Abstract
We have reported that a lack of RUNX3 function is causally associated with gastric carcinogenesis. We have also presented evidence that loss of Runx3 may be related to the genesis of intestinal metaplasia because expression of RUNX3 is reduced in some intestinal metaplasias, and some Runx3 −/– p53 −/– gastric epithelial cells differentiate into intestinal type cells in vivo. Recently several reports have indicated that blood cells play important roles in the gastric carcinogenesis. In the present study, we therefore examined whether Runx3 −/– p53 −/– gastric epithelial cells differentiate autonomously into intestinal type cells, or whether the presence of other cells is necessary for the differentiation in vitro. When Runx3 −/– p53 −/– gastric epithelial cells were cultured with collagen gels, they did not exhibit any morphogenesis and differentiated poorly. When cultured with fetal mouse gastric mesenchymes, the cells formed glandular structures and differentiated into surface mucous cells, but differentiation of intestinal type cells was never observed. When cultured with Matrigel, the cells formed glandular structures, and some cells differentiated into intestinal type cells in vitro. Reverse transcription–polymerase chain reaction analysis showed that the cells expressed stomach‐specific genes, and their levels decreased gradually during the culture. The cells expressed some intestine‐specific genes weakly at the start of culture, and their levels were increased with time in culture. We therefore conclude that Runx3 −/– p53 −/– gastric epithelial cells differentiate into intestinal type cells in combination with Matrigel in the absence of other cell types. Extracellular matrix, not blood cells, may play a role in the genesis of intestinal metaplasia. (Cancer Sci 2008; 99: 671–676)
Abbreviations:
- AB
alcian blue
- DMEM
Dulbecco's modified Eagle's medium
- EDTA
ethylene diamine tetracetic acid
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde 3‐phosphate dehydrogenase
- HID
high iron diamine
- IF
intrinsic factor
- Muc1
Mucin1
- Muc2
Mucin2
- PAS
periodic acid–Schiff
- PCR
polymerase chain reaction
- RT
reverse transcription
- SI
sucrase isomaltase.
Gastric cancer is one of the leading causes of cancer deaths worldwide.( 1 ) Gastric adenocarcinoma of the intestinal type is assumed to be formed in a stepwise fashion from chronic gastritis to atrophic gastritis, followed by intestinal metaplasia, leading to dysplasia and carcinoma.( 2 ) Intestinal metaplasia in the stomach represents the conversion of gastric type mucosa to a mucosa that closely resembles the intestinal one,( 3 ) and has been considered to be precancerous for gastric carcinogenesis,( 4 ) although there is controversy over whether intestinal metaplasia is a paraneoplastic change in gastric carcinogenesis.( 5 ) Several environmental factors, such as Helicobacter pylori infection,( 6 ) carcinogen treatment,( 7 ) and X‐ray irradiation,( 8 ) have been shown to induce intestinal metaplasia, but little is known about the molecular mechanism how gastric epithelial cells differentiate into intestinal type cells, and whether these factors are essential for the genesis of intestinal metaplasia. Recently, expression of Cdx2 has been demonstrated to be sufficient to induce intestinal metaplasias in the stomach in transgenic mice,( 9 , 10 ) but it remains to be solved how Cdx2 induces differentiation of intestinal type cells.
We have previously reported that Runx3, a runt domain transcription factor, is a major growth regulator of gastric epithelial cells, and that a lack of RUNX3 function is causally related to the genesis and progression of human gastric cancer.( 11 ) We have also found that expression of RUNX3 is reduced in some intestinal metaplasias in human stomachs,( 11 ) and some Runx3 −/– p53 −/– gastric epithelial cells differentiate into intestinal type cells in vivo,( 12 ) suggesting that a lack of RUNX3 function is also related to the genesis of intestinal metaplasia. Recently, several reports have indicated that blood cells play important roles in gastric carcinogenesis. Houghton et al. showed that bone marrow‐derived cells repopulated the gastric mucosa, and contributed over time to metaplasia, dysplasia, and gastric cancer in bone marrow‐transplanted mice.( 13 ) Brenner et al. reported that loss of Runx3 function induced leukocyte infiltration in the gut, and suggested that this might induce a progressive hyperplasia of the gastric mucosa.( 14 )
To examine whether Runx3 −/– p53 −/– gastric epithelial cells differentiate autonomously into intestinal type cells, or whether other cells or factors are necessary for the differentiation, we cultured the cells in combination with various mesenchymal components, and their differentiation in vitro was investigated histochemically and molecular biologically. The results indicate that Runx3 −/– p53 −/– gastric epithelial cells differentiate into intestinal type cells in vitro in the absence of other cell types.
