Abstract
By representational difference analysis, we previously identified the rat Erc (Expressed in renal carcinoma) gene that was more abundantly expressed in the renal carcinoma tissues of Eker rats than in the rat normal kidney. In this study, we raised antibodies against the amino‐terminal portion of the rat Erc, and demonstrated the existence of a ∼30‐kDa secretory form in the supernatant of cultured cells derived from rat renal carcinoma. The enzyme‐linked immunosorbent assay (ELISA) system using these antibodies detected high concentrations of this form in the sera of Eker rats bearing renal carcinomas, and in the sera of rats transplanted with mesothelioma cells. Mesothelin, a human homolog of the rat Erc, was recently reported to be a serum marker of malignant mesothelioma. The prognosis of mesothelioma is poor and there is no effective treatment at present. There are several rat model systems of mesothelioma that may be promising tools in the development of an antimesothelioma treatment. We hope our ELISA to detect the soluble form of rat Erc/Mesothelin is useful in the rat model system to exploit the antimesothelioma therapy to be used in human cases. (Cancer Sci 2007; 98: 659–664)
Renal cell carcinoma (RCC) is the sixth leading cause of cancer death, and approximately 12 000 people die from this malignancy in a year in the USA.( 1 ) We previously isolated the rat Erc gene from the RCC tissue of Eker rats by representational difference analysis (RDA). The Erc is more preferentially expressed in RCC of the Eker rats than in rat normal kidney.( 2 )
The human Mesothelin gene, a homolog of the rat Erc, is also expressed abundantly in human cancers.( 3 , 4 ) Pastan et al. and Yamashita et al. implied that Erc/Mesothelin is associated with cell‐to‐cell adhesion.( 5 , 6 ) Both in rat and human clones, the signal peptide near the amino (NH2)‐terminus, the glycosylphosphatidylinositol (GPI) anchorage sequence near the carboxyl (COOH)‐terminus, and the furin recognition site in the middle, are conserved (Fig. 1). From these structures, the existence of the membrane‐bound form (COOH‐terminal part) and the secretory form (amino‐terminal part) is predicted.
Figure 1.

Amino acid alignment of the rat Erc (top) and human Mesothelin (bottom). Identical amino acids in two proteins are indicated by asterisks. Lines covering the signal peptide, cleavage sequence of furin, and recognition sequence for glycosylphosphatidylinositol (GPI)‐anchoring reaction are shown on the sequences. Peptide sequences used as the immunogen for No. 150 and No. 549 antibodies are shadowed.
Recently, Erc/Mesothelin and its related form were reported to be serum markers of malignant mesothelioma.( 7 , 8 , 9 , 10 , 11 , 12 ) At present, there is no effective treatment for mesothelioma, and the existing rat model systems of mesothelioma are promising tools to develop antimesothelioma therapy.
In this study, we raised multiple antibodies against the NH2‐ and COOH‐terminal portions of rat Erc/Mesothelin. The antibodies for the NH2‐terminus detected a ∼30‐kDa secretory form in the medium of cultured rat renal carcinoma cells. The enzyme‐linked immunosorbent assay (ELISA) system using these antibodies confirmed the high concentration of this form in the sera of Eker rats bearing renal cell carcinomas, and in the sera of rats transplanted with mesothelioma cells. We hope this ELISA for rat Erc/Mesothelin will be useful for the rat mesothelioma model systems in the exploration of an effective therapy to be used in human cases.
Materials and Methods
Reverse transcription‐polymerase chain reaction of the rat Erc transcript in cultured cell lines. Rat renal carcinoma cell lines ERC‐33 (a gift from A.G. Knudson, Fox Chase Cancer Center) and LK9dL (cloned in our laboratory) were established from the primary RCC of an Eker rat.( 2 , 6 , 13 ) These cell lines were maintained in TIL (tumor‐infiltrating lymphocytes) medium (IBL, Fujioka, Japan) supplemented with 10% fetal bovine serum. Total RNA was extracted from the cultured cell using RNeasy Mini (QIAGEN, Chatsworth, CA, USA), and cDNA was generated with a first‐strand cDNA synthesis kit (Amersham Biosciences, Piscataway, NJ, USA). Rat Erc cDNA was amplified by polymerase chain reaction (PCR) using forward (5′‐ACAGGATCCCAGACCCAGATCACAAGGACA‐3′) and reverse (5′‐GTGCTCGAGTCACCGCCGGAACCTTGGGTG‐3′) primers.
