Abstract
Adiponectin, a circulating peptide hormone produced in adipose tissue, has been shown to be reduced in the plasma of patients with cancer, suggesting that this adipokine may be mechanically involved in the pathogenesis of adiposity‐related carcinogenesis. In this study, we examined the expression of adiponectin receptors (AdipoR1 and AdipoR2) and assessed the function of adiponectin in gastric cancer. All of the six gastric cancer cell lines significantly expressed mRNA and protein of both receptors with variable levels. Addition of 30 µg/mL adiponectin potently induced apoptosis and inhibited the proliferation of AZ521 and HCG27. Down‐regulation of either AdipoR1 or AdipoR2 by specific siRNA significantly suppressed the growth inhibitory effects of adiponectin in both cell lines. Moreover, a local injection of adiponectin markedly inhibited the growth of AZ521 inoculated subcutaneously in nude mice. Similarly, the continuous intraperitoneal infusion of adiponectin effectively suppressed the development of peritoneal metastasis of AZ521. Adiponectin negatively regulates the progression of gastric cancer cells possibly through both AdipoR1 and AdipoR2. Although adiponectin was already reported to have antiangiogenic effects, our results suggest that the antitumor effect of adiponectin was, at least partially, dependent on the direct effects on tumor cells. (Cancer Sci 2007; 98: 1120–1127)
It is well known that increased body weight is a risk factor for the development of metabolic syndrome. Recently, obesity has also been shown to be associated with increased mortality for various cancers.( 1 ) Consistently, many studies have shown a positive association between adiposity and increased risk of cancer at multiple sites.( 2 , 3 , 4 , 5 , 6 , 7 ) In gastric cancer, obesity is reported to be associated especially with the development of adenocarcinoma in the cardiac region.( 8 , 9 , 10 ) This association is thought to be explained partly by the increased rate of gastroesophageal reflux disease as a result of increased intra‐abdominal pressure.( 11 , 12 , 13 ) However, the reflux theory cannot explain every aspect of the manifestation of gastric cancer, and the epidemiologic study raises a hypothesis that adipose tissue might be functionally involved in the development of gastric cancer.
Adipocytes express various secretory proteins, including angiotensinogen, plasminogen activator 1 (PAI‐1), acylation‐stimulating protein (ASP), interleukin‐6 (IL‐6), tumor necrosis factor‐α (TNF‐α), resistin and leptin.( 14 , 15 , 16 , 17 , 18 , 19 ) Adiponectin is a peptide hormone secreted exclusively by adipose tissue. Adiponectin acts as an antiatherogenic hormone through the inhibition of vascular smooth muscle cell proliferation, as well as macrophage functions.( 20 , 21 ) Adiponectin also has an antidiabetic action through the modulation of glucose and fatty acid metabolism and insulin sensitivity.( 22 , 23 , 24 ) In fact, the circulating level of adiponectin has been reported to be inversely related to body mass index (BMI)( 25 , 26 , 27 ) and to be reduced in conditions of insulin resistance such as visceral obesity and type 2 diabetes.( 24 )
Recent studies have shown that a reduced adiponectin level in plasma is significantly correlated with the risk of various cancers.( 28 , 29 , 30 , 31 ) In gastric cancer, we have additionally reported that the plasma adiponectin level is negatively correlated with tumor stage.( 30 ) Moreover, being overweight also has a positive link with poor outcome in patients with certain cancers including gastric cancer.( 32 , 33 , 34 ) This finding together with the epidemiologic results, raises the possibility that adiponectin may be functionally involved in cancer progression as well as carcinogenesis.
The receptors for adiponectin have been recently defined and designated as AdipoR1/R2.( 23 ) AdipoR1 is widely expressed and is abundant in skeletal muscle, whereas AdipoR2 is predominantly expressed in the liver. AdipoR1/R2 have been reported to mediate increased AMP kinase and peroxisome proliferator activated receptor (PPAR)‐α ligand activity, fatty acid oxidation, and glucose uptake by adiponectin.( 35 ) Recent reports have shown that mRNA of AdipoR1/R2 were significantly expressed in breast( 36 , 37 ) and prostate( 38 ) cancers. However, the expression of AdipoR1/R2 in other malignant cells has not been evaluated yet. In the present study therefore we investigated the adiponectin receptor expression in human gastric carcinoma cell lines and then examined the role of adiponectin in the development and peritoneal metastasis of gastric cancer.
Materials and Methods
Reagents and cell lines. Recombinant full‐length murine adiponectin was produced and purified as described previously.( 39 , 40 ) AntiClean Etox affinity columns (Sterogene Bioseparations, Carlsbad, CA, USA) were used to remove endotoxin contamination and endotoxin levels in the adiponectin preparations were confirmed to be undetectable by Limulus Amoebocyte Lysate (LAL) assay. Recombinant human epidermal growth factor (EGF) was obtained from Peprotech EC (London, UK). Six human gastric cell lines, MKN28, MKN45, MKN74, HGC27 (Riken Cell Bank, Tsukuba, Japan), MKN1 and AZ521 (American Type Culture Collection, Manassas, VA, USA), were maintained in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS) and penicillin/streptomycin at 37°C in a humidified atmosphere of 5% CO2 in air. Fluorescein isothiocyanate (FITC) conjugated annexin V and propidium iodide (PI) for flow cytometry were obtained from Roche Diagnostics (Basel, Switzerland).
Preparation of total RNA and northern blot analysis. Total RNA was isolated from the six carcinoma cell lines by the acid guanidine isothiocyanate/phenol/chloroform extraction method, as described by Chomczynski and Sacchi.( 41 , 42 ) In brief, RNA was separated by 1.0% agarose‐formaldehyde gel electrophoresis, transferred onto a nylon membrane (GeneScreen; Perkin‐Elmer Life Sciences, Inc., Boston, MA, USA), and hybridized with cDNA probes labeled with [32P]dCTP (Perkin‐Elmer Life Sciences) using a DNA labeling kit (Nippon Gene, Toyama, Japan).
