Abstract
Epidermal growth factor receptor (EGFR) mutations are a strong determinant of tumor response to gefitinib in non‐small cell lung cancer (NSCLC). We attempted to elucidate the feasibility of EGFR mutation detection in cells of pleural effusion fluid. We obtained 24 samples of pleural effusion fluid from NSCLC patients. The pleural effusion fluid was centrifuged, and the cellular components obtained were used for detection. EGFR mutation status was determined by a direct sequencing method (exons 18–21) and by the Scorpion Amplified Refractory Mutation System (ARMS) method. EGFR mutations were detected in eight cases. Three mutations were detected by both methods, and the other five mutations were detected by Scorpion ARMS alone. The mutations were detected by both methods in all four partial responders among the seven patients who received gefitinib therapy. Direct sequencing detected the mutations in only two of four cases with partial response. These results suggest that the DNA in pleural effusion fluid can be used to detect EGFR mutations. The Scorpion ARMS method appears to be more sensitive for detecting EGFR mutations than the direct sequencing method. (Cancer Sci 2006; 97: 642–648)
Lung cancer is a major cause of cancer‐related mortality worldwide and is expected to remain a major health problem for the foreseeable future.( 1 ) Targeting the epidermal growth factor receptor (EGFR) is one appealing strategy for the treatment of non‐small cell lung cancer (NSCLC), because EGFR has been found to be expressed, sometimes strongly, in NSCLC.( 2 ) Mutations of EGFR tyrosine kinase in NSCLC and hyper‐responsiveness to gefitinib, a selective EGFR tyrosine kinase inhibitor, have been reported.( 3 , 4 ) These mutations consist of small in‐frame deletions or substitutions clustered around the ATP‐binding site in exons 18, 19 and 21 of EGFR, and increase the affinity of the enzyme for ATP and gefitinib. Some investigators have subsequently found that EGFR mutations are a strong determinant of tumor response to EGFR tyrosine kinase inhibitor.( 5 , 6 , 7 ) Approximately 90% of the NSCLC‐associated EGFR mutations in two reports consisted of two major EGFR mutations (E746_A750del in exon 19 and L858R in exon 21).( 5 , 8 ) These investigators used surgical tissue to detect the EGFR mutations in their trials. As it is often difficult to obtain a tumor sample from patients with inoperable NSCLC, a method of detecting mutant EGFR, especially the two major mutations, in other specimens needs to be established.
Malignant pleural effusion is a common complication of lung cancer and is present in approximately 15% of lung cancer patients( 9 ) and in 10–50% of patients at the time of diagnosis.( 10 ) Approximately one‐half of NSCLC patients with pleural effusion are initially positive cytologically, and most of the effusions are ultimately determined to be malignant. As sampling of pleural effusion fluid is usually easy, non‐invasive and repeatable, we hypothesized that tumor cells in the pleural effusion fluid of NSCLC patients are a source of useful information on the status of the EGFR gene with regard to gefitinib response.
Genomic polymerase chain reaction (PCR) and the direct sequencing method have been used widely to detect EGFR mutations. It is well known that fusion between normal cells and tumor cells prevents detection of mutations in tumor cells by the direct sequencing method. Therefore it is necessary to enhance sensitivity for detection of EGFR mutations in a mixture of normal and tumor cells. We hypothesized that Scorpion Amplified Refractory Mutation System (ARMS) technology enhances sensitivity for detecting EGFR mutations. Scorpion primers are used in a fluorescence‐based method for specific detection of PCR products.( 11 ) A ‘scorpion’ consists of a specific probe sequence held in a hairpin loop configuration by complementary stem sequences on the 5′ and 3′ sides of the probe. A scorpion can be used in combination with ARMS to enable detection of single‐base mutations.( 11 , 12 ) The ARMS method was used for allele discrimination, and additional mismatches were introduced near the 3′ terminus of the primers to enhance specificity. A previous study showed that the ARMS method is superior to the direct sequencing method and the WAVE® (Transgenomic Inc., Cambridge, MA, USA) method for the detection of EGFR mutations.( 13 )
In the present study we attempted to detect major EGFR mutations in pleural effusion, and to find out whether the Scorpion ARMS method enhances sensitivity for detection of EGFR mutations in mixtures of DNA from normal cells and tumor cells.
