Abstract
The α‐emitter 213Bi is characterized by a high relative biological effectiveness. 213Bi‐immunoconjugates targeting tumor‐specific d9‐E‐cadherin have been proven to effectively kill tumor cells in a murine peritoneal carcinomatosis model. The aim of the present study was to optimize the efficacy of 213Bi‐radioimmunotherapy for disseminated gastric cancer in a mouse model of early‐ and advanced‐stage disease and to evaluate the long‐term toxicity of 213Bi‐immunoconjugates. For that purpose, nude mice were treated with different activities of 213Bi‐d9 monoclonal antibody (MAb) targeting d9‐E‐cadherin or unspecific 213Bi‐d8MAb at days 1 or 8 after inoculation of HSC45‐M2 gastric cancer cells expressing mutant d9‐E‐cadherin. Therapeutic efficacy was evaluated by monitoring survival for up to 300 days. Long‐term toxicity was evaluated by the survival of tumor‐free mice injected with 213Bi‐immunoconjugates, kidney function parameters and histopathological examination of kidneys. We showed that survival was significantly prolonged following treatment of mice with 213Bi‐immunoconjugates (0.37–22.2 MBq) at day 1 after tumor cell inoculation (P < 0.002). Therapy with 1.85 MBq of 213Bi‐d9MAb was most successful, defeating early‐stage disease in 87% of all cases. Treatment at day 8 after tumor cell inoculation was less efficient. Long‐term nephrotoxicity could only be observed following application of 22.2 MBq of 213Bi‐d9MAb, the highest activity applied in the therapy trials. As treatment with 1.85 MBq 213Bi‐d9MAb showed excellent therapeutic efficacy without any signs of acute or chronic toxicity, radioimmunotherapy with the α‐emitter 213Bi is a promising concept for treatment of early peritoneal carcinomatosis. (Cancer Sci 2007; 98: 1215–1222)
Gastric cancer, with an incidence of approximately 934 000 newly diagnosed cases worldwide in 2002, is one of the most virulent cancers with a lethal disease course of approximately 65% in developed countries and 80% in developing countries.( 1 ) This poor prognosis is based on early tumor cell dissemination into the peritoneal cavity. Patients showing intraperitoneal (i.p.) tumor cell spread usually die within 1 year of histologically proven R0‐resection. After the development of a clinically manifested peritoneal carcinomatosis, the clinical status of the patient deteriorates rapidly. Symptoms are subileus or ileus, or the development of ascites. As none of the therapeutic options available (such as chemotherapy) have proven to be effective, an efficient therapy is needed urgently. This therapy for disseminated cells that are not vascularized should therefore ideally be applied locoregionally.
Tumor‐specific cell surface antigens expressed by intraperitoneally disseminated tumor cells seem to be an excellent target for the successful treatment of peritoneal carcinomatosis via radioimmunotherapy. Mutations in the cell surface adhesion molecule E‐cadherin have been described in approximately 50% of diffuse‐type gastric cancer patients. Among these mutations, E‐cadherin lacking exon 9 (d9‐E‐cad) has been detected in 10% of diffuse‐type gastric cancers.( 2 ) A monoclonal antibody (d9MAb) has been developed specifically recognizing tumor cells expressing d9‐E‐cad.( 3 ) D9MAb labeled with the highly cytotoxic α‐emitter 213Bi therefore seems a promising agent for the treatment of small intracavitary tumor deposits and tumor cell clusters, especially after locoregional application.( 4 )
In fact 213Bi‐d9MAb has turned out to be a powerful weapon for the elimination of cells expressing mutant d9‐E‐cad in vitro and in a xenograft mouse model.( 5 , 6 , 7 ) Following i.p. injection of HSC45‐M2 gastric cancer cells expressing d9‐E‐cad, mice developing peritoneal carcinomatosis showed a mean survival of 23 days. Treatment of mice with 213Bi‐d9MAb at day 1 after tumor cell inoculation significantly prolonged survival. However, survival decreased with increasing 213Bi activity. A 22.2‐MBq 213Bi‐immunoconjugate activity was less effective than 7.4 MBq. Surprisingly 213Bi‐d8MAb not targeting d9‐E‐cad was almost as effective as specific 213Bi‐d9MAb in terms of prolongation of survival.( 7 )
Therefore, the aim of the present study was to evaluate the therapeutic efficacy of low 213Bi activities (0.37 and 1.85 MBq) of 213Bi‐d9MAb and 213Bi‐d8MAb applied at day 1 after tumor cell inoculation. In addition, the therapeutic efficacy of specific (d9MAb) and unspecific (d8MAb) 213Bi‐immunoconjugates was analyzed in an advanced stage of peritoneal carcinomatosis at day 8 after tumor cell inoculation. To evaluate the potential toxicity of 213Bi, activity distribution in the kidneys and histopathology of the kidneys were examined after injection of both free 213Bi and 213Bi‐immunoconjugates.
Materials and Methods
213Bi labeling of antibodies. The rat monoclonal antibody d9MAb (clone 6H8, subclass IgG2a) specifically binding to mutant d9‐E‐cadherin (d9‐E‐cad) was developed as described previously.( 8 ) The rat monoclonal antibody d8MAb (clone 7H1, subclass IgG2a) recognizes mutant E‐cadherin lacking exon 8 (d8‐E‐cad) and does not cross‐react with d9‐E‐cad.( 3 ) Therefore, d8MAb was used as an unspecific control MAb. Labeling of SCN‐CHX‐A″‐DTPA‐chelated antibodies with 213Bi eluted from a 225Ac/213Bi generator system (provided by the Institute for Transuranium Elements, Karlsruhe)( 9 , 10 ) was done as reported.( 11 ) Labeling yield and radiochemical purity of 213Bi‐immunoconjugates were analyzed by instant thin layer chromatography (Gelman Sciences, USA). The binding efficiency of 213Bi‐labeled antibodies was determined in a cell‐binding assay using HSC45‐M2 gastric cancer cells as described previously.( 7 )
Animals and tumor model. Female Swiss nu/nu mice (4–6 weeks old; Charles River/Iffa Credo, France) were kept at our animal care facility for 2 weeks before use. Mice were housed four to five per cage in a limited access area at a mean temperature of 23°C and a humidity of 50–60%, and had free access to food and water. All animal experiments were carried out in accordance with the guidelines for the use of living animals in scientific studies and the German Law for the protection of animals. The human gastric cancer cell line HSC45‐M2, which is targeted specifically by d9MAb due to expression of mutant d9‐E‐cad, was kindly provided by K. Yanagihara from the National Cancer Center Research Institute, Chuo‐ku, Tokyo.( 12 , 13 ) The number of d9‐E‐cad antigen molecules expressed on HSC45‐M2 cells determined by FACS was 3 × 105/cell.( 6 ) Xenografts were established by i.p. inoculation of 1 × 107 HSC45‐M2 cells in 0.5 mL Dulbecco's modified Eagle's medium into nude mice.