RT‐PCR of Adponectin. Real‐time polymerase chain reaction (RT‐PCR) was carried out using TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA, USA) to quantify the mRNAs of AdipoR1/R2.( 23 ) The primer sets and the probes for mAdipoR1/R2 were as follows: The forward primer for mAdipoR1 was 5′‐ACGTTGGAGAGTCATCCCGTAT‐3′, the reverse primer, 5′‐CTCTGTGTGGATGCGGAAGAT‐3′, and the probe, 5′‐CCTGCTACATGGCCACAGACCACCT‐3′; the forward primer for mAdipoR2 was 5′‐TCCCAGGAAGATGAAGGGTTTAT‐3′, the reverse primer, 5′‐TTCCATTCGTTCGATAGCATGA‐3′, and the probe, 5′‐ATGTCCCCGCTCCTACAGGCCC‐3′. The relative amount of each AdipoR transcript was normalized to the amount of cyclophilin transcript in the same cDNA.( 23 )
Western blot analysis of AdipoR1/R2. The protein fraction extracted from six gastric cancer cells was electrophoresed in sodium dodecyl sulphate (SDS)‐10% polyacrylamide gel for 45 min at 200 V. It was then transferred onto an Immobilon transfer membrane (Millipore, Bedford, MA, USA) for sequential incubation with 5% reconstituted non‐fat milk powder to block non‐specific sites, dilutions of rabbit polyclonal anti‐AdipoR1 and rabbit polyclonal anti‐AdipoR2 antibody( 23 ) and horseradish peroxidase‐labeled donkey antirabbit immunoglobulin G (IgG), before development with a standard enhanced chemiluminescence kit (Amersham, Buckinghamshire, UK).
Immunohistochemical study of AdipoR1/R2. Protein expression of AdipoR1/R2 was evaluated by immunohistochemical staining using affinity purified rabbit polyclonal antibodies against AdipoR1/R2 in surgically removed specimens of gastric cancer. Parts of the freshly resected gastric cancer tissues were immediately fixed with liquid nitrogen, and each tissue was cut into serial‐step sections 3 µm wide. After being rinsed in phosphate‐buffered saline (PBS), endogenous peroxidase activity was inhibited by incubation with 0.3% hydrogen peroxide in 100% methanol for 30 min. After three washes in PBS, the non‐specific reaction was blocked by incubation with PBS containing 5% skimmed milk for 30 min at room temperature, and then the sections were incubated with normal rabbit serum for 30 min. The sections were incubated overnight at 4°C in humid chambers with the primary antibody to AdipoR1/R2 at 1:1000 dilution. After three washes with PBS, the sections were incubated with biotinylated rabbit antirabbit immunoglobulin for 30 min. After another wash with PBS, the slides were treated with peroxidase‐conjugated streptavidin for 30 min, and developed by immersion in 0.01% H2O2 and 0.05% diaminobenzidine tetrahydrochloride for 3 min. Then, light counterstaining with Mayer's hematoxylin was carried out.
Proliferation assay. AZ521 and HGC27 cells (5 × 103 cells in 100 µL/well) were seeded in a 96‐well plate in DMEM containing 0.1% fatty acid‐free BSA with or without 100 ng/mL EGF. In each experiment, adiponectin was added every 24 h at various concentrations. After incubation at 37°C in 5% CO2 for 48 h, the number of living cells was measured using an MTS assay (Promega, Madison, WI, USA) according to the manufacturer's instructions. Briefly, MTS solution was added to each well, and the cells were incubated for an additional 3 h. The number of living cells was determined by measuring absorbance at 490 nm using a 96‐well Microplate Reader (Bio‐Rad, Hercules, CA, USA).
Detection of apoptosis by flow cytometry. AZ521 or HGC27 stained with FITC‐conjugated annexin V and PI for 5 min at room temperature. The population of annexin V(–) PI(–) viable cells and annexin V(+) apoptotic cells was evaluated by flow cytometry. Data were collected in a FACS Calibur (Becton‐Dickinson, Mountain View, CA, USA) and analyzed using CellQuest software (Becton Dickinson, Mountain View, CA, USA).
RNA interference. Two pairs of siRNAs were chemically synthesized, annealed and transfected into 60–70% confluent AZ521 and HGC27 using Lipofectamine Plus (Life Technologies, Taipei, Taiwan) as described previously.( 43 ) The sequences of the sense siRNAs were as follows:
Human AdipoR1: GGACAACGACUAUCUAUCUGCUACATT
Human AdipoR2: GGAGUUUCGUUUCAUGAUCGGTT
Unrelated dorphin RNA sequence was used as mock sRNA.
In vivo experiments. Five‐week‐old female BALB/c nude mice (Nihon CLEA, Tokyo, Japan) were used. AZ521 cells suspended in PBS, and 1 × 106 cells in 100 µL were implanted subcutaneously in the middle of the back of each animal as described previously.( 44 ) Adiponectin (5 or 50 µg per mouse) dissolved in 100 µL PBS was intratumorally injected every day from 3 days after tumor inoculation throughout the experimental period (n = 5 mice per group). Control animals were injected with the same amount of PBS. Visible tumors were measured using digital calipers at the indicated time points and tumor volume was calculated as reported elsewhere.( 44 ) After 22 days of treatment, mice were killed, and the tumor tissue removed. Tumor volume was calculated according to the formula: width2 × length × 0.52, as previously reported.( 45 )
In experiments to examine peritoneal metastasis, a single cell suspension of 5 × 106 AZ521 cells resuspended in 1 mL PBS was injected into the peritoneal cavity of each mouse with a 23‐gauge syringe. Three days later, an Alzet micropump (model 2004, Alza, Palo Alto, CA, USA) was inserted into the peritoneum of each mouse. The pump was used to continuously deliver the indicated doses of adiponectin or PBS in a total volume of 0.2 mL. On day 28, all mice were killed, and the number of macroscopic nodules on the peritoneal surface was counted. All of the in vivo experimental protocols were approved by the animal care committee of The University of Tokyo.
Measurement of microvessel density and detection of apoptosis. In the peritoneal metastasis model, disseminated tumors were dissected and fixed in 3% paraformaldehyde, dehydrated, and embedded in paraffin. Blood vessel endothelial cells were immunohistochemically detected using a rat antimouse CD31 (1:300; Pharmingen, San Diego, CA, USA) primary antibody. Apoptotic cells were detected by terminal deoxynucleotidyltransferase‐mediated dUTP nick end labeling (TUNEL) method using an ApopTag peroxidase in situ apoptosis detection kit (Chemicon, Temecula, CA, USA). These sections were photographed, and signals were quantified under a microscope (×200 magnification) in 10 fields randomly selected in five tumors per group.
