Abstract
l‐type large amino acid transporter (LAT) 1, the first light chain (lc) of cluster of differentiation 98 (CD98) to be identified, is associated with the heavy chain (hc) of CD98 and expressed on the surface of various tumor cells irrespective of their origin. Because LAT1 is a 12‐pass membrane protein and its possible immunogenic extracellular region is very small, specific monoclonal antibodies (mAb) had not been developed. We report the successful preparation and characterization of mAb recognizing the extracellular domain of human LAT1 protein. Two mAb were selected from hybridoma clones established by fusing mouse myeloma cells and spleen cells from rats immunized against RH7777 rat hepatoma cells expressing recombinant green fluorescent protein fused to human LAT1 protein. Designated SOL22 and SOL69, these mAb specifically reacted with the extracellular domain of LAT1 on cells transfected with cDNA of LAT1, but not with cells transfected with cDNA of other CD98 lc, namely, LAT2, y+LAT1, y+LAT2, and xCT amino acid transporters. These mAb immunoprecipitated 35‐ and 90‐kDa proteins under reducing conditions in extracts prepared from human HeLa tumor cells, indicating the existence of intermolecular disulfide bonds between cysteine residues in the 90‐kDa hc and 35‐kDa lc (LAT1). SOL22 and SOL69 mAb reacted with a wide variety of living unfixed human tumor cell lines, but were only weakly reactive with HEK293F human embryonic kidney cells and human peripheral blood cells. Comparative immunohistochemical analyses of normal human tissues with anti‐CD98 hc and anti‐LAT1 revealed LAT1 to be an excellent molecular target for antibody therapy, possibly even superior to CD98 hc. (Cancer Sci 2008; 99: 1000–1007)
- Abbreviations: CD98
cluster of differentiation 98
- BSA
bovine serum albumin
- ELISA
enzyme‐linked immunosorbent assay
- FBS
fetal bovine serum
- FITC
fluorescein isothiocyanate
- GAPDH
glyceraldehyde‐3‐phosphate dehydrogenase
- GFP
green fluorescent protein
- hc
heavy chain
- LAT
l‐type amino‐acid transporter
- lc
light chain
- mAb
monoclonal antibody
- MFI
mean fluorescence intensity
- PBS
phosphate‐buffered saline
- PE
phycoerythrin
- PFA
paraformaldehyde
- qRT‐PCR
quantitative reverse transcription–polymerase chain reaction
- SDS
sodium dodecylsulfate
- SLC
solute carrier
- T‐PBS
phosphate‐buffered saline containing 0.05% Tween 20.
Cluster of differentiation 98/4F2 is a heterodimeric protein with a relative molecular mass of 125 000 (GP125), comprising a 90‐kDa hc and 35‐kDa lc.(
1
,
2
,
3
) CD98 was originally identified as a cell‐surface antigen associated with the activation of lymphocytes(
2
) and is expressed on the basal layer of the squamous epithelium and a wide variety of tumors,(
4
) suggesting its functional involvement in lymphocyte activation, cell proliferation, and malignant transformation. In fact, mAb against rat and human CD98 hc inhibits the activation of lymphocytes and proliferation of tumor cells.(
5
,
6
) In addition, NIH3T3 and Balb3T3 cells transfected with cDNA of human and rat CD98 hc have shown various malignant phenotypes.(
7
,
8
,
9
) CD98 lc have been revealed to be amino acid transporters,(
3
,
10
) and multiple functions of CD98 hc, such as cell fusion,(
11
) regulation of β1 integrin activation,(
12
) and induction of apoptosis,(
13
) have been demonstrated. Transporters corresponding to the amino acid transport system L, y+L, 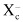 , and Asc have been shown to be CD98 lc, which require CD98 hc for their membrane‐based expression.(
3
,
9
,
14
) Six amino acid transporters (LAT1, LAT2, y+LAT1, y+LAT2, Asc‐1, and xCT) that belong to the SLC7 family, have been identified to be CD98 lc, and all CD98 lc are believed to be sorted to the plasma membrane via association with CD98 hc.(
15
,
16
,
17
,
18
,
19
,
20
,
21
)
, and Asc have been shown to be CD98 lc, which require CD98 hc for their membrane‐based expression.(
3
,
9
,
14
) Six amino acid transporters (LAT1, LAT2, y+LAT1, y+LAT2, Asc‐1, and xCT) that belong to the SLC7 family, have been identified to be CD98 lc, and all CD98 lc are believed to be sorted to the plasma membrane via association with CD98 hc.(
15
,
16
,
17
,
18
,
19
,
20
,
21
)
l‐type amino‐acid transporter 1 is a 12‐membrane pass non‐glycosylated protein that was first identified as CD98 lc associated with CD98 hc glycoprotein, and mediates Na+‐independent large amino acid transport (system L).( 3 , 22 ) It is reported that mRNA of LAT1 is expressed widely on tumor cells in addition to in the testis, ovary, and brain.( 3 , 23 , 24 , 25 ) However, because specific mAb recognizing the extracellular domain of native human LAT1 protein have not been obtained until now, the precise expression profile of LAT1 protein in normal and cancer cells remains unsolved. In the present paper, we report the successful production of specific mAb against human LAT1 protein, and discuss the specificity and usefulness of anti‐LAT1 mAb in cancer therapy.
Materials and Methods
Cell culture. Human leukemia cells (Molt‐4, Jurkat, Daudi, Raji, CCRF‐SB, K562, and U937), mouse myeloma cells (P3 × 63Ag8.653), and peripheral blood leukocytes from healthy volunteers were cultured in RPMI‐1640 medium (Sigma‐Aldrich, St Louis, MO, USA). Human tumor cell lines from the tongue (HEp2), larynx (HSC‐3), lung (A549), esophagus (TE‐3), breast (MCF‐7, SK‐Br‐3, ZR‐75–1, and MDA‐MB‐453), liver (HepG2, Hep3B, and HLF), pancreas (PK‐1 and PaCa‐1), stomach (MKN‐7 and MKN‐45), colon (SW1116, HT29, DU145, and LS‐174T), cervix (HeLa and ME180), prostate (PC‐3), kidney (ACHN and TOS‐1), and bladder (T24, J82, KU‐1, KK47, and MGH‐U1), glioblastoma cells (KNS‐42), melanoma cells (SK‐MEL‐37), neuroblastoma cells (Tagawa), HEK293F human embryonic kidney cells (Invitrogen, Carlsbad, CA, USA), Int407 embryonic intestine cells, RH7777 rat hepatoma cells (kindly donated by Mitsubishi Tanabe Pharma, Yokohama, Japan), and RenCa mouse renal carcinoma cells were cultured in Dulbecco's modified Eagle's medium (Sigma‐Aldrich). Cells were cultured in the media supplemented with 7% heat‐inactivated FBS (ICN Biomedicals, Aurora, OH, USA) in humidified CO2 incubators.
Animals. Female F344/N rats and male KSN nude mice, 6–8 weeks old, were purchased from Shimizu Animal Farm (Kyoto, Japan), and housed in a controlled environment at 22°C.
