Abstract
The aim of the present study was to characterize the expression pattern of tumor necrosis factor (TNF)‐α and its receptors in breast samples (benign diseases, in situ carcinomas and infiltrating carcinomas), and to compare these results with those obtained previously for interleukin‐6, p53 and p21 using the same samples in order to elucidate the effects of these cytokines on the proliferation–apoptosis equilibrium. Immunoexpression of TNF‐α and its receptors (TNFRI and TNFRII) were studied by western blotting and immunohistochemistry. The percentage of samples positive for TNF‐α and TNFRII was higher in in situ carcinoma than in benign breast diseases, and TNFRII was even higher in infiltrating tumors. The percentage of samples positive for TNFRI was similar in the three groups. For the three proteins and in the three patient groups, immunoreactions were observed in the peripheral cytoplasm. In the positive samples, immunostaining for TNF‐α was more intense in infiltrating tumors than in the other two patient groups, whereas immunostaining for both receptors was higher in in situ carcinoma than in benign breast diseases, and even higher in infiltrating tumors. Comparing the TNF‐α results with previous results for mtp53, p21 and interleukin‐6, we found an association between the expression of these four proteins and increasing malignancy. TNF‐α might be an important factor in breast cancer promotion as its proliferation and survival effects seems to be enhanced through the increased expression of TNFRII. Also, the pro‐apoptotic pathway of TNFRI could be inhibited by p21 (which appeared increased in breast cancer), altering TNFRI effects in promoting the expression of several factors, such interleukin‐6, which contribute to tumor promotion. (Cancer Sci 2006; 97: 1044–1049)
Tumor necrosis factor (TNF)‐α seems to exert a key role in promoting many tumors to breast cancer. TNF‐α is a 17‐kDa polypeptide that was first described as a serum‐derived substance that causes tumor cell death,( 1 ) and is involved in skin carcinogenesis and the spread of a variety of carcinomas and sarcomas. It can induce tumor necrosis by affecting tumor vascularization and initiating apoptotic cell death, but paradoxically, it also can promote cell proliferation.( 2 , 3 ) The action of TNF‐α is mediated by two distinct receptors, named TNFRI (55 kDa) and TNFRII (75 kDa), both of which show a similar affinity for TNF‐α in humans.( 4 , 5 , 6 ) TNFRI is the major mediator of most TNF‐α activities,( 7 ) including apoptosis.( 8 , 9 ) However, cell proliferation through Nuclear Factor kappa B(NF‐κB) transcription factor activation( 10 , 11 ) has also been reported in fibroblasts.( 12 ) TNFRII has been described to mediate proliferation in some cells by acting as a thymocyte.( 13 )
Another cytokine related to TNF‐α and cell proliferation is interleukin (IL)‐6. Recent studies have reported that high expression of IL‐6 is associated with proliferation markers in breast cancer.( 14 ) Both TNF‐α and IL‐6 could also be related to different factors that favor tumor progression, such as estrogen synthesis( 15 ) or the accumulation of mutated p53 (mtp53).( 16 )
The tumor suppressor gene p53 encodes a nuclear phosphoprotein that acts as a transcription factor, which is activated in response to TNF‐α stimuli.( 16 ) Activation of p53 stimulates genes, such as p21, that are associated with cell cycle arrest, DNA repair and apoptosis.( 17 ) Several reports have stated that overexpression of p21 in breast carcinoma has been associated with accumulation of mtp53,( 18 , 19 ) considered to be an indicator of poor prognosis.( 20 , 21 )
Expression of TNF‐α and its receptors has been reported in esophageal,( 22 ) prostatic,( 23 ) follicular thyroid,( 24 ) skin( 25 ) and ovarian( 26 ) cancers. TNF‐α has also been described as an important promoter of cancer in different breast cancer cell lines, such as MCF‐7( 27 ) and MCF10A.( 28 ) To our knowledge, no immunohistochemical analyses of TNF‐α and its receptors have been reported in human breast tissue. The aim of the present study was to characterize the expression patterns of TNF‐α and its receptors in benign conditions, in situ carcinomas, and infiltrating breast tumors by immunohistochemistry and western blotting, as well as to evaluate the relationships between these patterns and those of IL‐6, p21 and mtp53 (studied in our laboratory using the same samples as in the present study),( 22 , 29 ) to elucidate the effects of these cytokines on the proliferation–apoptosis equilibrium.
