Abstract
Psoralen plus ultraviolet A (PUVA) and narrowband ultraviolet B (UVB) are widely used in skin disease phototherapy. Recently, the efficacy of UVB therapy has been greatly improved by narrowband UVB, compared to conventional broadband UVB. The objectives of the current study were to evaluate the influence of UVB‐induced and PUVA‐induced oxidative stress on cultured keratinocytes. We analyzed 8‐hydroxy‐2′‐deoxyguanosine (8‐OH‐dG) in human keratinocytes (HaCaT cell line) using a high‐performance liquid chromatography system equipped with an electrochemical detector. Non‐irradiated human keratinocytes contained a baseline of 1.48 ± 0.22 (mean ± SD) 8‐OH‐dG per 106 deoxyguanosine (dG) residues in cellular DNA, which increased linearly with higher doses of UVB. When their abilities to induce 8‐OH‐dG were compared to each other, based on the minimal erythemal and therapeutically used doses, by irradiating them with broadband UVB at 100 mJ/cm2, the amount of 8‐OH‐dG increased to 3.42 ± 0.46 residues per 106 dG, while a narrowband UVB treatment at 1000 mJ/cm2, with biological effects comparable to those elicited by 100 mJ/cm2 broadband UVB, increased it to 2.06 ± 0.31 residues per 106 dG. PUVA treatment, with 100 ng/mL 8‐methoxypsoralen and 5000 mJ/cm2 UVA, increased the 8‐OH‐dG level to 4.52 ± 0.42 residues per 106 dG. When HaCaT cells treated with 2000 mJ/cm2 narrowband UVB were cultured and the amount of 8‐OH‐dG was monitored in the living cells, 65.6% of the residues were repaired 24 h after treatment. Our study provides a warning that widely used narrowband UVB and PUVA induce cellular oxidative DNA damage at the therapeutically used doses, although to a lesser degree than broadband UVB with the same clinically effective dose. (Cancer Sci 2006; 97: 99 – 105)
Eight‐hydroxy‐2′‐deoxyguanosine (8‐OH‐dG), also known as 7,8‐dihydro‐8‐oxo‐deoxyguanosine (8‐oxo‐dG),(
1
) has been proposed as a key biomarker of oxidative DNA damage relevant to carcinogenesis(
1
,
2
) and pathogenesis of autoimmune disorders.(
3
,
4
) This DNA damage is induced by the reactions of reactive oxygen species (ROS), such as hydrogen peroxide (H2O2), superoxide anions (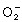 ), singlet oxygen and hydroxyl radicals (·OH).
), singlet oxygen and hydroxyl radicals (·OH).
Human skin is constantly exposed to environmental stresses, and is vulnerable to the effects of ROS generated by exposure to ultraviolet (UV) radiation.( 5 ) Yamamoto et al.( 6 ) reported that the formation of 8‐OH‐dG in DNA might be one of the mechanisms of daylight‐induced mutagenesis. In fact, irradiation with a fluorescent sun lamp or with UVB does induce 8‐OH‐dG in the epidermis of hairless mice.( 7 , 8 )
Parrish and Jaenicke( 9 ) found that 313 nm UVB radiation is the most effective wavelength for the treatment of psoriasis. This finding provided the impetus for developing the Philips TL‐01 fluorescent bulb, a narrowband UVB light source that produces a spectral emission between 310 and 315 nm. Narrowband UVB phototherapy has thus significantly improved the therapeutic efficacy of conventional broadband UVB (290–320 nm) phototherapy for skin diseases such as psoriasis, atopic dermatitis, vitiligo and others.( 10 , 11 , 12 , 13 )
Narrowband UVB is widely used in the treatment of skin disease, and the current trend toward the increased use of narrowband UVB phototherapy is justified.( 14 ) Its carcinogenic potential is judged to be substantially less than that of psoralen plus UVA (PUVA) photochemotherapy.( 15 ) Although the results of studies in mice indicate that narrowband UVB could induce more skin cancers than broadband UVB therapy,( 16 ) the participants in a workshop on the use of narrowband UVB in phototherapy concluded that the long‐term human cancer risk should be no greater than that with broadband phototherapy.( 17 )
When the DNA damage in keratinocytes induced by narrowband or broadband UVB was measured by single cell gel electrophoresis (comet assay), narrowband UVB produced less DNA damage than broadband UVB at equal doses.( 18 ) The formation of 8‐OH‐dG has also been reported in fibroblasts after UVA irradiation( 19 , 20 , 21 ) and in normal human epidermal keratinocytes after broadband UVB exposure.( 22 ) Using immunofluorescence staining methods, Budiyanto et al.( 23 ) observed that in both mouse skin and organ cultured human skin cells, 250 and 500 mJ/cm2 narrowband UVB yielded levels of cyclobutane pyrimidine (CPD/Py–Py) dimers similar to those induced by 25 and 50 mJ/cm2 broadband UVB, respectively, which have biological effects comparable to 250 and 500 mJ/cm2 narrowband UVB, respectively. However, the yields of 8‐OH‐dG after irradiation with 1000 and 3000 mJ/cm2 narrowband UVB were 1.5–3 times higher than those obtained using 100 and 300 mJ/cm2 broadband UVB, respectively.