Establishment of cell lines expressing GFP‐fused human LAT1 protein. GFP was fused genetically to the carboxy‐teminus of human LAT1 in a pAcGFP expression vector (BD Biosciences, Mountain View, CA, USA), and RH7777 rat hepatoma cells or HEK293F human embryonic kidney cells were transfected with LAT1‐GFP plasmids using Lipofectamine 2000 (Invitrogen), as suggested by the manufacturer, selected using culture media containing G418 (400 µg/mL; Nakalai, Kyoto, Japan), and clone‐sorted for cellular green fluorescence using a JSAN cell sorter (Bay Bioscience, Kobe, Japan).
Hybridomas. Female F344/N rats received subcutaneous and intraperitoneal injections (first and second immunizations) followed by a final intravenous injection of RH7777 cells expressing human GFP‐LAT1 (2.0 × 107) in each immunization at 3‐week intervals. The immune spleen cells (1.0 × 108) were fused with P3 × 63Ag8.653 mouse myeloma cells (3.0 × 107) using 50% polyethylene glycol 1540 (Roche, Penzberg, Germany). After the cell fusion, hybridoma cells were selected in RPMI‐1640 medium supplemented with 50× hypoxanthine, aminopterin, and thymidine (Invitrogen). Cell fusion was repeated three times with the use of freeze‐stocked spleen cells of immunized rats. Hybridomas were screened by testing antibodies in the culture supernatants by flow cytometry as described below, and were cloned by the limiting‐dilution method. Hybridoma cell clones (5.0 × 106) termed SOL22 and SOL69 were injected intraperitoneally into male KSN nude mice pretreated with 2,6,10,14‐tetramethylpentadecane (Pristane; Wako, Osaka, Japan). Ascites fluid was collected 10–20 days after the inoculation, and precipitated using 50%‐saturated ammonium sulfate. Further purification was carried out with Protein G sepharose (BD Healthcare, Uppsala, Sweden), because both mAb were determined to be IgG, with the analysis of the ascites fluid by SDS‐polyacrylamide gel electrophoresis followed by protein staining with Coomassie Brilliant Blue (ICN Biomedicals).
Flow‐cytometric analysis. Cells (3.0 × 105 in each sample), which were unfixed or fixed with PBS containing 4% PFA for 10 min, were suspended in 50 µL PBS containing 1% BSA (ICN Biomedicals), and mixed with an equal volume of purified mAb (10 µg/mL) for 1 h on ice. After being washed with PBS, cells were labeled with PE‐conjugated donkey antirat IgG (Jackson ImmunoResearch, West Grove, PA, USA) or FITC‐conjugated rabbit antirat immunoglobulin (DakoCytomation, Kyoto, Japan) for the screening of hybridomas, or FITC‐ or PE‐conjugated goat antirat IgG Fcγ for the selected mAb for 30 min on ice. After the washing of cells with PBS, the fluorescence intensity of individual cells was determined using a BD‐LSR flow cytometer (Becton‐Dickinson, Sunnyvale, CA, USA).
Two‐color immunostaining of cultured cells. Cells cultured in each well of eight‐well chamber slides (Falcon, Franklin Lakes, NJ, USA) were fixed with PBS containing 4% PFA for 10 min and treated sequentially with mouse and rat mAb (10 µg/mL in each) and 1:200‐diluted species‐specific FITC‐conjugated goat antimouse IgG (Jackson ImmunoResearch), with or without 1:200‐diluted species‐specific Texas Red‐conjugated goat antirat IgG (Jackson ImmunoResearch) in 1% BSA–PBS. Between each step, the cells were washed three times with PBS, and cell‐bound immunofluorescence was observed under a DMR fluorescent microscope (Leica Microsystems, Osaka, Japan).
Immunostaining of tissue sections. Human tonsil tissue (kindly donated by a laboratory student with IgA nephropathy at the surgical excision of a tonsil, and to otherwise be discarded) was fixed in 4% PFA in PBS and treated with sucrose solutions of gradually increasing strength (5–30%). Tissue sections (5 µm thick) were prepared using a CM1800 cryostat (Leica Microsystems), treated with Block Ace (Dainippon, Osaka, Japan) for 1 h, and incubated with purified mAb (10 µg/mL) diluted in 1% BSA–PBS overnight at room temperature. After a wash with PBS, endogenous peroxidase activity was inhibited by immersing sections in 3% H2O2–methanol for 5 min. After rinsing in PBS, the sections were incubated with biotinylated rabbit antirat IgG (Vector Laboratories, Burlingame, CA, USA) diluted 1:200 in 1% BSA–PBS for 1 h. After three washes with PBS, samples were treated with ABC reagent (Vector Laboratories) diluted 1 : 100 in 0.1% BSA–PBS for 45 min. After three more washes with PBS, the sections were incubated with 0.05% 3,3′‐diaminobenzidine (Dojin Chemicals, Kumamoto, Japan) and 0.01% H2O2 in 0.1 M Tris‐HCl (pH 7.4), and then counterstained with Methylgreen (Merk, Darmstadt, Germany). Samples were dehydrated with ethanol, cleared in xylene, and mounted in Permount (Fisher Scientific, Fair Lawn, NJ, USA). The location of antibody‐defined components was observed under an Axiolab microscope (Zeiss, Thornwood, NY, US) and photographed.
Cell‐surface biotin labeling of tumor cells and immunoprecipitation. Cells (5.0 × 106) were suspended in PBS (pH 8.0) containing 0.5 mg/mL sulfosuccinimidyl‐6′‐(biotinamide)‐6‐hexanamide hexanoate (EZ‐Link sulfo‐NHS‐LC‐LC‐Biotin; Pierce, Rockford, IL, USA), and incubated for 30 min at room temperature. Cells were washed with PBS three times, and treated with lysis buffer comprising 1% Nonidet P‐40, protease inhibitor cocktail (Nakalai, Nara, Japan), and benzonase (Merck, Whitehouse Station, NJ, USA) in PBS for 60 min on ice. Following centrifugation at 10 000g for 20 min, the supernatant was collected as the biotinylated cell lysate. Soluble antigens in the cell lysates were immunoprecipitated with primary antibody and rabbit antirat immunoglobulins (DakoCytomation) as secondary antibody followed by protein G sepharose 4 fast flow (GE Healthcare, Uppsala, Sweden). After the antigen‐bound sepharose beads were washed with lysis buffer, antigens were dissociated by the incubation of sepharose beads with SDS sample buffer for 5 min at 95°C. After centrifugation, antigens in the supernatant were examined by SDS‐polyacrylamide gel electrophoresis and transferred onto polyvinylidenefluoride membranes. The membranes were incubated with ABC elite reagent (Vector Laboratories). Blots were visualized with the use of 0.05% 3,3′‐diaminobenzidine (Wako) and 0.01% H2O2 in 0.1 M Tris‐HCl (pH 7.4).