Materials and Methods
Total or partial mastectomy specimens obtained from 65 women, who were clinically and histopathologically diagnosed with breast adenocarcinoma during 1998 in our hospital, were used for the present study. Twenty of these women (aged from 37 to 75 years; mean 51.23 years) presented with in situ carcinoma, and 45 women (aged from 40 to 77 years; mean 59.93 years) had infiltrating carcinoma. Tumor samples were compared with breast biopsies from 17 women (aged from 16 to 59 years; mean 43.8 years) with benign lesions including ductal and lobular hyperplasia, fibroadenoma and fibrocystic changes. We always used the normal regions in these biopsies. All infiltrative tumor samples were classified using the tumor size, lymphatic nodes and metastasis (TNM) system. Removal of tissues and the study of samples were approved by the Hospital's Ethics Committee and made with the consent of the patients’ relatives. Each sample was divided into two portions: one portion was processed immediately for immunohistochemistry, and the other portion was frozen in liquid nitrogen and maintained at −80°C for western blot analysis.
The primary antibodies used were goat antihuman TNF‐α, TNFRI and TNFRII (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
For western blot analysis, tissues were homogenized in extraction buffer (0.005 M Tris‐HCl, pH 8) with the addition of a protease inhibitor cocktail (10 mM iodoacetamide, 100 mM phenylmethyl sulfonic fluoride, 0.01 mg/mL soybean trypsin inhibitor and 1 µL/mL leupeptin) and phosphate inhibitors (10 mM sodium fluoride and 1 mM sodium orthovanadate) in the presence of 0.5% Triton X‐100. Homogenates were centrifuged for 10 min at 10 000 rpm(8700 g ). The protein concentration of supernatants was calculated using the Bradford method. Supernatants were then equilibrated with loading buffer (10% sodium dodecylsulfate [SDS] in Tris‐HCl [pH 8] containing 50% glycerol, 0.1 mM 2‐β‐mercaptoethanol and 0.1% bromophenol blue) at 50 µg/mL. The mixture was then denatured for 5 min at 100°C, and aliquots of 10 µL of homogenate were separated in SDS–polyacrylamide slab minigels (15% gels). Separated proteins were transferred in transfer buffer (25 mM Tris‐HCl, 192 mM glycine, 0.1% SDS and 20% methanol). Nitrocellulose membranes (0.2 µM), were blocked for 1 h with 1% donkey serum in Tris‐buffered saline, and incubated overnight at room temperature with the primary antibodies at 1:500 (TNF‐α), 1:1000 (TNFRI) and 1:250 (TNFRII) in TBS with 5% bovine serum albumin (BSA). After extensive washing with TBS/Tween‐20 (TBST), the membranes were incubated with rabbit antigoat (Dako, Barcelona, Spain) for 1 h at 1:2500 (diluted in TBS with 5% BSA) and then washed and incubated with the avidin–biotin–peroxidase (ABC) complex (Vector Laboratories, Burlingame, CA, USA) at 1:10 000 dilution. After an intensive wash, the filters were developed with an enhanced chemiluminescence kit, following the procedure described by the manufacturer (Amersham, Buckinghamshire, UK).
For immunohistochemical analysis, tissues were fixed for 24 h at room temperature in 0.1 M phosphate‐buffered saline 10% formaldehyde, dehydrated and embedded in paraffin. Sections (5‐µM thick) were processed using the ABC method. Following deparaffinization, sections were hydrated and incubated for 30 min in 0.3% H2O2 diluted in methanol to reduce endogenous activity. To retrieve the antigen, the sections were incubated with 0.1 M citrate buffer (pH 6) for 2 min in a conventional pressure cooker. After rinsing in TBS buffer, the slides were incubated with normal donkey serum at 10% in TBS for 30 min to prevent non‐specific binding of the first antibody. Thereafter, the primary antibodies were applied at a dilution of 1:125 (TNF‐α), 1:250 (TNFRI) and 1:50 (TNFRII), in TBS at room temperature overnight. Afterward, the sections were washed twice in TBS and then incubated with rabbit antigoat biotinylated immunoglobulin (Dako) at 1:500 in TBS. After 1 h of incubation with secondary antibody, the sections were incubated with a standard streptavidin–biotin complex (Vector Laboratories) at 1:1000 and developed with 3,3′‐diaminobenzidine (DAB), using the glucose oxidase–DAB–nickel intensification method.
Immunochemical procedure specificity was checked using negative and positive controls. For negative controls of immunoreactions, tissues of each type were incubated with preimmune serum at the same immunoglobulin concentration used for each antibody or with blocking peptides (Santa Cruz Biotechnology). As positive controls, histological sections (immunohistochemistry) of thymus (TNF‐α, its receptors and IL‐6) and skin (p21 and p53) were incubated with the same antibodies.