Polyclonal and monoclonal antibodies against the NH2‐terminal portion of rat Erc. A synthetic peptide NVNVLPRRSLERQRLLT, which corresponds to the N150‐T166 of amino acid sequences of rat Erc/Mesothelin (shadowed in Fig. 1) was coupled with thyroglobulin, and immunized to rabbits. Antisera of immunized rabbits were applied to thiol‐sepharose beads (Amersham Biosciences) coupled with synthetic peptide, and an IgG fraction was eluted. Eluted polyclonal IgG was designated as No. 150. As an immunogen for monoclonal antibodies, Histagged rat Erc protein was expressed in Eschericia coli using the QIAexpress system (QIAGEN) following the manufacturer's instruction. Briefly, rat Erc cDNA covering the ∼30‐kDa secretory form (NH2‐terminal part) was inserted into pQE‐30 in the same reading frame as the His tag, and introduced into E. coli M15 [pREP4]. After induction with IPTG (isopropylthio‐β‐D‐galactoside), E. coli was lyzed in 6 M GuHCl, and loaded onto a nickel‐nitrilotriacetic acid (Ni‐NTA) agarose. The His‐tagged Erc protein was eluted with imidazole, coupled with thyroglobulin, and then used for immunization of mice. Splenocytes of the mice were fused with a myeloma cell line X63‐Ag8.653. The supernatants of hybridoma cells were screened by their reactivity to the Erc protein used for immunization. Western blotting was used as a secondary screening, and hybridomas reacting with the ∼30‐kDa Erc protein in ERC‐33 cell lysate, but not with the LK9dL cell lysate were obtained. By the limiting dilution method, one hybridoma was chosen for this study and designated as 30F2.
Polyclonal antibody against the COOH‐terminal portion of rat Erc. A synthetic peptide IGDLKTEEDKSPVRDWLF, which corresponds to the I549‐F566 (shadowed in Fig. 1) of amino acid sequences of rat Erc/Mesothelin was coupled with thyroglobulin, and immunized to rabbits. Antibodies against this peptide were purified in the same way as for No. 150 (mentioned above) and the eluted polyclonal IgG was designated as No. 549.
Western blotting to detect the overexpressed and endogenous rat Erc in the cultured cells. Full‐length cDNA of the rat Erc/Mesothelin coding region was inserted into the pcDNA3.1(+) vector (Invitrogen, Carlsbad, CA, USA) to enable expression in Chinese hamster ovary (CHO)‐K1 cells. Transfection was carried out using FuGENE6 transfection reagent (Roche Diagnostics, Mannheim, Germany). After transient expression of the rat Erc/Mesothelin cDNA in the CHO‐K1 cells, both the culture supernatant (0.5 µL) and cell lysate (10 µg) were harvested. To detect the endogenous secretory form in the RCC cell lines (ERC‐33 and LK9dL), cells were seeded in 2 mL of TIL medium at a cell density of 105 cells/35‐mm plate and cultured for 3 days, and both the culture supernatant (10 µL) and cell lysate (40 µg) were harvested. The harvested sample was adjusted to be a solution containing 2% sodium dodecylsulfate (SDS), 10% glycerol, 50 mM TrisHCl (pH 6.8), 100 mM dithiothreitol and boiled. The sample was electrophoresed on a 10% Laemmli gel, and transferred to a nitrocellulose membrane, BA85 (Schleicher & Schuell Bioscience, Keene, NH, USA). The membrane was blocked in 1% skim milk in phosphate‐buffered saline (PBS) with 0.1% Tween 20 (PBS‐T) for 1 h at room temperature (rt), and was incubated with 1 µg/mL of primary antibodies (No. 150 and 30F2 for the NH2 terminus, and No. 549 for COOH‐terminus) in 1% skim milk in PBS‐T for 1 h at rt. Goat antirabbit Ig (for No.150 and No.549) or goat antimouse Ig (for 30F2) conjugated to peroxidase labeled‐dextran polymer (Envision K4002 or K4000) (DAKO, Carpinteria, CA, USA) were used as secondary antibodies and were diluted 50 times in 1% skim milk in PBS‐T. The reaction was at rt for 1 h. The rat Erc protein on each membrane was visualized by the ECL detection system (Amersham Biosciences).