Statistical analysis. All statistical calculations were carried out using StatView‐J 5.0 statistical software (SAS Institute, Cary, NJ, USA). Transfection efficiency and number of metastatic nodules were analyzed by anova. Differences with a P‐value of less than 0.05 were considered to be statistically significant.
Results
Expression of AdipoR1/R2 in human gastric cancer cells. First, we examined the expression of AdipoR1/R2 mRNAs in the six gastric cancer cell lines with Northern blot analysis. As shown in Fig. 1a, both AdipoR1/R2 were positively detected at 2.0 kb and 4.0 kb, respectively. RT‐PCR analysis also showed significant levels of expression of AdipoR1/R2 messages in all cell lines, although the expression levels varied among the six cell lines (Fig. 1b).
Figure 1.
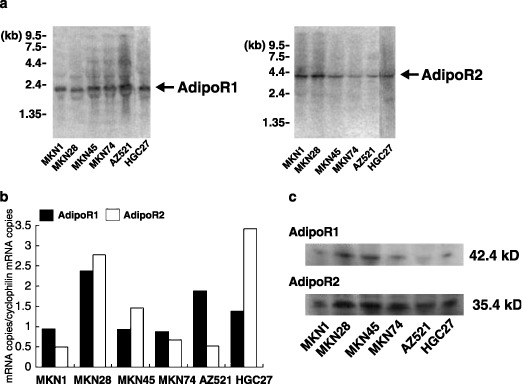
The expression of AdipoR1/R2 in human gastric cancer. (a) Northern blot analysis of AdipoR1 (left panel) and AdipoR2 (right panel) mRNA by different gastric cancer cells. Both receptors were detectable at 2.0 and 4.0 kb, respectively. (b) Reverse transcription‐polymerase chain reaction (RT‐PCR) was carried out on the total RNA as described in the material and methods section. The relative amounts of each AdipoR1/R2 transcript were normalized against the amount of cyclophilin transcript in the same cDNA.
Then, we examined the protein expression of these receptors by Western blotting using polyclonal antibodies against AdipoR1/R2 (Fig. 1c). Bands of AdipoR1 and R2 were detectable at 42.4 kDa and 35.4 kDa, respectively. Furthermore, the protein expression of AdipoR1 and AdipoR2 was confirmed by immunohistochemical staining of frozen sections of surgically resected tissue specimens. Surface epithelial cells in the normal part of the stomach did not show significant immunoreactivity for either AdipoR1 or R2, although some parietal cells showed slight immunoreactivity for Adipo R2 (Fig. 2a,c). In contrast, most of the gastric cancer cells were positively stained both for AdipoR1 and R2 (Fig. 2b,d).
Figure 2.
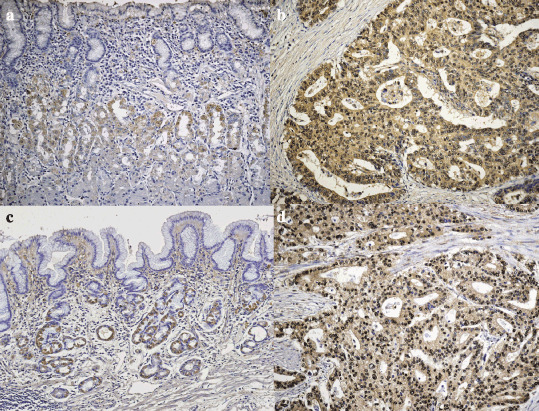
Immunostaining of AdipoR1/R2 in human gastric tisuue. Immunohistochemical staining of AdipoR1 (a; normal mucosa, b; carcinoma) and AdipoR2 (c; normal mucosa, d; carcinoma) using surgically resected gastric cancer tissues. AdipoR1 and R2 were positively detected in the cytoplasm as well as the cell membrane of carcinoma cells.
Adiponectin suppresses proliferation of AZ521 and HGC27 cells. Then, we examined the effects of recombinant adiponectin on proliferation. The addition of the adiponectin showed inhibitory effects on the proliferation of the six cell lines. Among them, the most prominent effects were observed in AZ521 and HGC27, which showed high expression of AdipoR1/R2 mRNA. At 48 h incubation, adiponectin showed no effects at concentrations in the order of ng/mL, but did inhibit growth at concentrations above 3–30 µg/mL (Fig. 3a). In AZ521, adiponectin slightly suppressed proliferation at 10 µg/mL, and the effect was significant at a concentration of 30 µg/mL (38 ± 3.8%, P < 0.05). In HCG27, the inhibitory effect of adiponectin was significant at 3 µg/mL (68 ± 4.1%, P < 0.05) and reached 16 ± 1.5% (P < 0.01) at 30 µg/mL. Even when the cancer cells were stimulated with 100 ng/mL EGF, the inhibition of growth by adiponectin was still effective at a concentration of 30 µg/mL in both cell lines (Fig. 3b).
Figure 3.
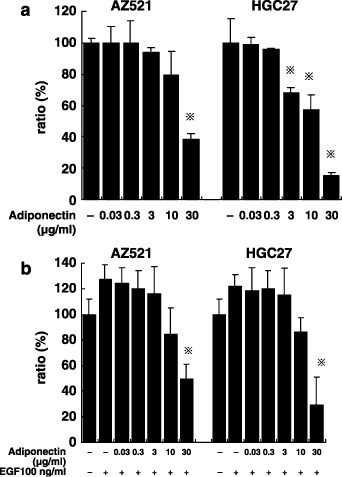
The effect of adiponectin on cell proliferation of AZ521 and HGC27. These cells were cultured with indicated concentrations of adiponectin in the absence (a) or presence (b) of 100 µM epidermal growth factor (EGF). After incubation for 120 h, cell proliferation was measured by a MTS assay. Data are expressed as a percentage of the control, where control indicates cell proliferation without adiponectin (100%). Columns indicate the mean of five different experiments carried out in triplicate; bars indicate SD. *P < 0.05, compared with control by one‐way anova combined with Bonferroni's test.