A comparative histological semiquantification of immunolabeling among the different groups of breast samples (in situ adenocarcinomas, infiltrating adenocarcinomas and benign fibrocystic lesions) was carried out for each of the four antibodies. Of each sample, six histological sections were selected at random. In each section, the staining intensity (optical density) per unit surface area was measured with an automatic image analyzer (Motic Images Advanced version 3.2; Motic China Group, Xiamen, China) in five microscopic fields per section, using the ×40 objective. Delimitation of stained surface areas was carried out manually using the image analyzer. For each positive immunostained section, one negative control section (the following in a series of consecutive sections) was also used, and the optical density of this control section was subtracted from that of the stained section. From the average values obtained for each breast, the mean ± SD for each breast group was calculated. The statistical significance between means of the different breast group samples was assessed using Fisher's exact test and the one‐way ANOVA test at P ≤ 0.05, by multiple pairwise comparisons of all of the values for each breast zone, for each specific antibody separately.
The values obtained for TNF‐α in the present study were compared with those obtained for IL‐6, p21 and mtp53 in published studies carried out in our laboratory previously using the same samples as in the present study. In order to test if the increase in TNF‐α expression was correlated with the expression of p53, p21 and IL‐6, we used Pearson's coefficient for each pair of values.
Results
Western blotting. For each antibody used, a single band was found at the corresponding molecular mass: TNF‐α, 17 kDa; TNFRI, 55 kDa; and TNFRII, 75 kDa (Fig. 1). These bands appeared in the three patient groups, except for TNFRII in benign lesions, which was undetectable using this technique.
Figure 1.

Western blot analysis of tumor necrosis factor (TNF)‐α, TNF receptor (TNFR) I and TNFRII after 15% sodium dodecylsulfate–polyacrylamide gel electrophoresis. BL, benign lesions; IC, infiltrating carcinoma; ISC, in situ carcinoma.
Immunohistochemistry. No immunoreaction was observed in negative controls incubated with preimmune serum, or using the antibodies preabsorbed with an excess of purified antigens. Staining of thymus sections (positive controls) was always positive for all antibodies used. For each antibody assayed, the percentage of positive cases and the immunostaining intensities are shown in Table 1.
Table 1.
Comparison of immunostaining intensities between groups of breast samples
| Group | No. cases | TNF‐α | TNFRI | TNFRII | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Positive cases | Optical density † | Positive cases | Optical density † | Positive cases | Optical density † | |||||
| n | % | n | % | n | % | |||||
| Benign lesions | 17 | 15 | 88.23 | 29 ± 6.5 a | 17 | 100 | 16.6 ± 5.75 a | 4 | 23.52 | 5.3 ± 4.9 a |
| In‐situ carcinomas | 20 | 20 | 100 | 20 | 100 | 10 | 50 | |||
| Lobular in situ | 8 | 8 | 100 | 30.3 ± 2.39 b | 8 | 100 | 30.9 ± 2.3 b | 4 | 50 | 31.7 ± 4.4 b |
| Ductal in situ | 12 | 12 | 100 | 33.3 ± 3.395 a | 12 | 100 | 27.7 ± 3.1 b | 6 | 50 | 27.9 ± 3.5 b |
| Infiltrating carcinomas | 45 | 34 | 75.55 | 45 | 100 | 35 | 77.7 | |||
| Lobular infiltrating | 21 | 17 | 80.9 | 45.8 ± 4.67 b | 21 | 100 | 37.8 ± 2.2 c | 18 | 85.71 | 37.8 ± 3.2 c |
| Ductal infiltrating | 24 | 17 | 70.83 | 34.8 ± 3.3 a | 24 | 100 | 35.5 ± 2.5 c | 17 | 70.83 | 31.9 ± 3.8 a |
Values are mean ± SD.
Values denoted by different superscripts are significantly different from each other. Those values sharing the same superscript are not statistically different from each other. Statistical analysis refers to each antibody separately. Significance was determined by multiple comparisons using Fisher's test at P ≤ 0.05. TNF, tumor necrosis factor; TNFR, TNF receptor.
In infiltrating tumor samples the immunoreaction to TNF‐α, as well as IL‐6, p53 and p21, was compared with several tumor parameters (nodal status, TNM system and Estrogen receptor [ER]/Progesterone receptor [PR] status) (Table 2).
Table 2.