Sandwich‐type ELISA. Aliquots (50 µL) of capturing antibodies (HH25 antihuman CD98hc human mAb, 10 µg/mL) were adsorbed passively in triplicate in the wells of polyvinylchrolide 96‐well plates (Sumitomo Bakelite, Tokyo, Japan) overnight at 4°C. Each well was treated with Block Ace for 3 h at 37°C to inhibit non‐specific binding of the staining reagents. HeLa cell lysate (50 µL; 3.0 × 107 cells/mL) containing detecting mAb (10 µg/mL) was added to each well and incubated overnight at 4°C. Following intensive washing with PBS containing 0.05% T‐PBS, 1:1000‐diluted species‐specific biotinylated donkey antimouse or antirat IgG (Jackson Immunoresearch) was added and incubated for 60 min at room temperature. After a wash with T‐PBS, 1:200‐diluted Elite ABC reagent in 0.1% BSA–PBS was added to each well, and the plates were incubated for 45 min at 37°C. Each well was washed intensively with T‐PBS and supplemented with 0.1 M citric‐acetate buffer (pH 6.0) containing 3,3′,5,5′‐tetramethylbenzidine (0.1 mg/mL) and 0.01% H2O2 (50 µL in each well). Color development in the wells was stopped after 5–10 min by the addition of 0.5 M H2SO4 (75 µL in each well), and the optical density of the solution at 450 nm was measured with a Model 550 microplate reader (Bio‐Rad, Hercules, CA, USA).
Real‐time qRT‐PCR. Total RNA was isolated from cells (5.0 × 106 cells) using an RNeasy Mini Kit (Qiagen, Tokyo, Japan), and cDNA was obtained from 1–5 µg of total RNA using a First‐Strand cDNA Synthesis kit (GE Healthcare). Real‐time qRT‐PCR was carried out with a PRISM 7700 (Applied Biosystems, Tokyo, Japan). Expression of LAT1 mRNA was normalized to the expression of GAPDH mRNA in each cell line.
Downregulation of LAT1 and CD98 hc protein expression in human cancer cells on treatment with mAb. HeLa cells were cultured without mAb, or with HBJ127, SOL22, or SOL69 for 24 h, and stained with SOL69 followed by FITC‐conjugated goat antirat IgG Fcγ (Jackson ImmunoResearch). Reactivity was analyzed using a BD‐LSR flow cytometer and data were shown by MFI, or observed under a DMR fluorescent microscope.
Results
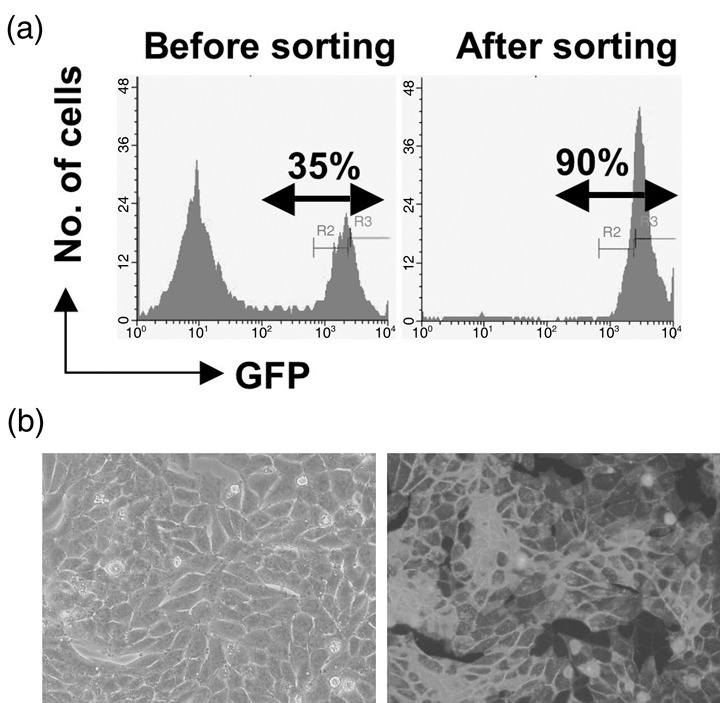
Production of mAb recognizing human LAT1 protein. LAT1, which transports neutral large amino acids, is significantly involved in the proliferation of tumor cells.( 26 ) Thus, LAT1 seems to be a promising molecular target for cancer therapy using mAb. In the present investigation, we have used a human LAT1‐expressing rat cell line as an immunogen, and the rat as an immunized animal. The total number of rat B cells is several times larger than that of the mouse, and the capacity to generate the antibody diversity of the rat is superior to that of mouse (Y. Kurosawa, pers. comm. 2007), although the composition of immunoglobulin genes between these two species is very similar.( 27 ) RH7777 rat hepatoma cells were transfected with cDNA of GFP‐fused human LAT1. Cells expressing higher levels of GFP‐LAT1 protein were concentrated by clone sorting, and the expression of LAT1‐GFP proteins was observed in almost all clone‐sorted transfectant cells (more than 90% of the total; Fig. 1a) and was characteristically located on the plasma membrane (Fig. 1b). Female F344/N rats were immunized with these GFP‐LAT1‐expressing RH7777 cells, and the rat showing the highest antibody titer was further boosted with transfectant cells, and polyethylene glycol‐mediated cell fusions were carried out to generate hybridoma cultures. In the present investigation, antibodies were assessed for reactivity with a 1:1 mixture of parent RH7777 cells and GFP‐LAT1‐expressing RH7777 cells to evaluate exactly the specificity of antibodies. Two hybridoma clones, designated SOL22 (γ2a, κ) and SOL69 (γ2a, κ), were selected from the screening of approximately 3000 wells of hybridoma cultures for a negative reaction to RH7777 cells and positive reaction to RH7777 cells expressing GFP‐human LAT1 protein, by indirect immunofluorescence followed by flow‐cytometric analysis. Both SOL22 and SOL69 reacted specifically with GFP‐LAT1‐expressing cells in a GFP expression‐dependent manner; however, the two mAb did not react with RH7777 cells expressing GFP‐CD44 (Fig. 2a). Furthermore, these two mAb also selectively reacted with RenCa mouse renal carcinoma cells transfected with GFP‐LAT1 (data not shown), demonstrating that SOL22 and SOL69 recognize human LAT1 protein.
Figure 1.
Establishment of an RH7777 rat hepatoma cell line expressing green fluorescent protein (GFP)‐human l‐type amino‐acid transporter (LAT) 1. (a) RH7777 cells were transfected with GFP‐human LAT1 cDNA using Lipofectamine 2000. The transfected cells were cultured in Dulbecco's modified Eagle's medium containing 7% fetal bovine serum and 400 µg/mL G418 for 2 weeks (left), and cells strongly expressing GFP‐human LAT1 proteins were clone sorted and expanded (right). (b) Phase‐contrast micrographs (left) and micrographs of ultraviolet‐excited green‐colored GFP‐LAT1 proteins (right) of clone‐sorted cells.
Figure 2.
Specific binding of the monoclonal antibodies (mAb) SOL22 and SOL69 to human l‐type amino‐acid transporter (LAT) 1. (a) HEK293F cells were transfected with cDNA of green fluorescent protein (GFP)‐human CD44 (left) or GFP‐human LAT1 (right), and were stained with SOL22 (upper panel) or SOL69 (lower panel), followed by phycoerythrin (PE)‐conjugated anti‐rat IgG Fcγ. (b) HEK293F cells were mock‐transfected or transfected with cDNA of GFP‐fused human cluster of differentiation 98 (CD98) heavy chain and various human CD98 light chains, and were stained with anti‐CD98 heavy chain rat mAb (HR35), SOL22, or SOL69 with PE‐conjugated anti‐rat IgG Fcγ.
