Abstract
The therapeutic effect of agonistic anti‐OX40 (CD134) monoclonal antibody (mAb) in combination with radiotherapy was evaluated in a murine lung cancer model. After intradermal transplantation of ovalbumin (OVA)‐transfected Lewis lung carcinoma, C57BL/6 mice were irradiated locally with a single dose of 20 Gy in combination with an intratumoral injection of anti‐OX40 mAb at 50 µg on day 4 after transplantation, which is when the major axis of the inoculated tumor reached a diameter of 7–9 mm. On days 8, 11, and 14, the tumor‐bearing mice were further treated with the same dose of anti‐OX40 mAb. Anti‐OX40 mAb in combination with radiotherapy prolonged survival and provided greater efficacy than either single treatment against well‐established tumors. An in vivo depletion study suggested that therapeutic immunity was mainly CD8+ T‐cell dependent. OX40+CD8+ T cells were augmented in draining lymph nodes obtained from irradiated mice compared with those from non‐irradiated mice. OVA‐major histocompatibility complex tetramer+ CD8+ T cells had been strongly recruited to the draining lymph nodes obtained from mice treated with anti‐OX40 mAb in combination with radiotherapy, and strong antigen‐specific cytotoxicity was confirmed by a 51Cr‐release assay. Moreover, a tumor‐rechallenge model indicated that this combination therapy induced durable tumor immunity. Thus, anti‐OX40 mAb in combination with radiotherapy may potentially help the management of patients with lung cancer. (Cancer Sci 2008; 99: 361–367)
OX‐40, also known as CD134, is a 50‐kDa type‐I membrane glycoprotein that belongs to the tumor necrosis factor receptor superfamily.( 1 ) OX‐40 is expressed on activated CD4+ and CD8+ T cells and has been shown to be the sole receptor for the OX‐40 ligand.( 1 ) The OX‐40–OX‐40 ligand interaction supplies a costimulatory signal for T‐cell proliferation in a CD28‐independent manner,( 2 ) and plays a major role in the pathogenesis of several autoimmune diseases,( 3 , 4 ) and graft‐versus‐host disease.( 5 )
In cancer studies, OX‐40 expression on tumor‐infiltrating lymphocytes correlates with better survival in several human cancers,( 6 , 7 ) suggesting that OX40 signals may play a critical role in establishing an antitumor immune response. In vivo, deliberate ligation of OX40 in tumor‐bearing mice induces tumor eradication,( 8 , 9 ) whereas in some of the models, administration of agonistic OX40 monoclonal antibody (mAb) alone is insufficient to induce tumor eradication.( 8 , 10 ) Our preliminary experiments also showed insufficient therapeutic outcomes using agonistic anti‐OX40 mAb alone. Therefore, the development of more powerful strategies is of paramount importance to augment the immunotherapeutic effects of agonistic anti‐OX40 mAb.
Ionizing radiotherapy is one of the core modalities for treatment of localized cancer. However, radiation can cause additional immunosuppressive effects in the cancer host, as irradiation directly induces bone marrow suppression, resulting in a reduction in the absolute number of cells responsible for immunity. Elevated levels of tissue‐derived transforming growth factor‐β( 11 ) and interleukin‐10( 12 ) caused by irradiation diminish the activity of T‐cell immunity specific for tumor antigens.( 13 ) In contrast, it has been shown that irradiation has a role in enhancing tumor immunogenicity and homing effector cells to the tumor site via induction of tumor apoptosis and upregulation of major histocompatibility complex (MHC), and costimulatory, and adhesive molecules on tumor cells.( 14 ) Under these circumstances, investigators have attempted to optimize the use of irradiation in concert with various immunological modalities, such as recombinant cytokines, cytokine‐gene‐transduced tumor or virus vaccinations, dendritic cells, agonistic anti‐CD40 mAb, ex vivo‐activated cells from draining lymph nodes (DLN), antigen‐specific T cells, and peritumoral injection of CpG ologodeoxynucleotide, in mouse experimental models.( 15 , 16 , 17 )
Based on cumulative previous knowledge, we focused on irradiation as an immunological partner of agonistic anti‐OX40 mAb therapy. In the present study, we evaluated the therapeutic effect of combining agonistic anti‐OX40 mAb and irradiation in a murine lung cancer model.
Materials and Methods
Mice. Wild‐type C57BL/6 mice were obtained from Charles River Japan (Yokohama, Japan). The mice were all female and used at 5–6 weeks of age. All animal experiments were approved by the ethics committee at our animal institute.