Materials and Methods
Runx3–/–p53–/– gastric epithelial cell lines. Establishment of a GIF‐11 Runx3 −/– p53 −/– gastric epithelial cell line has been described in previous papers.( 11 , 12 ) Briefly, 16.5‐day fetal mouse gastric epithelia were separated from attaching mesenchymes using 30 mM EDTA–Hanks’ solution, and epithelial tissues from a fetus were cultured separately on collagen gel. After several passages, the cells began to grow rapidly on plastic in DMEM (Sigma‐Aldrich, St Louis, MO, USA) supplemented with 10% FBS (Sigma‐Aldrich) in a humidified atmosphere of 5% CO2 in air. The cells were subcultured by treatment with trypsin–EDTA. In the present study, GIF‐11 cells were used at passage number 40–55.
Organ culture. The differentiation and morphogenesis of the cells in vitro was examined by culturing them in combination with various mesenchymal components, as shown in Figure 1. Detailed methods for combination with fetal mouse gastric mesenchymes,( 15 ) rat tail collagen gel,( 16 ) or Matrigel (BD Biosciences, Franklin Lakes, NJ, USA)( 17 ) have been described in previous papers. Briefly, a drop of collagen or Matrigel was placed on membrane filters (HAWP; Millipore, Carrigtwohill, County Cork, Ireland), and 104 cells were seeded on it using a cloning cup. They were cultured overnight, and on the next day (day 1) they were covered with mesenchymes from 16.5‐day fetal BALB/c mice (Charles River Japan, Yokohama, Japan) or another drop of collagen gel or Matrigel. Cells were cultured at the air–liquid interface by placing the filter with cells and mesenchymal components on a stainless steel grid, which was placed in a dish. The culture medium was DMEM with 10% FBS, and was changed once a week. After 1–6 weeks, cells with mesenchymal components were processed for histological and molecular biological analyses.
Figure 1.
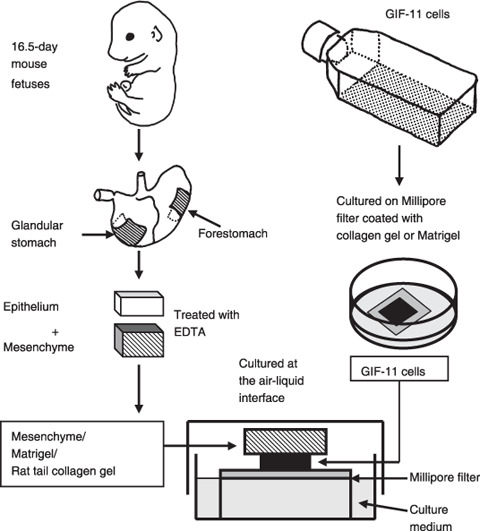
The method of organ culture. GIF‐11 cells were cultured on Millipore filters covered with a drop of collagen gel or Matrigel. The next day, the cells were covered with fetal mouse gastric mesenchymes or a drop of collagen gel or Matigel, and the cells with mesenchymal components were laid on a stainless steel grid in dishes, and were cultured at the air–liquid interface for up to 6 weeks.