Specificity of SOL22 and SOL69 mAb. We examined the precise specificity of mAb against various CD98 lc proteins, namely, LAT1, LAT2, y+LAT1, y+LAT2, and xCT. GFP‐tagged cDNA of five kinds of lc were transfected into HEK293F cells, and the reactivity of mAb against GFP‐tagged lc proteins transiently expressed on the surface of HEK293F cells was assessed by flow cytometry (Fig. 2b). Although SOL22 and SOL69 definitely reacted with cells expressing GFP‐LAT1 protein, neither mAb reacted with cells expressing other GFP‐CD98 lc proteins, demonstrating that both SOL22 and SOL69 specifically recognize LAT1 protein. Next, we examined the effect of overexpression of GFP‐CD98 hc protein on the reactivity of SOL22 and SOL69 with HEK293F cells (Fig. 2b). Transfection of GFP‐CD98 hc cDNA into HEK293F cells did not result in greater reactivity of SOL22 or SOL69, suggesting that the amount of endogenous CD98 hc protein in HEK293F cells is sufficient to bind and carry endogenous LAT1 protein to the cell surface.
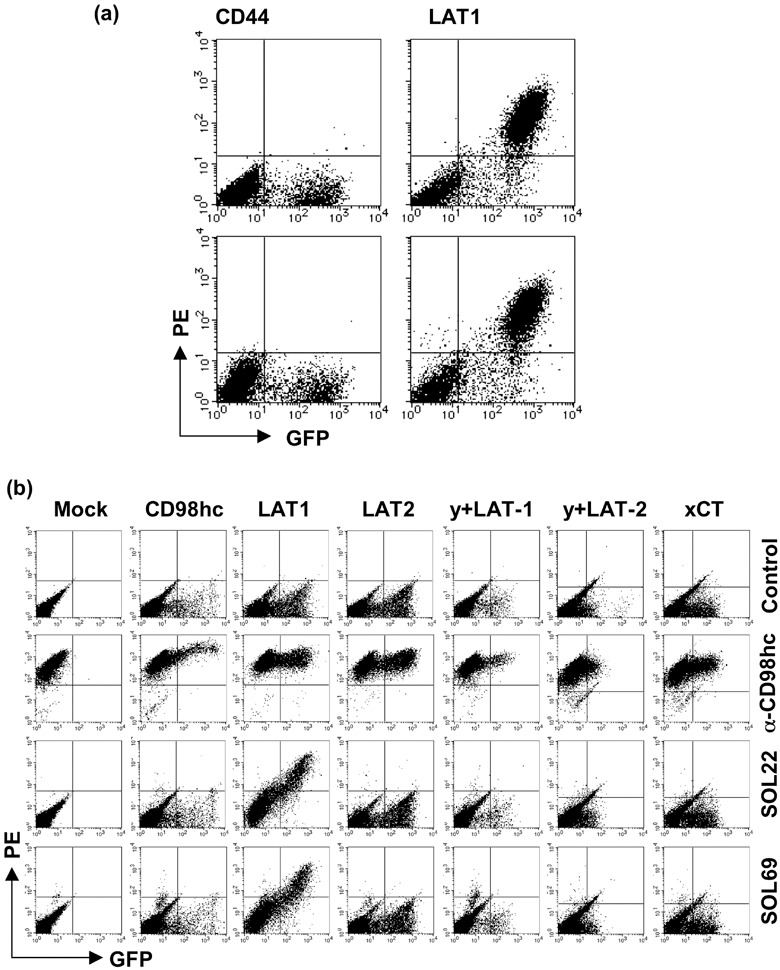
Expression of LAT1 in various human cell lines. We examined the reactivity of SOL22 and SOL69 with HeLa cells, the LAT1 mRNA of which is reported to be expressed at a high level( 26 ) and was confirmed in the present study (Fig. 3c). SOL22 and SOL69 definitely reacted with unfixed living HeLa cells (Fig. 3a), demonstrating that these mAb recognize native LAT1 protein, which is not fused to GFP. We next examined the reactivity of the SOL22 mAb with various human cell lines by flow cytometry, and showed reactivity with various cells based on the values of subtracted MFI obtained from the flow‐cytometric analysis. Although SOL22 reacted with various unfixed human cell lines (Fig. 3b, left), it did not react with mouse, rat, or chicken tumor cell lines (data not shown), suggesting that the mAb specifically recognizes the extracellular domain of human LAT1 protein. In addition, SOL22 also reacted with PFA‐fixed cells and reactivity was increased in many of the cell lines (Fig. 3b, right). In particular, SOL22 strongly reacted with unfixed living carcinoma cells (ZR‐75‐1 and MCF‐7 breast carcinomas, Hep3B hepatoma, PaCa‐1 pancreas carcinoma, ME180 and HeLa cervix carcinomas). K562, Daudi, and U937 leukemia cells also expressed high levels of LAT1 protein, as assessed by the immunostaining with SOL22. Almost the same result was obtained with the staining of tumor cells by SOL69 (data not shown). These results show that human LAT1 protein is expressed on the cell surface in various human cancer cells, irrespective of their origin. To substantiate the expression of LAT1 protein in various human cell lines, real‐time qRT‐PCR was carried out (Fig. 3c). The quantity of LAT1 mRNA in several cell lines was standardized using GAPDH and compared with that in HEK293F cells, because HEK293F cells express LAT2 bearing the function of amino acid transport instead of LAT1.( 17 ) In ZR‐75‐1, MCF‐7, ME180, and HeLa cells, the expression of LAT1 protein was well correlated with the expression of mRNA in these cells. In some cell lines, such as ACHN renal carcinoma and CCRF‐SB leukemia cells, however, reactivity with mAb was considerably weak, as expected from the LAT1 mRNA expression. However, the reactivity of anti‐LAT1 mAb with these tumor cells was elevated to levels comparable to that of the mRNA by the PFA fixation of these tumor cells.
Figure 3.
Expression of l‐type amino‐acid transporter (LAT) 1 protein and mRNA in various human cell lines. (a) Unfixed HeLa cells, and (b) unfixed and paraformaldehyde (PFA)‐fixed human cell lines were stained with SOL22, followed by fluorescein isothiocyanate (FITC)‐conjugated anti‐rat IgG Fcγ, and analyzed by flow cytometry. Dotted lines show control cells without the first monoclonal antibody (mAb), while solid lines show cells stained with mAb (a). A statistical analysis was carried out using CellQuest software, and mean fluorescence intensity (MFI) was subtracted from values without the first antibody (b). (c) Real time quantitative reverse transcription–polymerase chain reaction was carried out. The expression of LAT1 mRNA was normalized to the expression of glyceraldehyde‐3‐phosphate dehydrogenase mRNA in each cell line.