Adiponectin induces apoptosis of gastric cancer cells. In order to examine the mechanisms of the inhibitory effects of adiponectin, we examined the apoptotic index by annexin V‐PI staining using flow cytometry (Fig. 4a). The addition of adiponectin (30 µg/mL) to AZ521 for 6 h significantly increased the annexin V(+) PI(–) population as compared with the controls, and incubation for 24 h resulted in a marked increase in the annexin V(+) PI(+) population (Fig. 4a). More than 60% of AZ521 and HGC27 were annexinV(+) after 24 h of treatment with 30 µg/mL adiponectin, as compared to those without adiponectin (Fig. 4b). This clearly indicates that adiponectin inhibits the proliferation of gastric cancer cells mostly through the induction of apoptosis.
Figure 4.
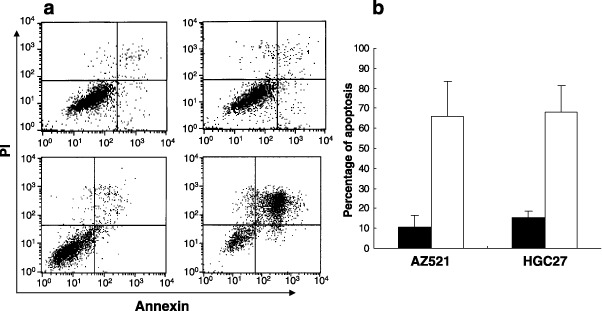
Apoptosis of AZ521 cancer cell with adiponectin. (a) AZ521 cell was cultured with (lower panel) or without (upper panel) 30 µg/mL adiponectin for 6 h (left panel) and 24 h (right panel), and then was double‐stained by Annexin‐V (x‐axis) and PI (y‐axis). The representative FACS profile was shown. (b) AZ521 and HGC 27 were cultured for 24 h with (white bar) or without (black bar) 30 µg/mL adiponectin for 24 h and Annexin‐V positive percentages were calculated as apoptotic cells. Columns indicate the mean of three different experiments performed in triplicate; bars indicate SD.
Inhibition of cell proliferation is dependent on AdipoR1/R2. We next examined the inhibitory effects of adiponectin in those cells in which AdipoR1 or AdipoR2 was specifically down‐regulated by siRNA. The addition of siRNA to AdipoR1 and R2 strongly suppressed the mRNA expression of corresponding receptors after 48 h treatment as evaluated RT‐PCR (Fig. 5a), as well as Western blotting (Fig. 5b) in AZ521. In this experimental condition, inhibition of AdipoR1 or AdipoR2 significantly restored the adiponectin‐induced growth inhibition. In AZ521 treated with control siRNA, 30 µg/mL adiponectin induced 33.6 ± 3.1% growth inhibition, whereas the effect was significantly restored by treatment with siRNA of AdipoR1 or AdipoR2 (Fig. 5c). In the same experimental condition, siRNA of AdipoR1 strongly abolished the effects of adiponectin to inhibit HGC27 proliferation, although the effect of siRNA of AdipoR2 was less prominent (Fig. 5c).
Figure 5.
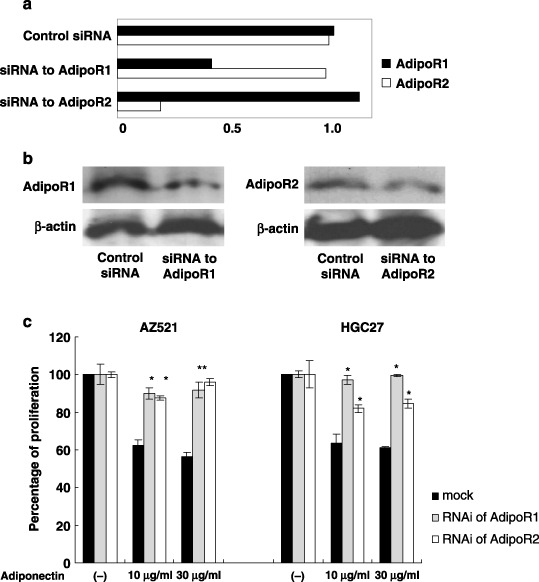
The effect of adiponectin in AdipoR1/R2 down‐regulated cells. AZ521 was cultured with 3 nM siRNA of AdipoR1/R2 or control siRNA for 48 h. (a) The relative amounts of each AdipoR1/R2 mRNA against cyclophilin were measured with real‐time‐polymerase chain reaction (RT‐PCR). (b) The protein expressions of AdipoR1/R2 were examined with Western blotting. (c) AZ521 and HGC27 treated with the same condition described above were cultured with or without 10 or 30 µg/mL adiponectin for additional 24 h and then the cell proliferation was evaluated as described in the legend of Figure 3. Columns indicate the mean of five studies performed in triplicate; bars indicate SD. *P < 0.05, compared with control by one‐way ANOVA combined with Bonferroni's test.
Suppression of subcutaneous tumor growth and peritoneal dissemination of AZ521. In order to examine the antitumor activity of adiponectin in vivo, we examined the growth of AZ521 inoculated subcutaneously or intraperitoneally in nude mice. In the subcutaneous model, repeated intratumorous administration of adiponectin from 3 days after tumor inoculation resulted in significant suppression of tumor growth. As shown in Figure 6, a low dose of adiponectin (5 µg per mouse) induced a slight but significant reduction of tumor volume after 3 weeks. More impressively, an 80% reduction of tumor volume was observed in mice treated with a high dose of adiponectin (50 µg per mouse).
Figure 6.
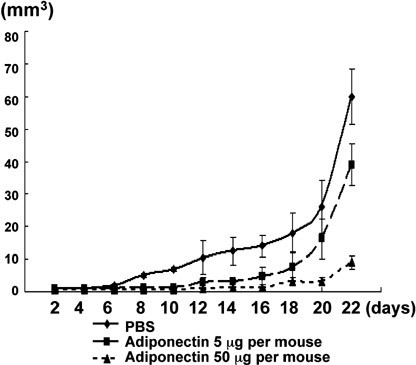
Growth inhibition of subcutaneous tumor by the treatment with adiponectin. AZ521 (1 × 106 in 100 µL phosphate‐buffered saline [PBS]) were subcutaneously implanted in nude mice. Adiponectin (5 µg or 50 µg per mouse) dissolved in 100 µL of PBS or PBS alone were intratumorally injected every day from 3 days after tumor inoculation throughout the experiment. Dots and bars indicate mean and SD in five mice treated with high or low concentrations of adiponectin and PBS in a representative one in three different sets of experiments.