Comparison of the expression of interleukin (IL)‐6, tumor necrosis factor (INT)‐α, mutated (mt) p53, p21 and ER with nodal status, the tumor size, lymphatic nodes and metastasis (TNM) system and Estrogen receptor (ER)/Progesterone receptor (PR) status in breast‐infiltrating carcinoma samples
| Variable | Cases (45) | TNF‐α (34 † ) | IL‐6 (39 † ) | mtp53 (30 † ) | p21 (38 † ) | |
|---|---|---|---|---|---|---|
| n | % | |||||
| Nodal status | ||||||
| Negative | 15 | 33.3 | 15 | 14 | 9 | 13 |
| Positive | 30 | 66.7 | 19 (P = 0.3575) | 25 (P = 0.822) | 21 (P = 0.805) | 25 (P = 1.000) |
| TNM | ||||||
| T1 | 14 | 31.2 | 10 | 13 | 8 | 13 |
| T2 | 19 | 42.2 | 13 | 18 | 13 | 14 |
| T3 | 7 | 15.5 | 7 | 6 | 6 | 7 |
| T4 | 5 | 11.1 | 4 (P = 0.944) | 2 (P = 0.805) | 3 (P = 0.949) | 4 (P = 0.956) |
| ER | ||||||
| Negative | 6 | 13.4 | 3 | 3 | 6 | 5 |
| Positive | 39 | 86.6 | 31 (P = 0.404) | 36 (P = 0.494) | 24 (P = 0.526) | 33 (P = 1.000) |
| PR | ||||||
| Negative | 12 | 26.7 | 8 | 10 | 8 | 9 |
| Positive | 33 | 73.3 | 26 (P = 0.799) | 29 (P = 1.000) | 22 (P = 1.000) | 29 (P = 0.804) |
Number of positive cases. Only positive samples in each group were compared.
TNF‐α. In the samples positive for TNF‐α, labeling was always observed in the cytoplasm of epithelial cells. No differences were found in the percentage of positive cases between the different groups. Immunoreactions to TNF‐α were similar in benign lesions (Fig. 2A), in situ carcinomas (Fig. 2B) and ductal infiltrating carcinomas, and more intense in lobular infiltrating carcinomas (Fig. 2C) (Table 1).
Figure 2.
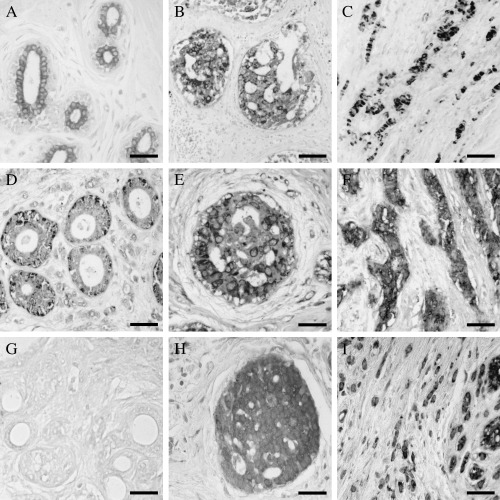
Immuhistochemistry of tumor necrosis factor (TNF)‐α. Similar immunostaining was found in (A) benign lesions and (B) in situ carcinomas. (C) In infiltrating cases the lobular carcinomas showed high intensity of immunoreaction, than in the other groups. (D) Immunohistochemistry of TNF receptor (TNFR) I localized in the peripheral cytoplasm. Weak immunostaining was found in benign lesions. (E) In in situ patients the immunostaining was more intense than in benign diseases. (F) The most intense immunoexpression was found in infiltrating cases. Immunohistochemistry of TNFRI localized in the peripheral cytoplasm: (G) Immunohistochemistry was negative in the major of benign lesion group. The expression increased in both (H) in situ and (I) infiltrating tumors. The highest expression was found in the infiltrating breast cancer. Scale bar: (A,D,G) 30 µm, (B,E) 25 µm and (C,F,H,I) 20 µM.
TNFRI. All samples (100%) were positive to TNFRI. Labeling was observed in the peripheral cytoplasm of epithelial cells (Fig. 2D; Table 1). In both groups of tumors (in situ [Fig. 2E] and infiltrating [Fig. 2F] carcinomas), immunoreactions were more intense than in benign lesions. In infiltrating tumors, immunostaining was more intense than in in situ tumors.
TNFRII. In samples positive for TNFRII, labeling was observed in the epithelial cell cytoplasm. The percentage of cases showing positive immunoreaction to TNFRII increased with the severity of the lesion: 23.52% of benign fibrocystic lesions (Fig. 2G), 50% of in situ tumors (Fig. 2H) and 77.7% of infiltrating tumors (Fig. 2I). In the two patient later groups, immunostaining intensity also increased with the severity of the lesion: higher in in situ tumors than in benign fibrocystic lesions and even higher in infiltrating tumors (Table 1).
Comparison between TNF‐α and IL‐6, p53 and p21. Comparison of the immunoexpression of TNF‐α with those of IL‐6, p53 and p21 (values obtained in previous studies) in the three groups of breast samples (benign lesions, in situ ductal and lobular tumors, and infiltrating ductal and lobular tumors) is shown in Table 3.
Table 3.
