Abstract
Inhibitor of DNA binding (Id) proteins are essential for cell differentiation, proliferation, migration, invasion and angiogenesis. Recently, they have been shown to correlate with less differentiated phenotypes, high malignant potential and poor clinical outcome in various kinds of tumors. In an attempt to develop new strategies for the treatment of peritoneal metastasis of gastric cancer, we prepared an Id1, 3 double‐knockdown gastric cancer cell line, MKN45, by RNA interference and investigated its effects on the development of metastatic nodules in the peritoneal cavity. Both cell proliferation and migration capabilities were decreased in Id1, 3 double‐knockdown cells, as was their ability to bind to laminin, which could be explained by the decreased expression of integrin α6. These are important steps in the metastatic process. In a mouse model, the number of peritoneal metastatic nodules formed by Id1, 3 double‐knockdown cells was reduced compared to mock‐transfected control cells, as was the size of individual tumors. In this study, we clearly demonstrated that Id1, 3 double‐knockdown significantly impaired the ability of gastric cancer cells to form peritoneal metastasis. Id should be considered an ideal target for the treatment and prevention of gastric cancer, and RNA interference is an attractive and promising strategy to achieve it. (Cancer Sci 2005; 96: 784–790)
Abbreviations:
- BSA, bovine serum albumin; ECM
extracellular matrix
- HBSS
Hanks’ Balanced Salt Solution
- HLH
helix‐loop‐helix
- Id
inhibitor of DNA binding
- PBS
phosphate‐buffered saline.
Gastric cancer is one of the most commonly occurring cancers, with a high incidence in some Asian countries, including Japan, and in European countries. Although the mortality rate of gastric cancer has declined in recent years, it remains the second cause of cancer death worldwide. The majority of cases are detected at an advanced stage, which contributes to poor prognosis. The 5‐year survival rate in such cases is approximately 20%.( 1 ) The most frequent cause of death of gastric cancer patients is peritoneal metastasis, which is difficult to diagnose preoperatively and to cure completely by surgery or chemotherapy.( 2 ) Therefore, the development of new therapeutic/preventive strategies for peritoneal metastasis of gastric cancer is desired.
The inhibitor of DNA binding (Id) proteins belong to helix‐loop‐helix (HLH) transcriptional regulatory factors. They form heterodimers with basic HLH proteins, such as E proteins, and inactivate their functions by inhibiting their binding to DNA. It is also known that Id proteins inactivate Rb and Ets proteins. Id proteins are essential for cell differentiation, proliferation, cell‐cycle progression, migration, invasion, cell fate, and angiogenesis.( 3 , 4 , 5 )
The Id family has four members, namely Id1 to Id4. The different family members localize to different chromosomes, are expressed at different patterns and have different functions.( 6 , 7 ) Deregulated expression of Id proteins was revealed in various tumors, such as ovarian, breast, prostate, colorectal and gastric cancers.( 8 , 9 , 10 ) Id1 is one of the most investigated members of the Id family, and has been shown to associate with undifferentiation, tumor malignancy, invasion and poor prognosis in several tumors.( 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 ) In gastric cancer, Id1 overexpression significantly correlated with poorer differentiation, more advanced stages, high metastatic potential, as well as more aggressive behavior of tumor cells.( 10 )
Among the Id family members, Id1 and Id3 are the more extensively overexpressed in various tumor types,( 19 , 20 ) including gastric cancer,( 10 ) and are expressed in an overlapping pattern. Id1 and Id3 inactivate Ets1 and Ets2 by binding them. This leads to the inhibition of p16 expression and consequently allows phosphorylation of Rb.( 21 ) Both Id1 and Id3 are required for the angiogenesis in tumor tissues,( 22 ) and knockdown of Id1 and Id3 has been shown to inhibit angiogenic processes.( 23 ) Additionally, tumor progression and metastasis were inhibited in Id1+/–, Id3−/– mice.( 22 )
Here, we focused on Id1 and Id3 as the targets for treatment of peritoneal metastasis of gastric carcinoma, and hypothesized that the reduction of Id1, 3 could impair the aggressiveness of gastric cancer. To confirm it, by way of RNA interference, Id1, 3 double‐knockdown gastric cancer cells were prepared, and their effects on metastatic potential were investigated.
Materials and Methods
Reagents and antibodies
Collagens type I, III, IV were from Nitta Gelatin (Osaka, Japan), and fibronectin and laminin from Sigma (St Louis, MO, USA). The mouse antibodies against human integrins α1, αV and β4, as well as the anti‐CD44 were from BD Pharmingen (San Jose, CA, USA). The mouse antibodies against integrins α2, α5, and α6 were from Immunotech (Marseille, France), and those against integrins α3, α4 and β1 were from DAKO Japan (Kyoto, Japan). The fluorescein‐isothiocyanate (FITC)‐labeled goat anti‐mouse IgG was from BD Pharmingen, as was the PE‐labeled mouse IgG of unrelated specificity. The unlabeled mouse IgG of unrelated specificity was from DAKO Japan.
