Abstract
Papillomavirus E6 oncoproteins transform mammalian cells through interaction with cellular proteins. Bovine papillomavirus type 1 E6 (BE6) interacts with three previously described cellular targets: the E6AP E3 ubiquitin ligase, the calcium-binding protein E6BP (also known as ERC-55), and paxillin, which is a focal adhesion adapter protein. BE6 interacts strongly with each of these proteins in vitro, binding to similar peptide sequences found in E6AP, E6BP, and paxillin. To determine which BE6 interactions are necessary for transformation by BE6, we used a novel selection strategy for temperature-sensitive BE6 mutants in yeast that could discriminate in their interaction between E6AP, E6BP, and paxillin. All BE6 mutants that retained transforming ability retained association with paxillin, while some mutants that were transformation positive failed to interact with E6AP or E6BP. This study demonstrates that oncogene mutants that are temperature sensitive for transformation can be selected in yeast and that the induction of anchorage-independent cell proliferation by BE6 does not require strong association of BE6 with either E6AP or E6BP. Of particular interest is the identification of a BE6 mutant that interacts strongly with the acidic charged leucine motifs of E6AP, E6BP, and paxillin but is devoid of transformation activity, thereby genetically identifying a second essential transformation function in BE6 that is independent of interaction with acidic charged leucine motifs.
The papillomavirus E6 oncoproteins are small zinc-binding proteins that do not have identified intrinsic enzymatic activity. E6 proteins are thought to act as adapter proteins, thereby altering the function of E6-associated cellular proteins. This model for E6 function is best supported by observations of human papillomavirus type 16 (HPV-16) E6 (16E6), which can alter the metabolism of the p53 tumor suppressor through association with a cellular E3 ubiquitin ligase called E6AP (7). HPV-16 E6 interacts with an 18-amino-acid sequence in E6AP (8), and in an as yet ill-defined fashion the E6AP-16E6 complex binds to p53, inducing the ubiquitin-dependent degradation of the trimolecular complex. 16E6 apparently functions as an adapter protein in the complex with p53, since E6AP does not interact with p53 in the absence of E6 and since the degradation of p53 requires both E6 and E6AP (8).
Targeted degradation of p53 has been observed in HPV types that are associated with anogenital cancers but has not been reported in other HPV types or with E6 genes from animal papillomaviruses. While these E6 proteins do not degrade p53, it is likely that some features are similar in the interactions of these E6 oncoproteins with their target cellular proteins. This has recently been illustrated in studies of bovine papillomavirus type 1 E6. Bovine papillomavirus type 1 E6 (BE6) has been described as interacting with three cellular proteins: E6AP (13), E6BP (also known as ERC-55) (4), and paxillin (17, 20). BE6 binds to similar peptide sequences found on each of these three proteins (3, 20). Unlike 16E6, where the binding to E6AP induces the degradation of p53, BE6 binding to E6AP has not been shown to induce the degradation of p53, and the in vivo interactions of BE6 with paxillin or E6BP have as yet unknown consequences. Since E6AP, E6BP, and paxillin all interact with BE6 through similar peptide sequences, how can we distinguish the protein interactions responsible for transformation by BE6 from interactions that are of unknown significance? This study describes the isolation of BE6 mutants that discriminate in their interactions between E6AP, E6BP, and paxillin.
Mutational analysis of the E6 oncoproteins has been hampered by the sensitivity of these oncoproteins to disruptive mutations. A previous mutational analysis of BE6 indicated that most BE6 mutants selected in yeast to be defective for one function of BE6 such as transcriptional activation were also defective for all other functions (transformation or interaction with E6AP). Mutants in which these functions were independent, while infrequent, could be isolated (13). This indicates that BE6 may have a complex structure that is sensitive to mutation. Disruptive mutations that are defective in more than one BE6 function are not useful for interpreting the significance of protein interactions. In this study, to obtain a panel of mutants with more subtle phenotypes, we selected for temperature-sensitive (ts) BE6 mutants in yeast. The recovered BE6 mutants were analyzed for transformation and protein interactions in vivo and in vitro with E6AP, E6BP, and paxillin. Some of the recovered mutants have ts transformation phenotypes. Interestingly, some of the recovered BE6 mutants discriminate in their binding to E6AP, E6BP, and paxillin. Only paxillin retained interactions in vitro with all transformation-positive BE6 mutants.