Comparison of the immunoexpression of tumor necrosis factor (TNF)‐α, interleukin (IL)‐6, mutated (mt) p53 and p21 in three groups of breast samples
| Variable | IL‐6 | mtp53 | p21 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | P‐value | Positive | Negative | P‐value | Positive | Negative | P‐value | |||||||
| n | % | n | % | n | % | n | % | n | % | n | % | ||||
| Benign fibrocystic lesions (n = 17) | |||||||||||||||
| Positive | 6 | 35.3 † | 9 | 52.9 | 11 | 64.7 † | 4 | 23.5 | 12 | 70.6 † | 3 | 17.6 | |||
| Negative | 0 | 2 | 11.4 | 1 | 5.8 | 1 | 5.8 | 1 | 5.8 | 1 | 5.8 | ||||
| TNF‐α | 0.514 | 0.514 | 0.426 | ||||||||||||
| In situ carcinoma (n = 20) | |||||||||||||||
| Positive | 10 | 50 ‡ | 10 | 50 | 17 | 85 ‡ | 3 | 15 | 15 | 75 ‡ | 5 | 25 | |||
| Negative | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| TNF‐α | NA | NA | NA | ||||||||||||
| Infiltrating carcinoma (n = 45) | |||||||||||||||
| Positive | 32 | 71.1 § | 2 | 4.4 | 31 | 68.8 § | 3 | 6.6 | 32 | 71.1 § | 2 | 4.4 | |||
| Negative | 8 | 17.7 | 3 | 6.6 | 6 | 13.3 | 5 | 11.1 | 9 | 20 | 2 | 4.4 | |||
| TNF‐α | 0.085 | 0.014 | 0.247 | ||||||||||||
Three patients were simultaneously positive for the four proteins (TNF‐α, IL‐6, mtp53 and p21).
‡ Eight patients were simultaneously positive for the four proteins.
§ Thirty patients were simultaneously positive for the four proteins. NA, not applicable.
Only 35.3% (six patients) of benign breast lesions were positive for TNF‐α and IL‐6; 64.7% (11 patients) of benign breast lesions were positive for TNF‐α and mtp53; and 70.6% (12 patients) showed expression of TNF‐α and p21. In the benign breast lesion group only three patients (17.64%) showed simultaneous expression of the four proteins studied.
The relationship between TNF expression and the expression of the other proteins appeared slightly increased in in situ breast carcinomas, as 50% (10 patients) were positive for TNF‐α and IL‐6; 85% (17 patients) had both TNF‐α and mtp53 expression; and 75% (15 patients) had TNF‐α and p21 expression. In the in situ tumor group only eight patients (40%) showed simultaneous expression of the four proteins studied. Most of infiltrating tumors were positive for TNF‐α and IL‐6 (71.1%), TNF‐α and mtp53 (68.8%) or TNF‐α and p21 (71.1%). Furthermore, we found that 30 patients (66.6%) showed simultaneous expression of the four proteins. Using Pearson's coefficient, we compared the expression of these proteins among the three pathology groups statistically and found that the increase in TNF‐α expression was correlated with an increase in both mtp53 (r = 0921) and p21 expression (r = 0953).
Discussion
In the present study, we detected TNF‐α and TNFRI in more than 85% of cases with benign breast pathologies, whereas TNFRII was detected in a low number of cases (23.5%). However, for both receptors, the immunostaining intensities were scant, and this suggests that TNF‐α has little effect on benign breast pathologies. Several authors have described the presence of TNF‐α in different benign hyperplasias, including prostatic,( 23 ) gallbladder mucosa,( 30 ) gastric( 31 ) and endometrial( 32 ) hyperplasias. However, the effects mediated by TNF‐α in these hyperplasias would be slight, as expression of both receptor types was scant.
In in situ breast carcinomas, TNF‐α seems to be more active than in benign breast pathologies because: (i) besides the higher percentage of patients that were positive to both TNF‐α and TNFRI (100%), 50% of patients were also positive for TNFRII; and (ii) although immunostaining intensity for TNF‐α was similar to that found in benign breast pathologies, immunoexpression of the two receptors was increased.
In infiltrating tumors, TNF‐α seems to be even more active than in in situ tumors because: (i) the percentage of patients positive for TNFRII rose to 77.7%; and (ii) immunostaining optical densities of TNF‐α and its two receptors were significantly higher for lobular carcinomas (although not for ductal carcinomas). These data suggest a role for TNF‐α in tumor progression in breast cancer. Meng et al.( 33 ) described high levels of TNF‐α in malignant breast epithelial cells, and suggested that TNF‐α may promote tumor growth. The elevated expression of TNFRII in infiltrating breast cancer might be related to the inhibition of apoptosis or proliferation by this receptor,( 34 ) which would have a survival or proliferation effect. TNFRII functions are not yet well know. However, the activity of this receptor has been related to NF‐κB activation and the production of cytokines such as IL‐6. Therefore, TNFRII expression would be a bad prognosis factor in breast cancer.( 35 ) Our infiltrating tumor samples also showed a higher proliferation index than the other two groups in the study (data not show), and the cause of this could be the TNF‐α high effect throughout TNFRII.