Expression of LAT1 protein in human normal cells and tissues. We next examined the reactivity of mAb with human peripheral blood leukocytes (Fig. 4a). SOL22 reacted weakly with monocytes, whereas SOL69 reacted only faintly with monocytes and granulocytes. In this context, CD98 hc is reported to be expressed most abundantly on the surface of monocytes in peripheral blood cells. The differences in reactivity of the two anti‐LAT1 mAb against leukocytes might be due to a difference in the LAT1 protein epitope that they recognize, as positive reactions were observed with the combination of SOL22 and SOL69, but were not observed with the combination of SOL22 and SOL22, or SOL69 and SOL69 in sandwich‐type ELISA. These two mAb did not react with resting small lymphocytes at all, and of interest, they did not react with lymphocytes irrespective of the activation of cells with TPA and A23187 or anti‐CD3 mAb. The excellent specificity of the anti‐LAT1 mAb was further verified with an immunohistochemical analysis using a human tonsil tissue (Fig. 4b). The anti‐LAT1 mAb faintly or insignificantly stained the epithelial tissue of the tonsil, and did not stain lymphoblastoid cells in the germinal center of the tonsil tissue at all, although anti‐CD98 hc mAb definitely stained lymphoblastoid cells in this region, in addition to cells in the basal layer of the epithelium. These results from the immunostaining of human normal cells strongly indicate that LAT1 is an excellent molecular target for cancer therapy, as a single molecule or as a molecular complex associated with CD98 hc, as we have demonstrated that the association between exogenous CD98 hc and endogenous CD98 lc at the cell surface is necessary for the anchorage‐independent growth induced by the transfection of CD98 hc.( 7 , 8 , 9 ) In contrast to the extremely weak expression of LAT1 in normal cells, LAT1 is expressed strongly on a wide variety of human cancer cells (Fig. 4b). Contrary to LAT1, expression of LAT2 on the cell surface in many normal tissues is reported.( 28 )
Figure 4.
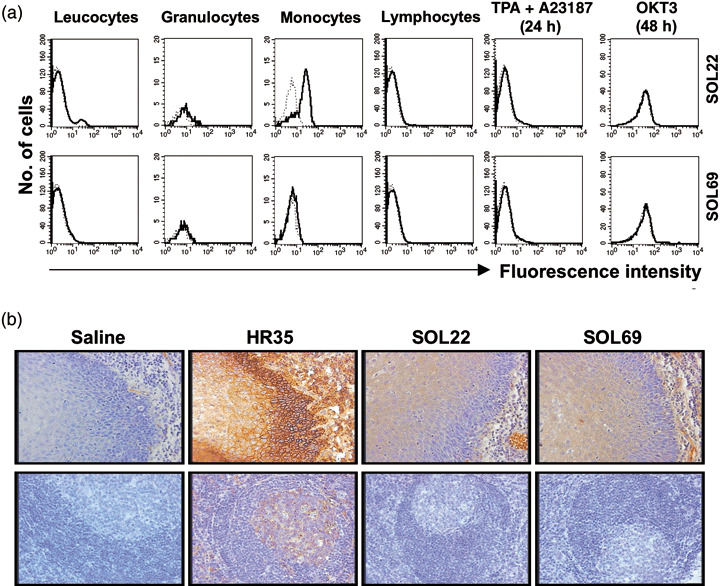
Reactivity of monoclonal antibodies (mAb) with human normal cells and tissues. (a) Peripheral blood leucocytes (PBL) was isolated by Ficoll gradient and cells were stained with SOL22 or SOL69 with fluorescein isothiocyanate‐conjugated anti‐rat IgG Fcγ. The population of lymphocytes, monocytes, and granulocytes was gated by forward and side scatters from the total PBL population. Activated lymphocytes were obtained from lymphocytes cultured with TPA and A23187 for 24 h, or with OKT3 for 48 h. (b) Human tonsil tissues were stained with anti‐cluster of differentiation 98 heavy chain mAb, SOL22, or SOL69 mAb by an immunoperoxidase method, and nuclei were counterstained with hematoxylin.
Colocalization of mAb‐defined antigen with CD98 hc. Because LAT1 was reported to be located on the cell surface by forming a heterodimer with CD98 hc, we compared the localization of SOL22‐ and SOL69‐defined antigens with CD98 hc (Fig. 5a). These mAb‐defined antigens were located on the cell membrane of HeLa cells (green), and mAb‐defined antigens were shown to colocalize with CD98 hc (red) on the cell membrane (merge). Moreover, the association of mAb‐defined antigens and CD98 hc was demonstrated by sandwich‐type ELISA using an anti‐CD98 hc mAb, combined with SOL22 and SOL69 (Fig. 5b). Antigens captured with the anti‐CD98 hc human mAb (HH25) in the lysate of HeLa cells were detected by the anti‐CD98 hc rat mAb (HR35) recognizing a different epitope from the antigen‐capturing mAb. Importantly, positive reactions were also observed with the combination of anti‐CD98 hc and SOL22 or SOL69, indicating that the mAb‐defined antigen was CD98 lc (LAT1) associated with CD98 hc. Next, the molecular weights of mAb‐defined antigens were determined in an immunoprecipitation experiment, because a specific protein band was not detected by western blotting with the mAb. As shown in Figure 5c, SOL22 and anti‐CD98hc mAb (4F2) immunoprecipitated 90‐ and a 35‐kDa proteins in extracts prepared from cell surface‐biotinylated HeLa tumor cells under reducing conditions. In this experiment, a sharp 35‐kDa band with SOL22 and a broad 35–40‐kDa band with the 4F2 mAb were detected, suggesting the association of CD98 hc with LAT1 and other CD98 lc in HeLa cells. By the immunoprecipitation with SOL22 or 4F2 followed by immunoblotting with anti‐CD98 hc mAb (HH25), an approximately 90‐kDa CD98 hc protein was detected, suggesting an association between CD98 hc and SOL22‐defined LAT1 (Fig. 5d). All of these results have shown that the mAb SOL22 certainly recognizes human LAT1 protein associating with CD98 hc.
Figure 5.