Cell culture and transfection
Human gastric cancer cell line, MKN45, was purchased from the Japanese Cancer Research Resource Bank (Osaka, Japan). Tumor cells were cultured in RPMI‐1640 medium (Sigma) containing 10% fetal calf serum and 1% antibiotics/antimycotic (i.e. 100 U/mL penicillinG, 100 µg/mL streptomycin sulfate, 250 ng/mL amphotericinB; Life Technologies, Grand Island, NY, USA) in an atmosphere of 37°C in 5% CO2, and passaged by treating with 0.02% EDTA in phosphate‐buffered saline (PBS) and 0.25% trypsin when achieving confluence. The Id1, 3 double‐knockdown MKN45 cells were obtained by the RNA interference procedure. shRNA for Id1 was TCCCAAAGAATCATGAAAGTCGCCAGTTCAAGAGACTGGCGACTTCATGATTCTTTT, and for Id3 was TCGGATCCAACCACTGCTACTCCCGCCTGTTCAAGAGACAGGCGGGAGTAGCA GTGGTTTTTTGGAAAAGCTTGG, both with 3′single strand overhangs for ligation into the RNA expression vector. psiRNA‐hH1‐neo (InvivoGen, San Diego, CA, USA) was the vector for Id1 and pSilencer 2.0‐U6 (Ambion, Austin, TX, USA) for Id3, containing H1 or U6 RNA polymerase III promoter, respectively. Both vectors were transfected into MKN45 at a concentration of 10 ng/5 × 106 cells using Effectene Transfection Reagent (Qiagen, Hilden, Germany) and empty vectors were used as the control vector (mock transfectants). Successfully transfected cells were selected by a long‐term culture in a selection medium containing 50–100 µg/mL G418. To examine whether Id1 and/or Id3 shRNA induce IFN‐γ expression, we measured the expression levels of IFN‐γ by reverse transcription polymerase chain reaction. IFN‐γ mRNA expression of Id1, 3 double‐knockdown cells was at the same level as mock transfectants and parental cells (data not shown).
Western blotting
Tumor cells were cultured until subconfluence, washed twice with PBS and lyzed with 500 µL/dish lysis buffer, which consisted of 1% Tween‐20 in Tris‐saline (150 mM NaCl in 50 mM Tris‐HCl, pH 7.6) containing protease inhibitors (1 mM ethylene diamine tetraacetic acid, 0.5 mM diisopropyl fluorophosphates, 0.5 mM phenylmethylsulfonyl fluoride, 1 µL/mL Nα‐p‐tosyl‐L‐lysine chlormethyl ketone, 0.1 µL/mL pepstain) for 1 h at 4°C. Lyzed cells were scraped, collected in 1.5 mL tubes and centrifuged at 12 500 g for 5 min. Supernatants were recovered as the protein samples, and were stored frozen until use. Protein concentration was measured by the BCA Protein Assay kit (Pierce Chemical, Rockford, IL, USA). Samples were subjected to 12.5% sodium dodecylsulfate polyacrylamide gel electrophoresis. Proteins in gels were transferred to immobilon membrane (Millipore, Bedford, MA, USA) by blotter. Five percent skim milk (blocking solution) was loaded over the membrane, and incubated for 30 min at room temperature with agitation. The membranes were then incubated with the rabbit anti‐human Id1 or Id3 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 1 h at 37°C with agitation. After washing with 0.1% Tween‐20 in Tris‐saline three times, the membranes were incubated with biotin‐labeled anti‐rabbit IgG for 1 h at room temperature with agitation. After the membranes were washed another three times, ABC solution (Vector Laboratory, Burlingame, CA, USA) was added, and further incubation was performed at room temperature for 30 min, with agitation. Finally, the membranes were washed three times and, for color development, DAB solution (DAKO Japan) was used. The protein expression levels were quantified using OptiQuant Software version 3 (Packard Instrument Co., Meriden, CT, USA).
Proliferation assay
Tumor cells suspended in culture medium were placed in a 96‐well plate (1 × 103 cells/100 µL/well) and cultured for 6 days in an atmosphere of 5% CO2, at 37°C. Viable cells were evaluated at day 1, 3 and 6 by the MTS assay (Promega, Madison, WI, USA), according to the manufacturer's recommendations. The proliferation rate was determined as the ratio to the value obtained at day 1.