MATERIALS AND METHODS
Plasmids.
Maltose-binding protein (MBP) fusions to paxillin have been described previously (20). Glutathione S-transferase fusions to E6AP and E6BP were the gifts of John Huibregtse and Elliot Androphy, respectively. Yeast two-hybrid reagents and random mutagenesis of BE6 in yeast have been previously described (13).
Cell lines, transfections, and culture conditions.
Mouse C127 cell lines were maintained in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum, glutamine, and antibiotics. For cell transformation assays, wild-type BE6 and BE6 mutants cloned in pBabe-puro were packaged as retroviruses in the packaging cell line BOSC by transient transfection (14) and used to infect murine C127 cells. Infected cells were selected in puromycin-containing medium for 21 days and then seeded into agar to assay for anchorage independence as previously described (19). Anchorage independence at 32, 37, and 39.5°C was assessed by culturing the cells at the assay temperature overnight, seeding into soft agar, and evaluating for colony formation 14 days later for cultures at 37 and 39.5°C and 21 days later for cultures at 32°C (due to the slower cell division at 32°C).
In vitro translation and in vitro binding assays.
In vitro coupled transcriptions and translations were performed as previously described (13). For in vitro binding assays, approximately 1 μg of maltose-binding protein or glutathione S-transferase fusion immobilized on agarose beads was suspended in 150 μl of LSAB buffer (100 mM Tris-HCl [pH 8], 100 mM NaCl, 1% Nonidet P-40, 0.1% nonfat dried milk, 2 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride) (4) together with 5 μl of rabbit reticulocyte lysate, programmed to express the indicated proteins. The samples were incubated for 4 h at 4°C, and the beads were washed three times with 1.0 ml of the binding buffer. Retained proteins were eluted with sodium dodecyl sulfate sample buffer, resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (15% polyacrylamide), fluorographed with salicylate (2), and subjected to autoradiography. Radioactive proteins were quantitated by gel scanning with a Packard Instant Imager.
RESULTS
Isolation of BE6 mutants ts for association with E6AP in yeast.
BE6 can act as a transcriptional activation domain in yeast and mammalian cells when fused to a heterologous DNA-binding domain (11). A fusion of BE6 to the lexA DNA-binding domain confers a selectable phenotype in yeast through activation of a lexA-responsive reporter gene (13). We had previously isolated BE6 mutants defective for transcriptional activation to ascertain the role of transcriptional activation in transformation by BE6. Most of the resulting mutants were defective for multiple functions of BE6 (transcriptional activation, transformation, and interaction with E6AP), with only rare mutants that dissociated transcriptional activation from transformation (13). We reasoned that BE6 mutants that are ts for transcriptional activation might have more subtle phenotypes in functions not related to transcriptional activation, such as interaction with proteins implicated in transformation by BE6.