Immunohistochemistry of rat Erc in Eker rat RCC. Four‐micrometer‐thick tissue sections were prepared from formalin‐fixed, paraffin‐embedded RCC tissue of an Eker rat. After deparaffinization, the tissue sections were heated in 10 mM citrate buffer (pH 6.0) for antigen retrieval, and treated with 3% hydrogen peroxide. Then, the sections were incubated with a primary antibody, no. 549 (raised against the COOH‐terminal of rat Erc) 1 µg/mL in PBS‐T at rt overnight. As a secondary antibody, Envision K4002 (DAKO), without dilution, was applied to the tissue sections. Diaminobenzidine (DAB) was used as the substrate for peroxidase.
Establishment of ELISA to detect the NH2‐terminal secretory form of rat Erc. As a standard protein for the ELISA system, the NH2‐terminal secretory form of the rat Erc protein was purified from culture supernatant of CHO‐K1 cell transfected with a full‐length rat Erc/Mesothelin cDNA in pcDNA3.1(+) vector, by using Formyl‐Cellulofine Column (Research Biochemicals, East Falmouth, MA, USA) coupled with a 30F2 antibody. The concentration of purified protein was measured by Protein Assay (Bio‐Rad, Tokyo, Japan). The purity of the protein was demonstrated densitometrically by using a Densitograph (ATTO, Tokyo, Japan) and gel‐filtration chromatography (data not shown). Immuno Module Plate (Nalge Nunc, Rochester, NY, USA) was coated with 30F2 antibody (10 µg/mL in 0.1 M carbonate buffer, pH 9.5) at 4°C overnight, and then blocked with 1% bovine serum albumin in PBS containing 0.05% NaN3 at 4°C overnight. Sample and standard protein (purified recombinant NH2‐terminal secretory form) were diluted with 1% Triton‐X in PBS, added to each well and incubated at 37°C for 1 h. After nine washes with washing buffer, 100 µL of 4 µg/mL horseradish peroxidase‐labeled No. 150 antibody was added to each well and incubated for 30 min at 4°C. After nine washes with washing buffer, 100 µL of tetramethyl benzidine buffer as a substrate was added to each well and incubated for 30 min at room temperature in the dark. Color development was stopped by the addition of 100 µL of stop solution (1 N H2SO4). Optic density at 450 nm of each sample was measured.
Quantification of the NH2‐terminal secretory form of rat Erc in the sera of Eker rats by ELISA. Sixteen Eker rats aged approximately 1 year were killed. Serum was frozen at −80°C and the kidney tissue was fixed in 10% neutralized formalin. RCC was macroscopically recognized in seven Eker rats. The serum level of the NH2‐terminal secretory form was quantified by the ELISA system.
Intra‐peritoneal transplantation of mesothelioma cells to rats and quantification of the NH2‐terminal secretory form of Erc in serum. The rat mesothelioma cell line MeET‐4, established by Kuwahara et al.( 14 ) was cultured in Dulbeco's modified Eagle's medium with 10% fetal bovine serum (FBS). The cells (3 × 107) were harvested at the growth stage, and transplanted into the peritoneal cavity of 8‐week‐old female Wister rats. At 10 and 17 days after transplantation, the rats were killed and the serum concentration of the NH2‐terminal secretory form of Erc was measured. The serum of a rat without transplantation was used as a control (0 day).