Reagents and monoclonal antibodies. Phycoerythrin (PE)‐conjugated anti‐OX40 mAb was obtained from Serotec (Kidlington, UK). PE‐conjugated tetramer of H‐2Kb‐restricted ovalbumin (OVA)257–264 (SIINFEKL) molecules (OVA‐MHC tetramer) was purchased from MBL (Nagoya, Japan). Fluorescein isothiocyanate (FITC)‐labeled control IgG, anti‐H‐2Kb mAb, anti‐I‐Ab mAb, anti‐CD4 mAb, and FITC‐labeled anti‐CD8 mAb were obtained from BD Pharmingen (San Diego, CA, USA). Anti‐CD4 mAb‐conjugated microbeads and anti‐CD8 mAb‐conjugated microbeads for the MACS system were purchased from Miltenyi Biotec (Bergisch Gladbach, Germany). A hybridoma that secretes agonistic anti‐OX40 mAb (clone OX86) was obtained from European Collection of Cell Cultures (Salisbury, UK). A hybridoma that produces isotype‐matched control IgG (clone AIIB2 developed by Dr CH Damsky) was purchased from the Developmental Studies Hybridoma Bank (Iowa City, IA, USA) developed under the auspices of the National Institute of Child Health and Human Development and maintained by the University of Iowa Department of Biological Sciences (Iowa City, IA, USA). The antibodies purified from these hybridomas included a negligible amount of endotoxin (<20 pg/mL) confirmed by kinetic turbidimetric assay (Toxinometer MT‐358; Wako Pure Chemical Industries, Osaka, Japan).
Cell culture. The Lewis lung carcinoma (LLC) cell line is a mouse lung carcinoma of C57BL/6 origin. The LLC‐OVA cell line was derived from LLC by transfection with chicken OVA cDNA cloned into the expression vector pac‐NEO‐OVA, which was kindly provided by Dr M Bevan (Seattle, WA, USA).( 18 ) Cells were cultured in Iscove's Modified Dulbecco's medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Invitrogen), l‐glutamine, 25 mM HEPES buffer, 0.05 mM 2‐mercaptoethanol, penicillin, and streptomycin. YAC‐1 and MBL‐2 lymphoma were maintained in RPMI‐1640 medium (Sigma‐Aldrich, St Louis, MO, USA) supplemented with 10% fetal bovine serum, 2 mM l‐glutamine, 0.05 mM 2‐mercaptoethanol, HEPES, penicillin, and streptomycin.
Treatment protocol. Each mouse was injected intradermally in the right flank at day 0 with 1 × 106 LLC‐OVA tumor cells in 150 µL phosphate‐buffered saline (PBS). The mice bearing LLC‐OVA were irradiated locally with a single dose of 20 Gy in combination with anti‐OX40 mAb (50 µg) in 100 µL PBS on day 4, when the major axis of the inoculated tumor had reached a diameter of approximately 7–9 mm. On days 8, 11, and 14, the tumor‐bearing mice were further treated with the same dose of anti‐OX40 mAb (50 µg). Tumor size was measured in three dimensions using calipers every second day, and tumor volume was calculated using the following formula: tumor volume = 0.4 × [average diameter] (mm) × [height (mm)]2.( 19 )
Local irradiation. Mice bearing LLC‐OVA were anesthetized and immobilized in a plastic syringe with a hole through which we could pull out the target tumor. Mice were put under an 8 cm‐thick lead shield with a 10‐mm × 10‐mm hole. Local irradiation was then carried out with a single dose of 20 Gy (60Co source, 1 Gy/min; Toshiba, Tokyo, Japan) through the hole, at the Central Institute of Isotope Science, Hokkaido University.
Cell depletion. Hybridoma cell lines (GK1.5, 2.43, NK1.1) were obtained from the American Type Culture Collection (Manassas, VA, USA). Mice were depleted of CD4+, CD8+, or natural killer (NK) cells by an intraperitoneal injection of 1 mL of a 1:10 dilution of GK 1.5 ascites supernatant, 2.43 ascites supernatant, or NK 1.1 ascites supernatant, respectively, on day 2 (2 days prior to the first administration of anti‐OX40 mAb with irradiation) and subsequently every 4 days for a total of four inoculations. The amount of depletion was confirmed by flow cytometry and the specific depletion was 95–100% for each group (data not shown).
Frequency detection of OX40+ T cells in DLN. Tumor‐bearing mice were irradiated or non‐irradiated on day 4 and then killed on day 8. Cells derived from DLN were then stained with PE‐conjugated anti‐OX40 mAb followed by FITC‐conjugated anti‐CD4 or CD8 mAb. Stained cells were assayed on a FACSCalibur (BD Biosciences, San Diego, CA, USA), and the data were analyzed with CellQuest software (BD Biosciences).
Tetramer staining. Tumor‐bearing mice were treated with the same schedule as described in the treatment protocol, and then killed at 48 h after the final injection of anti‐OX40 mAb. Cells derived from DLN were then subjected to tetramer staining. We modified the procedure slightly for use of the OVA‐MHC tetramer. Briefly, lymphocytes from DLN were washed twice in PBS, stained with 2 µL OVA‐MHC tetramer per 1 × 106 cells, and then incubated at 4°C for 15 min. The cells were again washed in PBS and stained with FITC‐conjugated anti‐CD8 mAb at 4°C for 15 min. Stained cells were assayed on a FACSCalibur, and the data were analyzed with CellQuest software.