Radioimmunotherapy with 213Bi‐immunoconjugates. A total of 178 female Swiss nu/nu mice were divided into 16 groups of 6–15 mice each. Mice were treated with a single i.p. injection of 0.37, 1.85, 7.4 or 22.2 MBq of specific 213Bi‐d9MAb or unspecific 213Bi‐d8MAb in 0.5 mL phosphate‐buffered saline at days 1 or 8 after inoculation of 1 × 107 HSC45‐M2 cells. Tumor cell distribution at day 1 after inoculation corresponds to single tumor cell dissemination in the peritoneal cavity of patients after resection of the primary solid tumor. Tumor cell distribution at day 8 mimics advanced micronodular peritoneal carcinomatosis.( 4 ) Controls were treated with saline or non‐labeled d9MAb. Mice were observed up to 300 days or killed as soon as ascites, large tumor burden or cachexia had developed, as visualized by magnetic resonance imaging (MRI). Survival was calculated by the method of Kaplan–Meier. Differences between groups were analyzed using the log‐rank test with a significance level of P < 0.05. Median survival and 95% confidence intervals are presented.
Evaluation of 213Bi toxicity
Alterations in bodyweight. Bodyweight was recorded daily within the first week after tumor cell inoculation or injection of 213Bi‐immunoconjugates, and weekly thereafter until week 8.
Survival of tumor‐free mice after injection of 213Bi‐immunoconjugates. Two groups of nude mice received i.p. injection of 7.4 MBq or 22.2 MBq 213Bi‐d9MAb without previous tumor cell inoculation. Mice were followed until signs of disease had developed.
Liver and kidney function parameters. Blood urea nitrogen (BUN), creatinine, glutamate oxaloacetate transaminase (GOT) and alkaline phosphatase (AP) concentrations were determined in the serum of mice using a serum analyzer (Vitros System 250 Chemistry; Johnson‐Johnson, Clinical Diagnostics, USA) before therapy, at different time points after therapy and at the time of sacrifice. Age‐matched untreated nude mice served as controls for the analysis of serum chemistry.
Histopathological examination of kidneys. Mice were killed by exsanguination following carbon dioxide anesthesia and asphyxiation. The kidneys were fixed immediately in phosphate‐buffered formaldehyde (3.8%) at pH 7.4 for 24 h, transferred to 70% ethanol and embedded in paraffin. Sections (2–3 µm) were stained with hematoxylin and eosin and evaluated using a light microscope.
Autoradiographic localization of 213Bi in the kidney following i.p. injection of free 213Bi or 213Bi‐d9MAb. To estimate kidney accumulation of free 213Bi released from 213Bi‐d9MAb conjugates following therapy autoradiographic images and native frozen renal sections were taken simultaneously after injection of both free 213Bi and 213Bi‐d9MAb. For that purpose a high‐resolution digital imager for the autoradiographic analysis of tissue distribution of α‐and β‐particle‐emitting isotopes was used (µ‐imager; Biospace Measures, France). Female Swiss nu/nu mice were injected intraperitoneally with 7.4 MBq of either free 213Bi or 213Bi‐d9MAb in 875 µL of PBS each. After 45 min mice were killed and the kidneys and muscles were frozen quickly and embedded in Tissue Tek (Sakura, Netherlands). Frozen sections (20 µm) (Kryostat HM500M, Microm, Germany) of the organs were mounted on Superfrost plus slides (Menzel, Germany), covered with scintillation foil and exposed in the µ‐imager. In addition to these images native renal sections of each sample were taken.
Results
213Bi labeling of antibodies and tumor cell binding of 213Bi‐immunoconjugates. Labeling of SCN‐CHX‐A″‐DTPA‐chelated d9MAb and d8MAb with the α‐emitter 213Bi resulted in maximum specific activities of 1.48 GBq/mg antibody. The radiochemical purity of the 213Bi‐labeled antibody fractions varied between 95 and 99% following gel filtration. The in vitro stability of 213Bi‐immunoconjugates in PBS at room temperature exceeded four half lives of 213Bi (t1/2 = 46 min). In human serum stability was slightly reduced. At 3 and 6 h after the start of incubation, 90 and 88%, respectively of 213Bi were bound to the antibody. Binding efficiencies of specific 213Bi‐d9MAb (25% binding) and unspecific 213Bi‐d8MAb (2% binding) toward HSC45‐M2 cells differed significantly.
Radioimmunotherapy with 213Bi‐immunoconjugates. Animals inoculated with 1 × 107 HSC45‐M2 gastric cancer cells for evaluation of the therapeutic efficacy of 213Bi‐immunoconjugates were killed as soon as they had developed ascites and bulky i.p. tumors as shown by MRI (Fig. 1). Controls that had not received any therapy displayed a median survival of 19 days after tumor cell inoculation. Treatment of mice at day 1 after tumor cell inoculation with unlabeled d9MAb resulted in a median survival of 38 days, not being significantly different from untreated animals. The same held true for therapy with 0.37 MBq of unspecific 213Bi‐d8MAb (median survival 37 days). In contrast, all other groups of mice treated with 213Bi‐immunoconjugates at day 1 after tumor cell inoculation survived significantly longer than untreated controls or mice that had received unlabeled d9MAb. Therapy with 1.85 MBq 213Bi‐d9MAb was most efficient in terms of prolongation of survival. At the end of the observation period (i.e. 300 days after inoculation of tumor cells) 87% (13/15) of the mice were still alive and showed no signs of disease. Surprisingly, treatment with 1.85 MBq of the unspecific 213Bi‐d8MAb was nearly as efficient as 213Bi‐d9MAb: 75% (9/12) of mice survived longer than 300 days. Therefore, median survival could not be calculated for both groups. 213Bi‐immunoconjugate activities both lower and higher than 1.85 MBq proved to be less efficient concerning prolongation of survival. Treatment with 0.37 MBq 213Bi‐d9MAb resulted in a median survival of 99 days. After therapy with 7.4 MBq of specific 213Bi‐d9MAb or unspecific 213Bi‐d8MAb median survival was 213 or 161 days, respectively. Treatment of animals with 22.2 MBq 213Bi‐immunoconjugates at day 1 after cell inoculation was almost as efficient with specific d9MAb as it was with unspecific d8MAb. The median survival of mice was 119 and 129 days after treatment with d9MAb and d8MAb, respectively (Fig. 2; Table 1). Though treatment with specific 213Bi‐d9MAb turned out to be slightly more efficient than with unspecific 213Bi‐d8MAb in general, the difference between d9MAb and d8MAb was not significant in either case.
