated into the epithelial cell layer (Fig. 3c). Many various inflammatory cells, such as neutrophils, macrophages, plasma cells (Fig. 3d) and lymphocytes (Fig. 3e), were permeated lamina propria.
Figure 3.
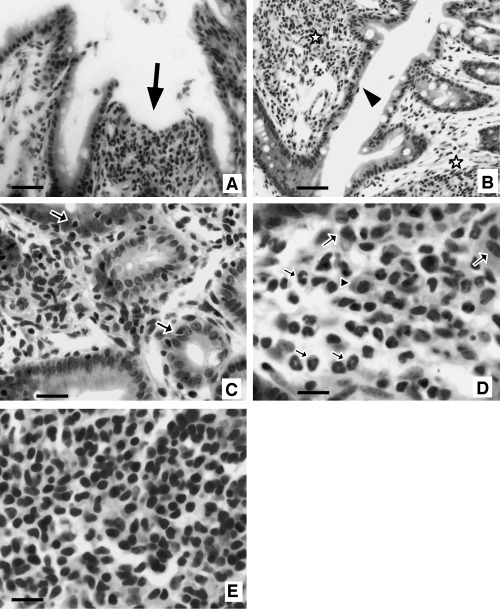
Pathological findings in the colon of CD45RBhighCD4+ T cell‐transferred mice (hematoxylin–eosin staining). (a,b) Low power view of colon mucosa. (a) Open ulcer (arrow), which is characterized by sloughing of epithelial cells and leukocyte infiltration in lamina propria is observed. (b) Regenerated epithelial cells with mild dysplasia and goblet cell depression are prominent (arrowhead). Marked or moderate inflammatory cell infiltration and fibrosis are apparent in subepithelial tissue (asterisk). (c) Inflammatory cell infiltration in epithelial cell layer (arrow). (d,e) High power view of infiltrated inflammatory cells in lamina propria. (d) Many neutrophils (small arrow), plasma cells (arrowhead), macrophages (large arrow), and (e) lymphocytes permeate in lamina propria layer. Bars: b, 50 µm; a,c, 25 µm; d,e, 10 µm.
Accumulation of 8‐nitroguanine, 8‐oxodG and expression of iNOS in colon tissues of the IBD mouse model. In the CD45RBhighCD4+ T cell‐transferred mouse, significant 8‐nitroguanine accumulation was induced in the nuclei and the cytoplasm of epithelial cells, and was also present in infiltrated cells supposed to be inflammatory cells in lamina propria (Fig. 4a,d). 8‐Nitroguanine was formed in most of 8‐oxodG‐immunoreactive nuclei of epithelial cells and infiltrated cells (Fig. 4b,c). When the sections were pretreated with RNase, 8‐nitroguanine immunoreactivity was more clearly observed in the nuclei of epithelial cells (Fig. 4g). This result suggests that 8‐nitroguanine was formed in genomic DNA. iNOS was expressed in the cytoplasm of epithelial cells and infiltrated cells in the lamina propria of the IBD mouse model (Fig. 4e). iNOS was expressed mainly in 8‐nitroguanine immunoreactive epithelial cells (Fig. 4f). However, no or little 8‐nitroguanine, 8‐oxodG and iNOS were observed in non‐treated control mice (Fig. 4h–j).
Figure 4.