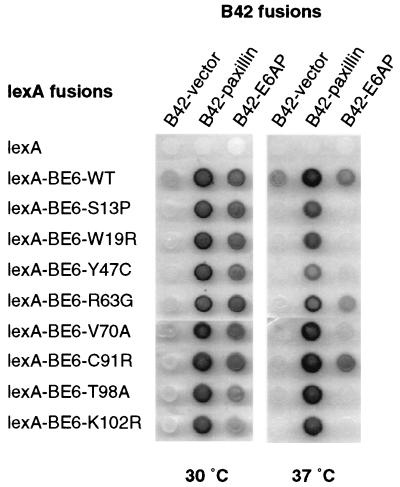
We randomly mutagenized only the BE6 portion of a lexA-BE6 fusion in yeast by gap repair mutagenesis (12) and screened the resulting colonies for ts transcriptional activation of a lexA-responsive lacZ reporter gene as previously described (13). Of 5,000 screened colonies, 9 were ts for transcriptional activation by visual screening of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) plates incubated at 37 and 30°C. DNA sequencing confirmed that each mutant contained a single point mutation resulting in a predicted single amino acid change. Interestingly, two of the BE6 ts mutants contained substitutions within zinc fingers (BE6-W19R and BE6-C91R) and one isolate contained a conservative substitution in an amino acid position that is invariant in all papillomavirus E6 proteins (K102R). The plasmids were reintroduced into yeast and tested in yeast two-hybrid assay for interaction with E6AP and paxillin at 30 and 37°C (Fig. 1). Wild-type BE6 and all of the BE6 ts mutants retained their interactions with both E6AP and paxillin at 30°C (although mutants T98A and K102R had reduced interactions at 30°C). At 37°C, wild-type BE6 and all of the ts mutants retained their interaction with paxillin but only the R63G and C91R mutants retained their interaction with E6AP. Interaction with E6BP was not analyzed by the yeast two-hybrid assay because we were unable to detect an interaction between lexA-BE6 and E6BP in the lexA-based yeast two-hybrid system (results not shown).
FIG. 1.
Interaction of the fusion proteins of E6AP and paxillin with BE6 ts mutants in vivo. The indicated BE6 fusions to the lexA DNA-binding domain and paxillin or E6AP fusions to the B42 transactivation domain were introduced into the yeast strain EGY48 containing a lexA-responsive lacZ reporter. Interaction between lexA and B42 fusion proteins is indicated by a blue color on galactose plates containing X-Gal. Identical plates were incubated overnight at either 30 or 37°C and then immediately photographed.
BE6 mutants that are ts in yeast can be ts for transformation in mammalian cells.
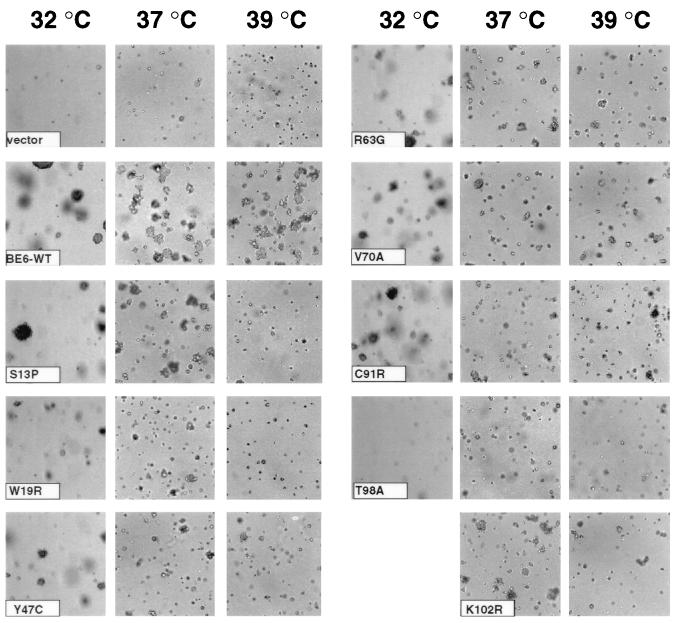
We reasoned that some BE6 mutants that were ts for transcriptional activation in yeast might be ts for transformation in mammalian cells. Like BE6, the p53 tumor suppresser is sensitive to mutational disruption, and p53 mutants that are ts in mammalian cells retain this phenotype in yeast (5, 15). We expressed wild-type BE6 and yeast-selected BE6 mutants in murine C127 cells by retrovirus infection and tested the resulting pooled BE6-expressing C127 colonies for the induction of anchorage-independent cell growth at 32, 37, and 39°C (Fig. 2). All E6 molecules including wild-type BE6 were reduced in colony-forming efficiency at 32°C compared to 37°C for unknown reasons. All of the BE6 mutants had some reduction in transformation compared to wild-type BE6 at all tested temperatures in that the colonies were smaller and/or less frequent than those of wild-type BE6. One mutant (BE6-T98A) was markedly reduced for transformation at all temperatures, while five mutants (BE6-Y47C, BE6-R63G, BE6-V70A, BE6-C91R, and BE6-K102R) retained significant transformation at all tested temperatures. Although these mutants were still positive for transformation at 39°C, their colony sizes were somewhat reduced compared to the colony sizes induced by wild-type BE6. Two mutants (BE6-S13P and BE6-W19R) were ts for induction of anchorage-independent growth (Fig. 2), producing no colonies at 39°C. None of the mutants displayed an enhanced cold-sensitive phenotype for transformation (results not shown).