Histological methods. Cells with mesenchymal components were fixed with cold 95% ethyl alcohol, embedded in paraffin, sectioned at 5 µm, and stained with PAS–hematoxylin. Some were also stained with AB–neutral red, or HID–AB to detect sulfated mucosubstances, as described previously.( 12 )
RT‐PCR. Glandular stomach and duodenal tissues were obtained from 3‐month‐old male mice. Total RNA was extracted from the cells and tissues using Trizol (Invitrogen, Carlsbad, CA, USA), according the manufacturer's instructions. Approximately 1 µg of total RNA from each tissue was reverse transcribed to cDNA using SuperScript II Reverse Transcriptase (Invitrogen), and the resulting cDNA was subjected to 21–36 cycles of PCR. The following oligonucleotide primer sets were used for the PCR reactions (expected product size in parentheses): Mucin1 (700 bp), 5′‐GGCTCAGCCACCAGTCCA‐3′ (sense) and 5′‐TGGGCAGAGGGAGGGAACT‐3′ (antisense); Sox2 (465 bp), 5′‐GCCCAGTAGACTGCACATGG‐3′ (sense) and 5′‐GCGAACTCCCTGCGAAGCG‐3′ (antisense); IF (600 bp), 5′‐GAACTGGTCACCTTCAAGC‐3′ (sense) and 5′‐AGCAGATCAACCCCTCTCA‐3′ (antisense); Mucin2 (339 bp), 5′‐ACACTCAGCACACCAACCAA‐3′ (sense) and 5′‐GGTAATCTCCTCCACCAGCA‐3′ (antisense); Cdx2 (300 bp), 5′‐AGTCGATACATCACCATCAGGA‐3′ (sense) and 5′‐AGAGAATTCTCACTGGGTGACAGTGGAGTT‐3′ (antisense); SI (376 bp), 5′‐TAGCTGCAGATGACAACCAAAC‐3′ (sense) and 5′‐TCAGGAATTCAAACTGATGGTG‐3′ (antisense); GAPDH (415 bp), 5′‐GACCACAGTCCATGCCATCAC‐3′ (sense) and 5′‐GTCCACCACCCTGTTGCTGTA‐3′ (antisense). The PCR products were separated by electrophoresis on 2.0% agarose gels in 1× TAE buffer, and detected by ethidium bromide staining. GAPDH mRNA controlled for equal sample loading.
Results
GIF‐11 Runx3–/–p53–/– gastric epithelial cells attached closely to each other on Matrigel. GIF‐11 Runx3 −/– p53 −/– gastric epithelial cells proliferated rapidly in cell culture on plastic, with an approximate doubling time of 24–30 h. They did not form a cell sheet on plastic, but individual cells often exhibited a criss‐cross pattern or piling up (Fig. 2a). When cultured on collagen gel, GIF‐11 cells showed similar morphologies as on plastic, but some cells attached closely to each other to form small cell masses (Fig. 2b). When cultured on Matrigel, almost all GIF‐11 cells soon attached very closely to each other to form cell masses where cell boundaries could not be easily found (Fig. 2c).
Figure 2.
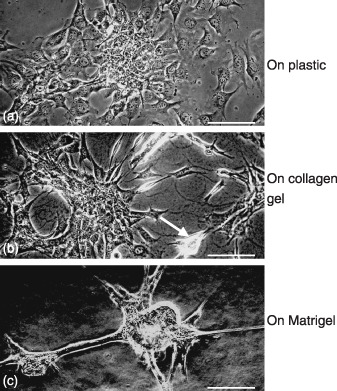
Phase contast micrographs of GIF‐11 cells cultured on (a) plastic, (b) collagen gel, and (c) Matrigel for 1 day. (b) Some cells on the collagen gel attach closely to each other to form small cell masses (arrow), (c) whereas on Matrigel, much greater cell masses are formed. Scale bars = 100 µm.
GIF‐11 cells differentiated into both gastric and intestinal type cells in organ culture. When cultured with collagen gel, GIF‐11 Runx3 −/– p53 −/– cells formed a simple cuboidal cell layer on the surface of the gel after 2 weeks (Fig. 3b), and they became stratified after 3 weeks (Fig. 3c). Some cells migrated into the gel, but none of the cells exhibited morphogenesis, and no differentiation was observed during culture, except that weak PAS‐positive materials were found between the cells (Fig. 3c).
Figure 3.
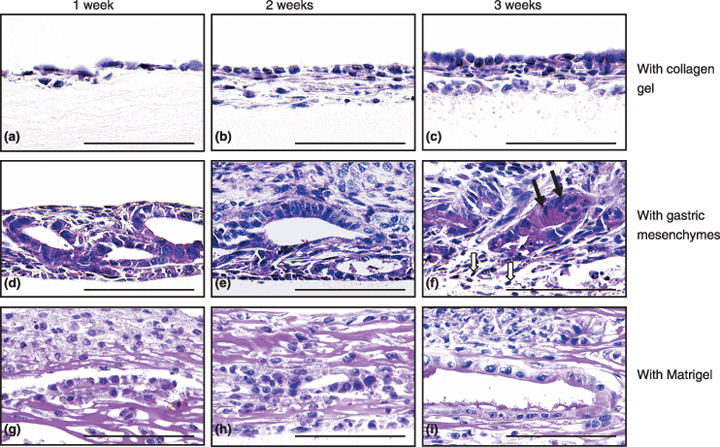
Morphogenesis and differentiation in vitro of GIF‐11 cells cultured with (a–c) collagen gels, (d–f) gastric mesenchymes, and (g–i) Matrigel, for (a,d,g) 1, (b,e,h) 2, and (c,f,i) 3 weeks. Sections are stained with periodic acid–Schiff (PAS) and hematoxylin. (f) Closed arrows show surface mucous cells characterized by large PAS‐positive materials and elongated nuclei. Some fibroblasts die during long‐term culture, and open arrows in (f) show the nuclei of dying cells. No leukocytes can be found in the mesenchyme in organ culture. Scale bars = 100 µm.