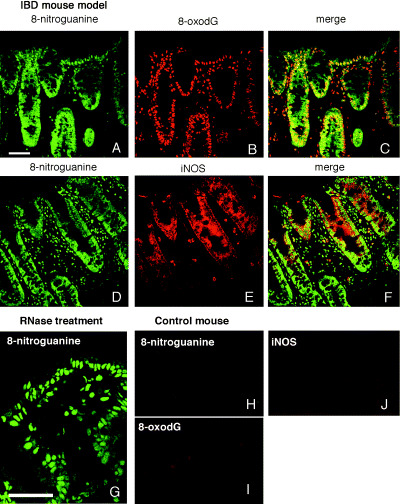
Formation of 8‐oxo‐7,8‐dihydro‐2′‐deoxyguanosine (8‐oxodG), 8‐nitroguanine and inducible nitric oxide synthase (iNOS) in the mouse model of inflammatory bowel disease. (a,d) 8‐Nitroguanine is accumulated in the nuclei and the cytoplasm of epithelial cells. 8‐Nitroguanine is also present in infiltrated cells. (b) 8‐oxodG formation is observed in the nuclei of epithelial cells and the infiltrated cells in lamina propria, (c) 8‐oxodG and 8‐nitroguanine co‐localized in the nuclei of most epithelial cells and infiltrated cells, (e) iNOS is expressed in the cytoplasm of epithelial cells and infiltrated cells in lamina propria, and (f) iNOS and 8‐nitroguanine colocalized in the cytoplasm of many epithelial cells and some infiltrated cells. The colon tissue sections were pretreated with ribonuclease and then immunohistochemical analysis was performed. (g) 8‐Nitroguanine immunoreactivity is more clearly observed in the nuclei of epithelial cells. (h) Little or no immunoreactivity of 8‐nitroguanine, (i) 8‐oxodG, and (j) iNOS was observed in control group mice. Scale bars: 50 µm.
Electron microscopic immunohistochemistry further confirmed that 8‐nitroguanine was accumulated mainly in the nucleus of the colon epithelial cells (Fig. 5). Immunogold particles were spread in the cytoplasm, and weak immunoreactivity was observed in mitochondria. No immunoreactivity was observed when 8‐nitroguanine antibody was omitted (data not shown).
Figure 5.
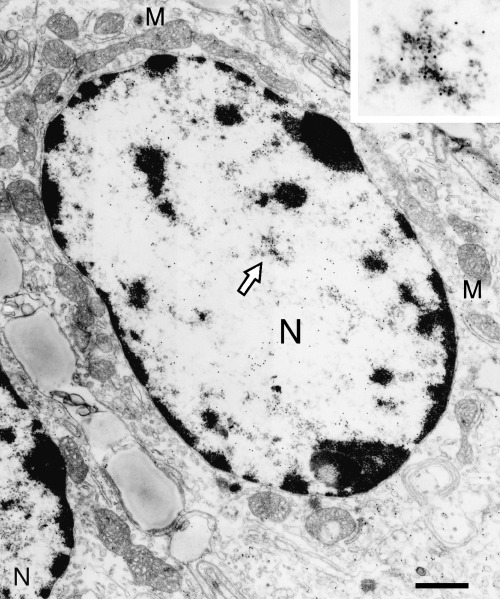
Immunoelectron micrograph of 8‐nitroguanine immunoreactivity in colon epithelial cell of the mouse model of inflammatory bowel disease. 8‐Nitroguanine is accumulated mainly in the nucleus (N) of the colon epithelial cells. Immunogold particles were spread in the cytoplasm, and weak immunoreactivity was observed in mitochondria (M). Inset, the magnification of the area indicated by an arrow. Scale bar: 1 µm.
Expression of PCNA and p53 in colon tissues of the IBD mouse model. In the IBD mouse model, intense PCNA was expressed mainly in the nuclei of many glandular epithelial cells, particularly in the lower part of the glands, whereas 8‐nitroguanine localized more widely in the mucosa. PCNA was expressed partially in the nuclei of 8‐nitroguanine‐immunoreactive epithelial cells (Fig. 6a–c). In the IBD mouse model, significant p53 was accumulated in the nuclei of regenerated epithelial cells. p53 was also expressed in some infiltrated cells. p53 expression was overlapped with 8‐nitroguanine (Fig. 6d–f). The four mice of IBD model that we used by transfer of CD45RBhighCD4+ T cells showed the similar results of histopathological analysis and immunohistostaining. No or little expression of PCNA and p53 was observed in non‐treated control mice (data not shown).
Figure 6.