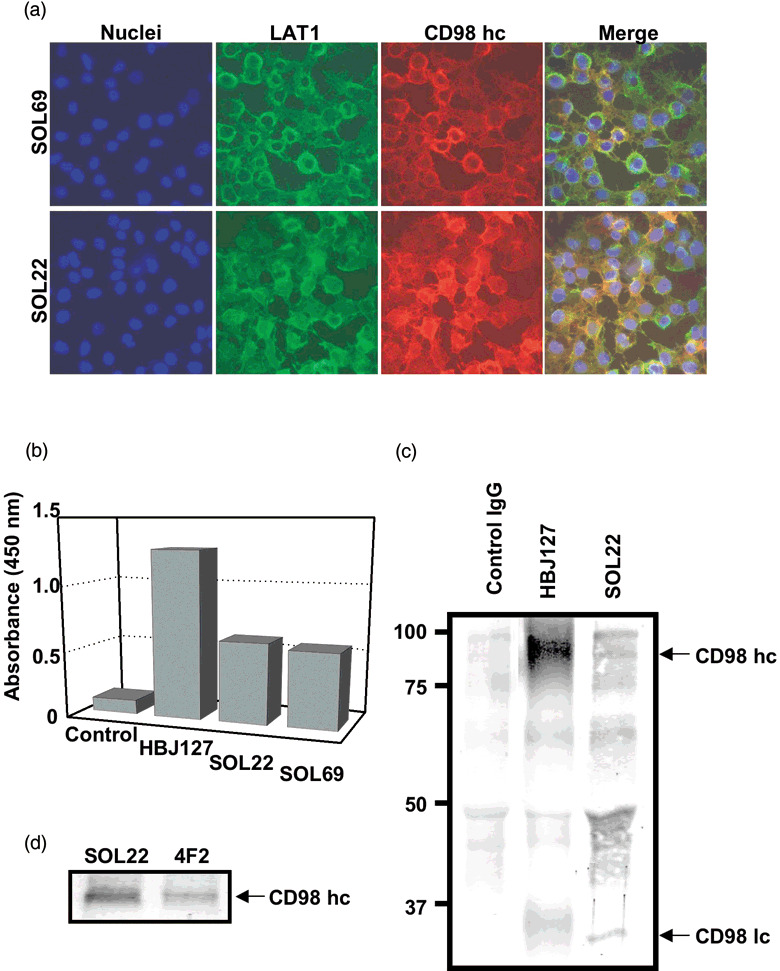
Association of cluster of differentiation 98 (CD98) heavy chain (hc) with the antigen recognized by SOL22 and SOL69. (a) HeLa cells were stained with SOL22 (upper) or SOL69 (lower) with species‐specific fluorescein isothiocyanate (FITC)‐conjugated anti‐rat IgG, and simultaneously stained with HBJ127 anti‐CD98 hc with species‐specific TexasRed‐conjugated anti‐mouse IgG. (b) Sandwich‐type enzyme‐linked immunosorbent assay was carried out with a combination of anti‐human CD98 hc human monoclonal antibody (mAb) (HH25) as the capture mAb, and anti‐human CD98 hc mouse mAb (HBJ127), SOL22, or SOL69 as the detecting mAb using HeLa cell lysates. (c) The lysates of biotin‐labeled HeLa cells were immunoprecipitated with SOL22 or anti human CD98hc mAb (4F2), subjected to sodium dodecylsulfate–polyacrylamide gel electrophoresis (SDS‐PAGE), and blotted onto polyvinylidene difluoride (PVDF) membranes, and proteins were visualized using Elite ABC and substrates. (d) The lysates of HeLa cells were immunoprecipitated with SOL22 or 4F2, subjected to SDS‐PAGE, and blotted onto PVDF membrane, and proteins were immunostained with anti‐CD98 mAb (HH25). LAT, l‐type amino‐acid transporter; lc, light chain.
Downregulation of LAT1 and CD98hc proteins in human cancer cells after treatment with mAb. Because LAT1 dysfunction caused by mAb might lead to growth inhibition or cell death through a lack of amino acids, we examined the effect of mAb on HeLa cells. Addition of SOL22 to the culture of HeLa cells caused the internalization of LAT1 and CD98 hc proteins from the cell surface (Fig. 6a,b).
Figure 6.
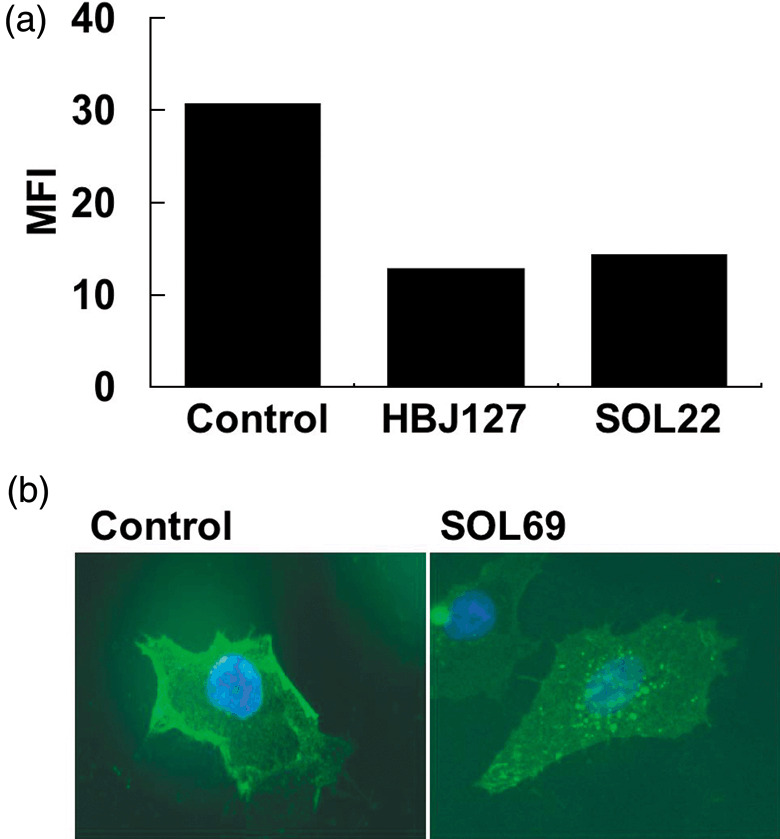
Downregulation of cluster of differentiation 98 (CD98) expression by anti‐l‐type amino‐acid transporter 1 monoclonal antibody (mAb). (a) HeLa cells were cultured without mAb (control), or with HBJ127 or SOL22 for 24 h, and stained with SOL69 followed by fluorescein isothiocyanate (FITC)‐conjugated anti rat IgG Fcγ. Reactivity was analyzed by flow cytometry, and the data are shown as mean fluorescence intensity (MFI). (b) HeLa cells cultured under standard conditions (control, left) or cultured with SOL69 (10 µg/mL) for 24 h (right) were stained with SOL69 followed by FITC‐conjugated anti rat IgG Fcγ, and observed under a fluorescence microscope.