Cytotoxicity assay. Tumor‐bearing mice were treated with the same schedule as described in the treatment protocol, and then killed at 48 h after the final injection of anti‐OX40 mAb. The lymphocytes in the DLN were extracted from 12 mice treated with the combination of anti‐OX40 mAb and radiotherapy (approximately 1.25 × 108 cells harvested), and from three mice from each of the other treatments, which provided sufficient numbers of cells to examine and the cytolytic activity of the lymphocytes as assessed using standard 51Cr‐release assays. The tumor‐specific cytotoxicity of the cells was determined using LLC‐OVA as the target cells. MBL‐2, YAC‐1, and parental LLC cells were used as the controls. Expression of MHC class I molecule on LLC and LLC‐OVA was inducible with 100 ng/mL interferon (IFN)‐γ for 72 h in vitro (Fig. 1); we therefore pretreated the two cell lines with the same dose and treatment period of IFN‐γ before using them as target cells in the 51Cr‐release assay. All LLC‐OVA and LLC cells were MHC class II negative despite pretreatment with IFN‐γ (Fig. 1). The tests were run in triplicate at the indicated effector : target ratios (E : T). The percentage of specific 51Cr release was calculated using the following formula: ([sample release – background release]/[maximum release – background release]) × 100.
Figure 1.
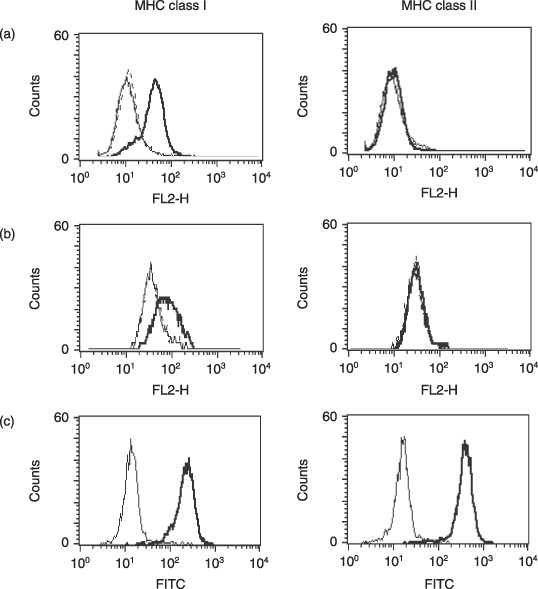
Expression of major histocompatibility complex (MHC) molecules on (a) Lewis lung carcinoma (LLC)‐ovalbumin, (b) LLC, and (c) MBL‐2 cells. (a,b) Simple, bold, and dotted lines indicate the expression levels of anticontrol IgG, and anti‐MHC molecule with or without pretreatment of 100 ng/mL interferon (IFN)‐γ for 72 h, respectively. (c) Simple and bold lines indicate the expression levels of anticontrol IgG and anti‐MHC molecule without pretreatment of IFN‐γ, respectively. FITC, fluorescein isothiocyanate.
Statistical analysis. Differences between treatment groups were analyzed using an unpaired two‐tailed Student's t‐test. Comparisons of survival among groups were calculated using the Wilcoxon rank‐sum test. All statistics were analyzed using Statview software (SAS Institute, Cary, NC, USA).
Results
Combination of anti‐OX40 mAb and radiotherapy induces a high rate of complete cure and prolongs survival. To test the therapeutic potential of anti‐OX40 mAb in combination with local radiotherapy, LLC‐OVA‐bearing mice were treated as described in Materials and Methods and then tumor growth and survival rate were compared with those of the single‐treatment groups. LLC‐OVA growth was marginally inhibited by radiotherapy alone (20 Gy) or anti‐OX40 mAb alone (50 µg) (Fig. 2a). However, none of the mice were cured. In contrast, six of the eight mice treated with anti‐OX40 mAb in combination with radiotherapy exhibited complete remission (Fig. 2). Local radiotherapy with 20 Gy was well tolerated by complete shielding of the entire body except for the tumor site. In parallel with these results, all mice treated with either single method died by day 80, whereas mice treated with anti‐OX40 mAb in combination with radiotherapy were alive with long‐term tumor eradication (Fig. 2b). These results suggest that anti‐OX40 mAb in combination with radiotherapy is efficient in tumor elimination, leading to long‐term survival with acceptable feasibility.
Figure 2.
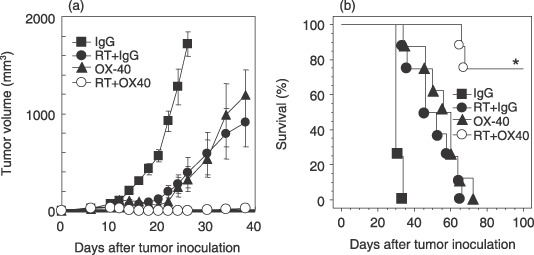
Anti‐OX40 monoclonal antibody (mAb) in combination with radiotherapy induces a high rate of complete remission and prolongs survival. Lewis lung carcinoma‐ovalbumin cells (1 × 106) were inoculated intradermally into C57BL/6 mice. When the tumor diameter of the major axis reached 7–9 mm on day 4, the tumor‐bearing mice were treated with the following protocols. (a) Tumor‐bearing mice were treated with control IgG on days 4, 8, 11, and 14 (closed square), or local radiotherapy (20 Gy) on day 4 with control IgG on days 4, 8, 11, and 14 (RT + IgG: closed circle), anti‐OX40 mAb alone on days 4, 8, 11, and 14 (50 µg, closed triangle), or radiotherapy (20 Gy) on day 4 with intratumoral injection of anti‐OX40 mAb on days 4, 8, 11, and 14 (50 µg, open circle). Tumor volume was calculated as described in Materials and Methods. Data are presented as the mean ± SE of eight mice in each experimental group. (b) The survival of mice following various protocols is shown. Similar results were obtained in three separate experiments. *P = 0.001 versus the anti‐OX40 mAb alone group, and P < 0.001 versus the RT + IgG group.