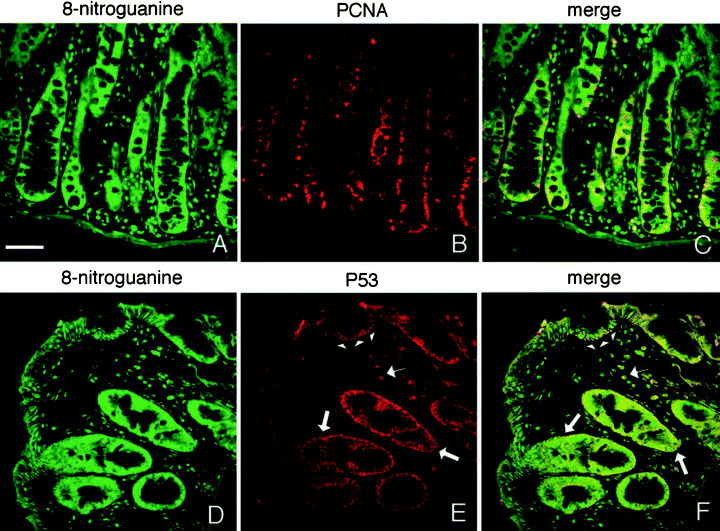
Proliferating cell nuclear antigen (PCNA) and p53 expression in the mouse model of inflammatory bowel disease. (a–c) Double immunostaining for 8‐nitroguanine and PCNA. (a,d) 8‐Nitroguanine localizes widely in the mucosa, (b) intense PCNA is expressed in the nuclei of many glandular epithelial cells and infiltrated cells in lamina propria, (c) PCNA is expressed partially in the nuclei of 8‐nitroguanine‐immunoreactive epithelial cells, (d–f) double immunostaining for 8‐nitroguanine and p53, (e) significant p53 expression is observed in the nuclei of regenerated epithelial cells (arrowhead) and some infiltrated cells (small arrow). p53 is also induced in the cytoplasm of many epithelial cells in lower portions of the gland (large arrow). (f) p53 is expressed in most of 8‐nitroguanine‐immunoreactive epithelial cells. Scale bar: 50 µm.
Discussion
To examine the mechanism of carcinogenesis mediated by IBD, we produced an IBD mouse model by transfer of CD45RBhighCD4+ T cells to SCID mice. Flow cytometry revealed that CD45RBhighCD4+ T cells were successfully isolated from mouse spleen. The intestine was shortened and the inflammatory response was observed in CD45RBhighCD4+ T cell‐transferred mice, similarly to the previous report. (22) Our present pathological findings of this mouse model were similar to those seen in IBD patients. 27 , 28 We confirmed that this mouse model and human IBD shared the findings of the chronic inflammatory response in mucosal lesion, ulceration and the appearance of regenerative epithelial cells with dysplasia. Therefore, this model is suitable for investigation on inflammation‐associated carcinogenesis in IBD patients. Recently, several animal models for colitis‐related colon carcinogenesis have been reported. Colonic neoplasms developed in mice treated with a genotoxic colonic carcinogen, azoxymethane, followed by dextran sodium sulfate (DSS). (29) A colitis‐associated neoplasia model in p53 deficient mice, by treatment with DSS, showed 100% incidence of neoplasias. (30) IBD mice, deficient for the mdr1a gene, spontaneously developed colitis. (31) Our IBD mouse model is more consistent with the pathogenesis of human IBD than other models, since our model was induced by immunological abnormality‐mediated inflammation, which is independent of chemicals and genetic deficiency.