Migration assay
Migration of tumor cells was evaluated by a modification of the wound closure assay described previously.( 24 , 25 ) Briefly, tumor cells were cultured in 100 mm culture dishes until a confluent monolayer formed. Linear wounds of similar width were produced on the monolayers, by scratching the monolayers with plastic tips. Phorbol 12‐myristate 13‐acetate (Sigma) was added at 100 nM to stimulate the tumor cells to migrate. To exclude affection by the cell proliferation, mitomycin C was added to the medium at a concentration of 5 µg/mL. The cells were allowed to migrate for 3 days, then washed with PBS and fixed with 4% formaldehyde in PBS. The width of the wounds was measured at predetermined points. The wound closure rate was calculated as the ratio between the wound width at day 3 and that at day 0.
Flow cytometry
Tumor cells were removed by trypsinization, washed twice with PBS and suspended in sample buffer (0.1% BSA‐0.1% NaN3‐PBS). Mouse antibodies against human integrin α1, α2, α3, α4, α5, α6, αV, β1 and β4 were added to the samples at a final concentration of 1–10 µg/mL. The samples were incubated at 4°C for 30 min, washed twice with washing buffer (0.2% BSA‐0.1% NaN3‐PBS), then FITC‐labeled goat anti‐mouse IgG was added at the appropriate concentration. After another 30‐min incubation at 4°C, the samples were washed twice, resuspended in sample buffer and analyzed in the flow cytometer (FACS Calibur; Becton‐Dickinson, San Jose, CA, USA). The mouse IgG of unrelated specificity was always used as the negative control for the primary antibody. Additionally, PE‐labeled mouse antibody against CD44 was tested, without the secondary antibody. In this case, the negative control was the PE‐labeled mouse IgG of unrelated specificity.
Adhesion assay
Adhesion assay was performed to evaluate the ability of cancer cells to bind extracellular matrix (ECM) proteins and mesothelial cells. Mesothelial cells were isolated from the omentum of patients undergoing an abdominal procedure, after informed consent was obtained. Cells were cultured in the same medium described above for MKN45 cells.
For the assay, mesothelial cells were seeded (5 × 104 cells/well) into collagen type I‐coated 96‐well microtiter plates in culture medium, and allowed to grow until confluence. To evaluate the cancer cells’ ability to bind ECM proteins, the wells of 96‐well plates were precoated with 30 µg/mL collagen type I, III, IV, 5 µg/mL fibronectin, laminin, or PBS (negative control) at 4°C overnight.
The wells were blocked with 0.2% BSA‐RPMI‐1640 at 37°C for 1 h to avoid non‐specific binding. Tumor cells were stained by Calcein (Dojindo, Kumamoto, Japan) in a 37°C water bath for 1 h then washed twice with PBS. The stained cells were suspended in 0.2% BSA‐RPMI‐1640 and placed in the precoated 96‐well plate (1 × 105 cells/100 µL/well). The cells were centrifuged at 190 g for a few seconds to sediment the cells on ECM, and cultured at 37°C for 10 min to allow them to adhere. The tumour cells placed on the mesothelial cells were not centrifuged but cultured at 37°C for 30 min. The wells were gently washed twice with 0.2% BSA‐RPMI‐1640 to remove any non‐adhered cells. Fluorescence of the adhered cells was measured in the fluorometer (Terascan VP; Minervatech, Tokyo, Japan) and analyzed. Positive unwashed wells, which contained labeled cells, and negative wells, which were cell‐free, were also prepared. The adhesion rate was calculated as the ratio between experimental wells and the positive wells, after subtraction of the negative wells.
In vivo proliferation model
Six‐week‐old female Balb/cA Jcl‐nu/nµ mice were purchased from the Saitama Experimental Animal Supply Company (Sugitomachi, Japan). Experiments were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of the University of Tokyo.
The mice were divided into three groups: parental; mock control; and Id1, 3 double‐knockdown. Each group consisted of three mice. Cultured tumor cells were removed by trypsinization, washed twice with PBS, suspended in Hanks’ Balanced Salt Solution (HBSS; Invitrogen, Carlsbad, CA, USA) and injected subcutaneously into the back of the mice (5 × 106 cells/0.2 mL/mouse). Tumor size was measured at days 6, 10, 13 and 19. The mice were killed on day 19. Tumor volume was calculated by the following formula: (short diameter)2 × (long diameter)/2.
In vivo peritoneal metastasis model
The mice were divided, as above, into three groups: parental; mock control; and Id1, 3 double‐knockdown. Each group consisted of four mice. Tumor cells were collected, washed twice with PBS, suspended in HBSS and injected into the peritoneal cavity of mice (1 × 107 cells/1 mL/mouse). The mice were killed at day 17, and the size and number of metastatic nodules in the peritoneal cavity was counted by the naked eye as described previously.( 26 )
Statistical analysis
To analyze the results of these experiments, we used the non‐repeated measures anova in combination with Scheffé's test. Significant differences were considered at P value <0.05.