Tumor eradication by anti‐OX40 mAb in combination with radiotherapy is mainly CD8+ T‐cell dependent. To identify the immune cells mediating the antitumor effects, we analyzed the antitumor effects of anti‐OX40 mAb in combination with radiotherapy on established LLC‐OVA tumors in mice depleted of CD4+, CD8+, or NK cells. Figure 3 shows that the therapeutic effect of anti‐OX40 mAb in combination with radiotherapy was completely abrogated in mice depleted of CD8+ T cells, and all of these mice died by day 80, whereas mice with CD4+ T‐cell depletion showed barely a reduction in the effect of anti‐OX40 mAb in combination with radiotherapy. Tumor growth was mildly suppressed upon depletion of NK cells, and survival showed a marginal difference compared with mice treated with control IgG in combination with radiotherapy (P = 0.092), suggesting that NK cells might be dispensable for killing tumor cells in this system. These findings suggest that anti‐OX40 mAb in combination with radiotherapy is able to eradicate LLC‐OVA cells mainly through the generation of a CD8+ T‐cell response.
Figure 3.
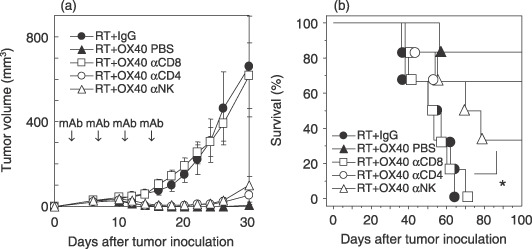
Depletion of CD8+ T cells abrogates the therapeutic effect of anti‐OX40 monoclonal antibody (mAb) in combination with radiotherapy. The Lewis lung carcinoma‐ovalbumin‐bearing mice were treated with anti‐OX40 mAb in combination with radiotherapy in CD4+‐, CD8+‐, or natural killer (NK) cell‐depleted mice. (a) Antitumor activities and (b) survival induced by treatment with or without antibody depletion are shown. αCD8 denotes depletion by anti‐CD8 mAb. The bars indicate the mean ± SE of six mice in each experimental group. *P = 0.092 versus the RT + IgG group. Similar results were obtained in three separate experiments. PBS, phosphate‐buffered saline; RT, radiotherapy.
Irradiation augments OX40+ T cells in DLN. To elucidate the killing mechanism in this system, we first investigated whether anti‐OX40 mAb therapy acted directly on LLC‐OVA cells. OX40 expression on LLC‐OVA cells was not detected (data not shown). The ratio of cell death of LLC‐OVA cells cultured with anti‐OX40 mAb (500 µg/mL = concentration of in vivo administration) for 4 days was similar to that of cells cultured with a control mAb for the same period. This was confirmed by flow cytometry using propidium iodide staining to measure DNA fragmentation (data not shown). Furthermore, absolute viable cell numbers of LLC‐OVA cells cultured with both antibodies for 4 days were also similar, which was confirmed by trypan blue staining (data not shown).
In this model, we found that the tumor started to shrink after administration of the second round of anti‐OX40 mAb on day 4 after irradiation plus first anti‐OX40 administration. In the following experiment, we used this timing based on the presumption that the immunological environment, including the proportion of OX40+ T cells in DLN, might change, thereby causing a synergic effect to occur. Thus, we compared the OX40+ T cells in DLN between irradiated and non‐irradiated mice on day 8. Both groups of mice were examined for the proportion and absolute number of OX40+ T cells in total cells from DLN by flow cytometric analysis. Figure 4a shows representative data from the analysis. The proportions of OX40+CD4+ and OX40+CD8+ T cells of the total cells from DLN extracted from a non‐irradiated mouse were 2.14 and 0.52%, respectively, whereas those from an irradiated mouse were 3.59 and 0.85%, respectively. Pooled analysis of the data from five individual mice revealed that the proportion and absolute number of either OX40+CD4+ or OX40+CD8+ T cells was significantly augmented in DLN obtained from irradiated mice compared with those from non‐irradiated mice (P = 0.002 for OX40+CD4+ T cells, P = 0.02 for OX40+CD8+ T‐cells in Fig. 4b; P = 0.02 for OX40+CD4+ T cells, P = 0.02 for OX40+CD8+ T cells in Fig. 4c). This result suggests that irradiation might play a role in expanding endogenous OX40+ T cells in DLN.
Figure 4.