FIG. 2.
Temperature-dependent transformation by BE6 mutants selected for temperature-dependent transcriptional activation. Transformation results reflect the induction of anchorage-independent growth in 0.3% agarose by BE6 mutants relative to wild-type BE6. Equal numbers of pooled puromycin-resistant mouse C127 cells 21 days after transfection with either BE6 or BE6 mutants expressed from the retroviral expression plasmid pBabe-puro were seeded at 5 × 104 cells per ml into agar as previously described (19). Cultures were scored for anchorage independence 14 days later at 37 and 39°C and 21 days later at 32°C. Shown are ×100-magnified photographs of anchorage-independent colonies. The 32°C culture of BE6-K102R is not shown in this assay but was similar in transformation efficiency to wild-type BE6 in other assays at 32°C.
In vitro binding of BE6 to paxillin, but not binding to E6AP or E6BP, correlates with transformation by BE6.
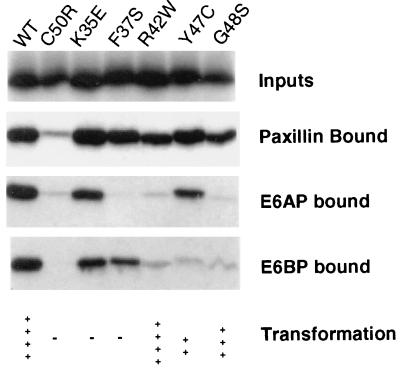
Previous studies have shown that BE6 mutants that are defective for transformation may fail to interact with E6AP, E6BP, or paxillin (4, 20). As discussed above, this may reflect the sensitivity of BE6 to mutational disruption. It may also be a consequence of the similarity in peptide-binding sequences found in each of the three proteins that interact with BE6 (Fig. 3). Since E6AP, paxillin, and E6BP all interact with BE6 through similar peptide sequences, mutations in BE6 that disrupt interaction with one protein are likely to disrupt interaction with each of these proteins. Figure 1 demonstrated that some of the BE6 ts mutants lost their interaction in yeast with E6AP while retaining their interaction with paxillin. However, interactions of lexA-BE6 fusion proteins in yeast might not reflect interactions observed with native BE6 molecules. This has been occasionally observed previously with BE6 mutants that interact well in yeast two-hybrid assays yet fail to interact in in vitro binding assays (13, 20). Also, variation in the binding conditions used in different laboratories has made the relative binding of BE6 mutants in vitro to E6AP, E6BP, and paxillin difficult to compare. To determine if some BE6 mutants might discriminate in their interactions between E6AP, E6BP, and paxillin and to compare these interactions under the same conditions, we tested the relative binding of wild-type and mutant BE6 molecules to immobilized E6AP, E6BP, and paxillin in vitro by using binding conditions described for the interaction of BE6 with E6BP (4). We screened the binding of the mutants described in this study as well as of the mutants isolated in an earlier study (13). All of the ts mutants except BE6-Y47C bound E6AP, paxillin, and E6BP identically to wild-type BE6 at 4°C (results not shown). We were unable to perform the in vitro binding assays at 37 or 39.5°C due to degradation of the in vitro-translated BE6 molecules under the binding conditions at 37 and 39.5°C (results not shown). We tested BE6 mutants that were able to induce cellular transformation for discrimination in the interaction between paxillin, E6AP, and E6BP; these mutants are illustrated in Fig. 4, and the quantified data are given in Table 1. Wild-type BE6 bound to all three proteins in vitro. A mutant with a disruptive mutation of a zinc finger of BE6 (BE6-C50R) was utilized as a negative control for nonspecific binding, because it is transformation negative and fails to interact with E6AP, E6BP, and paxillin. None of the transformation-positive mutants failed to interact with paxillin.