In the intraperitoneal inoculation model, mice received intraperitoneal injections of AZ521 cells, and 3 days later PBS or adiponectin was injected intraperitoneally using a micropump inserted into the peritoneal cavity of each mouse. Then, we examined whether continuous injection of adiponectin resulted in inhibition of peritoneal dissemination, by counting the macroscopic nodules of peritoneal dissemination at 2 weeks after tumor inoculation. In the control group, metastatic nodules were observed on the peritoneal surface, especially in the vicinity of the stomach or intestine and pelvis, whereas only a few nodules were detected on the surface of the parietal peritoneum (Fig. 7b). As shown in Fig. 7a, the total number of metastatic nodules was significantly reduced in the adiponectin group (3.3 ± 2.8) (P < 0.05) as compared to the control group (8.8 ± 5.7). Moreover, each metastatic nodule was much smaller in the adiponectin group, and thus, the total volume of metastatic nodules on the peritoneum in the adiponectin group was greatly reduced to 14% of that in the control group (22.5 ± 25.5 mm3 vs 189.1 ± 152.6 mm3, P < 0.05) (Fig. 7a). The intraperitoneal infusion did not cause apparent side‐effects with no significant change of total weight.
Figure 7.
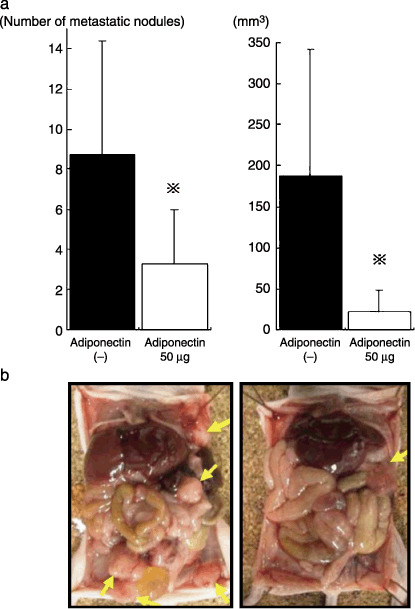
Inhibition of peritoneal metastasis by continuous intraperitoneal infusion of adiponectin. AZ521 (5 × 106 in 1 mL phosphate‐buffered saline [PBS]) was injected into the peritoneal cavity of each mouse, and 3 days later, 50 µg adiponectin or PBS were continuously infused into peritoneal cavity using an Alzet micropump. (a) On day 14, all mice were killed, and the number (left) and volume (right) of macroscopic nodules on the peritoneal surface were calculated. Columns and bars indicate mean and SD of five mice in a representative one in three different sets of experiments. (b) The representative peritoneal cavity of adiponectin (right) or PBS (left) injected mice. Arrow indicates peritoneal metastatic tumor.
Adiponectin reduces tumor vessels density and increases apoptotic cancer cells in peritoneal tumors. Finally, we evaluated the effects of adiponectin on neovascularization and apoptosis in peritoneal metastases. The vessel density in adiponectin‐treated tumors was significantly reduced to almost half of that in control tumors (Fig. 8a–c). Moreover, adiponectin treatment markedly increased the number of apoptotic tumor cells to 260% of controls (Fig. 9a–c).
Figure 8.
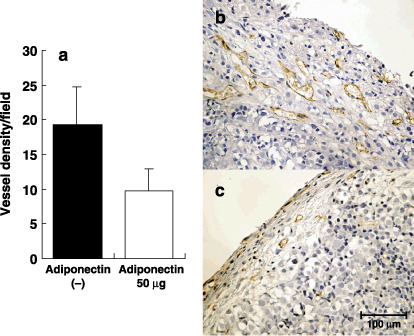
Inhibition of neovascularization in peritoneal tumors by adiponectin. (a) In peritoneal tumors, endothelial cells were immunohistochemically detected using a rat antimouse CD31 and vascular density were quantified by counting CD31 positive capillary in eight different fields randomly selected in four different tumors under ×400 magnification. Columns indicate the mean; bars indicate SD. Immunohistochemical expression of blood vessel endothelial cells in disseminated tumor with treated PBS (b) and adiponectin (c). Bar, 100 µm.
Figure 9.
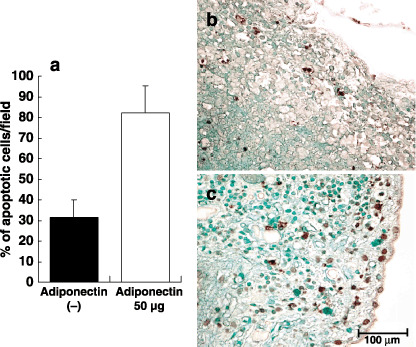
Induction of tumor apoptosis cells in peritoneal tumors by adiponectin. (a) In peritoneal tumors, apoptotic cells were immunohistochemically detected by the terminal deoxynucleotidyltransferase‐mediated dUTP nick end labeling (TUNEL) method and the number of apoptotic cells was quantified by counting TUNEL‐positive cells in eight different fields randomly selected in four different tumors under ×400 magnification. Columns indicate the mean; bars indicate SD. Apoptotic cells in tumors treated with phosphate‐buffered saline (PBS) (b) or adiponectin (c) were visualized as green staining. Bar, 100 µm.