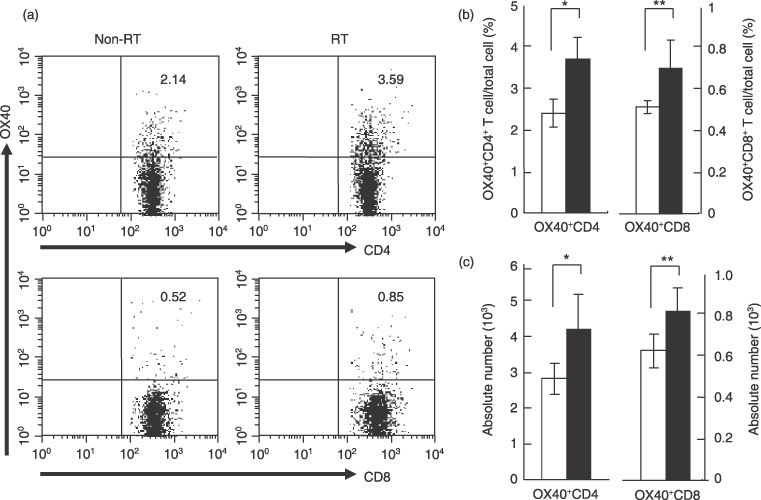
Irradiation augments OX40+ T cells in draining lymph nodes (DLN). Lewis lung carcinoma‐ovalbumin cells (1 × 106) were inoculated intradermally into C57BL/6 mice, and irradiated or non‐irradiated on day 4. DLN obtained from both mice on day 8 were labeled with phycoerythrin‐conjugated anti‐OX40 mAb, followed by staining with fluorescein isothiocyanate‐conjugated anti‐CD4 or CD8 mAb. (a) Representative data from flow cytometric analysis. The numbers in each graph indicate the percentage of OX40+ cells in total cells from DLN. (b,c) Flow cytometric analysis pooled from five separate analyses. Data are presented as the mean ± SE. White and black bars indicate non‐irradiated mice (non‐radiotherapy (RT)) and irradiated mice (RT), respectively. (b) Proportion of OX40+ cells in DLN from the non‐RT and RT groups. *P = 0.002; **P = 0.02. (c) Absolute number of OX40+ cells in DLN from the non‐RT and RT groups. *P = 0.02; **P = 0.02.
Anti‐OX40 mAb in combination with radiotherapy efficiently recruits antigen‐specific CD8+ T cells into DLN. Next we examined whether anti‐OX40 mAb in combination with radiotherapy allowed the efficient recruitment of OVA‐MHC tetramer+ CD8+ T cells into DLN. Representative data are shown in Figure 5a. The proportion of OVA‐MHC tetramer+ CD8+ T cells of the total cells was 0.07% in a control mouse, 0.21% in a mouse treated with radiotherapy and control IgG, 0.47% in a mouse treated with anti‐OX40 mAb alone, and 0.88% in a mouse treated with anti‐OX40 mAb and radiotherapy. The frequency of OVA‐MHC tetramer+ CD8+ T cells in total cells in DLN from mice treated with anti‐OX40 mAb in combination with radiotherapy was significantly higher compared with those from mice who underwent other treatments (P = 0.005 vs anti‐OX40 mAb alone group; Fig. 5b).
Figure 5.
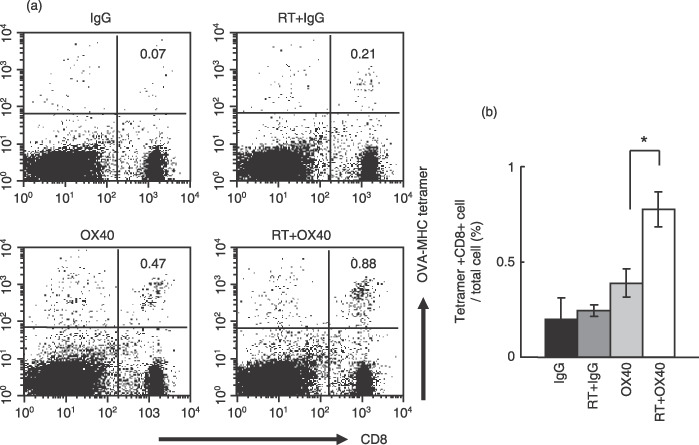
Ovalbumin (OVA)‐major histocompatibility complex (MHC) tetramer+ CD8+ cells were efficiently mobilized into draining lymph nodes (DLN) from mice treated with anti‐OX40 monoclonal antibody (mAb) in combination with radiotherapy. The Lewis lung carcinoma‐OVA‐bearing mice were treated with the protocols as described in Materials and Methods. DLN obtained from treated mice were labeled with phycoerythrin‐conjugated OVA‐MHC tetramer, followed by staining with fluorescein isothiocyanate‐conjugated anti‐CD8 mAb. (a) Representative data from flow cytometric analysis. The numbers in each graph indicate the percentage of OVA‐MHC tetramer+ cells in total cells from DLN. (b) Flow cytometric analysis pooled from four separate analyses. Data are presented as the mean ± SE. *P = 0.005. RT, radiotherapy.
To investigate whether lymphocytes in DLN from mice treated with anti‐OX40 mAb in combination with radiotherapy contain tumoricidal effectors, we tested the cytolytic activity by 51Cr‐release assay. Whole lymphocytes from DLN from mice treated with anti‐OX40 mAb in combination with radiotherapy showed highest cytotoxicity against LLC‐OVA cells compared with those from mice who underwent other treatments (Fig. 6a). Next, CD4+ and CD8+ T cells from DLN from mice treated with anti‐OX40 mAb in combination with radiotherapy were sorted using the MACS system and each population was then analyzed for killing activity. CD8+ T cells without stimulation ex vivo showed highest cytotoxicity against LLC‐OVA (Fig. 6b). These CD8+ T cells lysed LLC‐OVA but not LLC, YAC‐1, or MBL‐2 cells, whereas CD4+ T cells did not kill LLC‐OVA (Fig. 6c). These findings demonstrate that anti‐OX40 mAb in combination with radiotherapy augments the recruitment of antigen‐specific CD8+ T cells into the DLN, possibly leading to the direct eradication of tumor cells in vivo.