Results
Id1, 3 double‐knockdown in MKN45
The expressions of Id1 and Id3 were analyzed by Western blot, and the expression levels compared among parental, mock‐transfected control and double‐knockdown cells. As shown in Fig. 1, Id1 and Id3 proteins were strongly expressed in the parental MKN45 cells. Mock‐transfected cells expressed similar levels, but, as expected, the expression level of Id1 in double‐knockdown cells was reduced to approximately 65% of that in mock control cells. In turn, the expression level of Id3 in double‐knockdown cells was reduced to 60%.
Figure 1.
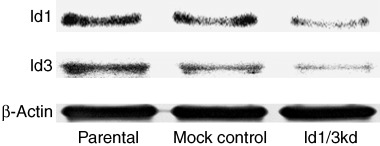
Western blot analysis of Id1 and Id3 proteins in MKN45 gastric cancer cells. Strong expressions of Id1 and Id3 proteins in parental and mock‐transfected control cells were observed. Both Id1 and Id3 expression levels were reduced in Id1, 3 double‐knockdown cells. β‐actin was used as an internal control.
Id1, 3 double‐knockdown inhibits the proliferative activity of MKN45
As shown in Figure 2a, the proliferative activity of Id1, 3 double‐knockdown cells at day 6 decreased by approximately 12% of mock‐transfected control cells, and the difference, although small, was statistically significant.
Figure 2.
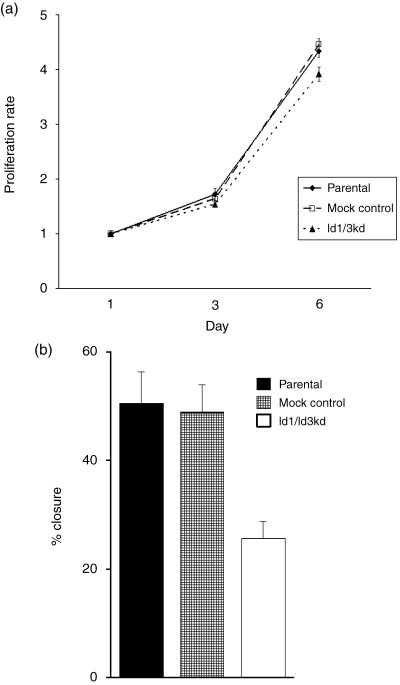
(a) Proliferation assay of MKN45 cells. At day 6 from the incubation of cancer cells, the proliferative activity of Id1, 3 double‐knockdown cells was slightly decreased compared with mock control cells, but the difference was significant (P < 0.001). The difference between parental and control cells was not significant. (b) Migration assay of MKN45 cells. The wound closure rate of Id1, 3 double‐knockdown cells was significantly decreased compared to that of mock control cells (P < 0.001). The difference between parental and control cells was not significant.
Id1, 3 double‐knockdown inhibits the migratory activity of MKN45
As shown in Figure 2b, the migratory activity of Id1, 3 double‐knockdown cells was decreased when compared with mock‐transfected control cells, as assessed by the decreased ability of wound closure. Compared with mock‐transfected cells, the wound closure rate was reduced to up to 52.5% in Id double‐knockdown cells (49% and 26%, respectively). In other words, Id1, 3 double‐knockdown impaired the ability of gastric cancer cells to migrate.
Decreased expression of integrin α6 on Id1, 3 double‐knockdown MKN45
The cell surface expression of transmembrane receptors was examined by flow cytometry, and the expression levels were compared among the transfected and parental cells. Integrin α2, α3, α5 α6, αV, β1, and CD44 were expressed in the parental MKN45 cells. As shown in Figure 3, the expression levels of these receptors were similar among these cells, except for integrin α6, which was reduced to approximately 80% of mock‐transfected control cells. Integrin α5 expression was weak, and α1, α4 and β4 could not be detected in any of the Id1, 3 double‐knockdown cells. The experiment was repeated using another Id1, 3 double‐knockdown gastric cancer cell line, MKN74, and a reduction of α6 expression to approximately 80% of the mock control cells was found in Id1, 3 double‐knockdown cells (data not shown).
Figure 3.
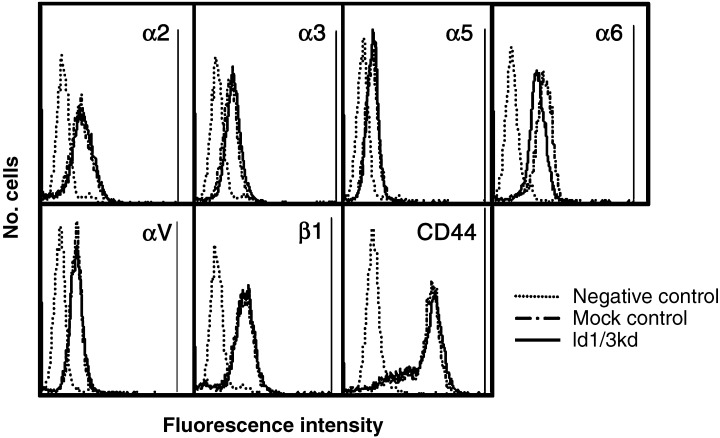
Flow cytometric analysis of the expression of transmembrane receptors. The expression levels of integrins α2, α3, α5, α6, αV, β1, and CD44 in parental cells was confirmed as the same as in mock control cells. The expression of integrin α6 was reduced in Id1, 3 double‐knockdown cells.