Discussion
Cluster of differentiation 98 is a heterodimeric membrane protein composed of CD98 hc (recently classified as SLC3A) and CD98 lc (recently classified as SLC7). The CD98lcs belong to a family of 12‐pass transmembrane proteins containing LAT1 (SLC7A5), LAT2 SLC7A8), y+LAT1 (SLC7A7), y+LAT2 (SLC7A6), Asc‐1 (SLA7A10), and xCT (SLC7A11), although CD98 hc is a single monomorphic type II glycoprotein. LAT1, which transports neutral large amino acids, is significantly involved in the proliferation of tumor cells;( 26 ) therefore, LAT1 seems to be a promising molecular target for cancer therapy using mAb. However, the preparation of an anti‐LAT1 mAb recognizing native LAT1 protein is difficult, because of the extremely hydrophobic 12‐pass membrane structure of this transporter. For this reason, LAT1 has been characterized mainly based on its amino acid‐transporting activity and by the amount of LAT1 mRNA. Despite efforts to produce anti‐LAT1 mouse mAb using synthetic peptides and bacterial recombinant proteins as immunogens, we could not obtain an anti‐LAT1 mAb recognizing the extracellular domain of living tumor cells. In the present investigation, we used a human LAT1‐expressing rat cell line as an immunogen, and the rat as an immunized animal, as its antibody repertoire is superior to that of mouse species. We successfully obtained the mAb SOL22 and SOL69 specifically recognizing the extracellular domain of human LAT1 protein. We examined the precise specificity of mAb against various CD98 lc proteins, and demonstrated that SOL22 and SOL69 could distinguish LAT1 from the other four CD98 lc. Although SOL22 and SOL69 reacted with a wide variety of living unfixed human tumor cell lines, irrespective of their origin, some cell lines showed a decrease in reactivity with the mAb after the fixation of tumor cells, indicating that our anti‐LAT1 mAb recognized the native or discontinuous epitope of LAT1 protein. We propose that the mAb could not efficiently access LAT1 protein epitopes in some cell lines, possibly because of the complicated structural topology of LAT1 protein affected by differences in surrounding molecules in a given cell line, as the reactivity of the mAb was often up‐ or downregulated by the treatment of these cells with fixing solutions containing PFA. SOL22 and SOL69 were only weakly reactive with HEK293F human embryonic kidney cells, human peripheral blood cells, and cells in human tonsil tissues. Furthermore, these two mAb did not react with lymphocytes irrespective of the activation of cells. Although the expression of CD98 hc in lymphocytes is upregulated by various activation stimuli, as is the case with many oncoproteins, the expression of LAT1 was not affected in the process of lymphocyte activation. A different lc other than LAT1 might be involved in the activation of lymphocytes associated with CD98 hc. Thus, LAT1 is considered to be an excellent molecular target for mAb therapy, possibly even superior to CD98 hc. Unlike typical receptor‐type oncoproteins, such as members of the HER family with restricted tumor distribution, LAT1 is overexpressed on the cell surface of almost all tumor cells irrespective of tissue of origin. Therefore, we expect that LAT1‐based antibody therapy will be applied to various types of human cancers. Recently, specific chimeric or humanized mAb against the extracellular domain of HER2,( 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 ) HER1,( 37 , 38 ) and CD20( 39 , 40 , 41 , 42 , 43 , 44 , 45 ) have been introduced for the treatment of breast cancer, colorectal cancer, and B cell malignancies, respectively. From our present analyses, cancer therapy with anti‐LAT1 mAb seems to be promising. It was also revealed that the expression of LAT1 protein is not necessarily consistent with that of the mRNA in various human tumor cell lines, suggesting that a precise evaluation of mAb reactivity with tumor specimens is indispensable for successful clinical outcomes with therapy using anti‐LAT1 mAb. The effects of anti‐LAT1 mAb on the incorporation of large and neutral amino acids and the growth of tumor cells are now under investigation; however, we have already confirmed that the mAb SOL22 and SOL69 could induce internalization of LAT1 and CD98 hc from the cell surface, suggesting possible effects on cellular amino acid incorporation. The chimerization and humanization of SOL22 and SOL69 mAb and analysis of antitumor effects including antibody‐dependent cellular cytotoxicity and complement‐dependent cytotoxicity of these mAb are currently underway.
Acknowledgments
This work was supported in part by the ‘Academic Frontier’ Project for Private Universities: matching fund subsidy from the Ministry of Education, Culture, Sports, Science, and Technology, 2005–07.
References
- 1. Broer S, Broer A, Hamprecht B. The 4F2hc surface antigen is necessary for expression of system 1‐like neutral amino acid‐transport activity in C6‐BU‐1 rat glioma cells: evidence from expression studies in Xenopus laevis oocytes. Biochem J 1995; 312: 863–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Haynes BF, Hemler ME, Mann DL et al . Characterization of a monoclonal antibody (4F2) that binds to human monocytes and to a subset of activated lymphocytes. J Immunol 1981; 126: 1409–14. [PubMed] [Google Scholar]
- 3. Kanai Y, Segawa H, Miyamoto K, Uchino H, Takeda E, Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98). J Biol Chem 1998; 273: 23 629–32. [DOI] [PubMed] [Google Scholar]
- 4. Masuko T, Abe J, Yagita H, Hashimoto Y. Human bladder cancer cell‐surface antigens recognized by murine monoclonal antibodies raised against T24 bladder cancer cells. Jpn J Cancer Res 1985; 76: 386–94. [PubMed] [Google Scholar]
- 5. Yagita H, Masuko T, Hashimoto Y. Inhibition of tumor cell growth in vitro by murine monoclonal antibodies that recognize a proliferation‐associated cell surface antigen system in rats and humans. Cancer Res 1986; 46: 1478–84. [PubMed] [Google Scholar]
- 6. Yagita H, Masuko T, Takahashi N, Hashimoto Y. Monoclonal antibodies that inhibit activation and proliferation of lymphocytes. I. Expression of the antigen on monocytes and activated lymphocytes. J Immunol 1986; 136: 2055–61. [PubMed] [Google Scholar]
- 7. Hara K, Kudoh H, Enomoto T, Hashimoto Y, Masuko T. Malignant transformation of NIH3T3 cells by overexpression of early lymphocyte activation antigen CD98. Biochem Biophys Res Commun 1999; 262: 720–5. [DOI] [PubMed] [Google Scholar]
- 8. Hara K, Kudoh H, Enomoto T, Hashimoto Y, Masuko T. Enhanced tumorigenicity caused by truncation of the extracellular domain of GP125/CD98 heavy chain. Oncogene 2000; 19: 6209–15. [DOI] [PubMed] [Google Scholar]
- 9. Shishido T, Uno S, Kamohara M et al . Transformation of BALB3T3 cells caused by over‐expression of rat CD98 heavy chain (HC) requires its association with light chain: mis‐sense mutation in a cysteine residue of CD98HC eliminates its transforming activity. Int J Cancer 2000; 87: 311–16. [DOI] [PubMed] [Google Scholar]
- 10. Mastroberardino L, Spindler B, Pfeiffer R et al . Amino‐acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature 1998; 395: 288–91. [DOI] [PubMed] [Google Scholar]
- 11. Ohgimoto S, Tabata N, Suga S et al . Molecular characterization of fusion regulatory protein‐1 (FRP‐1) that induces multinucleated giant cell formation of monocytes and HIV gp160‐mediated cell fusion. FRP‐1 and 4F2/CD98 are identical molecules. J Immunol 1995; 155: 3585–92. [PubMed] [Google Scholar]
- 12. Fenczik CA, Sethi T, Ramos JW, Hughes PE, Ginsberg MH. Complementation of dominant suppression implicates CD98 in integrin activation. Nature 1997; 390: 81–5. [DOI] [PubMed] [Google Scholar]