In the present study, we have demonstrated that inflammation can promote the formation of 8‐nitroguanine as well as 8‐oxodG in the colon epithelium of the IBD mouse model. The formation of 8‐nitroguanine and 8‐oxodG was strongly observed in the epithelial cells and slightly in stromal inflammatory cells. Both the light and electron microscopic immunohistochemistry revealed that 8‐nitroguanine was formed in the nuclei and cytoplasm. In the colon tissue pretreated with RNase, 8‐nitroguanine formation was more clearly observed in the nuclei of epithelial cells. These results suggest that 8‐nitroguanine is formed mainly in genomic DNA. iNOS was highly observed in the inflamed epithelium. Relevantly recent studies have shown that iNOS is expressed in the epithelial cells in colitis patients. 11 , 32 , 33 Our results and these reports suggest that the colon epithelium, as well as inflammatory cells, is an important source of NO. Overproduction of NO plays a crucial role in an enormous variety of pathological processes including cancer. (34) In addition to macrophages (35) and neutrophils, (36) the colonic epithelial cells themselves have the ability to produce superoxide anion (O2 • −). 37 , 38 , 39 , 40 O2 • − is dismutated to H2O2, which induces metal‐dependent 8‐oxodG formation. (10) NO reacts with O2 • − to produce ONOO−, which can induce 8‐oxodG, (41) and 8‐nitroguanine formation. 16 , 42 The formation of 8‐oxodG is known to cause G → T transversion. 43 , 44 8‐Nitroguanine undergoes spontaneous depurination, leading to the formation of apurinic sites in DNA, (45) which may induce base substitution, such as G → T transversion. (46) Therefore, 8‐nitroguanine, formed via NO generation particularly from the epithelial cells, is a putative mutagenic DNA lesion and may contribute to IBD carcinogenesis in addition to 8‐oxodG. (47)
In the mouse model of IBD, dysplasia and PCNA expression were observed in colon epithelium, suggesting that IBD promotes cell proliferation via inflammation‐mediated DNA damage. PCNA‐labeling indices was significantly higher in high‐grade than in low‐grade dysplasia in IBD patients. (48) Therefore, colitis‐mediated DNA damage, followed by epithelial cell proliferation, may promote colonic carcinogenesis. p53 is important in the cellular response to DNA damage. (49) Several studies have demonstrated the p53 tumor suppressor gene expression during the procession to ulcerative colitis‐associated carcinoma. 50 , 51 Recently, it has been reported that a high concentration of NO can induce p53 protein accumulation. 52 , 53 Our results are noteworthy, in that high levels of 8‐nitroguanine and p53 were accumulated in the same inflamed epithelium. Therefore, 8‐nitroguanine formation and resulting p53 accumulation may promote IBD‐associated colonic carcinogenesis.
In conclusion, 8‐nitroguanine may not only be a promising biomarker for inflammation, but also a useful indicator for the risk of inflammation‐mediated carcinogenesis.
Acknowledgments
This work was supported by Grants‐in‐Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan.
References
- 1. Podolsky DK. Inflammatory bowel disease. N Engl J Med 2002; 347: 417–29. [DOI] [PubMed] [Google Scholar]
- 2. Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol 2003; 3: 521–33. [DOI] [PubMed] [Google Scholar]
- 3. Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420: 860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ekbom A, Helmick C, Zack M, Adami HO. Increased risk of large‐bowel cancer in Crohn's disease with colonic involvement. Lancet 1990; 336: 357–9. [DOI] [PubMed] [Google Scholar]