Changes in the ability of Id1, 3 double‐knockdown MKN45 to bind ECM proteins
The ability of the three types of cells to bind ECM proteins, namely collagen types I, III, IV, fibronectin and laminin, was examined. As shown in Table 1, the parental and mock‐transfected cells adhered strongly to all the ECMs. The adhesion rate was similar in all groups, except for the binding of Id1, 3 double‐knockdown cells to laminin, which was decreased to approximately 58% of mock‐transfected control cells. This result is compatible with the down‐regulation of integrin α6, the receptor for laminin, on double‐knockdown cells.
Table 1.
Adhesion assay of parental, mock control and Id1, 3 double‐knockdown human gastric cancer MKN45 cells
| Parental (%) | Mock control (%) | Id1/3kd | P value | |
|---|---|---|---|---|
| Collagen‐I | 89.2 ± 5.9 | 90.8 ± 0.6 | 87.6 ± 1.0 | 0.568 |
| Collagen‐II | 77.8 ± 0.6 | 77.7 ± 4.7 | 79.6 ± 3.2 | 0.786 |
| Collagen‐IV | 61.2 ± 2.6 | 61.2 ± 2.5 | 61.3 ± 1.7 | 0.999 |
| Fibronectin | 4.8 ± 2.3 | 4.3 ± 3.2 | 5.7 ± 1.8 | 0.805 |
| Laminin | 39.2 ± 1.6 | 38.3 ± 3.2 | 22.3 ± 1.9 | 0.001 |
| Mesothelial | 26.7 ± 0.7 | 26.6 ± 4.0 | 26.3 ± 0.8 | 0.988 |
| Control | 1.7 ± 1.5 | 0.6 ± 0.5 | 3.5 ± 2.8 | 0.248 |
The adhesion percentages are shown as the mean ± SD in triplicate wells. P values compare Id1, 3 double‐knockdown cells and mock control cells. The binding abilities of these cells to extracellular matrix (ECM) proteins were similar, except for laminin, which was significantly decreased in Id1, 3 knockdown cells compared with mock control cells. In contrast, there were no differences in the ability of these cells to bind human mesothelial cells. The differences between parental and control cells were not significant for any of the ECM proteins tested.
Id1, 3 double‐knockdown does not affect the ability to bind mesothelial cells
As shown in Table 1, the adhesion of Id1, 3 double‐knockdown cells to human mesothelial cells was not significantly different from that of control cells.
Id1, 3 double‐knockdown decreased tumor growth in vivo
The ability of parental and mock‐transfected cells to form tumors when subcutaneously implanted into mice was evaluated. Tumor growth was observed approximately 6 days after implantation, and the tumor size was measured at days 6, 10, 13, and 19 (Fig. 4). Compared with mock‐transfected control cells, the Id1, 3 double‐knockdown cells grew slowly. The difference was statistically significant at day 6 and day 10, but not significant at day 13 and day 19. These data were similar to that of the in vitro proliferation assay.
Figure 4.
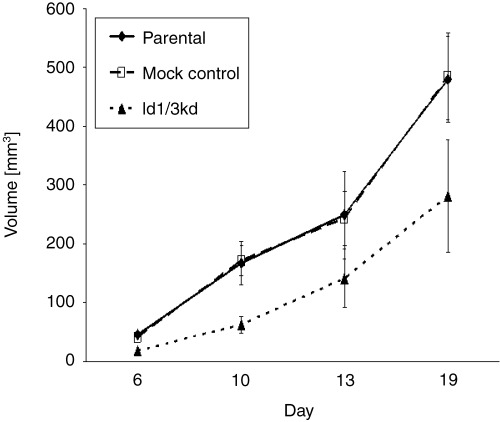
In vivo proliferation model. The tumor growth of Id1, 3 double‐knockdown cells was slow compared with mock control cells. The difference was statistically significant at day 6 (P < 0.001) and day 10 (P = 0.008), but not at day 13 (P = 0.178) or day 19 (P = 0.060). The difference between parental and control cells was not significant.
Id1, 3 double‐knockdown reduced peritoneal metastatic nodules in vivo
The ability of parental and mock‐transfected cells to form metastatic nodules was evaluated in an in vivo model of peritoneal metastasis. Peritoneal metastatic nodules developed preferentially around the vessels of the intestinal mesentery (Fig. 5a). The total number of metastatic nodules was significantly reduced in the Id1, 3 double‐knockdown cells (Fig. 5b), as was the size of the individual nodules, when compared with mock‐transfected control cells. The difference was especially evident when nodules with more than 2.0 mm in diameter were analyzed (Fig. 5b). The results were statistically significant.