When combined with fetal gastric mesenchymes, GIF‐11 cells migrated into the mesenchymal tissue and formed glandular structures after 1 week (Fig. 3d), suggesting that fibroblastic cells are needed for their morphogenesis. The cells exhibited few differentiated characteristics at the start of culture, but after 1–2 weeks, PAS‐positive mucus was found on their luminal surface, indicating that the cells differentiated into mucus‐secreting cells (Fig. 3e). After 3 weeks, some cells differentiated into surface mucous cells with elongated nuclei and large PAS‐positive, AB‐negative, and HID‐negative mucus droplets in their cytoplasm (Fig. 3f). The cells soon degenerated, and no live cells were found after 4–5 weeks (data not shown). Differentiation of intestinal type cells was never found when GIF‐11 cells were cultured with gastric mesenchymes.
When cultured with Matrigel, some GIF‐11 cells migrated into the gel to form glandular structures after 1–2 weeks (Fig. 3g,h). The gland‐forming cells exhibited polarity, with PAS‐positive mucus localized on their apical surface after 3 weeks (Fig. 3i). They did not differentiate into typical surface mucous cells, found in cultures with gastric mesenchymes. Rather, some cells differentiated into intestinal type cells, which were first found after 4 weeks by the presence of goblet cells, with large PAS‐positive materials (Fig. 4g). Histochemical analysis showed that these granules were AB− positive (Fig. 4h) and HID− positive (Fig. 4i), indicating that the cells contained sulfated mucins, which are found in intestinal epithelia (Fig. 4d,f) but not in gastric epithelia (Fig. 4c,e). After 6 weeks, most cells contained PAS‐positive mucus (Fig. 4j), but small PAS‐positive granules were AB− negative and HID− negative, and large PAS‐positive granules were AB− positive and HID− positive, indicating that some cells differentiate into gastric type mucus cells whereas others differentiate into intestinal type goblet cells (Fig. 4j–l).
Figure 4.
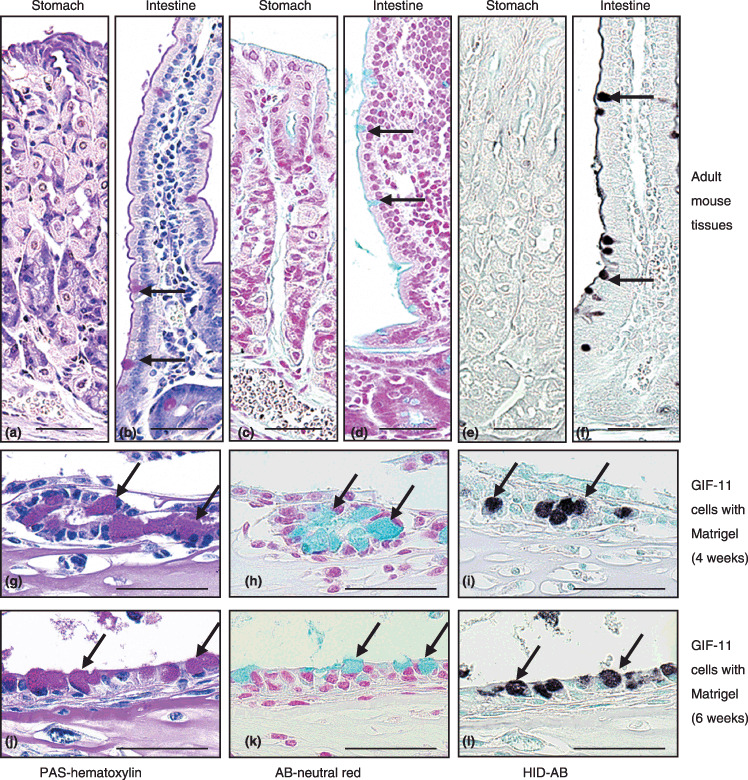
Differentiation of mucus‐containing cells in an adult mouse (a,c,e) stomach and (b,d,f) small intestine, and in GIF‐11 cells cultured with Matrigel for (g–i) 4 and (j–l) 6 weeks. Periodic acid–Schiff (PAS) and hematoxylin staining shows that (g,j) some GIF‐11 cells contain large PAS‐positive mucus droplets (arrows), (b) found specifically in goblet cells in the intestine (arrows). Alcian blue (AB)‐neutral red and high iron diamine (HID)‐AB staining shows that (h,k) the mucus droplets in GIF‐11 cells are AB‐positive (arrows) and (i,l) HID‐positive (arrows), (d,f) as is the case for mucus in goblet cells, (c,e) whereas mucus in gastric epithelial cells is negative for both stains. Scale bars = 50 µm.