Figure 1.
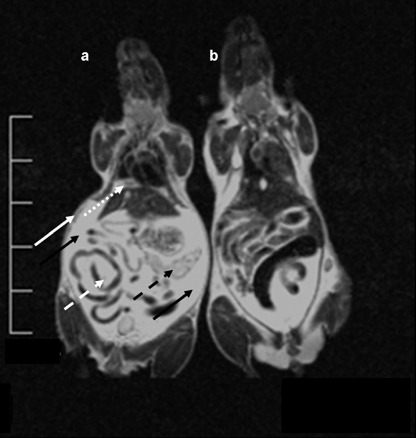
Magnetic resonance imaging (MRI) of disseminated tumor growth (ventro‐dorsal view). MRI carried out with a 1.5 Tesla MRT (Philips, the Netherlands) using a wrist coil. For the scan the 2D Turbospin Echo T2 program was chosen with a scan time of 11 min and a repetition time TR 2500 ms. (a) Mouse 3 weeks after tumor cell inoculation: the diaphragm is covered with a thick layer of tumor cells (dotted white arrow). Knotty metastases are visible at the caudal mesentery (dashed white arrow) and the peritoneal serous membrane (white arrow). The pancreas is heavily infiltrated with tumor cells (dashed black arrow). The peritoneal cavity is filled with ascites (black arrows). (b) Control mouse without tumor cells.
Figure 2.
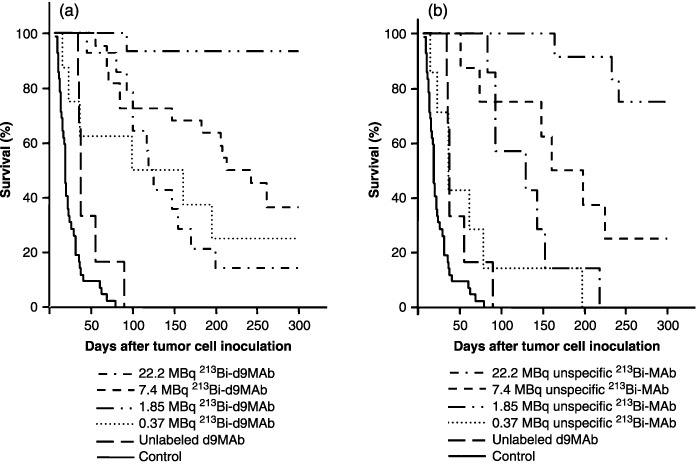
Survival of nude mice following 213Bi immunotherapy at day 1 after inoculation of 1 × 107 HSC45‐M2 gastric cancer cells. Mice were injected with saline (untreated controls), unlabeled d9MAb or 0.37, 1.85, 7.4 or 22.2 MBq of (a) specific 213Bi‐d9MAb or (b) unspecific 213Bi‐d8MAb.
Table 1.
Median survival of mice after intraperitoneal inoculation of HSC45‐M2 tumor cells and injection of different activities of 213Bi‐immunoconjugates
| Treatment | Median survival following therapy 1 day after tumor cell inoculation | Median survival following therapy 8 days after tumor cell inoculation | ||
|---|---|---|---|---|
| Days | 95% confidence interval | Days | 95% confidence interval | |
| Untreated control | 19 | 17–21 | 19 | 17–21 |
| Unlabeled d9MAb | 38 | 35–41 | ND | |
| 0.37 MBq d9MAb | 99 | 74–169 | ND | |
| 0.37 MBq d8MAb | 37 | 15–55 | ND | |
| 1.85 MBq d9MAb | – † | – | ND | |
| 1.85 MBq d8MAb | – ‡ | – | ND | |
| 7.4 MBq d9MAb | 213 | 162–264 | 73 | 44–102 |
| 7.4 MBq d8MAb | 161 | 92–230 | 45 | 13–77 |
| 22.2 MBq d9MAb | 119 | 104–134 | 86 | 19–153 |
| 22.2 MBq d8MAb | 129 | 37–221 | 47 | 26–90 |
87% of the animals were still alive after 300 days.
75% of the animals were still alive at 300 days. ND, not determined.
Treatment of mice with 213Bi‐immunoconjugates at day 8 after tumor cell inoculation was less efficient than treatment on day 1 in terms of prolongation of survival. However, 213Bi‐d9MAb conjugates were significantly superior to 213Bi‐d8MAb conjugates both at activities of 7.4 and 22.2 MBq. Following therapy with 7.4 MBq 213Bi‐d9MAb median survival was 73 days. With the same activity of unspecific 213Bi‐d8MAb median survival decreased to 45 days (P = 0.03). Mice treated with 22.2 MBq 213Bi‐d9MAb at day 8 after tumor cell inoculation showed a median survival of 86 days. Therapy with 22.2 MBq of unspecific 213Bi‐d8MAb was significantly less efficient, with a median survival of 47 days (P = 0.04) (Fig. 3; Table 1).
Figure 3.
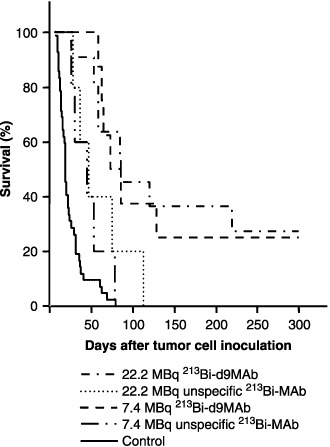
Survival of nude mice following 213Bi immunotherapy at day 8 after inoculation of 1 × 107 HSC45‐M2 gastric cancer cells. Mice were injected with saline (untreated controls), 7.4 or 22.2 MBq of specific 213Bi‐d9MAb or unspecific 213Bi‐d8MAb.