Results
Rat Erc expressed in cultured cell lines. A CHO cell transfected with the rat Erc cDNA was used as the control of Western blotting to confirm the specificity of the antibody used in this study. The No. 150 and 30F2 antibodies, both of which were raised by the immunization with the NH2‐terminal part of rat Erc, detected the ∼30‐kDa secretory form in the sup of the Erc‐transfected CHO cells but not in that of the parent CHO cells (Fig. 2, lanes 1, 2 in ‘30F2(N)’ and ‘No. 150(N)’). In the cellular lysates, these antibodies detected both the ∼70‐kDa full‐length form and the ∼30‐kDa secretory form in the Erc‐transfected CHO cell only (lanes 3, 4). The No. 549 antibody, which recognizes the COOH‐terminus of rat Erc, detected the ∼70‐kDa full‐length form in the cellular lysate of the Erc‐transfected CHO cell (Fig. 2, lanes 3, 4 in ‘No.549(C)’). No. 549 did not detect the ∼30‐kDa form in the supernatant (data not shown). We then examined the endogenous expression of Erc in cultured cells derived from the rat RCC, ERC‐33 and Lk9dL. At first we checked the amount of the Erc transcripts in the cellular lysates by reverse transcription‐PCR (RT‐PCR). The rat Erc transcript was much more abundantly expressed in the ERC‐33 than in Lk9dL (Fig. 3, lanes 7, 8 in ‘RT‐PCR’). In Western blotting, the 30F2 antibody detected the ∼30‐kDa secretory form in ERC33 supernatant (Fig. 3, lane 6 in ‘30F2(N)’), and both the ∼70‐kDa full‐length form and the ∼30‐kDa secretory form in the ERC33 cellular lysate (lane 8). These proteins were not detected in the sup or lysate of Lk9dL (lanes 5, 7). Western blotting using the No. 150 antibody also showed the same expression pattern in ERC33 (data not shown).
Figure 2.
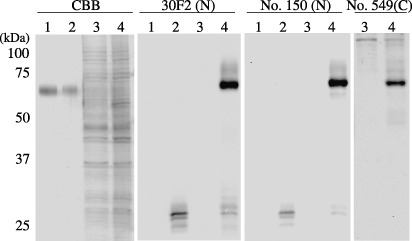
Detection of the overexpressed rat Erc in the cultured cells. The culture medium 0.5 µL (lanes 1, 2) or cellular lysate 10 µg (lanes 3, 4) of CHO cell transfected with a mock vector (lanes 1, 3) or of an Erc‐expression vector (lanes 2, 4) were loaded. Protein on the gel was stained with Coomassie Brilliant Blue (CBB), or transferred to nitrocellulose membrane and incubated with 30F2, No. 150, or No. 549 antibody, respectively. Note that the single band in lanes 1, 2 in a CBB‐stained gel is bovine serum albumin in the culture medium.
Figure 3.
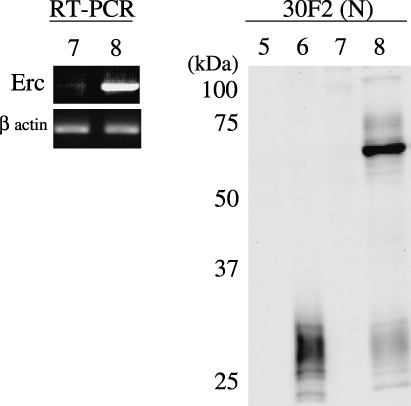
Endogenous expression of the rat Erc in the cultured cells. Left panel, transcripts of the Erc (a 806 bases fragment) or β actin in the cellular lysate of Lk9dL (lane 7) or of ERC33 (lane 8) was amplified by reverse transcription‐polymerase chain reaction (RT‐PCR). Right panel, the culture medium 10 µL (lanes 5, 6) or cellular lysate 40 µg (lanes 7, 8) of Lk9dL (lanes 5, 7) or of ERC33 (lanes 6, 8) were loaded. Protein on the gel was transferred to nitrocellulose membrane and incubated with 30F2 antibody.
Rat Erc expressed in RCC of Eker rats. Figure 4 shows the heterogeneous expression patterns of rat Erc in the RCC tissue of an Eker rat, detected by the No. 549 antibody. In the left part of the picture, the Erc was mainly expressed in the cytoplasm, while in the right, the protein was mainly expressed in the cellular membrane. In the middle part, there was no expression of the protein, despite this area also being of RCC.
Figure 4.