Patients and Methods
Patients
We studied NSCLC patients who had a pleural effusion at the time of diagnosis. The diagnosis of NSCLC was based on histological or cytological findings. This study was approved by the Institutional Review Boards of the National Cancer Center Hospital and Kanazawa University Hospital, and written informed consent was obtained from all participants. The patient record consisted of age, sex, smoking habit, histological type of NSCLC and treatment. The response of the patients treated with gefitinib was evaluated in accordance with the ‘Response Evaluation Criteria in Solid Tumors (RECIST)’ guidelines.( 14 ) No research results were entered into the patient's record or released to the patient or their physician.
Collection of pleural effusion fluid and cell separation
The pleural effusion fluid was collected from patients in heparinized tubes between 29 March and 25 November 2005. No particular collection method was used. Pleural effusion fluid (1 mL) was centrifuged at 250 g for 10 min, and the cell pellet was stored at −80°C until use.
DNA extraction
DNA was extracted from the stored cell pellets using a Qiamp DNA Mini Kit (Qiagen, Hilden, Germany) according to the protocol for tissue samples in the manufacturer's instructions. The DNA obtained was eluted in 50 µL of sterile bidistilled buffer, and the concentration and purity of the extracted DNA were assessed by spectrophotometry. The extracted DNA was stored at −20°C until use.
PCR amplification and direct sequencing
Exons 18, 19, 20 and 21 of the EGFR gene were amplified by PCR. The primers were designed based on a report by Lynch et al.( 3 ) Genomic PCR of 20 ng of template DNA was carried out in 25‐µL volumes containing 0.75 IU of Ampli Taq Gold DNA polymerase (Roche Molecular Systems, Branchburg, NJ, USA), 2.5 µL of PCR buffer, 0.8 µM dNTP, 0.5 µM of each primer, and different concentrations of MgCl2, depending on the polymorphic marker. The first PCR analyses were carried out in a volume of 25 µL for 25 cycles, consisting of a denaturation step at 94°C for 45 s, a primer annealing step at 58°C for 30 s, and an elongation step at 72°C for 30 s. The final step at 72°C was extended for 10 min. Nested PCR was carried out for 20 cycles under the same conditions as the first PCR. Sequencing of each sample was carried out in duplicate using an ABI prism 310 (Applied Biosystems, Foster City, CA, USA). The sequences were compared with the GenBank‐archived human sequence for EGFR (accession number AY588246).
Scorpion ARMS for the detection of E746_A750del and L858R
We used an EGFR ScorpionTM Kit (DxS, Manchester, UK), which combines the two technologies ARMS and Scorpion, to detect mutations in real‐time PCR reactions. All reactions were carried out in 25‐µL volumes with 1 µL of template DNA, 7.5 µL of reaction buffer mix, 0.6 µL of primer mix and 0.1 µL of Taq polymerase. Real‐time PCR was carried out using SmartCycler® II (Cepheid, Sunnyvale, CA, USA) under the following conditions: initial denaturation at 95°C for 10 min, 50 cycles of 95°C for 30 s, and 62°C for 60 s with fluorescence reading (set to FAM that allows optical excitation at 480 nm and measurement at 520 nm) at the end of each cycle. Data analysis was carried out using Cepheid SmartCycler software (version 1.2b). The threshold cycle (Ct) was defined as the cycle at the highest peak of the second derivative curve, which represents the point of maximum curvature of the growth curve. Positive results were defined as Ct 45 and maximum fluorescence intensity 50. Analysis of each sample was carried out in duplicate. The EGFR Scorpion Kit is intended for detection of the two major somatic mutations in EGFR. These mutations consist of an in‐frame deletion in exon 19 (E746_A750del) and a point mutation in exon 21 (L858R). There are two types of E746_A750del, with starting points at nucleotide positions 2235 and 2236 (NM_005228). The assay can detect both types of E746_A750del. Other deletion patterns in exon 19 and other mutations in the tyrosine kinase domain of EGFR, which are also associated with sensitivity of lung cancers to gefitinib, can not be detected using this assay.