Figure 5.
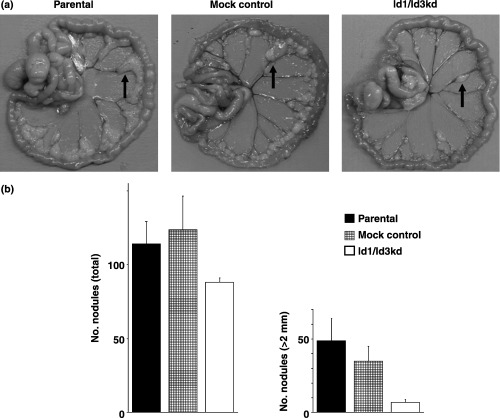
In vivo peritoneal metastatic model. (a) Metastatic nodules formed on the intestinal mesentery. The arrows show the nodules. (b) The total number of metastatic nodules was reduced in Id1, 3 double‐knockdown cells compared with mock control cells (P = 0.038). The number of nodules with a diameter more than 2.0 mm was also reduced significantly (P = 0.013). The difference between parental and control cells was not significant.
Discussion
We focused on Id proteins as the target for the treatment of peritoneal metastasis of gastric cancer, due to the following reasons. First, Id proteins regulate the expressions of several important genes related to tumorigenesis and cancer progression.( 4 , 27 , 28 ) Second, most of the normal mature adult tissues do not express the Id protein, and therefore, specific targeting of cancer cells is a realistic possibility. Third, only partial reduction of Id levels seems to be sufficient to significantly reduce tumor invasion and metastasis, as shown by the systemic targeting of Id1 expression in tumor‐bearing mice( 13 ) and tumor xenografts grown in Id‐knockdown models.( 22 ) And finally, peritoneal metastasis is frequently associated with poorer differentiated gastric cancers, which also show high levels of Id protein expression.( 10 )
By way of RNA interference, we obtained stable transfectants of Id1, 3 double‐knockdown of MKN45, which is a cell line derived from a patient with poorly differentiated adenocarcinoma. MKN45 was previously reported to express Id1 and Id3 at high levels.( 10 ) Id1 and Id3 are overexpressed in various tumor types more extensively than the other two family members. Most of the gastric cancer cell lines express high levels of Id1 and Id3, but the expression of Id2 is weaker and not uniform.( 10 ) Id1 and Id3 are known to be evolutionally closely related and have similar promoter sequences. They also have overlapping biochemical functions and can compensate each other. Knockdown of either Id1 or Id3 resulted in only small inhibition of human umbilical vein endothelial cell activation, and inhibition of both was necessary to strongly inhibit it.( 23 ) And tumor growth was inhibited more effectively in Id1+/–, Id3−/– mice than in Id1+/–, Id3+/+ mice.( 22 ) Therefore, we concluded that inhibition of either Id would be insufficient to obtain the antitumor effect, and prepared double‐knockdown cells for the experiments.
Using an in vivo model of peritoneal metastasis in nude mice, we tested the ability of Id1, 3 double‐knockdown cells to form tumor nodules in the peritoneal cavity. For the experiments, mock‐transfectants and the parental cells were used as controls. Compared to control cells, Id1, 3 double‐knockdown MKN45 cells had a decreased ability to form peritoneal metastatic nodules, and additionally, the size of the metastatic nodules was significantly reduced.
Similar to hematogenous metastasis, peritoneal dissemination develops through multiple steps, including invasion and migration of cancer cells through the gastric wall, and once in the peritoneal cavity, cancer cells adhere to mesothelial cells, which causes retraction of mesothelial cells, exposing the basal membrane. Cancer cells adhere to the basal membrane, destroy the sub‐basal membrane tissue and invade the surrounding tissue. Cancer cells then recruit endothelial cells to develop angiogenesis and grow to form the metastatic nodules.
To identify the mechanisms regulating the decreased ability of Id1, 3 double‐knockdown cells to form peritoneal metastasis, the various steps of the metastatic process were investigated in vitro.
Id1, 3 double‐knockdown MKN45 cells had decreased abilities to proliferate and migrate. Compared to mock‐transfectants and parental cells, the proliferative activity of the double‐knockdown cells was reduced by approximately 10% (12%), and the migratory property by approximately 50% (48%). Also, when implanted into the flank of nude mice, growth of Id1, 3 double‐knockdown cells was impaired compared to control cells.
Next, the ability of Id1, 3 double‐knockdown cells to adhere to ECM proteins was investigated. The ability of Id1, 3 double‐knockdown cells to bind laminin was significantly impaired, but not in collagens or fibronectin, which could be explained by the decreased expression of integrin α6, observed by flow cytometry.