FIG. 3.
Charged leucine interaction motifs that interact with BE6. Partial conceptual translations of E6AP (GenBank L07557) and ERC-55 (GenBank I37371) are shown at the top, with translations of the amino-terminal 15 amino acids of human paxillin (U14588). The E6-binding motif of ERC-55 is from reference 3, and the E6-binding motif of E6AP is from reference 8. The LD motifs of paxillin are from reference 1, and the motif for binding of BE6 to LD1 is from reference 20. BE6 interacts with the individual LD2, LD3, and LD4 motifs but weakly with LD5 (R. Wade and S. Vande Pol, unpublished data). The start position of the indicated peptide is shown at the left. Conserved hydrophobic and charged residues are boxed.
FIG. 4.
In vitro association of BE6 and BE6 mutants with E6AP, paxillin, and E6BP. Binding of in vitro-translated BE6 and BE6 mutants to GST-E6AP, GST-E6BP, and MBP-paxillin fusions immobilized on agarose beads was performed as described in Materials and Methods. The results of a single representative experiment are shown, with quantitation of this and two additional experiments shown in Table 1. Transformation results shown below the gels are the results of at least three separate assays for each mutant and are expressed as anchorage independence relative to wild-type (wt) BE6 at 37°C. ++++, colonies of equivalent size to and at least 50% of the frequency of wild-type BE6; +++, colonies of at least 20% of the frequency of and similar size to wild-type BE6; ++, colonies distinctly smaller than wild-type BE6 that arise at less than 10% the frequency of wild-type BE6; −, no colonies above that seen with the empty vector pBabe-puro. Pooled puromycin-resistant colonies transfected with wild-type BE6 induced-anchorage independent colonies at 55 to 70% efficiency in these assays. The results shown for BE6 mutants K35E, R42W, F37S and C50R are from references 13 and 20.
TABLE 1.
Interaction of BE6 and BE6 mutants with paxillin, E6AP, and E6BP in vitro
| BE6 genotypea | % in vitro bindingb to:
|
Transformationc | ||
|---|---|---|---|---|
| Paxillin | E6AP | E6BP | ||
| BE6-WT | 100 | 100 | 100 | ++++ |
| BE6-C50R | 0 | 0 | 0 | − |
| BE6-K35E | 170 | 31 | 31 | − |
| BE6-F37S | 149 | −0.04 | 23 | − |
| BE6-R42W | 75 | 2.1 | 4.1 | ++++ |
| BE6-Y47C | 91 | 12 | 0.16 | ++ |
| BE6-G48S | 59 | 3.4 | 0.15 | +++ |
Wild-type BE6 (BE6-WT) and BE6 mutants with single code amino acid substitutions.
In vitro binding of in vitro-translated and 35S-labeled BE6 proteins was performed as described in Materials and Methods. Binding to GST-E6AP, GST-E6BP, or MBP-paxillin is shown. Results are the average of three separate assays for interaction of BE6 and each mutant with E6AP, E6BP, and paxillin and are relative to that of wild-type BE6 (BE6-WT) normalized to 100%, and the disruptive zinc finger mutant BE6-C50R, normalized to 0% (BE6-C50R is used to account for nonspecific interactions). The mean percentage of input counts bound in the three assays for wild type BE6 was 26% by GST-E6AP, 8.8% by GST-E6BP, and 27% by MBP-paxillin.
Transformation criteria are as described in the legend to Fig. 4.