Experiments comparing the detection of E746_A750del in mixtures of wild‐type and E746_A750del DNA by direct sequencing and Scorpion ARMS
We used the standard DNA included in the EGFR Scorpion Kit to confirm sensitivity for the detection of E746_A750del. The following DNA mixtures were prepared: 10, 100, 1000 and 10 000 pg E746_A750del DNA, and 10 000 pg wild‐type DNA with 10, 100, 1000 and 10 000 pg E746_A750del DNA. These DNA mixtures were used to evaluate the sensitivity of direct sequencing and Scorpion ARMS. The results obtained using Scorpion ARMS were quantified using a standard curve generated by plotting the Ct against the log of the amount of DNA contained in the known standards. The linear correlation coefficient (R 2) values and the formulas for the slopes were calculated. To validate this assay we carried out the assay using plasmid DNA derived from the PCR products of A431 cells, which are known to contain wild‐type DNA, PC‐9 cells, which are known to contain E746_A750del, and 11‐18 cells, which are known to contain L858R. The plasmid DNA was subcloned into a cloning Topo® vector (Invitrogen, Carlsbad, CA, USA). The experiments were carried out at a copy number of 107.
Results
Patients and pleural effusion specimens
Twenty‐four patients with NSCLC were enrolled in the present study (Table 1). There were 11 women (45.8%) and 10 never‐smokers (41.7%). The histological diagnosis was adenocarcinoma in 23 patients and unclassified NSCLC in the other patient. NSCLC was diagnosed cytologically in the pleural effusion samples in 22 of the patients. There were no malignant cells in the pleural effusion fluid of the other two patients. The age range was 39–82 years (median 62 years). Seven patients were treated with gefitinib (250 mg/day) and their response was evaluated. The volume of the pleural effusion fluid collected from the patients ranged from 30 to 280 mL. DNA from cell pellets was extracted for all 24 samples at concentrations ranging from 3.2 to 335.5 ng/µL.
Table 1.
Patient characteristics and epidermal growth factor receptor mutation status
| No. | Age (years) | Sex | Smoking history | Histology | Response to gefitinib | EGFR mutation | |
|---|---|---|---|---|---|---|---|
| Direct sequencing | Scorpion ARMS | ||||||
| 1 | 62 | F | Never | ADC | PR | Wild type | E746_A750del |
| 2 | 40 | F | Never | ADC | SD | Wild type | Wild type |
| 3 | 39 | F | Never | ADC | PD | Wild type | Wild type |
| 4 | 69 | M | Former | ADC | – | Wild type | Wild type |
| 5 | 72 | F | Never | ADC | – | Wild type | Wild type |
| 6 | 66 | F | Never | ADC | – | Wild type | Wild type |
| 7 | 56 | M | Current | ADC | – | Wild type | Wild type |
| 8 | 61 | M | Former | ADC | – | Wild type | Wild type |
| 9 | 65 | M | Former | ADC | PD | Wild type | Wild type |
| 10 | 80 | F | Never | ADC | – | Wild type | E746_A750del |
| 11 | 82 | M | Current | NSCLC | – | Wild type | Wild type |
| 12 | 57 | F | Former | ADC | – | Wild type | Wild type |
| 13 | 55 | M | Former | ADC | – | Wild type | Wild type |
| 14 | 67 | M | Former | ADC | – | Wild type | Wild type |
| 15 | 61 | M | Never | ADC | PR | Wild type | E746_A750del |
| 16 | 65 | M | Former | ADC | PR | E746_A750del † | E746_A750del |
| 17 | 65 | F | Former | ADC | – | Wild type | L858R |
| 18 | 48 | F | Never | ADC | – | Wild type | L858R |
| 19 | 61 | M | Current | ADC | – | Wild type | Wild type |
| 20 | 60 | M | Current | ADC | PR | E746_A750del ‡ | E746_A750del |
| 21 | 63 | F | Never | ADC | – | E746_A750del ‡ | E746_A750del |
| 22 | 54 | M | Former | ADC | – | Wild type | Wild type |
| 23 | 49 | M | Current | ADC | – | Wild type | Wild type |
| 24 | 66 | F | Never | ADC | – | Wild type | Wild type |
Type of mutation: †2236–2250del; ‡2235–2249del (NM_005228). –, Patient did not receive gefitinib; ADC, adenocarcinoma; NSCLC, non‐small cell lung cancer; PD, progressive disease; PR, partial response; SD, stable disease.