Discussion
Adiponectin has recently attracted much attention in relation to the pathogenesis and treatment of the symptoms of metabolic syndrome such as cardiovascular disorders and diabetes.( 35 ) In mice, adiponectin replacement has been shown to decrease hyperglycemia and hypertriglyceridemia, restore insulin sensitivity, and alleviate fatty liver disease.( 36 , 37 , 46 , 47 ) Adiponectin has also been reported to have direct antiatherosclerotic effects through the inhibition of adhesion molecule expression of endothelial cells and lipid accumulation in macrophages.( 48 , 49 ) Moreover, depletion of the adiponectin gene has been shown to greatly increase neointimal formation in response to external vascular cuff injury in mice.( 49 , 50 )
These studies suggest that adiponectin might be involved in the pathogenesis of various diseases associated with such cellular functions as proliferation, and migration as well as metabolism. In fact, adiponectin attenuated DNA synthesis, cell proliferation, and migration induced by several growth factors in cultured smooth muscle cells( 51 ) and endothelial cells.( 52 , 53 ) These findings strongly suggest that adiponectin can elicit anticancer effects, since tumor growth is largely dependent on angiogenesis. In fact, Brakenhielm et al. demonstrated that adiponectin inhibited the growth of mouse fibrosarcoma in vivo, and they proposed the possibility that adiponectin could be used as an antiangiogenic drug.( 53 )
In this study, the size of subcutaneous gastric cancer transplanted in nude mice was reduced by 80% when treated with intratumorous infusion of adiponectin, which is consistent with the results in syngenic fibrosarcoma. We found that adiponectin was effective to suppress only when administrated at a high concentration. However, the treatment of such a high dose of adiponectin did not produce marked adverse effects or significant weight change (data not shown). Since adiponectin is abundantly produced from adipose tissue and the plasma concentration of adiponectin is µg/mL level, this adipockine is supposed to elicit biological effects basically with such high concentrations. Moreover, our in vitro study provided clear evidence that adiponectin strongly inhibited the proliferation of AZ521 and HGC27 through the induction of apoptosis. The concentration of functional adiponectin for gastric cancer cells was 10–30 µg/mL, which is almost the same as that observed in smooth muscle cells and endothelial cells.( 21 , 53 ) Recently, the same effect in vitro has been reported in a breast cancer cells.( 54 ) The exact mechanism of adiponectin to induce apoptosis is currently being investigated.
In our experiments, the effect of adiponectin was not significant at 3 µg/mL, but was prominent at 30 µg/mL. The concentration appears to be physiologically relevant, since previous studies in humans have shown that the mean circulating adiponectin level in plasma is 7–12 µg/mL in healthy patients, but is changed by various pathological conditions such as obesity, metabolic syndrome, cardiovascular disease, and cancer.( 25 , 26 , 30 , 55 , 56 , 57 ) Furthermore, plasma adiponectin levels have been reported to be significantly reduced in patients with various cancers.( 28 , 29 , 30 , 31 ) This raises the possibility that regulation of the adiponectin concentration in serum or tumor stroma may have significant effects on the behavior of malignant cells. The results of our experiments could well explain why people with obesity, which is often associated with reduced plasma adiponectin level, have a higher risk of developing cancers.
So far, two receptors for adiponectin, AdipoR1/R2, have been recently cloned in humans.( 23 ) AdipoR1 is known to be ubiquitously expressed at the highest levels in skeletal muscle, while AdipoR2 is predominantly expressed in skeletal muscle and liver.( 23 ) AdipoR2 mRNA expression has been reported to be significantly reduced in liver biopsies of patients with nonalcoholic fatty liver disease (NASH).( 58 ) However, the tissue distribution and pathophysiological function of these two receptors are not fully understood. In our studies, we found that both AdipoR1 and AdipoR2 were widely expressed at considerable levels in gastric carcinoma cells. Moreover, adiponectin‐induced growth inhibition was significantly abrogated by down‐regulation of either AdipoR1 or AdipoR2 by specific siRNAs. These data suggest that the reduction in proliferation by adiponectin is dependent on both AdipoR1/R2, although the dependency on AdipoR1/R2 may vary among cell types. The expression level of each receptor in tumor cells may critically determine their biological characteristics.
In gastric cancer, peritoneal metastasis is one of the most important factors determining the prognosis of patients, and no reliable treatment has been developed to control the poor outcome.( 59 , 60 , 61 ) In this study therefore we examined intraperitoneal infusion of adiponectin as a possible treatment strategy for peritoneal dissemination of gastric cancer. In this therapeutic model, continuous adiponectin infusion into peritoneal cavity caused significant antitumor effects on peritoneal metastatic nodules, with reduced microvascular density and increased apoptotic tumor cells. In this mice model, the plasma concentration of adiponectin did not show significant difference by the intraperitoneal adiponectin infusion (data not shown), suggesting that maintenance of high adiponectin levels in the peritoneal cavity may be critical for suppression of peritoneal metastasis. Thus far, there has been no information on the physiological concentration of adiponectin in the peritoneal cavity. However, it can be presumed that a considerable amount of adiponectin may be present physiologically in the intra‐abdominal space, since there is abundant visceral adipose tissue in the peritoneal cavity that is capable of producing this adipocytokine. The role of intraperitoneal adiponectin in the development of peritoneal metastasis is an intriguing issue for future study.
In summary, this study demonstrates that a physiologically present adipokine, adiponectin, has direct antitumor effects presumably through AdipoR1/R2 being expressed on gastric cancer cells. Although adiponectin was already reported to have antiangiogenic effects, our results suggest that adiponectin can potently regulate the growth of cancer cells as well as endothelial cells, and that the antitumor effect observed in vivo was, at least partly dependent on the direct effects on tumor cells. Adipose tissues are strongly suggested to have positive roles in tumor development and progression through the production of many cytokines such as adiponectin. Investigation of the interaction between tumors and fat cells may provide a clue to elucidate unknown, but critically important, mechanisms in tumor biology.