Taking these findings together, the inhibitory effect of Id1, 3 double‐knockdown cells on the ability of gastric cancer cells to form peritoneal metastatic nodules was speculated to be dependent on their decreased ability to bind laminin at the metastatic site, in addition to their decreased proliferative activity and reduced migratory properties. Laminin is one of the important structural components of the basal membrane, and a decreased ability to interact with it would result in reduced invasiveness and dissemination. In the peritoneal cavity, laminin is present in the underlying basal membrane of mesothelial cells, and for cancer cells to bind it, mesothelial cells have to retract, exposing the underlying membrane. Therefore, the ability of gastric cancer cells to directly bind mesothelial cells, which was unaffected in double‐knockdown cells, does not appear to be sufficient for the formation of metastatic nodules.
The size of the disseminated peritoneal nodules formed by the Id1, 3 double‐knockdown cells was smaller compared to those formed by the mock‐transfectant and parental cells. This can be partially explained by the decreased proliferative activity, but the possibility of an impaired ability to induce angiogenesis cannot be discarded. In fact, both Id1 and Id3 are required for angiogenesis in adult tissues, and the knockdown of Id1 and Id3 inhibited the angiogenic processes, shown by the defective endothelial cell vasculature in tumor xenografts and the inability to support the growth or metastasis of three different tumors.( 22 ) Without Id expression, angiogenic blood vessels, which support tumor growth, cannot branch and sprout from pre‐existing vessels to form new capillaries.
Recently, using antisense technology to target endogenous Id1 gene expression, Fong et al.( 13 ) could clearly demonstrate the significant inhibition of not only the invasiveness of breast cancer cells in vitro, but also their ability to metastasize in vivo. Additionally, systemic targeting of Id1 by antisense Id1 plasmid was effective in significantly inhibiting hematogenous metastasis of breast cancer.( 13 )
In conclusion, Id proteins are essential for both cancer cells and endothelial cells, and also play a pivotal role in the development of peritoneal metastasis of gastric cancer. A reduction of functional Id levels was shown to be enough to strongly affect tumor phenotypes, and consequently, the targeting of Id proteins would be a novel strategy for the prevention and treatment of gastric cancer. Multiple strategies can be used to target the functional activities of intracellular proteins for cancer therapy, including antisense oligonucleotides, antigene or ribozyme gene transfer, small molecules or peptides. The RNA interference method, used in the present study, also proved to be effective for Id targeting, and studies are ongoing in our laboratory on the effectiveness of siRNA vaccines for the treatment of peritoneal metastasis of gastric cancer.
Acknowledgments
This study was supported partly by a Grant‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, partly by a grant from the Ministry of Health, Labour and Welfare of Japan, and partly by a grant from the Sankyo Foundation of Life Science.
References
- 1. Roder DM. The epidemiology of gastric cancer. Gastric Cancer 2002; 5 (Suppl. 1): 5–11. [DOI] [PubMed] [Google Scholar]
- 2. Fujimura T, Ishii K, Oyama K et al. A new scoring system for peritoneal metastasis in gastric cancer. Gastric Cancer 2003; 6: 146–52. [DOI] [PubMed] [Google Scholar]
- 3. Israel MA, Hernandez MC, Florio M et al. Id gene expression as a key mediator of tumor cell biology. Cancer Res 1999; 59: 1726s–30s. [PubMed] [Google Scholar]
- 4. Norton JD. ID helix‐loop‐helix proteins in cell growth, differentiation and tumorigenesis. J Cell Sci 2000; 113 (Pt 22): 3897–905. [DOI] [PubMed] [Google Scholar]
- 5. Norton JD, Atherton GT. Coupling of cell growth control and apoptosis functions of Id proteins. Mol Cell Biol 1998; 18: 2371–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Riechmann V, van Cruchten I, Sablitzky F. The expression pattern of Id4, a novel dominant negative helix‐loop‐helix protein, is distinct from Id1, Id2 and Id3. Nucleic Acids Res 1994; 22: 749–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Riechmann V, Sablitzky F. Mutually exclusive expression of two dominant‐negative helix‐loop‐helix (dnHLH) genes, Id4 and Id3, in the developing brain of the mouse suggests distinct regulatory roles of these dnHLH proteins during cellular proliferation and differentiation of the nervous system. Cell Growth Differ 1995; 6: 837–43. [PubMed] [Google Scholar]