Evaluation of toxicity of 213Bi‐immunoconjugates
Alterations in bodyweight.
Mice that had not been inoculated with tumor cells showed a steady increase in bodyweight up to 120% within 56 days. In contrast, bodyweight was reduced by 10% within 2 days of inoculation with 1 × 107 HSC45‐M2 cells. However, following 213Bi therapy, loss of bodyweight was compensated within 14 days. The application of the different activities of 213Bi‐immunoconjugates (0.37–22.2 MBq) per se did not negatively influence bodyweight within the observation period of 8 weeks.
Survival of tumor‐free mice after injection of 213Bi‐immunoconjugates. After injection of 22.2 MBq 213Bi‐d9MAb, survival of mice that had not received tumor cells and of those that had been inoculated with 1 × 107 HSC45‐M2 gastric cancer cells 1 day before 213Bi‐d9MAb application was almost identical. The median survival was 128 days in the case of tumor‐free mice and 119 days in the case of mice that had been inoculated with tumor cells. In contrast, after injection of 7.4 MBq 213Bi‐d9MAb survival of mice without preceding tumor cell inoculation was significantly longer than survival of mice that had received HSC45‐M2 cells (P < 0.004). Within the 300‐day observation period only one out of nine tumor‐free mice died, whereas eight out of 12 mice that had been inoculated with tumor cells died (Fig. 4). Following autopsy none of the tumor‐free mice showed any signs of organ toxicity. These results indicate that activities of 213Bi‐immunoconjugates as high as 22.2 MBq cause chronic toxicity in mice, whereas 7.4 MBq does not.
Figure 4.
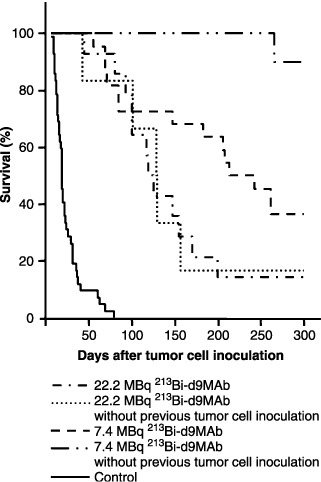
Survival after treatment with 213Bi‐d9MAb of nude mice that had received no HSC45‐M2 gastric cancer cells or had been inoculated with 1 × 107 HSC45‐M2 gastric cancer cells 1 day before therapy. Mice were injected with saline (untreated controls), 7.4 or 22.2 MBq of specific 213Bi‐d9MAb.
Liver and kidney function parameters. The levels of GOT and AP, indicators of liver function, remained constant within the observation period in all different treatment groups. Therefore it can be excluded that therapy with 213Bi‐immunoconjugates causes liver toxicity. In the first weeks after treatment with 213Bi‐immunoconjugates no symptoms of nephrotoxicity could be observed. Within months chronic renal failure developed only in animals that had been treated with the highest 213Bi‐activity (22.2 MBq) resulting in a significant increase in BUN levels compared to pretreatment values (39 ± 5 vs 17 ± 3 ng/dL). Monitoring of serum creatinine could not confirm 213Bi‐induced kidney damage; however, sensitivity seemed to be too small for evaluation of toxicity as has been reported earlier.( 14 ) No other signs of organ toxicity were observed in any treatment group.
Histopathological findings. No pathological findings were seen in liver and intestines of treated mice at every activity. Toxicity was observed only in the kidney after injection of 22.2 MBq 213Bi‐immunoconjugates. Macroscopically in five of six mice the kidneys appeared ochre and swollen with the renculi clearly visible. Four of these animals had developed sterile ascites without tumor cells. The histopathology was characterized by progressive hyalinization of glomerula with sclerosis associated with hydronephrosis and tubular atrophy 5 months after injection of 22.2 MBq 213Bi‐immunoconjugate. Moreover, interstitial scaring was seen as found in chronic forms of radiation nephritis (Fig. 5). Following application of 7.4 MBq 213Bi‐immunoconjugate, one of 10 mice developed swollen kidneys. However, histopathological analysis of tissue sections did not confirm pathological alterations.
Figure 5.
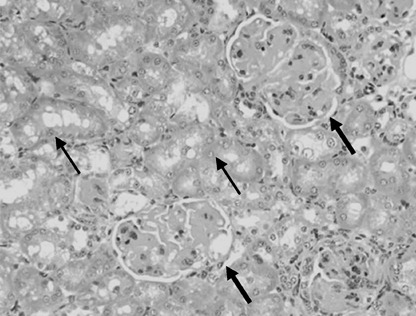
Histopathology of kidneys following intraperitoneal application of 22.2 MBq 213Bi‐d9MAb. The section shows hyalinization of glomerula with glomerula sclerosis (thick arrows) and edema of tubular epithelial cells (thin arrows); HE (160×).
Autoradiographic localization of 213Bi in the kidney. Renal localization of 213Bi 45 min after i.p. injection of free 213Bi or 213Bi‐d9MAb into nude mice was examined by ex vivo autoradiography of frozen renal sections combined with muscle (M. quadriceps femoris) as a negative control using a µ‐imager. The renal localization patterns of 213Bi revealed a clearly localized distribution being identical following i.p. injection of either free 213Bi or 213Bi‐d9MAb conjugates. The strongest signal was found in the renal cortex and the outer zone of the medulla, whereas little activity could be detected in the inner medulla and the renal pelvis. In muscle no accumulation of 213Bi could be detected (Fig. 6). These results suggest that kidney toxicity following injection of high activities of 213Bi‐d9MAb is due to free 213Bi released from 213Bi‐d9MAb conjugates.
Figure 6.
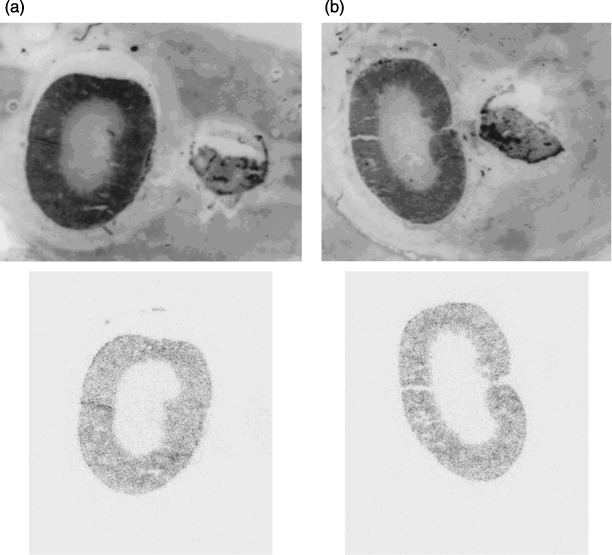
Renal localization of 213Bi via ex vivo autoradiography. At 45 min after intraperitoneal injection of 7.4 MBq (a) free 213Bi or (b) 213Bi‐d9MAb into tumor‐free nude mice. 213Bi localization was analyzed in frozen renal sections using a µ‐imager. Strong accumulation of 213Bi in the renal cortex was independent of the application of free 213Bi or 213Bi coupled to the antibody. Above, native frozen section; below, autoradiographic image.