Figure 6.
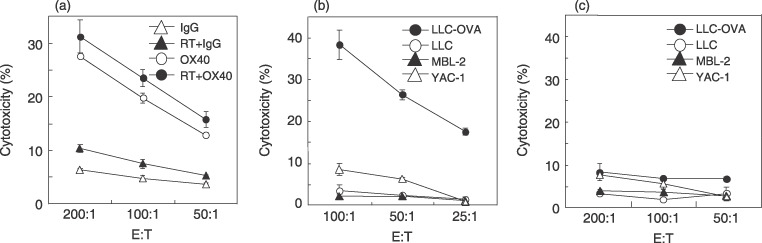
Tumor‐specific cytotoxic lymphocyte were generated by anti‐OX40 monoclonal antibody (mAb) in combination with radiotherapy. The Lewis lung carcinoma (LLC)‐ovalbumin (OVA)‐bearing mice were treated with the protocols as described in Materials and Methods. The lymphocytes in draining lymph nodes (DLN) were harvested from the treated mice. Harvested cells were used in (a) 8‐h and (b,c) 4‐h 51Cr‐release assays. Similar results were obtained in three separate experiments. Data are presented as the mean ± SE of triplicate samples. (a) Cytolytic activity by whole DLN cells from mice tested against LLC‐OVA. Cytolytic activity of (b) CD8+ and (c) CD4+ T cells in DLN from mice treated with anti‐OX40 mAb in combination with radiotherapy were tested against LLC‐OVA (closed circle), LLC (open circle), MBL‐2 (closed triangle), and YAC‐1 (open triangle). E : T indicates the effector : target ratio; RT, radiotherapy.
Anti‐OX40 mAb in combination with radiotherapy induces immunological memory. To determine whether long‐lasting tumor immunity was induced in mice cured by anti‐OX40 mAb in combination with radiotherapy, a tumor‐rechallenge experiment was carried out. Surviving mice were rechallenged with viable LLC‐OVA cells (1 × 106) contralaterally at 50–90 days after confirmation of complete remission by the initial anti‐OX40 mAb in combination with radiotherapy. Although none of the mice without prior treatment rejected the inoculated tumors, eight of nine survivors (89%) after treatment with anti‐OX40 mAb in combination with radiotherapy were able to resist the tumor rechallenge and remained disease free for an additional 100 days (Fig. 7). This result suggests that anti‐OX40 mAb in combination with radiotherapy leads to the development of immunological memory for tumor eradication.
Figure 7.
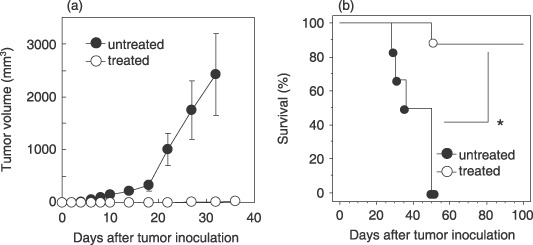
Anti‐OX40 monoclonal antibody (mAb) in combination with radiotherapy induces immunological memory. Mice 50–90 days after complete remission by anti‐OX40 mAb in combination with radiotherapy (treated mice) or aged‐matched untreated mice were injected intradermally with viable 1 × 106 Lewis lung carcinoma‐ovalbumin cells each in contralateral (left) flanks. (a) Antitumor activities and (b) survival induced by tumor rechallenge are shown. The bars indicate the mean ± SE of nine treated mice (open circles) and six untreated mice (closed circles). Data are presented as the mean ± SE. Similar results were obtained in two separate experiments. *P = 0.003.
Discussion
OX40 signals are known to play a critical role in establishing an antitumor immune response. However, the administration of agonistic OX40 mAb alone has not always been sufficient to induce tumor eradication. In the present study, we induced a high rate of complete cure against murine lung carcinoma by combined intratumoral administration of anti‐OX40 mAb and local radiotherapy. The antitumor immunity induced in our system was powerful to the point that CD8+ T cells in DLN extracted from mice with anti‐OX40 mAb, in combination with radiotherapy, strongly lysed LLC‐OVA without stimulation with dendritic cells pulsed with tumor lysate or OVA peptide ex vivo.
The role of OX40 in the enhancement of CD4+ T‐cell function has been well characterized. OX40 costimulation increases CD4+ T‐cell proliferation and effector functions.( 20 , 21 , 22 ) OX40 costimulation can also prevent or reverse CD4+ T‐cell tolerance( 23 ) and contribute to the generation of memory CD4+ T cells through promotion of the antiapoptotic proteins Bcl‐2 and Bcl‐XL.( 24 ) However, the tumor eradication demonstrated in the present study was primarily CD8+ T‐cell dependent.