Sensitivity of direct sequencing and EGFR Scorpion for detection of E746_A740del
Preliminary experiments were carried out to evaluate the sensitivities of direct sequencing and the EGFR Scorpion Kit. When direct sequencing was used to detect E746_A750del in the standard E746_A750del DNA samples (10–10 000 pg), the mutation was detected at amounts as low as 10 pg. When diluted standard E746_A750del DNA was mixed with standard wild‐type DNA at ratios from 1:1 to 1:1000, E746_A750del was detected by direct sequencing at ratios as low as 1:10.
When E746_A750del DNA was detected with Scorpion ARMS, all curves for standard E746_A750del DNA (10–10 000 pg) and the primer set for detection of E746_A750del increased for up to 45 cycles (Fig. 1A). When wild‐type standard DNA and distilled water were used as negative controls, the curves did not increase, and remained flat at 50 cycles (Fig. 1A). When diluted standard E746_A750del DNA was mixed with wild‐type DNA in ratios from 1:1 to 1:1000, all curves that indicated the presence of E746_A750del increased for up to 45 cycles (Fig. 1B). Standard curves in the range of measured amounts in this study were linear with R 2 values of 0.997 and 0.987. Both slopes of the curves were almost parallel (Fig. 1C). The Ct of diluted standard E746_A750del DNA mixed with wild‐type DNA was almost the same as for standard E746_A750del DNA. Although the peak fluorescence levels of diluted standard E746_A750del DNA mixed with wild‐type DNA were lower than without the wild‐type DNA standard, the presence of E746_A750del was clearly detected at the ratio of 1:1000. Curves of DNA containing E746_A750del at amounts up to 10 pg were unaffected by interfusion of wild‐type DNA.
Figure 1.
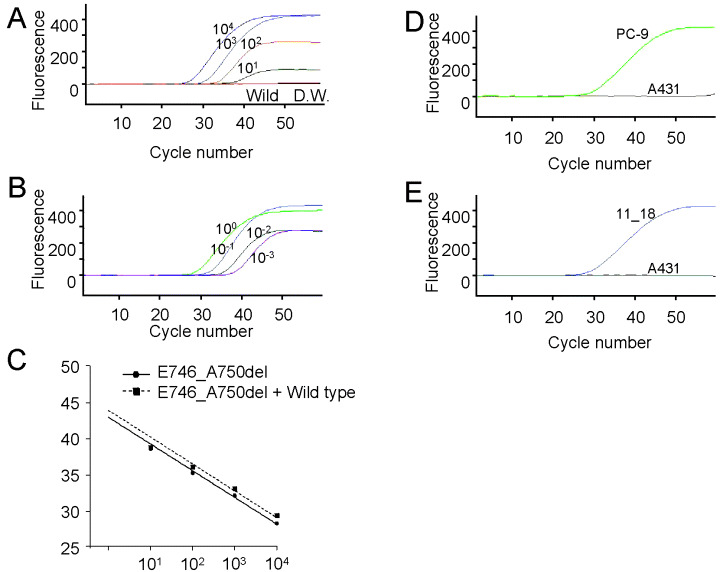
Sensitivity for detection of the E746_A750del and L858R mutations with the epidermal growth factor receptor (EGFR) Scorpion Kit. (A) Standard E746_A750del DNA was used at various volumes: 10 000 pg (104), 1000 pg (103), 100 pg (102) and 10 pg (101). Standard wild‐type DNA (Wild) and distilled water (DW), as negative controls, were used in the same experiment. (B) Standard E746_A750del DNA (10–10 000 pg) was mixed with 10 000 pg of standard wild‐type DNA at ratios of 1 : 1 (10), 1 : 10 (10−1), 1 : 100 (10−2) and 1 : 1000 (10−3). (C) Standard curves were obtained by plotting the threshold cycle (Ct) of each curve (shown in Fig. 1A,B) against the log of the standard DNA volume. Detection of E746_A750del and L858R in plasmid DNA derived from lung cancer cell lines. (D) PC‐9 with E746_A750del DNA and A431 with wild‐type DNA. (E) 11‐18 with L858R DNA and A431.