Tumor necrosis factor‐α has shown contrary effects in different carcinomatous tissues and conditions. It has been reported that TNF‐α can exert either pro‐apoptotic or survival and proliferation effects, so it is necessary to clarify the role that TNF‐α is playing in the different tissues and pathological conditions. It seems that, in normal breast tissue, TNF‐α regulates cell proliferation through its pro‐apoptotic effects, but in breast cancer, both the inhibition of the TNFRI apoptotic pathway and the increase in TNFRII survival and proliferation effects might be related to enhanced cell proliferation. This effect could be mediated by promoting the expression of other cytokines such as IL‐6. In this way, it has been reported that the TNFRI apoptotic pathway can be inhibited by accumulation of p21, at the ask‐1 level, and directed toward Ap1 activation and expression of survival‐related genes( 36 ) by NF‐κB activation, as occurs in other tissues such as prostate.( 37 ) We found high levels of p21 in these breast tumors, so TNF‐α could have an effect on survival and proliferation.
In order to find a dominant target for therapy, it should be taken into account that breast cancer is a heterogeneous disease in which different growth factors, cytokines, oncogenes and other mitogenic signals may be involved to effect uncontrolled apoptosis and cell proliferation. Only when all of these factors become well known can a positive therapy be found. Ueno et al. studied the local expression of several cytokines, such as IL‐6 and TNF‐α, in primary breast cancer tissue and showed the relevance of these proteins in crucial tumor processes such as angiogenesis and interactions with the immune system.( 38 ) Evaluation of TNF‐α, its receptors and other factors, such as IL‐6, p53 accumulation and subcellular location of p21, might also be significant in the assessment of its malignancy.
Mommers et al. has suggested that accumulation of mtp53 or p21 in benign breast lesions is associated with an increased risk of progression to breast pathology,( 39 ) and Garcia‐Tuñon et al. suggested that IL‐6 is more active in tumors than in non‐neoplastic tissue.( 14 ) In two previous studies, carried out in the same patients studied here, we reported that immunoexpression of mtp53, p21( 29 ) and IL‐6( 14 ) was increased in in situ carcinoma with regard to the values found in benign breast diseases, and that expression was even higher in breast‐infiltrating tumors. Comparison of these results with those of the present study suggests that, in benign breast diseases, TNF‐α expression is associated with mtp53 and p21 expression (from one‐half to two‐thirds of patients were positive for the three immunostains), whereas the association of TNF‐α with IL‐6 was low (17.6% of patients; P = 0.514). In in situ carcinoma patients, the association of TNF‐α expression with mtp53 and p21 expression was higher than in benign breast diseases (more than two‐thirds of patients), and the association with IL‐6 was 40% of patients.
In infiltrating tumors, TNF‐α expression was highly associated with mtp53 (P = 0.0141) and p21 (P = 0.247) expression, and with IL‐6 (P = 0.085). Also, there was a close relationship with ER expression. Therefore, the association between TNF‐α immunoexpression and those of mtp53 and p21 was observed not only in infiltrating carcinomas but also in in situ carcinomas and benign breast diseases, but the close association with IL‐6 and mtp53 is characteristic only of infiltrating carcinomas, coinciding with the highest levels of cell proliferation found (data not show).
Conclusion
We conclude that TNF‐α in breast cancer could have a key role in tumor progression, as in our patients there was an association between TNF‐α, the expression of tumor markers (mp53 and p21), IL‐6 and increasing infiltrating tumor capacity. Therefore, an evaluation of TNF‐α and IL‐6 as therapeutic targets in breast cancer development and progression would be useful.
Acknowledgments
Grants from Comunidad Autonoma de Madrid and the University of Alcalá (CAM‐UAH2005/085) supported this work.