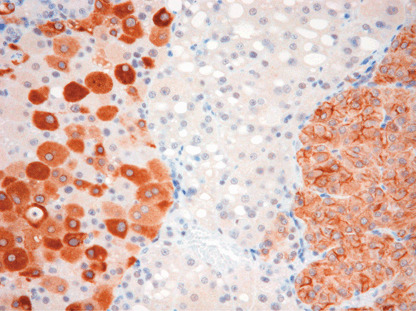
Expression of the rat Erc in renal cell carcinoma (RCC) tissue of Eker rat, detected by No. 549 antibody.
Establishment of the ELISA system for the NH2‐terminal secretory form of rat Erc protein. Optic density at 450 nm of a recombinant NH2‐terminal secretory form was measured by the ELISA system using two antibodies, No. 150 and 30F2, and the result exhibited a linear log/log plot over the range between 1.25 and 80 ng/mL of a recombinant protein (Fig. 5). The assay was repeated four times, and the mean ± standard deviation (SD) of optic density was 0.039 ± 0.0050, 0.073 ± 0.0127, 0.130 ± 0.0026, 0.252 ± 0.0162, 0.445 ± 0.0039, 0.815 ± 0.0456, abd 1.524 ± 0.0341 for 1.25, 2.5, 5.0, 10.0, 20.0, 40.0, and 80.0 ng/mL of the recombinant protein, respectively. The ELISA was able to quantify the secretory form of rat Erc in the supernatant of cultured RCC cells; approximately 60 ng/mL in ERC‐33 supernatant (Fig. 6). Its amount in LK9dL supernatant was lower than the sensitivity level of this ELISA system.
Figure 5.
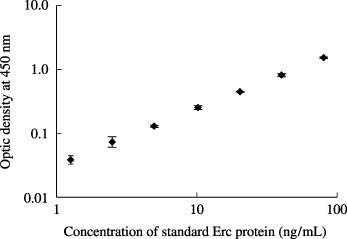
A standard curve of the enzyme‐linked immunosorbent assay (ELISA) to quantify the NH2‐terminal soluble form of rat Erc. The affinity purified ∼30‐kDa protein was used as a standard. The assay was repeated four times, and the mean ± standard deviation (SD) is shown.
Figure 6.
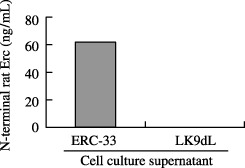
Quantification of the NH2‐terminal rat Erc in the supernatant of cultured cell (ERC‐33 and LK9dL) by ELISA.
Quantification of the NH2‐terminal secretory form of rat Erc in the sera of Eker rats by ELISA. Kidneys of 16 Eker rats were examined, and seven showed the macroscopic RCC. In all nine RCC‐negative rats, the NH2‐terminal secretory form in serum was lower than 12 ng/mL, whereas it was higher than 20 ng/mL in four (328, 316, 323, 345) of seven RCC‐bearing rats (Fig. 7). Average concentrations of the NH2‐terminal secretory form in serum was also higher in seven RCC‐bearing rats than in nine RCC‐negative rats (23.5 ng/mL vs 8.9 ng/mL, P = 0.03).
Figure 7.
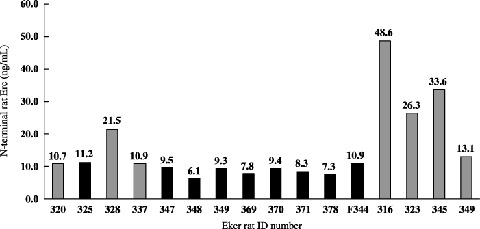
The NH2‐terminal Erc in the sera of Eker rat quantified by enzyme‐linked immunosorbent assay (ELISA). Sera of 16 Eker rats were used. Numbers above each bar indicate the concentration. Gray or black bars indicate Eker rats with or without renal cell carcinoma (RCC), respectively.
Quantification of the NH2‐terminal secretory form in the sera of rats transplanted with mesothelioma cells. The serum concentration of the NH2‐terminal form of Erc was 882 ng/mL or 1797 ng/mL in a rat killed 10 days or 17 days after the intraperitoneal transplantation of MeET‐4. That of the control mouse was less than 10 ng/mL (Fig. 8).
Figure 8.