Discussion
Peritoneal dissemination is the most frequently observed type of recurrence in patients with abdominal cancer following curative surgery, originating from the dissemination of free peritoneal tumor cells shed from the primary tumor. Therefore, cytological examination of the peritoneal lavage fluid is important for prediction of peritoneal recurrence in gastric cancer patients.( 15 , 16 ) The presence of free peritoneal tumor cells identifies high‐risk patients who could benefit from new therapeutic strategies to prevent peritoneal recurrence. The locoregional application of appropriate carriers labeled with highly cytotoxic α‐emitters binding selectively to free peritoneal cells would be an effective strategy to destroy these tumor cells.( 17 , 18 , 19 , 20 , 21 , 22 ) Alpha‐emitter immunoconjugates have proven to be powerful therapeutic agents in animal experiments after intravenous or intracavitary application,( 23 , 24 , 25 , 26 , 27 , 28 , 29 ) and in clinical trials.( 30 , 31 ) Because α‐particles transfer their energy within a tissue range of only 60–80 µm leading to a linear energy transfer of approximately 100 keV/µm, they can effectively destroy single tumor cells. The α‐emitter 213Bi was chosen for our experimental therapeutic studies because of its availability from a 225Ac/213Bi generator system,( 9 , 10 ) its short half‐life of only 46 min as well as fast and efficient coupling to chelated d9MAb. Fast and effective targeting of the i.p. tumor cell burden can be attained following i.p. application of the immunoconjugate. Selective binding increases the cytotoxic potential toward tumor cells and tumor cell clusters while minimizing the irradiation of adjacent normal tissue.
The i.p. application of 213Bi‐d9MAb specifically targeting d9‐E‐cad expressed on gastric cancer cells at days 1 or 8 after tumor cell inoculation in nude mice can serve as a model for the described clinical situation of early‐stage and advanced‐stage peritoneal carcinomatosis, respectively. Treatment of nude mice with 213Bi‐immunoconjugates 1 day after i.p. inoculation of HSC45‐M2 gastric cancer cells significantly prolonged median survival at 213Bi‐activities of 1.85, 7.4 and 22.2 MBq. However, the therapeutic efficacy of specific 213Bi‐d9MAb was not significantly superior to unspecific 213Bi‐d8MAb at any of the 213Bi activities assayed. Only at 0.37 MBq did nude mice survive considerably longer following treatment with 213Bi‐d9MAb (median survival 99 days) compared with 213Bi‐d8MAb (median survival 37 days). However, survival following application of 0.37 MBq 213Bi‐d9MAb was significantly shorter than survival after injection of 1.85 or 7.4 MBq 213Bi‐immunoconjugates. Therefore, 213Bi‐d9MAb activities as low as 0.37 MBq only cause a temporary reduction in tumor cell mass that is insufficient as a cure. Excellent therapeutic efficiency without any signs of toxicity was obtained after application of 1.85 MBq 213Bi‐d9MAb. This injected activity can realistically be transferred to the treatment of patients, corresponding to approximately 1.5 GBq.
In contrast to the results obtained with 213Bi‐immunotherapy 1 day after inoculation of tumor cells, the therapeutic efficacy of specific 213Bi‐d9MAb 8 days after tumor cell inoculation was significantly better than of unspecific 213Bi‐d8MAb both at 7.4 and 22.2 MBq. However, median survival did not exceed 100 days in any case. This suggests that 8 days after tumor cell inoculation the tumor mass is too widespread to be completely eliminated, even after application of 22.2 MBq 213Bi‐d9MAb immunoconjugates. Improvement of therapeutic efficacy at an advanced stage of the disease has been obtained using double applications of 213Bi‐d9MAb at days 8 and 15 after inoculation of tumor cells.( 32 )
Our results further indicate that a significant difference in terms of therapeutic efficacy following treatment of mice with specific 213Bi‐d9MAb and unspecific 213Bi‐d9MAb can only be observed if the ratio of 213Bi‐immunoconjugates to cellular antigens is comparatively low, especially in the case of treatment at day 8 after tumor cell inoculation. At a comparatively high excess of 213Bi‐immunoconjugates compared to antigens with treatment 1 day after i.p. tumor cell inoculation, no significant difference in survival of mice was obtained between the specific d9MAb and the unspecific d8MAb 213Bi‐immunoconjugates. These findings are in agreement with results reported by Elgqvist et al.( 33 ) following treatment of nude mice bearing i.p. OVCAR‐3 tumors with 400 kBq of specific 211At‐MX35 F(ab′)2 or non‐specific 211At‐Rituximab F(ab′)2. Therapeutic efficacy decreased with increasing tumor size, and differences in therapeutic efficacy between specific and non‐specific 211At‐immunoconjugates were not observed in early‐stage but only in advanced‐stage disease. Also, in nude mice bearing human pancreatic carcinoma xenografts expressing high levels of HER2, therapy with 18.5 MBq of specific 213Bi‐herceptin and unspecific 213Bi‐HuIgG showed almost identical efficacy. Both 213Bi‐labeled antibodies increased the survival of animals; 50% of the animals that received injections of the specific 213Bi‐herceptin and unspecific 213Bi‐HuIgG were still alive at 98 and 107 days, respectively.( 34 )
Due to the short range of α‐particles the crossfire effect of α‐particles targeted to tumor cells is negligible. For complete elimination of xenografted tumor cells it seems inevitable to apply an excess of 213Bi‐immunoconjugates compared to the number of antigens expressed by the tumor cells. Application of an excess of 213Bi‐immunoconjugates results in an increase of 213Bi‐imunoconjugates that are not bound to tumor cells. Thus, the unspecific crossfire effect, that is, irradiation of tumor cells that are not targeted by 213Bi‐immunoconjugates, also increases. As has been shown in in vitro studies, massive crossfire explains the almost identical therapeutic efficacies of specific (d9MAb) and unspecific (d8MAb) 213Bi‐immunoconjugates following application of an excess of 213Bi‐immunoconjugates compared to cellular antigens.( 6 ) Reduction of 213Bi‐immunoconjugate activity concentration again results in significant differences in terms of cell killing between specific and unspecific immunoconjugates.