The signals of plasmid DNA derived from the PC‐9 cells and 11‐8 cells were detected at approximately the same Ct values (E746_A750del, 28.6; L858R, 29.2) and, as expected, when plasmid DNA derived from A431 was used, the curve did not increase and remained flat after 50 cycles (Fig. 1D,E).
Detection of EGFR mutations by direct sequencing
EGFR mutations in three of the 24 patients (12.5%) were detected by direct sequencing (Table 1). All three were heterozygous, and E746_A750del was detected in all three of them. Figure 2 shows the wave figures of the nucleotide sequence obtained by direct sequencing of part of exon 19 in two patients (patient no. 10, Fig. 2A; patient no. 21, Fig. 2C). The data for patient no. 10 was judged to represent wild‐type EGFR (Fig. 2A). That of patient no. 21 showed a mixture of wild‐type and 2235–2249del sequences (Fig. 2C).
Figure 2.
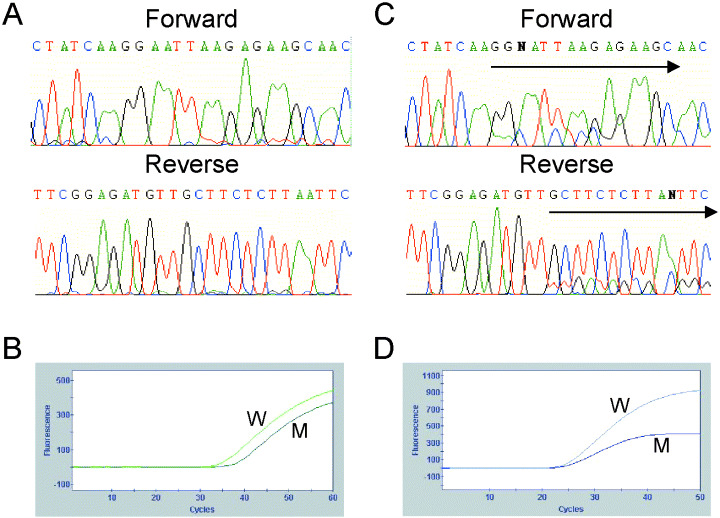
Results of direct sequencing and the Scorpion Amplified Refractory Mutation System (ARMS) method in patient no. 10 (A,B) and patient no. 21 (C,D). (A) The wave figure represents wild‐type epidermal growth factor receptor (EGFR). (B) Two ascending curves, indicating that wild type and deletion mutation in exon 19 were detected. (C) The two waves start to overlap at the starting points of the arrows. These features mean that the nucleotide sequence of the EGFR gene in this patient has a heterozygous deletion. The deletion removed amino acids 746–750 (E746_A750del). (D) Two ascending curves, indicating that wild type and deletion mutation in exon 19 were detected.
Mutation analysis using the Scorpion ARMS method
EGFR mutation status in all samples was analyzed using the EGFR Scorpion Kit. As wild‐type curves were detected in all patients, we concluded that no sample was too small to be detect by the Scorpion ARMS method and that it would be possible to determine the EGFR mutation status based on the results. Curves for an EGFR mutation were detected in eight of the 24 patients (33.3%; Table 1). In six of these eight patients, curves indicating the presence of a deletion mutation in exon 19 were detected (Fig. 2B,D), and curves for the other two patients indicated the presence of L858R in exon 21.