Unlike paxillin, E6AP failed to interact in this assay with either BE6-R42W or BE6-G48S, indicating that a strong interaction with E6AP is dispensable for the induction of anchorage-independent cell growth. Similarly, E6BP failed to interact with the transformation-positive mutants BE6-R42W, BE6-G48S, and BE6-Y47C. While paxillin and E6BP bound to BE6-F37S, E6AP did not. Since all BE6-R42W, BE6-Y47C, and BE6-G48S mutants are positive for transformation, strong interactions of BE6 with E6AP or E6BP are dispensable for induction of anchorage-independent cell growth by BE6.
While E6AP and E6BP contain a single charged leucine motif shown to interact with E6, paxillin has five charged leucine motifs within motifs termed LD motifs. BE6 principally interacts with the LD1 and LD4 motifs in vitro, and mutation of the LD1 motif in vivo blocks the interaction of paxillin with BE6 (18, 20). Although the results are not shown in Fig. 4 or Table 1, we have analyzed the interaction of BE6 mutants with the LD1 motif of paxillin and found that it has similar interactions to those of the full-length MBP-paxillin fusion.
Included in the binding analysis of Fig. 4 are two additional mutants that are defective for transformation: BE6-K35E and BE6-F37S. The F37S mutant further illustrates the finding that BE6 mutants can clearly discriminate in its interaction with the similar charged leucine motifs found in E6AP, E6BP, and paxillin, since the F37S mutant interacts well with paxillin and E6BP but poorly with E6AP. The BE6-K35E mutant is unaltered in interactions compared to wild-type BE6 but is completely defective for transformation (13), indicating that the K35E mutation disrupts a BE6 function that is essential for transformation yet independent of the interaction with acidic charged leucine motifs found on paxillin, E6AP, or E6BP.
DISCUSSION
If an association between an E6 protein and a cellular protein is found, how can the significance of this interaction be established? We know that BE6 binds to cellular proteins containing acidic charged leucine motifs, but how do we assess the role of these multiple interactions? Both yeast two-hybrid assays and coimmune precipitation of highly overexpressed BE6 are biased toward the isolation of abundant proteins containing an interacting charged leucine motif and do not necessarily identify the cellular interactions responsible for transformation, which may involve a low-abundance protein. This is more likely, considering the very low levels of BE6 expression in stably transformed cells compared to expression levels in overexpression experiments.
The interaction of E6AP with cancer-associated mucosal HPV types is of firmly established significance due to the degradation of p53 in vitro and in vivo and the established role of p53 in cell cycle regulation, apoptosis, and cancer development. The significance of the HPV E6-p53 association is further supported by the fact that p53 is targeted by oncoproteins of DNA tumor viruses distantly related to papillomaviruses. In the last year, however, additional cellular targets of the HPV E6 protein have been proposed: the Bcl-2 family member Bak (16), the GAP protein E6TP1 (6), and the replication factor Mcm7 (9, 10). Each of these proteins contains leucine-rich interaction motifs with some similarity to the charged leucine motifs examined in this study (S. Vande Pol, unpublished observations). Null cell lines for each of these gene products might be used to evaluate the significance of these interactions if that was possible, but since HPVs only replicate within differentiated human keratinocytes, such an evaluation program would be technically very challenging. The selection of HPV E6 mutants that differ in their interaction with cellular proteins, as has been done in this study, is one way to assess the role of these interactions.