One explanation of this finding is the augmentation of OX40+CD8+ T cells seen in the DLN from irradiated mice compared with those from non‐irradiated mice. Previous studies have shown that OX40 expression is upregulated on T cells when they encounter antigens.( 1 ) Thus, we believe that in the present study administration of anti‐OX40 mAb after radiotherapy stimulated OX40+CD8+ T cells and induced antigen‐specific CD8+ T‐cell expansion and tumor eradication. Augmentation of antigen‐specific CD8+ T cells was demonstrated by an increase in the frequency of both OVA‐MHC tetramer+ CD8+ T cells and high cytotoxicity against LLC‐OVA of CD8+ T cells in DLN. This mechanism might explain the synergistic effect of agonistic anti‐OX40 mAb and radiotherapy.
The most important thing in the present study was that the independent increase in antigen‐specific CD8+ T cells by anti‐OX40 mAb in combination with radiotherapy seemed to abrogate the requirement for CD4+ T‐cell help. Other studies have supported our findings that OX40 can directly costimulate antigen‐specific CD8+ T cells both in vivo ( 25 , 26 ) and in vitro. ( 27 ) However, it has been often described that an optimum effector response generally requires the participation of both CD4+ and CD8+ T cells,( 28 ) which was also observed in a tumor eradication model using anti‐OX40 mAb.( 8 ) Our data now raise questions about the role of OX40 costimulation of CD4+ T cells for the generation of immunological memory.
In DLN from mice treated with anti‐OX40 mAb alone, OVA‐MHC tetramer+ T cells and lymphocytes with relatively high cytotoxicity against LLC‐OVA were detected. However, anti‐OX40 mAb monotherapy did not completely cure LLC‐OVA tumors, as anti‐OX40 mAb in combination with radiotherapy did. A correlation between inhibition of tumor growth and the frequency of tetramer+ cells has been reported to be occasionally lacking.( 29 , 30 ) This lack of correlation in the present study might be due to the non‐irradiated tumors secreting higher immunosuppressive cytokines than the irradiated tumors. It is possible that these cytokines regulate OVA‐MHC tetramer+ CD8+ T cells mobilized into tumors from DLN, or reduce the long‐term killing activity of CD8+ T cells. Further investigation is required to elucidate the characteristic differences of CD8+ T cells within DLN or tumors between the anti‐OX40 mAb in combination with radiotherapy and the non‐irradiated groups.
In conclusion, antiagonistic OX40 mAb therapy in combination with radiotherapy may assist in managing lung cancer.
Acknowledgments
We thank Ms Takae Ohyama (Institute for Animal Experimentation at Hokkaido University) for her kind support in breeding the mice used in this experiment. We thank Dr Takashige Abe for technical advice regarding irradiation.
References
- 1. Sugamura K, Ishii N, Weinberg AD. Therapeutic targeting of the effector T‐cell co‐stimulatory molecule OX40. Nat Rev Immunol 2004; 4: 420–31. [DOI] [PubMed] [Google Scholar]
- 2. Akiba H, Oshima H, Takeda K et al . CD28‐independent costimulation of T cells by OX40 ligand and CD70 on activated B cells. J Immunol 1999; 62: 7058–66. [PubMed] [Google Scholar]
- 3. Higgins LM, McDonald SAC, Whittle N, Crockett N, Shields JG, MacDonald TT. Regulation of T cell activation in vitro and in vivo by targeting the OX40–OX40 ligand interaction: Amelioration of ongoing inflammatory bowel disease with an OX40–IgG fusion protein, but not with an OX40 ligand–IgG fusion protein. J Immunol 1999; 162: 486–93. [PubMed] [Google Scholar]
- 4. Weinberg AD, Wegmann KW, Funatake C, Whitham RH. Blocking OX‐40/OX‐40 ligand interaction in vitro and in vivo leads to decreased T cell function and amelioration of experimental allergic encephalomyelitis. J Immunol 1999; 162: 1818–26. [PubMed] [Google Scholar]
- 5. Blazar BR, Sharpe AH, Chen AI et al . Ligation of OX40 (CD134) regulates graft‐versus‐host disease (GVHD) and graft rejection in allogeneic bone marrow transplant recipients. Blood 2003; 101: 3741–8. [DOI] [PubMed] [Google Scholar]
- 6. Ladanyi A, Somlai B, Gilde K, Fejos Z, Gaudi I, Timar J. T‐cell activation marker expression on tumor‐infiltrating lymphocytes as prognostic factor in cutaneous malignant melanoma. Clin Cancer Res 2004; 10: 521–30. [DOI] [PubMed] [Google Scholar]
- 7. Petty JK, He K, Corless CL, Vetto JT, Weinberg AD. Survival in human colorectal cancer correlates with expression of the T‐cell costimulatory molecule OX‐40 (CD134). Am J Surg 2002; 183: 512–18. [DOI] [PubMed] [Google Scholar]
- 8. Kjaergaard J, Tanaka J, Kim JA, Rothchild K, Weinberg A, Shu S. Therapeutic efficacy of OX‐40 receptor antibody depends on tumor immunogenicity and anatomic site of tumor growth. Cancer Res 2000; 60: 5514–21. [PubMed] [Google Scholar]