RT‐PCR analysis on the differentiation of GIF‐11 cells cultured with collagen gel or Matrigel. The differentiation of GIF‐11 cells cultured with collagen gel or Matrigel was further examined by analyzing the expression of Cdx2, IF, Muc1, Muc2, SI, and Sox2. Control experiments with gastrointestinal tissues of adult mice showed that Muc1, Sox2, and IF were expressed exclusively by gastric tissues, whereas Muc2, Cdx2, and SI were expressed specifically by intestinal tissues (Fig. 5, left panel), consistent with previous reports.( 18 ) We thus used these six genes as region‐specific markers to specify how gastric epithelial cells differentiate into intestinal type cells in culture.
Figure 5.
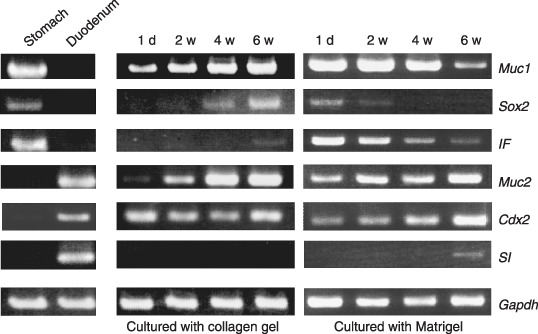
Reverse transcription–polymerase chain reaction analysis on the differentiation of GIF‐11 cells combined with collagen gel (middle panel) or Matrigel (right panel) for 1 day (1 d), 2 weeks (2 w), 4 weeks (4 w), and 6 weeks (6 w). Mucin1 (Muc1), Sox2, intrinsic factor (IF), Mucin2 (Muc2), Cdx2, and sucrase isomaltase (SI) are used as tissue‐specific markers for differentiation, as Muc1, Sox2, and IF are expressed explicitly by gastric (stomach) epithelial cells, and Muc2, Cdx2, and SI are expressed specifically by intestinal (duodenum) epithelial cells (left panel). Gapdh, glyceraldehyde 3‐phosphate dehydrogenase.
In cell culture on plastic (data not shown) and also when cultured with collagen gel, GIF‐11 cells expressed Muc1 and Cdx2 strongly at the start of culture when they proliferated rapidly. When cultured with collagen gel, the cells also expressed Sox2, IF, and Muc2, indicating that the cells exhibit intermediate characteristics between gastric and intestinal epithelial cells. The levels of Muc1, Sox2, IF, and Muc2 increased gradually during culture, whereas the level of Cdx2 did not increase (Fig. 5, middle panel). We suppose that GIF‐11 cells tend to differentiate into both gastric and intestinal type cells in culture with collagen gel, but that the cells could not differentiate fully, possibly because some essential factors are not contained in the gel.
When cultured with Matrigel, GIF‐11 cells expressed IF, Muc1, and Sox2, marker genes for gastric epithelial cells, at the start of culture, indicating that they exhibit characteristics of gastric epithelial cells when combined with Matrigel. They also expressed Muc2 strongly and Cdx2 faintly, indicating that the cells express some intestinal type genes, even when they do not exhibit any morphological characteristics of intestinal type cells. When cultured with Matrigel, the level of gastric type gene expression gradually decreased, whereas that of intestinal type gene expression increased progressively. The expression of Sox2 decreased greatly after 2 weeks, and it could not be detected after 4 weeks. In contrast, that of Cdx2 was greatly increased after 4 weeks, and reached a maximum level after 6 weeks. The expression of SI could not be detected at the start of culture, and was first noted after 4 weeks when differentiation of intestinal type cells was first noted. Its level was greatly increased after 6 weeks (Fig. 5, right panel). These results indicate that the potency to differentiate into intestinal and gastric type cells gradually increased and decreased, respectively, when the cells were cultured with Matrigel, and that factors contained in Matrigel may facilitate the differentiation of intestinal type cells.