Comparison of detection of the two major mutations by the two methods
In the present study EGFR mutations were detected in eight patients. In three of them (nos 16, 20 and 21) the EGFR mutations were detected by both methods, whereas in the other five (nos 1, 10, 15, 17 and 18) they were detected by the Scorpion ARMS method alone. No patients were found to have EGFR mutations by direct sequencing alone. EGFR mutations were not detected using either direct sequencing or the Scorpion ARMS method in two samples that were not diagnosed cytologically as NSCLC.
EGFR mutation status and clinical manifestations
EGFR mutations were detected more frequently in the samples from women (5/11, 45.5% of women; 3/13, 23.1% of men) and non‐smokers (5/10, 50.0% of non‐smokers; 3/14, 21.4% of smokers) (Table 2). Four of the seven patients who received gefitinib therapy had a partial response, one had stable disease, and the other three patients had progressive disease. All four patients with a partial response had EGFR mutations (Table 3). Evaluation of mutation status by the direct sequencing method revealed mutations in two of the four patients with partial response, whereas Scorpion ARMS revealed mutations in all four patients with partial response. Mutation status determined by Scorpion ARMS was superior to mutation status determined by direct sequencing for predicting responsiveness to gefitinib. No EGFR mutations were detected in patients with stable disease or progressive disease.
Table 2.
Frequency of epidermal growth factor receptor (EGFR) mutations in pleural effusion from patients with non‐small cell lung cancer according to sex and smoking history
| Variable | Direct sequencing | Scorpion ARMS | ||
|---|---|---|---|---|
| + | – | + | – | |
| Sex and EGFR mutant state | ||||
| Female | 1 | 10 | 5 | 6 |
| Male | 2 | 11 | 3 | 10 |
| Smoking history and EGFR mutant state | ||||
| Non‐smoker | 1 | 9 | 5 | 5 |
| Smoker | 2 | 13 | 3 | 11 |
+, Mutation positive; –, no mutation; ARMS, Amplified Refractory Mutation System.
Table 3.
Frequency of epidermal growth factor receptor (EGFR) mutations in pleural effusion from patients with non‐small cell lung cancer according to response to gefitinib
| Variable | Direct sequencing | Scorpion ARMS | ||
|---|---|---|---|---|
| + | – | + | – | |
| Partial response | 2 | 2 | 4 | 0 |
| Stable/progressive disease | 0 | 3 | 0 | 3 |
The response to gefitinib was evaluated in all seven patients treated with gefitinib. +, Mutation positive; –, no mutation.
Discussion
The present study yielded two major findings. The first is that EGFR mutations, especially E746_A750 del and L858R, were detected in DNA from pleural effusion fluids, and the second is that the Scorpion ARMS method may be more sensitive for detecting EGFR mutations than the direct sequencing method. Patients with EGFR mutations may be misdiagnosed as not having any mutations if direct sequencing alone is used. Three patients were concluded to have mutations using both methods, but the other four patients were concluded to have mutations by the Scorpion ARMS method alone. As all four of these patients had partial responses to gefitinib, the results strongly suggest a correlation between mutation status and clinical responsiveness to gefitinib, although further clinical study is needed to make a definite conclusion. EGFR mutation status determined by the Scorpion ARMS method reflected responsiveness to gefitinib more accurately than direct sequencing.
Direct sequencing is currently the routine method used to detect EGFR mutations in tumor samples, and no standard method of detection of EGFR mutations in tumor specimens except surgical tissues has been established. The results of our small study lead us to conclude that the EGFR Scorpion Kit is superior to direct sequencing for detection of EGFR mutations, especially the two major mutations (deletion mutations in exon 19 and L858R), as predictive markers. As our preliminary experiment showed that the sensitivity of Scorpion ARMS for detection of EGFR mutations is superior to the sensitivity of direct sequencing when a mixture of wild‐type and mutant DNA is used, we infer from these results that the differences in sensitivity for detection in the four patients with the mutations were attributable to the density of tumor cells in the pleural effusion fluid.