- 9. Weinberg AD, Rivera MM, Prell R et al . Engagement of the OX‐40 receptor in vivo enhances antitumor immunity. J Immunol 2000; 164: 2160–9. [DOI] [PubMed] [Google Scholar]
- 10. Kjaergaard J, Peng L, Cohen PA, Drazba JA, Weinberg AD, Shu S. Augmentation versus inhibition: effects of conjunctional OX‐40 receptor monoclonal antibody and IL‐2 treatment on adoptive immunotherapy of advanced tumor. J Immunol 2001; 167: 6669–77. [DOI] [PubMed] [Google Scholar]
- 11. Canney PA, Dean S. Transforming growth factor β: a promoter of late connective tissue injury following radiotherapy? Br J Radiol 1990; 63: 620–3. [DOI] [PubMed] [Google Scholar]
- 12. Broski AP, Halloran PF. Tissue distribution of IL‐10 mRNA in normal mice: Evidence that a component of IL‐10 expression is T and B cell‐independent and increased by irradiation. Transplantation 1994; 27: 582–92. [PubMed] [Google Scholar]
- 13. Horwitz DA, Zheng SG, Gray JD. The role of the combination of IL‐2 and TGF‐β or IL‐10 in the generation and function of CD4+ CD25+ and CD8+ regulatory T cell subsets. J Leuko Biol 2003; 74: 471–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Friedman EJ. Immune modulation by ionizing radiation and its implications for cancer immunotherapy. Curr Pharm Des 2002; 8: 1765–80. [DOI] [PubMed] [Google Scholar]
- 15. Demaria S, Bhardwaj N, McBride WH, Formenti SC. Combining radiotherapy and immunotherapy: a revived partnership. Int J Rad Oncol Biol Phys 2005; 63: 655–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Honeychurch J, Glennie MJ, Johnson PWM, Illidge TM. Anti‐CD40 monoclonal antibody therapy in combination with irradiation results in a CD8 T‐cell‐dependent immunity to B‐cell lymphoma. Blood 2003; 102: 1449–57. [DOI] [PubMed] [Google Scholar]
- 17. Yokouchi H, Chamoto K, Wakita D et al . Combination tumor immunotherapy with radiotherapy and Th1 cell therapy against murine lung carcinoma. Clin Exp Metastasis 2007; 24: 533–40. [DOI] [PubMed] [Google Scholar]
- 18. Moore MW, Carbone FR, Bevan MJ. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell 1998; 54: 777–85. [DOI] [PubMed] [Google Scholar]
- 19. Chamoto K, Wakita D, Narita Y et al . An essential role of antigen‐presenting cell/T‐helper type 1 cell–cell interactions in draining lymph node during complete eradication of class II‐negative tumor tissue by T‐helper type 1 cell therapy. Cancer Res 2006; 66: 1809–17. [DOI] [PubMed] [Google Scholar]
- 20. Gramaglia I, Weinberg AD, Lemon M, Croft M. Ox‐40 ligand: a potent costimulatory molecule for sustaining primary CD4 T cell responses. J Immunol 1998; 161: 6510–17. [PubMed] [Google Scholar]
- 21. Gramaglia I, Jember A, Pippig SD, Weinberg AD, Killeen N, Croft M. The OX40 costimulatory receptor determines the development of CD4 memory by regulating primary clonal expansion. J Immunol 2000; 165: 3043–50. [DOI] [PubMed] [Google Scholar]
- 22. Song J, So T, Cheng M, Tang X, Croft M. Sustained surviving expression from OX40 costimulatory signals drives T cell clonal expansion. Immunity 2005; 22: 621–31. [DOI] [PubMed] [Google Scholar]
- 23. Bansal‐Pakala P, Croft M. Breaking immunological tolerance through OX40 (CD134). Sci World J 2001; 1: 633–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rogers PR, Song J, Gramaglia I, Killeen N, Croft M. OX40 promotes Bcl‐xL and Bcl‐2 expression and is essential for long‐term survival of CD4 T cells. Immunity 2001; 15: 445–55. [DOI] [PubMed] [Google Scholar]
- 25. Bansal‐Pakala P, Halteman BS, Cheng MH, Croft M. Costimulation of CD8 T cell responses by OX40. J Immunol 2004; 172: 4821–5. [DOI] [PubMed] [Google Scholar]
- 26. Murata S, Ladle BH, Kim PS et al . OX40 costimulation synergizes with GM‐CSF whole‐cell vaccination to overcome established CD8+ T cell tolerance to an endogenous tumor antigen. J Immunol 2006; 176: 974–83. [DOI] [PubMed] [Google Scholar]
- 27. Taraban VY, Rowley TF, O’Brien L et al . Expression and costimulatory effects of the TNF receptor superfamily members CD134 (OX40) and CD137 (4‐1BB), and their role in the generation of anti‐tumor immune responses. Eur J Immunol 2002; 32: 3617–27. [DOI] [PubMed] [Google Scholar]
- 28. Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature 2001; 411: 380–4. [DOI] [PubMed] [Google Scholar]
- 29. Lee KH, Wang E, Nielsen MB et al . Increased vaccine‐specific T cell frequency after peptide‐based vaccination correlates with increased susceptibility to in vitro stimulation but does not lead to tumor regression. J Immunol 1999; 163: 6292–300. [PubMed] [Google Scholar]
- 30. Lee PP, Yee C, Savage PA et al . Characterization of circulating T cells specific for tumor‐associated antigens in melanoma patients. Nat Med 1999; 5: 677–85. [DOI] [PubMed] [Google Scholar]