For evaluation of 213Bi toxicity mice without tumor cells and mice that had been inoculated with tumor cells (1 × 107) were treated with 7.4 and 22.2 MBq 213Bi‐d9MAb, respectively. Following i.p. application of 22.2 MBq 213Bi‐d9MAb median survivals of mice without tumor cells (128 days) and of mice xenotransplanted with tumor cells (119 days) were almost identical (Fig. 4). This suggests that in both cases death of the mice was finally caused by 213Bi radiotoxicity and not by tumor spread. Accordingly, ascites that had developed in mice that had been inoculated with tumor cells was free of cells in any case thus most likely due to hypoproteinemia. Because white blood cell counts were in the normal range at the time of autopsy (R. Beck, unpublished data), non‐specific inflammatory reactions can also be excluded. Following application of 7.4 MBq 213Bi‐d9MAb survival was significantly different in tumor‐free mice and mice that had received i.p. injection of tumor cells: 90% of tumor‐free mice but only 36% of tumor‐bearing mice survived longer than 300 days (Fig. 4). Nevertheless, we presume that death of tumor xenotransplanted animals was predominantly caused by radiotoxicity due to the following consideration. The human signet ring carcinoma cell line HSC45‐M2 used to establish our peritoneal carcinomatosis model is characterized by secretion of lysozyme. Following inoculation of tumor cells, secreted lysozyme can cause lesions at the i.p. serous membrane, which facilitates transition of 213Bi‐d9MAb in the blood circulation. Results of biodistribution studies support this hypothesis: 1 h after i.p. application of 213Bi‐d9MAb 213Bi‐activity concentration in the blood was significantly higher in HSC45‐M2 tumor‐bearing mice (5.4 ± 1.5% injected dose [ID]/g) than in mice without previous tumor cell inoculation (1.4 ± 1.1% ID/g) (R. Beck, unpublished data). Nevertheless, 213Bi‐d9MAb therapy is unlikely to cause bone marrow toxicity because white blood cell counts returned to the normal range at least 40 days after therapy and were normal at the time of autopsy. Also, chromosomal aberrations in bone marrow cells could no longer be observed from day 3 after therapy.( 7 ) In a corresponding biodistribution study carried out with intraperitoneally inoculated MDA‐MB‐435S breast cancer cells that do not secrete lysozyme, no difference was observed concerning 213Bi‐activity concentration in the blood.( 4 )
In a substantial number of established radionuclide therapies the bone marrow is the dose‐limiting organ. Also, the kidneys are sensitive to irradiation and they turned out to be the dose‐limiting organs in 213Bi radioimmunotherapy. Following application of 22.2 MBq 213Bi‐immunoconjugates progressive glomerula sclerosis, tubular atrophy and interstitial scaring were observed, as found in chronic forms of radiation nephritis. These pathological alterations of internal irradiation of the kidneys are a consequence of various functional and morphological disturbances showing a clear time‐ and dose‐dependent course of the nephropathy. In humans the progressive reduction in renal function observed after kidney irradiation leads to glomerulosclerosis and tubulointerstitial fibrosis.( 35 )
As revealed by biodistribution studies,( 7 ) 3 h after i.p. injection of 213Bi‐immunoconjugates kidney accumulation of 213Bi was 5.4% ID/g tissue. Two possible mechanisms of 213Bi accumulation in the kidneys might explain the damage that was observed 5 months after therapy with 22.2 MBq 213Bi‐d9MAb. First, 213Bi released following transition of 213Bi‐immunoconjugates from the peritoneal cavity into the serum (B. Pfost, personal communication) can be deposited in inclusion bodies in renal tubular epithelial cells. Second, the tubules might selectively reabsorb 213Bi‐antibody fragments with molecular weights <40 kDa resulting from enzymatic degradation of 213Bi‐immunoconjugates. In a recent study a significant reduction in renal 213Bi uptake was achieved using the metal scavengers, 2,3‐dimercapto‐1‐propanesulfonic acid (DMPS) and N‐(2,3‐dimercaptopropyl)‐phthalamidic acid (DMPA), diuretic therapy with furosemide and chlorothiazide or competitive blockade of Bi binding sites in the renal tubular cells by non‐radioactive 209Bi.( 36 ) Renal uptake of radiolabeled peptides can be inhibited by gelatin‐based compounds like succinylated gelatin.( 37 )
Following 225Ac injection into mice the time course of kidney damage and pathological alterations in the kidneys were similar to the results obtained in our study with 213Bi‐d9MAb.( 38 ) Additionally, the authors noticed an increase in tubular and interstitial cell transforming growth factor (TGF)‐β1 peaking at 25 weeks after 225Ac application. Therefore, internally delivered 225Ac irradiation‐induced loss of tubular epithelial cells might trigger a chain of adaptive changes resulting in renal parenchymal damage accompanied by a loss of renal function.( 38 , 39 )
The nephropathological alterations observed following injection of 213Bi‐d9MAb can not be due to heavy metal cytotoxicity as only trace amounts of the metal Bi were injected (approximately 50 pg 213Bi).( 40 )
Nephrotoxic effects of 213Bi‐d9MAb immunotherapy in the nude mouse model could be observed only at a 213Bi‐activity of 22.2 MBq. This 213Bi‐d9MAb activity also reduced white blood cell counts and caused chromosomal aberrations in bone marrow cells. However, these changes proved not to be persistent: Pretreatment values of white blood cell counts were reached again between 14 and 21 days after 213Bi‐d9MAb injection and chromosomal aberrations could only be observed 1 day after therapy.( 7 ) 213Bi‐d9MAb therapy at day 1 after tumor cell inoculation using 1.85 MBq completely eliminated tumor cells, thus curing nearly all of the treated mice. Such low activities of 213Bi‐d9MAb did not cause any signs of toxicity. Therefore, 213Bi immunotherapy of peritoneal carcinomatosis carried out 24 h after surgery of the primary tumor is a promising concept for future application in patients. Early application of 213Bi‐d9MAb ensures homogenous distribution of the radioimmunoconjugate because postoperative adhesions impairing distribution do not develop until 3 days after surgery. Also, due to the short half‐life of 213Bi no problems with regard to radiation safety should arise during 213Bi immunotherapy.