- 5. Langholz E, Munkholm P, Davidsen M, Binder V. Colorectal cancer risk and mortality in patients with ulcerative colitis. Gastroenterology 1992; 103: 1444–51. [DOI] [PubMed] [Google Scholar]
- 6. Choi PM, Zelig MP. Similarity of colorectal cancer in Crohn's disease and ulcerative colitis: implications for carcinogenesis and prevention. Gut 1994; 35: 950–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich‐Pyest E, Urieli‐Shoval S, Galun E, Ben‐Neriah Y. NF‐κB functions as a tumour promoter in inflammation‐associated cancer. Nature 2004; 431: 461–6. [DOI] [PubMed] [Google Scholar]
- 8. Dijkstra G, Moshage H, Jansen PL. Blockade of NF‐κB activation and donation of nitric oxide: new treatment options in inflammatory bowel disease? Scand J Gastroenterol 2002;. (Suppl): 37–41. [DOI] [PubMed] [Google Scholar]
- 9. Ohshima H, Tatemichi M, Sawa T. Chemical basis of inflammation‐induced carcinogenesis. Arch Biochem Biophys 2003; 417: 3–11. [DOI] [PubMed] [Google Scholar]
- 10. Kawanishi S, Hiraku Y, Oikawa S. Mechanism of guanine‐specific DNA damage by oxidative stress and its role in carcinogenesis and aging. Mutat Res 2001; 488: 65–76. [DOI] [PubMed] [Google Scholar]
- 11. Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J 1996; 313: 17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tazawa H, Okada F, Kobayashi T, Tada M, Mori Y, Une Y, Sendo F, Kobayashi M, Hosokawa M. Infiltration of neutrophils is required for acquisition of metastatic phenotype of benign murine fibrosarcoma cells: implication of inflammation‐associated carcinogenesis and tumor progression. Am J Pathol 2003; 163: 2221–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. D’Inca R, Cardin R, Benazzato L, Angriman I, Martines D, Sturniolo GC. Oxidative DNA damage in the mucosa of ulcerative colitis increases with disease duration and dysplasia. Inflamm Bowel Dis 2004; 10: 23–7. [DOI] [PubMed] [Google Scholar]
- 14. Tardieu D, Jaeg JP, Deloly A, Corpet DE, Cadet J, Petit CR. The COX‐2 inhibitor nimesulide suppresses superoxide and 8‐hydroxy‐deoxyguanosine formation, and stimulates apoptosis in mucosa during early colonic inflammation in rats. Carcinogenesis 2000; 21: 973–6. [DOI] [PubMed] [Google Scholar]
- 15. Yermilov V, Rubio J, Becchi M, Friesen MD, Pignatelli B, Ohshima H. Formation of 8‐nitroguanine by the reaction of guanine with peroxynitrite in vitro. Carcinogenesis 1995; 16: 2045–50. [DOI] [PubMed] [Google Scholar]
- 16. Masuda M, Nishino H, Ohshima H. Formation of 8‐nitroguanosine in cellular RNA as a biomarker of exposure to reactive nitrogen species. Chem Biol Interact 2002; 139: 187–97. [DOI] [PubMed] [Google Scholar]
- 17. Akaike T, Okamoto S, Sawa T, Yoshitake J, Tamura F, Ichimori K, Miyazaki K, Sasamoto K, Maeda H. 8‐Nitroguanosine formation in viral pneumonia and its implication for pathogenesis. Proc Natl Acad Sci USA 2003; 100: 685–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pinlaor S, Yongvanit P, Hiraku Y, Ma N, Semba R, Oikawa S, Murata M, Sripa B, Sithithaworn P, Kawanishi S. 8‐Nitroguanine formation in the liver of hamsters infected with Opisthorchis viverrini . Biochem Biophys Res Commun 2003; 309: 567–71. [DOI] [PubMed] [Google Scholar]
- 19. Pinlaor S, Ma N, Hiraku Y, Yongvanit P, Semba R, Oikawa S, Murata M, Sripa B, Sithithaworn P, Kawanishi S. Repeated infection with Opisthorchis viverrini induces accumulation of 8‐nitroguanine and 8‐oxo‐7,8‐dihydro‐2′‐deoxyguanine in the bile duct of hamsters via inducible nitric oxide synthase. Carcinogenesis 2004; 25: 1535–42. [DOI] [PubMed] [Google Scholar]