The sensitivity of E6 proteins to disruptive mutations has rendered their genetic analysis difficult. While other viral oncoproteins such as adenovirus E1a or simian virus 40 TAg have a modular structure with discrete domains for interaction with cellular proteins, deletion analysis of the E6 proteins has not shown discrete domains on E6 responsible for transcriptional activation, transformation, or interaction with charged leucine motifs. To circumvent this problem, we isolated conditional mutants in yeast. Interestingly, two of these mutants (BE6-W19R and BE6-C91R) contained arginine substitutions within zinc finger domains. Arginine is not found at these positions within zinc fingers in natural E6 isolates. It is possible that such substitutions induce temperature-dependent zinc finger disruptions and that the same mutations isolated in BE6 are recreated in E6 proteins from other papillomavirus types to create temperature-dependent behavior in E6 oncogenes. Six of the eight BE6 ts mutants had temperature-dependent loss of interaction with E6AP in vivo by the yeast two-hybrid assay while retaining interaction with paxillin. This may reflect the stronger interaction of BE6 with paxillin than with E6AP in yeast and suggests the possibility that BE6, which is strongly associated with a charged leucine motif, can be stabilized by that association. We found that one of these yeast selected mutants (BE6-Y47C) was able to discriminate in binding between E6AP, E6BP, and paxillin in vitro at 4°C, in contrast to binding of the wild-type BE6 to these target proteins. In particular, mutants that interact poorly with E6AP or E6BP (BE6-R42W, BE6-Y47C, and BE6-G48S) could retain the ability to induce anchorage-independent cell proliferation. In contrast, all transformation-positive BE6 mutants retained interaction with paxillin in vitro. While this does not demonstrate that interaction with paxillin is necessary for transformation by BE6, it does demonstrate that strong interactions with E6AP or E6BP are not essential for transformation of murine C127 cells. However, it does not eliminate E6AP or E6BP as playing a role in the full papillomavirus replication cycle. Although we have previously seen the interaction of E6AP with BE6-R42W in vitro (13), the binding conditions used in the present study and in the previous study of E6AP-BE6 interactions (4) were more stringent. Our present results also do not eliminate an accessory role of E6BP or E6AP interaction with BE6 in transformation of C127 cells, since all of the mutants of BE6 analyzed in this study were reduced in transformation compared to wild-type BE6. However, strong interactions of BE6 with E6AP or E6BP are not essential for transformation by BE6.
This study defines a second transformation function for BE6 that is independent of association with acidic charged leucine motifs found on paxillin, E6AP, or E6BP. The BE6 K35E mutant interacts with all three of these proteins similar to wild-type BE6 in vitro (Table 1). However, BE6-K35E is defective for transformation (13). BE6 may act as an adapter protein similar to HPV-16 E6, where HPV-16 E6 interacts with the charged leucine motif of E6AP and then with p53 by using an as yet undefined mechanism. BE6 might bind to one cellular interacter such as paxillin through a charged leucine motif and to a second interacting protein independently of the charged leucine motif interaction. BE6 cannot interact with paxillin and E6AP simultaneously, indicating that BE6 may not interact with two separate acidic charged leucine motifs at once (20). Alternatively, the BE6-K35E mutation may disrupt the interaction of BE6 with a charged leucine motif that is quite different in structure from those found on paxillin, E6AP, or E6BP. Such a motif would be present on an as yet unidentified cellular protein that is also essential for transformation by BE6. In either instance, BE6-K35E defines a second essential transformation function for BE6.
ACKNOWLEDGMENTS
We thank John Huibregtse for the E6AP cDNA and Elliot Androphy for the GST-E6BP clone.
This work was supported by NIH grant CA69292 to S.B.V.