Discussion
In the present study, we found that GIF‐11 gastric epithelial cells differentiated poorly in combination with collagen gel, but that they formed glandular structures and differentiated into surface mucous cells when combined with fetal gastric mesenchymes, and that some of them differentiated into intestinal type cells when cultured with Matrigel. The results are consistent with a previous report where Runx3 −/– p53 −/– cells did not exhibit any morphogenetic potencies and differentiated poorly when cultured with collagen gel.( 12 ) RT‐PCR analysis of GIF‐11 cells combined with Matrigel showed that the cells expressed both gastric‐specific (IF, Muc1, and Sox2) and intestine‐specific (Muc2 and Cdx2) genes at the start of culture, and that the level of gastric type genes was decreased and the level of intestinal type gene expression was increased during culture, resulting in the differentiation of intestinal type cells. The results are consistent with a previous immunohistochemical analysis that showed that intestinal type cells differentiated from GIF‐11 cells in nude mice expressed Cdx2.( 12 ) There are several papers reporting differentiation of intestinal type cells in transgenic mouse stomachs,( 9 , 10 ) but it is not clear from these previous reports whether intestinal type cells transdifferentiated from gastric type cells, or undifferentiated cells residing in the gastric stem cells exhibited intestinal type differentiation in the mouse. The present study clearly showed that GIF‐11 cells expressing gastric‐specific genes differentiate into both surface mucous cells and intestinal type cells, depending on the culture conditions. To our knowledge, this is the first study showing that intestinal type cells differentiate from gastric type stem‐like cells in vitro. Tatematsu et al. suggested that gastric and intestinal mixed type intestinal metaplasia might be formed by differentiation of stem cells that can produce both gastric and intestinal type cells.( 5 ) Thus, the characteristics of GIF‐11 cells are similar to those of the stem cells suggested by Tatematsu et al.( 5 ) GIF‐11 cells may be useful for further studies to analyze how intestinal metaplasia is generated in the stomach, and how intestinal metaplasia is related to gastric carcinogenesis.
There are several papers suggesting that bone marrow‐derived cells or leukocytes play a role in the genesis of gastric hyperplasia and gastric cancer.( 13 , 14 ) In the present study, we used GIF‐11 cells, which were established from gastric epithelial cells of a Runx3 −/– p53 −/– mouse fetus using a technique that allows complete separation of epithelial and mesenchymal tissues without cross contamination.( 19 , 20 ) Thus, the present experiment clearly shows that Runx3 −/– p53 −/– gastric epithelial cells differentiate into intestinal type cells in the absence of blood cells. Concerning intestinal metaplasia induced by loss of function of Runx3, bone marrow‐derived cells and leukocytes do not seem to play a major part.
In the present investigation, we found that GIF‐11 Runx3 −/– p53 −/– cells expressed both gastric type and intestinal type genes at the start of culture when combined with collagen gel or Matrigel. The result indicates that the cells retain intermediate characteristics between gastric and intestinal type cells. We have previously found that the expression of RUNX3 is downregulated in some cases of intestinal metaplasia,( 11 ) and that differentiation of intestinal type cells is found only in Runx3 −/– p53 −/–, but not p53 −/– gastric epithelial cells.( 12 ) We also observed ectopic expression of Cdx2 in the stomach epithelium in Runx3 −/– mice while it was never expressed in wild‐type stomachs (K. Ito et al., unpublished observation, 2004). All of these results suggest that Runx3 plays an important role in the control of intestinal type genes in gastric epithelial cells. Taniuchi et al. have shown that the Runx protein is important for lineage specification in developing lymphocytes.( 21 ) Runx3 may also play an important role in the specification of gastric epithelial cells, and loss of Runx3 may destabilize the differentiation of gastric epithelial cells. We speculate that during culture with Matrigel, expression of Cdx2 in the destablized cells increased gradually by an unknown mechanism, and its level rose above a threshold to induce differentiation of intestinal type cells after 4 weeks.