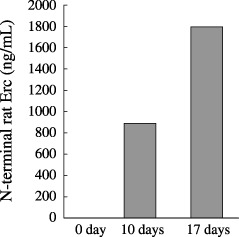
The NH2‐terminal Erc in the sera of rats transplanted with mesothelioma cells. Sera of Wister rats sacrificed 10 or 17 days after the MeET‐4 transplantation, or serum of a rat without transplantation (0 day) were used.
Discussion
We previously isolated the rat Erc, which is more abundantly expressed in RCC tissues of Eker rats than in the normal rat kidney.( 2 ) In this study, we established the ELISA system to detect the ∼30‐kDa secretory form of Erc, and demonstrated its concentration to be higher in the sera of RCC‐bearing Eker rats than in those of‐non‐RCC‐bearing rats (Fig. 7). We also showed that its serum concentration was much higher in rats transplanted with the mesothelioma cell MeET‐4( 14 ) (Fig. 8).
A human homolog of the rat Erc is Mesothelin/MPF (megakaryocyte potentiating factor).( 5 , 15 ) The gene expression analysis using methods such as cDNA microarray showed that Mesothelin is preferentially expressed in the ovarian or pancreatic cancers.( 3 , 4 ) These data imply that Erc/Mesothelin/MPF product is associated with progression of carcinogenesis although its function in detail is still unknown.
Mesothelin was identified as a cell surface antigen recognized by the monoclonal antibody K1 in human mesothelioma and ovarian carcinoma.( 5 , 16 , 17 ) MPF was independently identified as a soluble factor from the supernatant of cultured human pancreatic cancer cells.( 18 ) Subsequently cDNA cloning revealed Mesothelin and MPF are derived from a common ∼70‐kDa precursor.( 5 , 15 ) By the furin cleavage of the precursor, a ∼30‐kDa NH2‐terminal half (MPF) is released into the extracellular space and a ∼40‐kDa COOH‐terminal half remains attached to the cell surface by a GPI‐anchor.( 16 ) Another isoform, soluble Mesothelin‐related protein (SMR), is detected by antibodies recognizing the COOH‐terminal portion.( 9 ) SMR has the 82 bp insertion in mRNA as a result of an alternative splicing, causing the frame shift and changing the structure required for the attachment of GPI moiety. As expected, a 42–45 kDa SMR is not anchored to the membrane, and is released into the extracellular space.( 9 ) Recently SMR,( 10 , 11 ) a ∼30‐kDa NH2‐terminal half (MPF),( 7 , 8 ) and the other form of Mesothelin,( 12 ) detected by antibodies to the COOH‐terminal portion, were reported to be serum markers of malignant mesothelioma.
In this study, we raised antibodies to both the NH2‐ and COOH‐terminal portions of the rat Erc/Mesothelin. We confirmed the specificity of these antibodies by detecting the ∼70‐kDa or ∼30‐kDa proteins only in the Erc‐transfected CHO cell (Fig. 2, three right panels, lanes 2, 4). By using two antibodies for the NH2‐terminus (30F2 and No. 150), we detected the ∼30‐kDa protein in the supernatant, and both the ∼70‐kDa full‐length form and ∼30‐kDa protein in the cellular lysate of the Erc‐transfected CHO cell. We also confirmed the endogenous expression of these proteins in the cultured cells (Fig. 3, right panel, lanes 6, 8). In the cellular lysate, the ∼70‐kDa form was much more abundant than that of the ∼30‐kDa protein, and in the supernatant, the ∼70‐kDa form was not detected. The antibody recognizing the COOH‐terminus (No. 549) detected the ∼70‐kDa full‐length form in the cytoplasm, and, as expected, this antibody did not react to the ∼30 kDa secretory form. These data indicated that most of the ∼30‐kDa protein was excreted into the extracellular space by the furin cleavage, and that the ∼70‐kDa protein was not excreted.