To our knowledge detection of EGFR mutations in pleural effusion fluid has been described in one case report where the patient responded to gefitinib.( 15 ) Although our study did not confirm a correlation between mutation status and clinical responsiveness to EGFR tyrosine kinase inhibitors such as gefitinib, their results and our own in patients who received gefitinib therapy encourage us to conclude that EGFR mutation status determined in pleural effusion fluid may be useful for predicting responsiveness to EGFR tyrosine kinase inhibitors. The authors of the case report did not mention the possibility that normal cells may have prevented detection of EGFR mutations in tumor cells and that a patient with an EGFR mutation may be concluded not to have a mutation (false negative) as a result.
Some investigators have tried to increase the sensitivity of EGFR mutant detection. One attempt involved detection of EGFR mutations using a LightCycler PCR assay.( 16 ) SSCP assay is more sensitive than direct sequencing and is a more rapid method.( 17 ) Recently, two rapid and sensitive methods have been demonstrated: the peptide nucleic acid‐locked nucleic acid PCR clamp method,( 18 ) and the mutant‐enriched PCR assay.( 19 ) In these previous studies, EGFR mutations were detected in the presence of 1000‐fold and 2000‐fold wild‐type EGFR, respectively. Although the minimum detectable mutation volumes were not evaluated, the sensitivity of these methods seems to be comparable with that of the Scorpion ARMS method, and the sensitivity of these assays seems to be sufficient for clinical use. The latter study used various clinical samples, including 20 samples of pleural fluid. We have shown a relationship between EGFR mutation status in pleural fluids and the gefitinib response in a portion of the enrolled patients. The relationship in the remaining patients is currently being evaluated, and confirmation is expected in the very near future. As the Scorpion ARMS method is simple and very fast, it may be suitable for mutation screening. However, one limitation of the EGFR Scorpion Kit is that it is only able to detect mutations targeted by the Scorpion primers. It is known that deletion mutations in exon 19 have many variations in deleted nucleotides and addition of point mutations. The Scorpion ARMS method could detect mutations targeted by primers designed in advance and is capable of detecting the specific mutation E746_A750del in exon 19. E747_P753del insS and L747_T751del are minor variations of deletion mutations in exon 19 and could not be detected using this method in another study (data not shown). All EGFR mutations are not at these two sites; some are clustered around the ATP‐binding site in exons 18, 19 and 21.( 3 , 4 , 5 , 6 , 7 , 8 ) Although approximately 90% of NSCLC‐associated EGFR mutations consist of the two major EGFR mutations,( 5 , 8 ) other mutations may be misdiagnosed as negative mutation results using the Scorpion ARMS method. Moreover, a secondary mutation, a substitution of methionine for threonine at position 790 (T790M), leads to gefitinib resistance in NSCLC patients who have EGFR mutations and are responsive to gefitinib.( 20 , 21 ) These mutation states may also be critical factors for gefitinib therapy. Scorpion primers need to be designed to detect these mutations, and further study using these primers is required.
Our two initial aims, which were to detect two major EGFR mutations in pleural effusion fluid and to increase the sensitivity of detection of EGFR mutations in the mixtures of DNA from normal cells and tumor cells, were achieved in this study. As the next step, a prospective study of a large number of NSCLC patients with pleural effusion is likely to reveal a correlation between EGFR mutation state in pleural effusion fluids and clinical responsiveness to EGFR tyrosine kinase inhibitors, such as gefitinib.
Acknowledgments
We wish to thank Dr Stephan Little (Dxs, Manchester, UK) for providing the EGFR Scorpion Kit and technical assistance. H. Kimura received support from an awardee of a Research Resident Fellowship from the Foundation for Promotion of Cancer Research (Japan) for the 3rd Term Comprehensive 10‐Year Strategy for Cancer Control.