Silberg et al. found that ectopic expression of Cdx2 in the gastric epithelium is sufficient to cause transdifferentiation of gastric mucosa into intestinal type cells.( 9 ) They also found that SI was not activated in the transgenic mouse stomach, and speculated that other factors may be necessary for the full differentiation of intestinal epithelial cells. Mutoh et al. reported that all of the gastric mucosal cells except enterochromaffin‐like cells were completely replaced by intestinal type cells, including goblet cells and absorptive cells, in another transgenic mouse strain where Cdx2 was expressed in parietal cells under the control of the H + /K +‐ATPase promoter.( 10 ) In this case, parietal cells disappeared after approximately 6 weeks, and the pH in the stomach increased from 2 to more than 7. Watanabe et al. repeatedly suggested that elevation of pH in the gastric juice due to the loss of parietal cells in the fundic mucosa induced the development of intestinal metaplasia in the rat.( 22 , 23 ) Therefore, differentiation of intestinal type cells may be induced not by the expression of Cdx2, but by the loss of parietal cells in the transgenic stomach reported by Mutoh et al.( 10 ) We found in the present investigation that GIF‐11 Runx3 −/– p53 −/– cells expressed SI when cultured with Matrigel. Thus, not only the expression of Cdx2 but also the loss of Runx3 may be essential for the differentiation of intestinal type cells.
In the present study, we found that GIF‐11 gastric epithelial cells differentiated into surface mucous cells when cultured with gastric mesenchymes, but that they differentiated into intestinal type cells when combined with Matrigel. It remains to be solved why intestinal type differentiation could not be found in culture with gastric mesenchymes. It is possible that mesenchymes could not survive long culture periods up to 4 weeks when the differentiation of intestinal type cells was first noted, and that epithelial cells died before the differentiation of intestinal type cells in combination with mesenchymes. It is, however, more probable that Matrigel contains factors necessary to induce intestinal type differentiation of GIF‐11 cells, and that gastric mesenchymes secrete factors that inhibit intestinal type differentiation and promote gastric type differentiation of the cells. Consistent with this idea, we found that surface mucous cells were differentiated only when GIF‐11 cells were combined with gastric mesenchymes but not with Matrigel. Also, Tsukada et al.( 24 ) have reported the importance of gastric mesenchymes in inducing gastric epithelial differentiation in rat fetuses. Matrigel has been reported to contain various extracellular matrix and growth factors, including laminin, type IV collagen, and insulin‐like growth factor‐1.( 25 ) It is a problem for the future to identify factors that facilitate the differentiation of GIF‐11 cells into intestinal type cells.
Intestinal metaplasia is considered to be preneoplastic for gastric carcinogenesis. It is possible that metaplastic tissues are reverted to normal tissues to inhibit carcinogenesis if the deregulated system is normalized. We have previously reported that expression of RUNX3 is often silenced by hypermethylation of its promoter region in human gastric tissues.( 11 ) If the loss of Runx3 function plays an important role in the genesis of intestinal metaplasia, differentiation of metaplastic tissues into normal gastric tissues may be induced by reactivating the silenced gene by using reagents such as demethylating agents or histone deacetylation inhibitors. Further investigations are necessary to identify such chemicals that induce redifferentiation of metaplastic tissues. Our in vitro culture system would be very useful for these purposes.
Acknowledgments
We thank Ms Shigeko Shimizu for her skillful technical assistance. This work was supported by Grants‐in‐Aid from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and by the Agency for Science, Technology and Research, Singapore.
References
- 1. Pisani P, Parkin DM, Bray F, Ferlay J. Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer 1999; 83: 18–29. [DOI] [PubMed] [Google Scholar]
- 2. Yuasa Y. Control of gut differentiation and intestinal‐type gastric carcinogenesis. Nature Rev Cancer 2003; 3: 592–600. [DOI] [PubMed] [Google Scholar]
- 3. Stemmermann GN. Intestinal metaplasia of the stomach. Cancer 1994; 74: 556–64. [DOI] [PubMed] [Google Scholar]
- 4. Correa P. Human gastric carcinogenesis: a multistep and multifactorial process. Cancer Res 1992; 52: 6735–40. [PubMed] [Google Scholar]