References
- 1. Calle EE, Rodriguez C, Walker‐Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003; 348: 1625–38. [DOI] [PubMed] [Google Scholar]
- 2. Carroll KK. Obesity as a risk factor for certain types of cancer. Lipids 1998; 33: 1055–9. [DOI] [PubMed] [Google Scholar]
- 3. Bergstrom A, Pisani P, Tenet V, Wolk A, Adami HO. Overweight as an avoidable cause of cancer in Europe. Int J Cancer 2001; 91: 421–30. [DOI] [PubMed] [Google Scholar]
- 4. Peto J. Cancer epidemiology in the last century and the next decade. Nature 2001; 411: 390–5. [DOI] [PubMed] [Google Scholar]
- 5. Cleary MP, Maihle NJ. The role of body mass index in the relative risk of developing premenopausal versus postmenopausal breast cancer. Proc Soc Exp Biol Med 1997; 216: 28–43. [DOI] [PubMed] [Google Scholar]
- 6. Ji BT, Chow WH, Yang G et al. Dietary habits and stomach cancer in Shanghai, China. Int J Cancer 1998; 76: 659–64. [DOI] [PubMed] [Google Scholar]
- 7. Tuyns AJ, Kaaks R, Haelterman M, Riboli E. Diet and gastric cancer. A case‐control study in Belgium. Int J Cancer 1992; 51: 1–6. [DOI] [PubMed] [Google Scholar]
- 8. Vaughan TL, Davis S, Kristal A, Thomas DB. Obesity, alcohol, and tobacco as risk factors for cancers of the esophagus and gastric cardia: adenocarcinoma versus squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev 1995; 4: 85–92. [PubMed] [Google Scholar]
- 9. Vaughan TL, Farrow DC, Hansten PD et al. Risk of esophageal and gastric adenocarcinomas in relation to use of calcium channel blockers, asthma drugs, and other medications that promote gastroesophageal reflux. Cancer Epidemiol Biomarkers Prev 1998; 7: 749–56. [PubMed] [Google Scholar]
- 10. Lagergren J, Bergstrom R, Nyren O. Association between body mass and adenocarcinoma of the esophagus and gastric cardia. Ann Intern Med 1999; 130: 883–90. [DOI] [PubMed] [Google Scholar]
- 11. Fraser‐Moodie CA, Norton B, Gornall C, Magnago S, Weale AR, Holmes GK. Weight loss has an independent beneficial effect on symptoms of gastro‐oesophageal reflux in patients who are overweight. Scand J Gastroenterol 1999; 34: 337–40. [DOI] [PubMed] [Google Scholar]
- 12. Terry P, Lagergren J, Wolk A, Nyren O. Reflux‐inducing dietary factors and risk of adenocarcinoma of the esophagus and gastric cardia. Nutr Cancer 2000; 38: 186–91. [DOI] [PubMed] [Google Scholar]
- 13. Stein HJ, Kauer WK, Feussner H, Siewert JR. Bile reflux in benign and malignant Barrett's esophagus: effect of medical acid suppression and nissen fundoplication. J Gastrointest Surg 1998; 2: 333–41. [DOI] [PubMed] [Google Scholar]
- 14. Guerre‐Millo M. Adipose tissue and adipokines: for better or worse. Diabetes Metab 2004; 30: 13–9. [DOI] [PubMed] [Google Scholar]
- 15. Maeda K, Okubo K, Shimomura I, Mizuno K, Matsuzawa Y, Matsubara K. Analysis of an expression profile of genes in the human adipose tissue. Gene 1997; 190: 227–35. [DOI] [PubMed] [Google Scholar]
- 16. Spiegelman BM, Choy L, Hotamisligil GS, Graves RA, Tontonoz P. Regulation of adipocyte gene expression in differentiation and syndromes of obesity/diabetes. J Biol Chem 1993; 268: 6823–6. [PubMed] [Google Scholar]
- 17. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 1994; 372: 425–32. [DOI] [PubMed] [Google Scholar]
- 18. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor‐alpha: direct role in obesity‐linked insulin resistance. Science 1993; 259: 87–91. [DOI] [PubMed] [Google Scholar]
- 19. Shimomura I, Funahashi T, Takahashi M et al. Enhanced expression of PAI‐1 in visceral fat: possible contributor to vascular disease in obesity. Nat Med 1996; 2: 800–3. [DOI] [PubMed] [Google Scholar]
- 20. Yokota T, Oritani K, Takahashi I et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood 2000; 96: 1723–32. [PubMed] [Google Scholar]
- 21. Arita Y, Kihara S, Ouchi N et al. Adipocyte‐derived plasma protein adiponectin acts as a platelet‐derived growth factor‐BB‐binding protein and regulates growth factor‐induced common postreceptor signal in vascular smooth muscle cell. Circulation 2002; 105: 2893–8. [DOI] [PubMed] [Google Scholar]
- 22. Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen‐like factor, apM1 (AdiPose Most abundant Gene transcript 1). Biochem Biophys Res Commun 1996; 221: 286–9. [DOI] [PubMed] [Google Scholar]
- 23. Yamauchi T, Kamon J, Ito Y et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003; 423: 762–9. [DOI] [PubMed] [Google Scholar]
- 24. Diez JJ, Iglesias P. The role of the novel adipocyte‐derived hormone adiponectin in human disease. Eur J Endocrinol 2003; 148: 293–300. [DOI] [PubMed] [Google Scholar]
- 25. Arita Y, Kihara S, Ouchi N et al. Paradoxical decrease of an adipose‐specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 1999; 257: 79–83. [DOI] [PubMed] [Google Scholar]
- 26. Hotta K, Funahashi T, Arita Y et al. Plasma concentrations of a novel, adipose‐specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol 2000; 20: 1595–9. [DOI] [PubMed] [Google Scholar]
- 27. Yang WS, Lee WJ, Funahashi T et al. Plasma adiponectin levels in overweight and obese Asians. Obes Res 2002; 10: 1104–10. [DOI] [PubMed] [Google Scholar]
- 28. Petridou E, Mantzoros C, Dessypris N et al. Plasma adiponectin concentrations in relation to endometrial cancer: a case‐control study in Greece. J Clin Endocrinol Metab 2003; 88: 993–7. [DOI] [PubMed] [Google Scholar]
- 29. Miyoshi Y, Funahashi T, Kihara S et al. Association of serum adiponectin levels with breast cancer risk. Clin Cancer Res 2003; 9: 5699–704. [PubMed] [Google Scholar]
- 30. Ishikawa M, Kitayama J, Kazama S, Hiramatsu T, Hatano K, Nagawa H. Plasma adiponectin and gastric cancer. Clin Cancer Res 2005; 11: 466–72. [PubMed] [Google Scholar]
- 31. Goktas S, Yilmaz MI, Caglar K, Sonmez A, Kilic S, Bedir S. Prostate cancer and adiponectin. Urology 2005; 65: 1168–72. [DOI] [PubMed] [Google Scholar]