We constructed the ELISA system by using two antibodies 30F2 and No. 150, both of which were raised for the NH2‐terminal portion of rat Erc. The concentration of the ∼30‐kDa secretory form in the supernatant of two cell lines (ERC‐33 and LK9dL), quantified by this ELISA system (Fig. 6), was correlated with the result of RT‐PCR or Western blot (Fig. 3). We showed that the concentration of this secretory form was higher in the sera of Eker rats bearing RCC (Fig. 7). If the present ELISA system was used as the diagnostic test for RCC in Eker rats with a presumptive cut‐off value of 12 ng/mL, its sensitivity and specificity could be 71.4% (5/7) and 100.0% (9/9), respectively. We also showed that the ELISA system was useful for the rat model system of mesothelioma (Fig. 8).
Figure 4 shows that the Erc was expressed heterogeneously in RCC tissue. There was the Erc‐negative area even in the RCC. This result indicated that the Erc was not expressed in all RCC cases, and explained that the serum level of the soluble Erc was not high in some of the RCC bearing Eker rats (cases 320, 337 and 349 in Fig. 7).
Two rat RCC cell lines, ERC‐33 and LK9dL, used in this study are derived from primary Eker RCC, and ERC‐33 has more malignant characters than LK9dL. ERC‐33 can be cultured on non‐collagen‐coated culture plates but LK9dL can only be cultured on collagen‐coated culture plates, and the doubling time of ERC‐33 is much shorter than that of LK9dL. Furthermore, ERC‐33 implanted into subcutaneous tissue of the nude mice develop lung metastasis but LK9dL does not.( 19 ) The ∼30‐kDa secretory form was detected in the more proliferative ERC‐33, and this finding is also compatible with our idea that Erc/MPF/Mesothelin is associated with the progression of cancers, although the other factors may be involved in this process. This secretory form produced by neoplastic cells might act as an autocrine growth factor to promote the carcinogenesis, and this possibility must be examined in the future study.
Malignant mesothelioma is etiologically associated with asbestos exposure. The number of patients is increasing in Japan, but there is no effective treatment and its prognosis is poor at present. There are several rat models of mesothelioma,( 14 , 20 , 21 , 22 , 23 ) which are promising tools to develop and evaluate the antimesothelioma therapy. We established the rat ELISA system to detect the soluble form of Erc/MPF/Mesothelin, and demonstrated the high concentration of this form in the sera of rats bearing mesothelioma (Fig. 8). We hope this ELISA will be valuable to evaluate the new antimesothelioma therapy, using the rat model system of this disease.
Acknowledgments
This work was supported by a Grant‐in‐Aid for Cancer Research and Grants‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports, and Science and Technology of Japan and the Ministry of Health, Labor, and Welfare of Japan.
References
- 1. Jemal A, Murray T, Ward E et al. Cancer statistics, 2005. CA Cancer J Clin 2005; 55: 10–30. [DOI] [PubMed] [Google Scholar]
- 2. Hino O, Kobayashi E, Nishizawa M et al. Renal carcinogenesis in the Eker rat. J Cancer Res Clin Oncol 1995; 121: 602–5. [DOI] [PubMed] [Google Scholar]
- 3. Hough CD, Sherman‐Baust CA, Pizer ES et al. Large‐scale serial analysis of gene expression reveals genes differentially expressed in ovarian cancer. Cancer Res 2000; 60: 6281–7. [PubMed] [Google Scholar]
- 4. Argani P, Iacobuzio‐Donahue C, Ryu B et al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE). Clin Cancer Res 2001; 7: 3862–8. [PubMed] [Google Scholar]
- 5. Chang K, Pastan I. Molecular cloning and expression of a cDNA encoding a protein detected by the K1 antibody from an ovarian carcinoma (OVCAR‐3) cell line. Int J Cancer 1994; 57: 90–7. [DOI] [PubMed] [Google Scholar]