References
- 1. Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin‐induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci USA 1975; 72: 3666–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Idriss H, Naismith JH. TNF alpha and the TNF receptor superfamily: structure–function relationship(s). Microsc Res Tech 2000; 50: 184–95. [DOI] [PubMed] [Google Scholar]
- 3. Szlosarek PW, Balkwill FR. Tumour necrosis factor alpha: a potential target for the therapy of solid tumours. Lancet Oncol 2003; 4: 565–73. [DOI] [PubMed] [Google Scholar]
- 4. Loetscher H, Pan YC, Lahm HW et al. Molecular cloning and expression of the human 55 kD tumor necrosis factor receptor. Cell 1990; 61: 351–9. [DOI] [PubMed] [Google Scholar]
- 5. Smith CA, Davis T, Anderson D et al. A receptor for tumor necrosis factor defines an unusual family of cellular and viral proteins. Science 1990; 248: 1019–23. [DOI] [PubMed] [Google Scholar]
- 6. Fiers W. Tumor necrosis factor: Characterization at the molecular, cellular and in vivo level. FEBS Let 1991; 285: 199–212. [DOI] [PubMed] [Google Scholar]
- 7. Wiegmann K, Schutze S, Kampen E, Himmler A, Machleidt T, Kronke M. Human 55‐kDa receptor for tumor necrosis factor coupled to signal transduction cascades. J Biol Chem 1992; 267: 17 997–8001. [PubMed] [Google Scholar]
- 8. Engelmann H, Novick D, Wallach D. Two tumor necrosis factor‐binding proteins purified from human urine: Evidence for immunological cross‐reactivity with cell surface tumor necrosis factor receptors. J Biol Chem 1990; 265: 1531–6. [PubMed] [Google Scholar]
- 9. Flynn JL, Goldstein MM, Chan J et al. Tumor necrosis factor‐α is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 1995; 2: 561–72. [DOI] [PubMed] [Google Scholar]
- 10. Liu ZG, Hsu H, Goeddel DV, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF‐κB activation prevents cell death. Cell 1996; 87: 565–76. [DOI] [PubMed] [Google Scholar]
- 11. Ricote M, Royuela M, Garcia‐Tunon I, Bethencourt FR, Paniagua R, Fraile B. Pro‐apoptotic tumor necrosis factor‐α transduction pathway in normal prostate, benign prostatic hyperplasia and prostatic carcinoma. J Urol 2003; 170: 787–90. [DOI] [PubMed] [Google Scholar]
- 12. Tartaglia LA, Weber RF, Figari IS, Reynolds C, Palladino MA Jr, Goeddel DV. The two different receptors for tumor necrosis factor mediate distinct cellular responses. Proc Natl Acad Sci USA 1991; 88: 9292–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tartaglia LA, Goeddel DV. Two TNF receptors. Immunol Today 1992; 13: 151–3. [DOI] [PubMed] [Google Scholar]
- 14. Garcia‐Tuñon I, Ricote M, Ruiz A, Fraile B, Paniagua R, Royuela M. IL‐6, its receptors and its relationship with bcl‐2 and bax proteins in infiltrating and in situ human breast carcinoma. Histopathology 2005; 47: 82–9. [DOI] [PubMed] [Google Scholar]
- 15. Purohit A, Newman SP, Reed MJ. The role of cytokines in regulating estrogen synthesis: implications for the etiology of breast cancer. Breast Cancer Res 2002; 4: 65–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang Y, Kelso A, Cheers C. Cytokine production in the murine response to brucella infection or immunization with antigenic extracts. Immunology 1993; 80: 458–64. [PMC free article] [PubMed] [Google Scholar]
- 17. Yamada SD, Hickson JA, Hrobowski Y et al. Mitogen‐activated protein kinase kinase‐4 (MKK4) acts as a metastasis suppressor gene in human ovarian carcinoma. Cancer Res 2002; 62: 6517–723. [PubMed] [Google Scholar]
- 18. Diab SG, Yu YY, Hilsenbeck SG, Allred DC, Elledge RM. WAF1/CIP1 protein expression in human breast tumors. Breast Cancer Res Treat 1997; 43: 99–103. [DOI] [PubMed] [Google Scholar]
- 19. Johnson R, Spiegelman B, Hanahan D, Wisdom R. Cellular transformation and malignancy induced by ras requires c‐jun. Mol Cell Biol 1996; 16: 4504–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Isola J, Visakorpi T, Holli K, Kallioniemi OP. Association of overexpression of tumor suppressor protein p53 with rapid cell proliferation and poor prognosis in node‐negative breast cancer patients. J Natl Cancer Inst 1992; 84: 1109–14. [DOI] [PubMed] [Google Scholar]
- 21. Iwaya K, Tsuda H, Hiraide H et al. Nuclear p53 immunoreaction associated with poor prognosis of breast cancer. Jpn J Cancer Res 1991; 82: 835–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hubel K, Mansmann G, Schafer H, Oberhauser F, Diehl V, Engert A. Increase of anti‐inflammatory cytokines in patients with esophageal cancer after perioperative treatment with G‐CSF. Cytokine 2000; 12: 1797–800. [DOI] [PubMed] [Google Scholar]