- 32. McTiernan A. Obesity and cancer. the risks, science, and potential management strategies. Oncology (Williston Park) 2005; 19: 871–81; discussion (881–872): 885–76. [PubMed] [Google Scholar]
- 33. Stephenson GD, Rose DP. Breast cancer and obesity: an update. Nutr Cancer 2003; 45: 1–16. [DOI] [PubMed] [Google Scholar]
- 34. Moriwaki Y, Kunisaki C, Kobayashi S, Harada H, Imai S, Kasaoka C. Does body mass index (BMI) influence morbidity and long‐term survival in gastric cancer patients after gastrectomy? Hepatogastroenterology 2003; 50: 284–8. [PubMed] [Google Scholar]
- 35. Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev 2005; 26: 439–51. [DOI] [PubMed] [Google Scholar]
- 36. Dieudonne MN, Bussiere M, Dos Santos E, Leneveu MC, Giudicelli Y, Pecquery R. Adiponectin mediates antiproliferative and apoptotic responses in human MCF7 breast cancer cells. Biochem Biophys Res Commun 2006; 345: 271–9. [DOI] [PubMed] [Google Scholar]
- 37. Takahata C, Miyoshi Y, Irahara N, Taguchi T, Tamaki Y, Noguchi S. Demonstration of adiponectin receptors 1 and 2 mRNA expression in human breast cancer cells. Cancer Lett 2006. Nov 21; [Epub ahead of print]. [DOI] [PubMed]
- 38. Mistry T, Digby JE, Chen J, Desai KM, Randeva HS. The regulation of adiponectin receptors in human prostate cancer cell lines. Biochem Biophys Res Commun 2006; 348: 832–8. [DOI] [PubMed] [Google Scholar]
- 39. Yamauchi T, Kamon J, Waki H et al. The fat‐derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 2001; 7: 941–6. [DOI] [PubMed] [Google Scholar]
- 40. Berg AH, Du Combs TPX, Brownlee M, Scherer PE. The adipocyte‐secreted protein Acrp30 enhances hepatic insulin action. Nat Med 2001; 7: 947–53. [DOI] [PubMed] [Google Scholar]
- 41. Chomczynski P, Sacchi N. Single‐step method of RNA isolation by acid guanidinium thiocyanate‐phenol‐chloroform extraction. Anal Biochem 1987; 162: 156–9. [DOI] [PubMed] [Google Scholar]
- 42. Shida D, Kitayama J, Yamaguchi H et al. Lysophosphatidic acid (LPA) enhances the metastatic potential of human colon carcinoma DLD1 cells through LPA1. Cancer Res 2003; 63: 1706–11. [PubMed] [Google Scholar]
- 43. Miyagishi M, Taira K. U6 promoter‐driven siRNAs with four uridine 3′ overhangs efficiently suppress targeted gene expression in mammalian cells. Nat Biotechnol 2002; 20: 497–500. [DOI] [PubMed] [Google Scholar]
- 44. Eriksson A, Cao R, Pawliuk R et al. Placenta growth factor‐1 antagonizes VEGF‐induced angiogenesis and tumor growth by the formation of functionally inactive PlGF‐1/VEGF heterodimers. Cancer Cell 2002; 1: 99–108. [DOI] [PubMed] [Google Scholar]
- 45. Cao R, Wu HL, Veitonmaki N et al. Suppression of angiogenesis and tumor growth by the inhibitor K1‐5 generated by plasmin‐mediated proteolysis. Proc Natl Acad Sci USA 1999; 96: 5728–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L. Endogenous glucose production is inhibited by the adipose‐derived protein Acrp30. J Clin Invest 2001; 108: 1875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fruebis J, Tsao TS, Javorschi S et al. Proteolytic cleavage product of 30‐kDa adipocyte complement‐related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA 2001; 98: 2005–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ouchi N, Kihara S, Arita Y et al. Novel modulator for endothelial adhesion molecules: adipocyte‐derived plasma protein adiponectin. Circulation 1999; 100: 2473–6. [DOI] [PubMed] [Google Scholar]
- 49. Ouchi N, Kihara S, Arita Y et al. Adipocyte‐derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte‐derived macrophages. Circulation 2001; 103: 1057–63. [DOI] [PubMed] [Google Scholar]
- 50. Matsuda M, Shimomura I, Sata M et al. Role of adiponectin in preventing vascular stenosis. The missing link of adipo‐vascular axis. J Biol Chem 2002; 277: 37 487–91. [DOI] [PubMed] [Google Scholar]
- 51. Kubota N, Terauchi Y, Yamauchi T et al. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem 2002; 277: 25 863–6. [DOI] [PubMed] [Google Scholar]
- 52. Motoshima H, Wu X, Mahadev K, Goldstein BJ. Adiponectin suppresses proliferation and superoxide generation and enhances eNOS activity in endothelial cells treated with oxidized LDL. Biochem Biophys Res Commun 2004; 315: 264–71. [DOI] [PubMed] [Google Scholar]
- 53. Brakenhielm E, Veitonmaki N, Cao R et al. Adiponectin‐induced antiangiogenesis and antitumor activity involve caspase‐mediated endothelial cell apoptosis. Proc Natl Acad Sci USA 2004; 101: 2476–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kang JH, Lee YY, Yu BY et al. Adiponectin induces growth arrest and apoptosis of MDA‐MB‐231 breast cancer cell. Arch Pharm Res 2005; 28: 1263–9. [DOI] [PubMed] [Google Scholar]
- 55. Schondorf T, Maiworm A, Emmison N, Forst T, Pfutzner A. Biological background and role of adiponectin as marker for insulin resistance and cardiovascular risk. Clin Lab 2005; 51: 489–94. [PubMed] [Google Scholar]
- 56. Kumada M, Kihara S, Sumitsuji S et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol 2003; 23: 85–9. [DOI] [PubMed] [Google Scholar]
- 57. Trujillo ME, Scherer PE. Adiponectin – journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J Intern Med 2005; 257: 167–75. [DOI] [PubMed] [Google Scholar]
- 58. Kaser S, Moschen A, Cayon A et al. Adiponectin and its receptors in non‐alcoholic steatohepatitis. Gut 2005; 54: 117–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Maehara Y, Emi Y, Baba H et al. Recurrences and related characteristics of gastric cancer. Br J Cancer 1996; 74: 975–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Adachi Y, Oshiro T, Mori M, Maehara Y, Sugimachi K. Prediction of early and late recurrence after curative resection for gastric carcinoma. Cancer 1996; 77: 2445–8. [DOI] [PubMed] [Google Scholar]
- 61. Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg 2000; 87: 236–42. [DOI] [PubMed] [Google Scholar]