- 8. Chan AS, Tsui WY, Chen X et al. Downregulation of ID4 by promoter hypermethylation in gastric adenocarcinoma. Oncogene 2003; 22: 6946–53. [DOI] [PubMed] [Google Scholar]
- 9. Wang Q, Tsao SW, Fu S et al. Overexpression of Id‐1 in gastric adenocarcinoma: Implication for a novel diagnostic marker. Anticancer Res 2004; 24: 881–6. [PubMed] [Google Scholar]
- 10. Han S, Gou C, Hong L et al. Expression and significances of Id1 helix‐loop‐helix protein overexpression in gastric cancer. Cancer Lett 2004; 216: 63–71. [DOI] [PubMed] [Google Scholar]
- 11. Hu YC, Lam KY, Law S, Wong J, Srivastava G. Identification of differentially expressed genes in esophageal squamous cell carcinoma (ESCC) by cDNA expression array: Overexpression of Fra‐1, Neogenin, Id‐1, and CDC25B genes in ESCC. Clin Cancer Res 2001; 7: 2213–21. [PubMed] [Google Scholar]
- 12. Schindl M, Schoppmann SF, Strobel T et al. Level of Id‐1 protein expression correlates with poor differentiation, enhanced malignant potential, and more aggressive clinical behavior of epithelial ovarian tumors. Clin Cancer Res 2003; 9: 779–85. [PubMed] [Google Scholar]
- 13. Fong S, Itahana Y, Sumida T et al. Id‐1 as a molecular target in therapy for breast cancer cell invasion and metastasis. Proc Natl Acad Sci USA 2003; 100: 13543–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin CQ, Singh J, Murata K et al. A role for Id‐1 in the aggressive phenotype and steroid hormone response of human breast cancer cells. Cancer Res 2000; 60: 1332–40. [PubMed] [Google Scholar]
- 15. Schoppmann SF, Schindl M, Bayer G et al. Overexpression of Id‐1 is associated with poor clinical outcome in node negative breast cancer. Int J Cancer 2003; 104: 677–82. [DOI] [PubMed] [Google Scholar]
- 16. Ouyang XS, Wang X, Lee DT, Tsao SW, Wong YC. Over expression of ID‐1 in prostate cancer. J Urol 2002; 167: 2598–602. [PubMed] [Google Scholar]
- 17. Coppe JP, Itahana Y, Moore DH, Bennington JL, Desprez PY. Id‐1 and Id‐2 proteins as molecular markers for human prostate cancer progression. Clin Cancer Res 2004; 10: 2044–51. [DOI] [PubMed] [Google Scholar]
- 18. Wilson JW, Deed RW, Inoue T et al. Expression of Id helix‐loop‐helix proteins in colorectal adenocarcinoma correlates with p53 expression and mitotic index. Cancer Res 2001; 61: 8803–10. [PubMed] [Google Scholar]
- 19. Fong S, Debs RJ, Desprez PY. Id genes and proteins as promising targets in cancer therapy. Trends Mol Med 2004; 10: 387–92. [DOI] [PubMed] [Google Scholar]
- 20. Coppe JP, Smith AP, Desprez PY. Id proteins in epithelial cells. Exp Cell Res 2003; 285: 131–45. [DOI] [PubMed] [Google Scholar]
- 21. Ruzinova MB, Benezra R. Id proteins in development, cell cycle and cancer. Trends Cell Biol 2003; 13: 410–8. [DOI] [PubMed] [Google Scholar]
- 22. Lyden D, Young AZ, Zagzag D et al. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature 1999; 401: 670–7. [DOI] [PubMed] [Google Scholar]
- 23. Sakurai D, Tsuchiya N, Yamaguchi A et al. Crucial role of inhibitor of DNA binding/differentiation in the vascular endothelial growth factor‐induced activation and angiogenic processes of human endothelial cells. J Immunol 2004; 173: 5801–9. [DOI] [PubMed] [Google Scholar]
- 24. Mallo GV, Soubeyran P, Lissitzky JC et al. Expression of the Cdx1 and Cdx2 homeotic genes leads to reduced malignancy in colon cancer‐derived cells. J Biol Chem 1998; 273: 14030–6. [DOI] [PubMed] [Google Scholar]
- 25. Jeffers M, McDonald WF, Chillakuru RA et al. A novel human fibroblast growth factor treats experimental intestinal inflammation. Gastroenterology 2002; 123: 1151–62. [DOI] [PubMed] [Google Scholar]
- 26. Sako A, Kitayama J, Koyama H et al. Transduction of soluble Flt‐1 gene to peritoneal mesothelial cells can effectively suppress peritoneal metastasis of gastric cancer. Cancer Res 2004; 64: 3624–8. [DOI] [PubMed] [Google Scholar]
- 27. Sikder HA, Devlin MK, Dunlap S, Ryu B, Alani RM. Id proteins in cell growth and tumorigenesis. Cancer Cell 2003; 3: 525–30. [DOI] [PubMed] [Google Scholar]
- 28. Lasorella A, Uo T, Iavarone A. Id proteins at the cross‐road of development and cancer. Oncogene 2001; 20: 8326–33. [DOI] [PubMed] [Google Scholar]