- 5. Tatematsu M, Tsukamoto T, Inada K. Stem cells and gastric cancer: role of gastric and intestinal mixed intestinal metaplasia. Cancer Sci 2003; 94: 135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Takenaka Y, Tsukamoto T, Mizoshita T et al . Helicobacter pylori infection stimulates intestinalization of endocrine cells in glandular stomach of Mongolian gerbils. Cancer Sci 2006; 97: 1015–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tatematsu M, Furihata C, Katsuyama T et al . Independent induction of intestinal metaplasia and gastric cancer in rats treated with N‐methyl‐N′‐nitro‐N‐nitrosoguanidine. Cancer Res 1983; 43: 1335–41. [PubMed] [Google Scholar]
- 8. Watanabe H. Experimentally induced intestinal metaplasia in Wistar rats by X‐ray irradiation. Gastroenterology 1978; 75: 796–9. [PubMed] [Google Scholar]
- 9. Silberg DG, Sullivan J, Kang E et al . Cdx2 ectopic expression induces gastric intestinal metaplasia in transgenic mice. Gastroenterology 2002; 122: 689–96. [DOI] [PubMed] [Google Scholar]
- 10. Mutoh H, Hakamata Y, Sato K et al . Conversion of gastric mucosa to intestinal metaplasia in Cdx2‐expressing transgenic mice. Biochem Biophys Res Commun 2002; 294: 470–9. [DOI] [PubMed] [Google Scholar]
- 11. Li QL, Ito K, Sakakura C et al . Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell 2002; 109: 113–24. [DOI] [PubMed] [Google Scholar]
- 12. Fukamachi H, Ito K, Ito Y. Runx3 −/– gastric epithelial cells differentiate into intestinal type cells. Biochem Biophys Res Commun 2004; 321: 58–64. [DOI] [PubMed] [Google Scholar]
- 13. Houghton J, Stoicov C, Nomura S et al . Gastric cancer originating from bone marrow‐derived cells. Science 2004; 306: 1568–71. [DOI] [PubMed] [Google Scholar]
- 14. Brenner O, Levanon D, Negreanu V et al . Loss of Runx3 function in leukocytes is associated with spontaneously developed colitis and gastric mucosal hyperplasia. Proc Natl Acad Sci USA 2004; 101: 16 016–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fukamachi H, Mizuno T, Kim YS. Gland formation of human colon cancer cells combined with foetal rat mesenchymes in organ culture: an ultrastructural study. J Cell Sci 1987; 87: 615–21. [DOI] [PubMed] [Google Scholar]
- 16. Fukamachi H, Kim YS. Glandular structure formation of LS174T human colon cancer cells cultured with collagen gels. Dev Growth Differ 1989; 31: 107–12. [DOI] [PubMed] [Google Scholar]
- 17. Fukamachi H, McLachlan JA. Proliferation and differentiation of mouse uterine epithelial cells in primary serum‐free culture: Estradiol‐17‐β‐suppresses uterine epithelial proliferation cultured on a basement membrane‐like substratum. In Vitro Cell Dev Biol 1991; 27: 907–13. [DOI] [PubMed] [Google Scholar]
- 18. Kim BM, Buchner G, Miletich I et al . The stomach mesenchymal transcription factor Barx1 specifies gastric epithelial identity through inhibition of transient Wnt signaling. Dev Cell 2005; 8: 611–22. [DOI] [PubMed] [Google Scholar]
- 19. Fukamachi H, Ichinose M, Ishihama S et al . Fetal rat glandular stomach epithelial cells differentiate into surface mucous cells which express cathepsin E in the absence of mesenchymal cells in primary culture. Differentiation 1994; 56: 83–9. [DOI] [PubMed] [Google Scholar]
- 20. Matsubara Y, Ichinose M, Yahagi N et al . Hepatocyte growth factor activator: a possible regulator of morphogenesis during fetal development of rat gastrointestinal tract. Biochem Biophys Res Commun 1998; 253: 477–84. [DOI] [PubMed] [Google Scholar]
- 21. Taniuchi I, Osato M, Egawa T et al . Differential requirements for Runx proteins in CD4 repression and epigenetic silencing during T lymphocyte development. Cell 2002; 111: 621–33. [DOI] [PubMed] [Google Scholar]
- 22. Watanabe H, Naito M, Kawashima K, Ito A. pH‐related differentiation in the epithelia of the gastric mucosa of postnatal rats. Acta Path Jap 1985; 35: 569–76. [DOI] [PubMed] [Google Scholar]
- 23. Watanabe H, Okamoto T, Fudaba Y et al . Influence of gastric pH modifiers on development of intestinal metaplasia induced by X‐irradiation in rats. Jap J Cancer Res 1993; 84: 1037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tsukada S, Ichinose M, Yahagi S et al . Induction of precocious pepsinogen synthesis by glucocorticoids in fetal rat gastric epithelium in organ culture: Importance of mesenchyme for epithelial differentiation. Differentiation 1998; 62: 239–47. [DOI] [PubMed] [Google Scholar]
- 25. Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Sem Cancer Biol 2005; 15: 378–86. [DOI] [PubMed] [Google Scholar]