REFERENCES
- 1.Brown M C, Curtis M S, Turner C E. Paxillin LD motifs may define a new family of protein recognition domains. Nat Struct Biol. 1998;5:677–678. doi: 10.1038/1370. [DOI] [PubMed] [Google Scholar]
- 2.Chamberlain J P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979;98:132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- 3.Chen J J, Hong Y, Rustamzadeh E, Baleja J D, Androphy E J. Identification of an alpha helical motif sufficient for association with papillomavirus E6. J Biol Chem. 1998;273:13537–44. doi: 10.1074/jbc.273.22.13537. [DOI] [PubMed] [Google Scholar]
- 4.Chen J J, Ried C E, Band V, Androphy E J. Interaction of papillomavirus E6 oncogenes with a putative calcium binding protein. Science. 1995;269:529–531. doi: 10.1126/science.7624774. [DOI] [PubMed] [Google Scholar]
- 5.Flaman J M, Frebourg T, Moreau V, Charbonnier F, Martin C, Chappuis P, Sappino A P, Limacher I M, Bron L, Benhattar J, et al. A simple p53 functional assay for screening cell lines, blood, and tumors. Proc Natl Acad Sci USA. 1995;92:3963–3967. doi: 10.1073/pnas.92.9.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao Q, Srinivasan S, Boyer S N, Wazer D E, Band V. The E6 oncoproteins of high-risk papillomaviruses bind to a novel putative GAP protein, E6TP1, and target it for degradation. Mol Cell Biol. 1999;19:733–744. doi: 10.1128/mcb.19.1.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huibregtse J M, Scheffner M, Howley P M. Cloning and expression of the cDNA for E6-AP, a protein that mediates the interaction of the human papillomavirus E6 oncoprotein with p53. Mol Cell Biol. 1993;13:775–784. doi: 10.1128/mcb.13.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huibregtse J M, Scheffner M, Howley P M. Localization of the E6-AP regions that direct human papillomavirus E6 binding, association with p53, and ubiquitination of associated proteins. Mol Cell Biol. 1993;13:4918–4927. doi: 10.1128/mcb.13.8.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuhne C, Banks L. E3-ubiquitin ligase/E6-AP links multicopy maintenance protein 7 to the ubiquitination pathway by a novel motif, the L2G box. J Biol Chem. 1998;273:34302–34309. doi: 10.1074/jbc.273.51.34302. [DOI] [PubMed] [Google Scholar]
- 10.Kukimoto I, Aihara S, Yoshiike K, Kanda T. Human papillomavirus oncoprotein E6 binds to the C-terminal region of human minichromosome maintenance 7 protein. Biochem Biophys Res Commun. 1998;249:258–262. doi: 10.1006/bbrc.1998.9066. [DOI] [PubMed] [Google Scholar]
- 11.Lamberti C, Morrissey L C, Grossman S R, Androphy E J. Transcriptional activation by the papillomavirus E6 zinc finger oncoprotein. EMBO J. 1990;9:1907–1913. doi: 10.1002/j.1460-2075.1990.tb08317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muhlrad D, Hunter R, Parker R. A rapid method for localized mutagenesis of yeast genes. Yeast. 1992;8:79–82. doi: 10.1002/yea.320080202. [DOI] [PubMed] [Google Scholar]
- 13.Ned R, Allen S, Vande Pol S. Transformation by bovine papillomavirus type 1 (BPV-1) E6 is independent of transcriptional activation by E6. J Virol. 1997;71:4866–4870. doi: 10.1128/jvi.71.6.4866-4870.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pear W S, Nolan G P, Scott M L, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scharer E, Iggo R. Mammalian p53 can function as a transcription factor in yeast. Nucleic Acids Res. 1992;20:1539–1545. doi: 10.1093/nar/20.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas M, Banks L. Inhibition of Bak-induced apoptosis by HPV-18 E6. Oncogene. 1998;17:2943–2954. doi: 10.1038/sj.onc.1202223. [DOI] [PubMed] [Google Scholar]
- 17.Tong X, Howley P M. The bovine papillomavirus E6 oncoprotein interacts with paxillin and disrupts the actin cytoskeleton. Proc Natl Acad Sci USA. 1997;94:4412–4417. doi: 10.1073/pnas.94.9.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tong X, Salgia R, Li J L, Griffin J D, Howley P M. The bovine papillomavirus E6 protein binds to the LD motif repeats of paxillin and blocks its interaction with vinculin and the focal adhesion kinase. J Biol Chem. 1997;272:33373–33376. doi: 10.1074/jbc.272.52.33373. [DOI] [PubMed] [Google Scholar]
- 19.Vande Pol S, Howley P. Negative regulation of the bovine papillomavirus E5, E6, and E7 oncogenes by the viral E1 and E2 genes. J Virol. 1995;69:395–402. doi: 10.1128/jvi.69.1.395-402.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vande Pol S B, Brown M C, Turner C E. Association of bovine papillomavirus type 1 E6 oncoprotein with the focal adhesion protein paxillin through a conserved protein interaction motif. Oncogene. 1998;16:43–52. doi: 10.1038/sj.onc.1201504. [DOI] [PubMed] [Google Scholar]