- 23. De Miguel MP, Royuela M, Bethencourt FR, Santamaria L, Fraile B, Paniagua R. Immunoexpression of tumour necrosis factor‐α and its receptors 1 and 2 correlates with proliferation/apoptosis equilibrium in normal, hyperplasic and carcinomatous human prostate. Cytokine 2000; 12: 535–8. [DOI] [PubMed] [Google Scholar]
- 24. Zubelewicz B, Muc‐Wierzgon M, Wierzgon J. Genetic disregulation of the gene coding tumor necrosis factor alpha receptors (TNFαRs) in follicular thyroid cancer: preliminary report. J Biol Regul Homeost Agents 2002; 16: 98–104. [PubMed] [Google Scholar]
- 25. Scott KA, Arnott CH, Robinson SC et al. TNF‐α regulates epithelial expression of MMP‐9 and integrin αvβ6 during tumour promotion. A role for TNF‐α in keratinocyte migration? Oncogene 2004; 23: 6954–66. [DOI] [PubMed] [Google Scholar]
- 26. Rzymski P, Opala T, Wilczak M, Wozniak J, Sajdak S. Serum tumor necrosis factor alpha receptors, p55/p75 ratio and ovarian cancer detection. Int J Gynaecol Obstet 2005; 88: 292–8. [DOI] [PubMed] [Google Scholar]
- 27. Wang X, Li N, Li H et al. Silencing of human phosphatidylethanolamine‐binding protein 4 sensitizes breast cancer cells to tumor necrosis factor‐α‐induced apoptosis and cell growth arrest. Clin Cancer Res 2005; 11: 7545–53. [DOI] [PubMed] [Google Scholar]
- 28. Stuelten CH, DaCosta Byfield S, Arany PR, Karpova TS, Stetler‐Stevenson WG, Roberts AB. Breast cancer cells induce stromal fibroblasts to express MMP‐9 via secretion of TNF‐α and TGF‐β. J Cell Sci 2005; 118: 2143–53. [DOI] [PubMed] [Google Scholar]
- 29. García‐Tuñón I, Ricote M, Ruiz A, Fraile B, Paniagua R, Royuela M. Cell cycle control related proteins (p53, 21, and Rb) and transforming growth factor α (TGFα) in benign and carcinomatous (in situ and infiltrating) human breast: implications in malignant transformations. Cancer Invest 2006; 24: 119–25. [DOI] [PubMed] [Google Scholar]
- 30. Shi JS, Zhou LS, Han Y, Zhu AJ, Sun XJ, Yang YJ. Expression of tumor necrosis factor and its receptor in gallstone and gallbladder carcinoma tissue. Hepatobiliary Pancreat Dis Int 2004; 3: 448–52. [PubMed] [Google Scholar]
- 31. Oshima M, Oshima H, Matsunaga A, Taketo MM. Hyperplastic gastric tumors with spasmolytic polypeptide‐expressing metaplasia caused by tumor necrosis factor‐α‐dependent inflammation in cyclooxygenase‐2/microsomal prostaglandin E synthase‐1 transgenic mice. Cancer Res 2005; 65: 9147–51. [DOI] [PubMed] [Google Scholar]
- 32. Sukhikh GT, Zhdanov AV, Davydova MP et al. Disorders in cytokine gene expression in endometrial hyperplasia and effect of hormone therapy. Bull Exp Biol Med 2005; 139: 235–7. [DOI] [PubMed] [Google Scholar]
- 33. Meng L, Zhou J, Hironobu S, Suzuki T, Zeitoun K, Bulun S. TNFα and IL‐11 secreted by malignant breast epithelial cells inhibit adipocyte differentiation by selectively down‐regulating C/EBPα and PPAR‐γ: Mechanism of desmoplastic reaction. Cancer Res 2001; 61: 2250–5. [PubMed] [Google Scholar]
- 34. Dinarello C, Moldawer L. Proinflamatory and anti‐inflammatory cytokines in rheumatoid arthritis: A primer for clinicians. Thousand Oaks, CA: Amgen, 1999. [Google Scholar]
- 35. Mestiri S, Bouaouina N, Ben Ahmed S, Chouchane L. A functional polymorphism of the tumor necrosis factor receptor‐II gene associated with the survival and relapse prediction of breast carcinoma. Cytokine 2005; 30: 182–7. [DOI] [PubMed] [Google Scholar]
- 36. Asada M, Yamada T, Ichijo H et al. Apoptosis inhibitory activity of cytoplasmic p21 (Cip1/WAF1) in monocytic differentiation. EMBO J 1999; 18: 1223–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ricote M, Royuela M, Garcia‐Tunon I, Bethencourt FR, Paniagua R, Fraile B. Pro‐apoptotic tumor necrosis factor‐α transduction pathway in normal prostate, benign prostatic hyperplasia and prostatic carcinoma. J Urol 2003; 170: 787–90. [DOI] [PubMed] [Google Scholar]
- 38. Ueno T, Toi M, Saji H et al. Significance of macrophage chemoattractant protein‐1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res 2000; 6: 3282–9. [PubMed] [Google Scholar]
- 39. Mommers EC, Leonhart AM, Falix F et al. Similarity in expression of cell cycle proteins between in situ and invasive ductal breast lesions of same differentiation grade. J Pathol 2001; 194: 327–33. [DOI] [PubMed] [Google Scholar]