- 20. Ma N, Adachi Y, Hiraku Y, Horiki N, Horiike S, Imoto I, Pinlaor S, Murata M, Semba R, Kawanishi S. Accumulation of 8‐nitroguanine in human gastric epithelium induced by Helicobacter pylori infection. Biochem Biophys Res Commun 2004; 319: 506–10. [DOI] [PubMed] [Google Scholar]
- 21. Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C.B‐17 SCID mice. Int Immunol 1993; 5: 1461–71. [DOI] [PubMed] [Google Scholar]
- 22. Philippe D, Dubuquoy L, Groux H, Brun V, Chuoi‐Mariot MT, Gaveriaux‐Ruff C, Colombel JF, Kieffer BL, Desreumaux P. Anti‐inflammatory properties of the mu opioid receptor support its use in the treatment of colon inflammation. J Clin Invest 2003; 111: 1329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pinlaor S, Hiraku Y, Ma N, Yongvanit P, Semba R, Oikawa S, Murata M, Sripa B, Sithithaworn P, Kawanishi S. Mechanism of NO‐mediated oxidative and nitrative DNA damage in hamsters infected with Opisthorchis viverrini: a model of inflammation‐mediated carcinogenesis. Nitric Oxide 2004; 11: 175–83. [DOI] [PubMed] [Google Scholar]
- 24. Ma N, Ding X, Miwa T, Semba R. Immunohistochemical localization of taurine in the rat stomach. Adv Exp Med Biol 2003; 526: 229–36. [DOI] [PubMed] [Google Scholar]
- 25. Tsuruya K, Furuichi M, Tominaga Y, Shinozaki M, Tokumoto M, Yoshimitsu T, Fukuda K, Kanai H, Hirakata H, Iida M, Nakabeppu Y. Accumulation of 8‐oxoguanine in the cellular DNA and the alteration of the OGG1 expression during ischemia‐reperfusion injury in the rat kidney. DNA Repair (Amst) 2003; 2: 211–29. [DOI] [PubMed] [Google Scholar]
- 26. Takagishi Y, Ma N, Semba R, Yamamura H. Glutamate‐specific immunoreactivity in the jaundiced Gun rat cerebellum. Environ Med 1997; 41: 77–82. [Google Scholar]
- 27. Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol 2002; 20: 495–549. [DOI] [PubMed] [Google Scholar]
- 28. Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol 2003; 170: 3939–43. [DOI] [PubMed] [Google Scholar]
- 29. Tanaka T, Kohno H, Suzuki R, Yamada Y, Sugie S, Mori H. A novel inflammation‐related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci 2003; 94: 965–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fujii S, Fujimori T, Kawamata H, Takeda J, Kitajima K, Omotehara F, Kaihara T, Kusaka T, Ichikawa K, Ohkura Y, Ono Y, Imura J, Yamaoka S, Sakamoto C, Ueda Y, Chiba T. Development of colonic neoplasia in p53 deficient mice with experimental colitis induced by dextran sulphate sodium. Gut 2004; 53: 710–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Panwala CM, Jones JC, Viney JL. A novel model of inflammatory bowel disease: mice deficient for the multiple drug resistance gene, mdr1a, spontaneously develop colitis. J Immunol 1998; 161: 5733–44. [PubMed] [Google Scholar]
- 32. Hofseth LJ, Saito S, Hussain SP, Espey MG, Miranda KM, Araki Y, Jhappan C, Higashimoto Y, He P, Linke SP, Quezado MM, Zurer I, Rotter V, Wink DA, Appella E, Harris CC. Nitric oxide‐induced cellular stress and p53 activation in chronic inflammation. Proc Natl Acad Sci USA 2003; 100: 143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Singer II, Kawka DW, Scott S, Weidner JR, Mumford RA, Riehl TE, Stenson WF. Expression of inducible nitric oxide synthase and nitrotyrosine in colonic epithelium in inflammatory bowel disease. Gastroenterology 1996; 111: 871–85. [DOI] [PubMed] [Google Scholar]
- 34. Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer 2003; 3: 276–85. [DOI] [PubMed] [Google Scholar]
- 35. Kato M, Tokuyama K, Minakami H, Nagai A, Kozawa K, Goto H, Morikawa A, Kimura H. Increased superoxide radicals generation from alveolar macrophages in immature guinea‐pigs. Cell Biol Int 2002; 26: 829–32. [DOI] [PubMed] [Google Scholar]