References
- 1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55: 74–108. [DOI] [PubMed] [Google Scholar]
- 2. Franklin WA, Veve R, Hirsch FR et al. Epidermal growth factor receptor family in lung cancer and premalignancy. Semin Oncol 2002; 29: 3–4. [DOI] [PubMed] [Google Scholar]
- 3. Lynch TJ, Bell DW, Sordella R et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non‐small‐cell lung cancer to gefitinib. N Engl J Med 2004; 350: 2129–39. [DOI] [PubMed] [Google Scholar]
- 4. Paez JG, Janne PA, Lee JC et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004; 304: 1497–500. [DOI] [PubMed] [Google Scholar]
- 5. Pao W, Miller V, Zakowski M et al. EGF receptor gene mutations are common in lung cancers from ‘never smokers’ and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA 2004; 101: 13 306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shigematsu H, Lin L, Takahashi T et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 2005; 97: 339–46. [DOI] [PubMed] [Google Scholar]
- 7. Han SW, Kim TY, Hwang PG et al. Predictive and prognostic impact of epidermal growth factor receptor mutation in non‐small‐cell lung cancer patients treated with gefitinib. J Clin Oncol 2005; 23: 2493–501. [DOI] [PubMed] [Google Scholar]
- 8. Kosaka T, Yatabe Y, Endoh H et al. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res 2004; 64: 8919–23. [DOI] [PubMed] [Google Scholar]
- 9. Pass HI, Carbone DP, Johnson DH et al. Lung Cancer Principles and Practice, 3rd edn. Philadelphia: Lippincott, Williams & Wilkins; 2005; 291–303. [Google Scholar]
- 10. Fenton KN, David Richardson J. Diagnosis and management of malignant pleural effusions. Am J Surg 1995; 170: 69–74. [DOI] [PubMed] [Google Scholar]
- 11. Whitcombe D, Theaker J, Guy SP et al. Detection of PCR products using self‐probing amplicons and fluorescence. Nat Biotechnol 1999; 17: 804–7. [DOI] [PubMed] [Google Scholar]
- 12. Newton CR, Graham A, Heptinstall LE et al. Analysis of any point mutation in DNA. The amplification refractory mutation system (ARMS). Nucl Acids Res 1989; 17: 2503–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wookey A, Ellison G, Donald E et al. Comparison of methods for the detection of mutations in the epidermal growth factor receptor gene. Ann Meeting Am Assoc Cancer Res 2005; 96: 5287. [Abstract] [Google Scholar]
- 14. Therasse P, Arbuck SG, Eisenhauer EA et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205–16. [DOI] [PubMed] [Google Scholar]
- 15. Huang MJ, Lim KH, Tzen CY et al. EGFR mutations in malignant pleural effusion of non‐small cell lung cancer: a case report. Lung Cancer 2005; 49: 413–15. [DOI] [PubMed] [Google Scholar]
- 16. Sasaki H, Endo K, Konishi A et al. EGFR mutation status in Japanese lung cancer patients: genotyping analysis using LightCycler. Clin Cancer Res 2005; 11: 2924–9. [DOI] [PubMed] [Google Scholar]
- 17. Marchetti A, Martella C, Felicioni L et al. EGFR mutations in non‐small‐cell lung cancer: analysis of a large series of cases and development of a rapid and sensitive method for diagnostic screening with potential implications on pharmacologic treatment. J Clin Oncol 2005; 23: 857–65. [DOI] [PubMed] [Google Scholar]
- 18. Nagai Y, Miyazawa H, Huqun et al. Genetic heterogeneity of the epidermal growth factor receptor in non‐small cell lung cancer cell lines revealed by a rapid and sensitive detection system, the peptide nucleic acid‐locked nucleic acid PCR clamp. Cancer Res 2005; 65: 7276–82. [DOI] [PubMed] [Google Scholar]
- 19. Asano H, Toyooka S, Tokumo M et al. Detection of EGFR gene mutation in lung cancer by mutant‐enriched polymerase chain reaction assay. Clin Cancer Res 2006; 12: 43–8. [DOI] [PubMed] [Google Scholar]
- 20. Kobayashi S, Boggon TJ, Dayaram T et al. EGFR mutation and resistance of non‐small‐cell lung cancer to gefitinib. N Engl J Med 2005; 352: 786–92. [DOI] [PubMed] [Google Scholar]
- 21. Kwak EL, Sordella R, Bell DW et al. Irreversible inhibitors of the EGF receptor may circumvent acquired resistance to gefitinib. Proc Natl Acad Sci USA 2005; 102: 7665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]