- 6. Yamashita Y, Yokoyama M, Kobayashi E, Takai S, Hino O. Mapping and determination of the cDNA sequence of the Erc gene preferentially expressed in renal cell carcinoma in the Tsc2 gene mutant (Eker) rat model. Biochem Biophys Res Commun 2000; 275: 134–40. [DOI] [PubMed] [Google Scholar]
- 7. Onda M, Nagata S, Ho M et al. Megakaryocyte potentiation factor cleaved from Mesothelin precursor is a useful tumor marker in the serum of patients with mesothelioma. Clin Cancer Res 2006; 12: 4225–31. [DOI] [PubMed] [Google Scholar]
- 8. Shiomi K, Miyamoto H, Segawa T et al. Novel ELISA system for detection of N‐ERC/Mesothelin in the sera of mesothelioma patients. Cancer Sci 2006; 97: 928–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scholler N, Fu N, Yang Y et al. Soluble member(s) of the mesothelin/megakaryocyte potentiating factor family are detectable in sera from patients with ovarian carcinoma. Proc Natl Acad Sci USA 1999; 96: 11 531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Robinson BWS, Creaney J, Lake R et al. Mesothelin‐family proteins and diagnosis of mesothelioma. Lancet 2003; 362: 1612–6. [DOI] [PubMed] [Google Scholar]
- 11. Robinson BWS, Creaney J, Lake R et al. Soluble mesothelin‐related protein – a blood test for mesothelioma. Lung Cancer 2005; 49S1: S109–11. [DOI] [PubMed] [Google Scholar]
- 12. Hassan R, Remaley AT, Sampson ML. Detection and quantitation of serum mesothelin, a tumor marker for patients with mesothelioma and ovarian cancer. Clin Cancer Res 2006; 12: 447–53. [DOI] [PubMed] [Google Scholar]
- 13. Kobayashi T, Hirayama Y, Kobayashi E, Kubo Y, Hino O. A germline insertion in the tuberous sclerosis (Tsc2) gene gives rise to the Eker rat model of dominantly inherited cancer. Nat Genet 1995; 9: 70–4. [DOI] [PubMed] [Google Scholar]
- 14. Kuwahara M, Inui K, Sugimoto K et al. Establishment and characterization of spontaneous mesothelioma cell lines derived from F344 rats. Virchows Arch 1997; 431: 257–63. [DOI] [PubMed] [Google Scholar]
- 15. Kojima T, Oh‐eda M, Hattori K et al. Molecular cloning and expression of megakaryocyte potentiating factor cDNA. J Biol Chem 1995; 270: 21 984–90. [DOI] [PubMed] [Google Scholar]
- 16. Chang K, Pai LH, Batra JK, Pastan I, Willingham MC. Characterization of the antigen (CAK1) recognized by monoclonal antibody K1 present on ovarian cancers and normal mesothelium. Cancer Res 1992; 52: 182–6. [PubMed] [Google Scholar]
- 17. Chang K, Pastan I, Willingham MC. Isolation and characterization of a monoclonal antibody, K1, reactive with ovarian cancers and normal mesothelium. Int J Cancer 1992; 50: 373–81. [DOI] [PubMed] [Google Scholar]
- 18. Yamaguch N, Hattori K, Oeda M, Kojima T, Imai N, Ochi N. A novel cytokine exhibiting megakaryocyte potentiating activity from a human pancreatic tumor cell line HPC‐Y5. J Biol Chem 1994; 269: 805–8. [PubMed] [Google Scholar]
- 19. Fukuda T, Hirayama Y, Mitani H et al. Generation of metastatic variants of Eker renal carcinoma cell lines for experimental investigation of renal cancer metastasis. Jpn J Cancer Res 1998; 89: 1104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mekawa Y, Kurokawa Y, Takahashi M et al. Spontaneous tumors in F‐344/DuCrj rats. Gann 1983; 74: 365–72. [PubMed] [Google Scholar]
- 21. Maita K, Hirano M, Harada T et al. Spontaneous tumors in F‐344/DuCrj rats from 12 control groups of chronic and oncogenicity studies. J Toxicol Sci 1987; 12: 111–26. [DOI] [PubMed] [Google Scholar]
- 22. Craighead JE, Akley NJ, Gould LB, Libbus BL. Characteristics of tumors and tumor cells cultured from experimental asbestos‐induced mesotheliomas in rats. Am J Pathol 1987; 129: 448–62. [PMC free article] [PubMed] [Google Scholar]
- 23. Manning LS, Whitaker D, Murch AR et al. Establishment and characterization of five human malignant mesothelioma cell lines derived from pleural effusions. Int J Cancer 1991; 47: 285–90. [DOI] [PubMed] [Google Scholar]