- 36. Simmonds NJ, Allen RE, Stevens TR, Van Someren RN, Blake DR, Rampton DS. Chemiluminescence assay of mucosal reactive oxygen metabolites in inflammatory bowel disease. Gastroenterology 1992; 103: 186–96. [DOI] [PubMed] [Google Scholar]
- 37. Perner A, Andresen L, Pedersen G, Rask‐Madsen J. Superoxide production and expression of NAD(P)H oxidases by transformed and primary human colonic epithelial cells. Gut 2003; 52: 231–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Banfi B, Clark RA, Steger K, Krause KH. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem 2003; 278: 3510–3. [DOI] [PubMed] [Google Scholar]
- 39. Geiszt M, Lekstrom K, Witta J, Leto TL. Proteins homologous to p47phox and p67phox support superoxide production by NAD(P)H oxidase 1 in colon epithelial cells. J Biol Chem 2003; 278: 20006–12. [DOI] [PubMed] [Google Scholar]
- 40. Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 2004; 4: 181–9. [DOI] [PubMed] [Google Scholar]
- 41. Inoue S, Kawanishi S. Oxidative DNA damage induced by simultaneous generation of nitric oxide and superoxide. FEBS Lett 1995; 371: 86–8. [DOI] [PubMed] [Google Scholar]
- 42. Hsieh YS, Wang HC, Tseng TH, Chang WC, Wang CJ. Gaseous nitric oxide‐induced 8‐nitroguanine formation in human lung fibroblast cells and cell‐free DNA. Toxicol Appl Pharmacol 2001; 172: 210–6. [DOI] [PubMed] [Google Scholar]
- 43. Bruner SD, Norman DP, Verdine GL. Structural basis for recognition and repair of the endogenous mutagen 8‐oxoguanine in DNA. Nature 2000; 403: 859–66. [DOI] [PubMed] [Google Scholar]
- 44. Shibutani S, Takeshita M, Grollman AP. Insertion of specific bases during DNA synthesis past the oxidation‐damaged base 8‐oxodG. Nature 1991; 349: 431–4. [DOI] [PubMed] [Google Scholar]
- 45. Yermilov V, Rubio J, Ohshima H. Formation of 8‐nitroguanine in DNA treated with peroxynitrite in vitro and its rapid removal from DNA by depurination. FEBS Lett 1995; 376: 207–10. [DOI] [PubMed] [Google Scholar]
- 46. Loeb LA, Preston BD. Mutagenesis by apurinic/apyrimidinic sites. Annu Rev Genet 1986; 20: 201–30. [DOI] [PubMed] [Google Scholar]
- 47. Jourd’heuil D, Kang D, Grisham MB. Interactions between superoxide and nitric oxide: implications in DNA damage and mutagenesis. Front Biosci 1997; 2: d189–96. [DOI] [PubMed] [Google Scholar]
- 48. Kullmann F, Fadaie M, Gross V, Knuchel R, Bocker T, Steinbach P, Scholmerich J, Ruschoff J. Expression of proliferating cell nuclear antigen (PCNA) and Ki‐67 in dysplasia in inflammatory bowel disease. Eur J Gastroenterol Hepatol 1996; 8: 371–9. [DOI] [PubMed] [Google Scholar]
- 49. Levine AJ. p53, the cellular gatekeeper for growth and division. Cell 1997; 88: 323–31. [DOI] [PubMed] [Google Scholar]
- 50. Hurlimann J, Chaubert P, Benhattar J. p53 Gene alterations and p53 protein accumulation in infiltrating ductal breast carcinomas: correlation between immunohistochemical and molecular biology techniques. Mod Pathol 1994; 7: 423–8. [PubMed] [Google Scholar]
- 51. Harpaz N, Peck AL, Yin J, Fiel I, Hontanosas M, Tong TR, Laurin JN, Abraham JM, Greenwald BD, Meltzer SJ. p53 protein expression in ulcerative colitis‐associated colorectal dysplasia and carcinoma. Hum Pathol 1994; 25: 1069–74. [DOI] [PubMed] [Google Scholar]
- 52. Messmer UK, Ankarcrona M, Nicotera P, Brune B. p53 expression in nitric oxide‐induced apoptosis. FEBS Lett 1994; 355: 23–6. [DOI] [PubMed] [Google Scholar]
- 53. Ambs S, Hussain SP, Harris CC. Interactive effects of nitric oxide and the p53 tumor suppressor gene in carcinogenesis and tumor progression. FASEB J 1997; 11: 443–8. [DOI] [PubMed] [Google Scholar]