Acknowledgments
We would like to thank Martin W. Brechbiel at the Radioimmune and Inorganic Chemistry Section, NCI, NIH (Bethesda, MD, USA) for providing SCN‐CHX‐A″‐DTPA chelate and Kazuyoshi Yanagihara at the National Cancer Center Research Institute (Tokyo, Japan) for providing the HSC45‐M2 stomach cancer cell line. We also thank Christos Apostolidis from the Institute for Transuranium Elements (Karlsruhe, Germany) for the preparation of the 225Ac/213Bi generator systems. We are grateful to Elisabeth Kremmer at the Institute of Molecular Immunology, GSF (Munich, Germany) and Karl‐Friedrich Becker from the Institute of Pathology, Technische Universität München (Munich, Germany) for providing the antibodies d8MAb and d9MAb. We finally thank Ingrid Becker from the Institute of Pathology, Technische Universität München (Munich, Germany) for histopathological examination of kidney sections and Gjermund Henriksen from the Department of Nuclear Medicine, Technische Universität München (Munich, Germany) for introducing the µ‐imager. The work was supported by Deutsche Forschungsgemeinschaft (grants SE 962/2‐4, R. Senekowitsch‐Schmidtke).
References
- 1. Parkin MD, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55: 74–108. [DOI] [PubMed] [Google Scholar]
- 2. Handschuh G, Candidus S, Luber B et al . Tumor‐associated E‐cadherin mutations alter cellular morphology, decrease cellular adhesion, and increase cellular motility. Oncogene 1999; 18: 4301–12. [DOI] [PubMed] [Google Scholar]
- 3. Becker K‐F, Kremmer E, Eulitz M et al . Functional allelic loss detected at the protein level in archival human tumors using allele‐specific E‐cadherin monoclonal antibodies. J Pathol 2002; 197: 567–74. [DOI] [PubMed] [Google Scholar]
- 4. Senekowitsch‐Schmidtke R, Schuhmacher C, Becker K‐F et al . Highly specific tumor binding of a 213Bi‐labeled monoclonal antibody against mutant E‐cadherin suggests its usefulness for locoregional α‐radioimmunotherapy of diffuse‐type gastric cancer. Cancer Res 2001; 61: 2804–8. [PubMed] [Google Scholar]
- 5. Miederer M, Seidl C, Beyer G‐J et al . Comparison of the radiotoxicity of two alpha emitting immunoconjugates Terbium‐149 and Bismuth‐213 directed against a tumorspecific, Exon 9 deleted (d9) E‐cadherin adhesion protein. Radiat Res 2003; 159: 612–20. [DOI] [PubMed] [Google Scholar]
- 6. Seidl C, Schröck H, Seidenschwang S et al . Cell death triggered by alpha‐emitting 213Bi‐immunoconjugates in HSC45‐M2 gastric cancer cells is different from apoptotic cell death. Eur J Nucl Med Mol Imaging 2005; 32: 274–85. [DOI] [PubMed] [Google Scholar]
- 7. Huber R, Seidl C, Schmid E et al . Locoregional α‐radioimmunotherapy of intraperitoneal tumor cell dissemination using a tumor‐specific monoclonal antibody. Clin Cancer Res 2003; 9: 3922S–8S. [PubMed] [Google Scholar]
- 8. Becker K‐F, Kremmer E, Eulitz M et al . Analysis of E‐cadherin in diffuse‐type gastric cancer using a mutation‐specific monoclonal antibody. Am J Pathol 1999; 155: 1803–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Apostolidis C, Carlos‐Márquez R, Janssens W, Molinet R, Nikula T, Ouadi A. Cancer treatment using Bi‐213 and Ac‐225 in radioimmunotherapy. Nuclear News 2001; 44: 29–33. [Google Scholar]
- 10. Apostolidis C, Molinet R, Rasmussen G, Morgenstern A. Production of Ac‐225 from Th‐229 for targeted α therapy. Anal Chem 2005; 77: 6288–91. [DOI] [PubMed] [Google Scholar]
- 11. Nikula TK, Curcio MJ, Brechbiel MW, Gansow OA, Finn RD, Scheinberg DA. A rapid, single vessel method for preparation of clinical grade ligand conjugated monoclonal antibodies. Nucl Med Biol 1995; 22: 387–90. [DOI] [PubMed] [Google Scholar]
- 12. Yanagihara K, Ito A, Toge T, Numoto M. Antiproliferative effects of isoflavones on human cancer cell lines established from the gastrointestinal tract. Cancer Res 1993; 53: 5815–21. [PubMed] [Google Scholar]
- 13. Fukudome Y, Yanagihara K, Takeichi M, Ito F, Shibamoto S. Characterization of a mutant E‐cadherin protein encoded by a mutant gene frequently seen in diffuse‐type human gastric carcinoma. Int J Cancer 2000; 88: 579–83. [DOI] [PubMed] [Google Scholar]
- 14. Behr TM, Béhé M, Stabin MG et al . High‐linear energy transfer (LET) α versus low‐LET β emitters in radioimmuno‐therapy of solid tumors: therapeutic efficacy and dose‐limiting toxicity of 213Bi‐versus 90Y‐labeled CO17‐1A Fab′ fragments in a human colonic cancer model. Cancer Res 1999; 59: 2635–43. [PubMed] [Google Scholar]
- 15. Bryan RT, Cruickshank NR, Needham SJ et al . Laproscopic peritoneal lavage in staging gastric and oesophageal cancer. Eur J Surg Oncol 2001; 27: 291–7. [DOI] [PubMed] [Google Scholar]
- 16. Rosenberg R, Nekarda H, Bauer P, Schenck U, Hoefler H, Siewert JR. Free peritoneal tumour cells are an independent prognostic factor in curatively resected stage IB gastric carcinoma. Br J Surg 2006; 93: 325–31. [DOI] [PubMed] [Google Scholar]
- 17. McDevitt MR, Barendswaard E, Ma D et al . An α‐particle emitting antibody ([213Bi]J591) for radioimmunotherapy of prostate cancer. Cancer Res 2000; 60: 6095–100. [PubMed] [Google Scholar]