- 13. Warren AP, Patel K, McConkey DJ, Palacios R. CD98: a type II transmembrane glycoprotein expressed from the beginning of primitive and definitive hematopoiesis may play a critical role in the development of hematopoietic cells. Blood 1996; 87: 3676–87. [PubMed] [Google Scholar]
- 14. Nakamura E, Sato M, Yang H et al . 4F2 (CD98) heavy chain is associated covalently with an amino acid transporter and controls intracellular trafficking and membrane topology of 4F2 heterodimer. J Biol Chem 1999; 274: 3009–16. [DOI] [PubMed] [Google Scholar]
- 15. Segawa H, Fukasawa Y, Miyamoto K, Takeda E, Endou H, Kanai Y. Identification and functional characterization of a Na+‐independent neutral amino acid transporter with broad substrate selectivity. J Biol Chem 1999; 274: 19 745–51. [DOI] [PubMed] [Google Scholar]
- 16. Pineda M, Fernandez E, Torrents D et al . Identification of a membrane protein, LAT‐2, that co‐expresses with 4F2 heavy chain, an l‐type amino acid transport activity with broad specificity for small and large zwitterionic amino acids. J Biol Chem 1999; 274: 19 738–44. [DOI] [PubMed] [Google Scholar]
- 17. Rossier G, Meier C, Bauch C et al . LAT2, a new basolateral 4F2hc/CD98‐associated amino acid transporter of kidney and intestine. J Biol Chem 1999; 274: 34 948–54. [DOI] [PubMed] [Google Scholar]
- 18. Torrents D, Estevez R, Pineda M et al . Identification and characterization of a membrane protein (y+L amino acid transporter‐1) that associates with 4F2hc to encode the amino acid transport activity y+L: A candidate gene for lysinuric protein intolerance. J Biol Chem 1998; 273: 32 437–45. [DOI] [PubMed] [Google Scholar]
- 19. Pfeiffer R, Rossier G, Spindler B, Meier C, Kuhn L, Verrey F. Amino acid transport of y+L‐type by heterodimers of 4F2hc/CD98 and members of the glycoprotein‐associated amino acid transporter family. EMBO J 1999; 18: 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fukasawa Y, Segawa H, Kim JY et al . Identification and characterization of a Na+‐independent neutral amino acid transporter that associates with the 4F2 heavy chain and exhibits substrate selectivity for small neutral d‐ and l‐amino acids. J Biol Chem 2000; 275: 9690–8. [DOI] [PubMed] [Google Scholar]
- 21. Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem 1999; 274: 11 455–8. [DOI] [PubMed] [Google Scholar]
- 22. Christensen HN. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev 1990; 70: 43–77. [DOI] [PubMed] [Google Scholar]
- 23. Grudzinski W, Matula M, Sielewiesiuk J, Kernen P, Krupa Z, Gruszecki WI. Effect of 13‐cis violaxanthin on organization of light harvesting complex II in monomolecular layers. Biochim Biophys Acta 2001; 1503: 291–302. [DOI] [PubMed] [Google Scholar]
- 24. Sang J, Lim YP, Panzica M, Finch P, Thompson NL. TA1, a highly conserved oncofetal complementary DNA from rat hepatoma, encodes an integral membrane protein associated with liver development, carcinogenesis, and cell activation. Cancer Res 1995; 55: 1152–9. [PubMed] [Google Scholar]
- 25. Wolf DA, Wang S, Panzica MA, Bassily NH, Thompson NL. Expression of a highly conserved oncofetal gene, TA1/E16, in human colon carcinoma and other primary cancers: homology to Schistosoma mansoni amino acid permease and Caenorhabditis elegans gene products. Cancer Res 1996; 56: 5012–22. [PubMed] [Google Scholar]
- 26. Yanagida O, Kanai Y, Chairoungdua A et al . Human l‐type amino acid transporter 1 (LAT1): characterization of function and expression in tumor cell lines. Biochim Biophys Acta 2001; 1514: 291–302. [DOI] [PubMed] [Google Scholar]
- 27. Gibbs RA, Weinstock GM, Metzker ML et al . Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature 2004; 428: 493–521. [DOI] [PubMed] [Google Scholar]
- 28. Yoon JH, Kim IJ, Kim H et al . Amino acid transport system L is differently expressed in human normal oral keratinocytes and human oral cancer cells. Cancer Lett 2005; 222: 237–45. [DOI] [PubMed] [Google Scholar]
- 29. Burstein HJ, Kuter I, Campos SM et al . Clinical activity of trastuzumab and vinorelbine in women with HER2‐overexpressing metastatic breast cancer. J Clin Oncol 2001; 19: 2722–30. [DOI] [PubMed] [Google Scholar]
- 30. Carter P, Presta L, Gorman CM et al . Humanization of an anti‐p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci USA 1992; 89: 4285–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cobleigh MA, Vogel CL, Tripathy D et al . Multinational study of the efficacy and safety of humanized anti‐HER2 monoclonal antibody in women who have HER2‐overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 1999; 17: 2639–48. [DOI] [PubMed] [Google Scholar]
- 32. Slamon DJ, Leyland‐Jones B, Shak S et al . Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001; 344: 783–92. [DOI] [PubMed] [Google Scholar]
- 33. Tokuda Y, Watanabe T, Omuro Y et al . Dose escalation and pharmacokinetic study of a humanized anti‐HER2 monoclonal antibody in patients with HER2/neu‐overexpressing metastatic breast cancer. Br J Cancer 1999; 81: 1419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vogel CL, Cobleigh MA, Tripathy D et al . Efficacy and safety of trastuzumab as a single agent in first‐line treatment of HER2‐overexpressing metastatic breast cancer. J Clin Oncol 2002; 20: 719–26. [DOI] [PubMed] [Google Scholar]
- 35. Tokuda Y, Ohnishi Y, Shimamura K et al . In vitro and in vivo anti‐tumour effects of a humanised monoclonal antibody against c‐erbB‐2 product. Br J Cancer 1996; 73: 1362–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tokuda Y, Okumura A, Ohta M et al . A humanized anti‐c‐erbB‐2 monoclonal antibody for the treatment of breast cancer. Breast Cancer 1997; 4: 269–72. [DOI] [PubMed] [Google Scholar]
- 37. Cunningham D, Humblet Y, Siena S et al . Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan‐refractory metastatic colorectal cancer. N Engl J Med 2004; 351: 337–45. [DOI] [PubMed] [Google Scholar]
- 38. Chung KY, Shia J, Kemeny NE et al . Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol 2005; 23: 1803–10. [DOI] [PubMed] [Google Scholar]
- 39. Reff ME, Carner K, Chambers KS et al . Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood 1994; 83: 435–45. [PubMed] [Google Scholar]
- 40. Czuczman MS, Weaver R, Alkuzweny B, Berlfein J, Grillo‐Lopez AJ. Prolonged clinical and molecular remission in patients with low‐grade or follicular non‐Hodgkin's lymphoma treated with rituximab plus CHOP chemotherapy: 9‐year follow‐up. J Clin Oncol 2004; 22: 4711–16. [DOI] [PubMed] [Google Scholar]
- 41. Maloney DG, Grillo‐Lopez AJ, Bodkin DJ et al . IDEC‐C2B8: results of a phase I multiple‐dose trial in patients with relapsed non‐Hodgkin's lymphoma. J Clin Oncol 1997; 15: 3266–74. [DOI] [PubMed] [Google Scholar]
- 42. Maloney DG, Liles TM, Czerwinski DK et al . Phase I clinical trial using escalating single‐dose infusion of chimeric anti‐CD20 monoclonal antibody (IDEC‐C2B8) in patients with recurrent B‐cell lymphoma. Blood 1994; 84: 2457–66. [PubMed] [Google Scholar]
- 43. McLaughlin P, Grillo‐Lopez AJ, Link BK et al . Rituximab chimeric anti‐CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four‐dose treatment program. J Clin Oncol 1998; 16: 2825–33. [DOI] [PubMed] [Google Scholar]
- 44. Tobinai K, Kobayashi Y, Narabayashi M et al . Feasibility and pharmacokinetic study of a chimeric anti‐CD20 monoclonal antibody (IDEC‐C2B8, rituximab) in relapsed B‐cell lymphoma. The IDEC‐C2B8 Study Group. Ann Oncol 1998; 9: 527–34. [DOI] [PubMed] [Google Scholar]
- 45. Wilson WH. Chemotherapy sensitization by rituximab: experimental and clinical evidence. Semin Oncol 2000; 27: 30–6. [PubMed] [Google Scholar]