- 18. Ballangrud AM, Yang WH, Palm S et al . Alpha‐particle emitting atomic generator (Actinium‐225)‐labeled trastuzumab (herceptin) targeting of breast cancer spheroids: efficacy versus HER2/neu expression. Clin Cancer Res 2004; 10: 4489–97. [DOI] [PubMed] [Google Scholar]
- 19. Song YJ, Qu CF, Rizvi SM et al . Cytotoxicity of PAI2, C595 and herceptin vectors labelled with the alpha‐emitting radioisotope Bismuth‐213 for ovarian cancer cell monolayers and clusters. Cancer Lett 2006; 234: 176–83. [DOI] [PubMed] [Google Scholar]
- 20. Persson MI, Gedda L, Jensen HJ, Lundqvist H, Malmstrom PU, Tolmachev V. Astatinated trastuzumab, a putative agent for radionuclide immunotherapy of ErbB2‐expressing tumours. Oncol Rep 2006; 15: 673–80. [DOI] [PubMed] [Google Scholar]
- 21. Qu CF, Song EY, Li Y et al . Pre‐clinical study of 213Bi labelled PAI2 for the control of micrometastatic pancreatic cancer. Clin Exp Metastasis 2005; 22: 575–86. [DOI] [PubMed] [Google Scholar]
- 22. Rizvi SMA, Li Y, Song EYJ et al . Preclinical studies of Bismuth‐213 labeled plasminogen activator inhibitor type 2 (PAI2) in a prostate cancer nude mouse xenograft model. Cancer Biol Ther 2006; 5: 386–93. [DOI] [PubMed] [Google Scholar]
- 23. Imam SK. Advancements in cancer therapy with alpha‐emitters: a review. Int J Radiat Oncol Biol Phys 2001; 51: 271–8. [DOI] [PubMed] [Google Scholar]
- 24. Goldenberg DM, Sharkey RM. Advances in cancer therapy with radiolabeled monoclonal antibodies. Q J Nucl Med Mol Imaging 2006; 50: 248–64. [PubMed] [Google Scholar]
- 25. Couturier O, Supiot S, Degraef‐Mougin M et al . Cancer radioimmunotherapy with alpha‐emitting nuclides. Eur J Nucl Med Mol Imaging 2005; 32: 601–14. [DOI] [PubMed] [Google Scholar]
- 26. Kennel SJ, Lankford T, Davern S et al . Therapy of rat tracheal carcinoma IC‐12 in SCID mice: vascular targeting with [213Bi]‐MAb TES‐23. Eur J Canc 2002; 38: 1278–87. [DOI] [PubMed] [Google Scholar]
- 27. Borchardt PE, Yuan RR, Miederer M, McDevitt MR, Scheinberg DA. Targeted actinium‐225 in vivo generators for therapy of ovarian cancer. Cancer Res 2003; 63: 5084–90. [PubMed] [Google Scholar]
- 28. Milenic D, Garmestani K, Dadachova E et al . Radioimmunotherapy of human colon carcinoma xenografts using a 213Bi‐labeled domain‐deleted humanized monoclonal antibody. Cancer Biother Radiopharm 2004; 19: 135–47. [DOI] [PubMed] [Google Scholar]
- 29. Elgqvist J, Andersson H, Back T et al . Therapeutic efficacy and tumor dose estimations in radioimmunotherapy of intraperitoneally growing OVCAR‐3 cells in nude mice with 211At‐labeled monoclonal antibody MX35. J Nucl Med 2005; 46: 1907–15. [PubMed] [Google Scholar]
- 30. Jurcic JG, Larson SM, Sgouros G et al . Targeted α particle immunotherapy for myeloid leukemia. Blood 2002; 100: 1233–9. [PubMed] [Google Scholar]
- 31. Zalutsky MR, Pozzi OR. Radioimmunotherapy with alpha‐particle emitting radionuclides. Q J Nucl Med Mol Imaging 2004; 48: 289–96. [PubMed] [Google Scholar]
- 32. Bloechl S, Beck R, Seidl C, Morgenstern A, Schwaiger M, Senekowitsch‐Schmidtke R. Fractionated locoregional low‐dose radioimmunotherapy improves survival in a mouse model of diffuse‐type gastric cancer using a 213Bi‐conjugated monoclonal antibody. Clin Cancer Res 2005; 11: 7070S–4S. [DOI] [PubMed] [Google Scholar]
- 33. Elgqvist J, Andersson H, Bäck T et al . α‐Radioimmunotherapy of intraperitoneally growing OVCAR‐3 tumors of variable dimensions: outcome related to measured tumor size and mean absorbed dose. J Nucl Med 2006; 47: 1342–50. [PubMed] [Google Scholar]
- 34. Milenic DE, Garmestani K, Brady ED et al . Targeting of HER2 antigen for the treatment of disseminated peritoneal disease. Clin Cancer Res 2004; 10: 7834–41. [DOI] [PubMed] [Google Scholar]
- 35. Cohen EP, Robbins MEC. Radiation nephropathy. Semin Nephrol 2003; 23: 486–99. [DOI] [PubMed] [Google Scholar]
- 36. Leussink BT, Nagelkerke JF, Van De Water B et al . Pathways of proximal tubular cell death in bismuth nephrotoxicity. Toxicol Appl Pharmacol 2002; 180: 100–9. [DOI] [PubMed] [Google Scholar]
- 37. Vegt E, Wetzels JF, Russel FG et al . Renal uptake of radiolabeled octreotide in human subjects is efficiently inhibited by succinylated gelatin. J Nucl Med 2006; 47: 432–6. [PubMed] [Google Scholar]
- 38. Jaggi JS, Kappel BJ, McDevitt MR et al . Efforts to control the errant products of a targeted in vivo generator. Cancer Res 2005; 65: 4888–95. [DOI] [PubMed] [Google Scholar]
- 39. Jaggi JS, Seshan SV, McDevitt MR, LaPerle K, Sgouros G, Scheinberg DA. Renal tubulointerstitial changes after internal irradiation with α‐particle‐emitting actinium daughters. J Am Soc Nephrol 2005; 16: 2677–89. [DOI] [PubMed] [Google Scholar]
- 40. Jaggi JS, Seshan SV, McDevitt MR, Sgouros G, Hyjek E, Scheinberg DA. Mitigation of radiation nephropathy after internal α‐particle irradiation of kidneys. Int J Radiat Oncol Biol Phys 2006; 64: 1503–12. [DOI] [PubMed] [Google Scholar]