The ratio of Py–Py dimers to 8‐OH‐dG formation in cellular DNA after UVB irradiation is 80–100:1,( 19 , 24 ) while the ratio of psoralen‐adducts to 8‐OH‐dG formation by PUVA treatment is 25:1.( 25 ) Although UVB and PUVA treatments induce Py–Py dimers and psoralen‐DNA adducts, respectively, as major cellular DNA modifications, our study focused on the analysis of 8‐OH‐dG as a marker of cellular oxidative stress for the following reasons: (1) not only initiation but also chronic inflammation‐induced promotion and progression may be involved in UVB‐induced skin carcinogenesis;( 26 , 27 ) (2) antioxidants inhibit both UVB‐induced 8‐OH‐dG formation and carcinogenesis in mouse skin;( 28 , 29 ) and (3) in Ogg1 (8‐OH‐Gua glycosylase) knockout mice, UVB irradiation induced both 8‐OH‐dG formation and an increase in skin tumors, suggesting that 8‐OH‐dG is involved in UVB‐induced skin carcinogenesis.( 30 )
The purpose of the present study was to assess the oxidative stress induced by clinically used UV wavelengths, doses and apparatus. There has been no accurate analysis reported using a high‐performance liquid chromatography (HPLC) system equipped with an electrochemical detector (ECD) of 8‐OH‐dG in human keratinocytes irradiated with narrowband UVB. PUVA is another modality whose potential to form 8‐OH‐dG should be investigated, because PUVA is the most widely used phototherapy for skin diseases. However, the PUVA‐induced formation of 8‐OH‐dG has been reported only for human epidermoid carcinoma cells.( 31 )
In the present study, we quantified the 8‐OH‐dG formed in keratinocytes (HaCaT) after irradiation with clinically used doses of broadband and narrowband UVB, and PUVA. Our results provide information about the oxidative DNA damage‐inducing potencies of these three phototherapies and the repair of 8‐OH‐dG.
Materials and Methods
Cells and culture conditions
The HaCaT cell line( 32 ) was cultured in Dulbecco's Modified Eagle's Medium (DMEM; Nissui Pharmaceutical, Tokyo, Japan), supplemented with 10% fetal bovine serum, l‐glutamine (2 mM), 100 units/mL penicillin, 100 µg/mL streptomycin sulfate and sodium pyruvate (1 mM), and was maintained at 37°C in a humidified atmosphere containing 5% CO2 in air. Unless otherwise mentioned, all culture supplies were purchased from Gibco‐Invitrogen (Carlsbad, CA, USA).
Ultraviolet irradiation of cells
The cells were seeded into 100‐mm tissue culture dishes and allowed to attach for a period of 16–24 h at 37°C. Before UV irradiation, the culture medium was removed and 5 mL of phosphate‐buffered saline (PBS, pH 7.4) were placed over the monolayer, so that the depth of the solution was always 0.1 cm, to prevent cell drying and reflection of UV. A total of approximately 5 × 106 keratinocytes in a 100‐mm dish were exposed to UV irradiation at room temperature. Broadband UVB irradiation was applied at a wavelength range of 280–370 nm, peaking at 305 nm, using a bank of five FL.20SE.30 medical sun lamps (Toshiba, Tokyo, Japan) emitting mainly UVB, but also small amounts of UVA and UVC. The irradiation was 1.0 mW/cm2 at a distance of 33 cm, as measured with a radiometer (UVR‐3036/S; Toshiba). Narrowband UVB irradiation was carried out with a bank of four TL‐20 W/01 lamps (Philips, Eindhoven, Holland) at a wavelength range of 310–315 nm (emission maximum at 313 nm, almost monochromatic) housed in a luminaire (type UV801 KL‐1; Waldmann, Villingen‐Schwennigen, Germany). For PUVA treatment, keratinocytes were exposed to UVA produced by six 40‐watt CLEO lamps (Philips) at a wavelength range between 315 and 400 nm with a peak emission at 355–365 nm, housed in a Waldmann luminaire. The distance from the light source was maintained at 25 cm. The dosimetry was monitored with a UV meter (type 585200000; Waldmann) equilibrated for the UV sources according to the manufacturer's instructions. Control cells were incubated in PBS without irradiation. At several time points after irradiation, the adherent cells were harvested, washed with ice‐cold PBS and processed immediately for DNA isolation.
Cell viability
A portion of each cell suspension obtained from the control and irradiation experiments was used to determine cell viability. Cell viability was determined using the trypan blue dye exclusion test (0.4%) (Gibco‐BRL, Grand Island, NY, USA). Due to the toxicity of UV light, we collected the adherent cells immediately after irradiation unless otherwise mentioned. As damaged cells gradually became detached during the culture period, depending on the UV irradiance, only adherent cells with viability above 90% were subjected to the analysis in the time course experiments.
PUVA treatment
Stock solutions were prepared by dissolving crystalline 8‐methoxypsoralen (8‐MOP) (Sigma, St Louis, MO, USA) in absolute ethanol (100 µg/mL). Before UVA irradiation, 10 µL of the 8‐MOP stock solution were added to 10 mL of PBS for the keratinocyte culture. A final 8‐MOP concentration of 100 ng/mL was chosen, as the mean plasma concentration in humans receiving PUVA therapy is approximately 100 ng/mL.( 33 ) After an incubation at 37°C for 30 min in the dark, the cells were irradiated with UVA.
Determination of 8‐OH‐dG in cellular DNA
Cellular DNA was isolated using a DNA extractor WB kit containing NaI (Wako, Osaka, Japan).( 34 , 35 ) Desferal (deferoxamine mesylate; Sigma) was added to the lysis solution (1 mM) to prevent DNA oxidation.( 36 ) The isolated DNA was digested with 8 units of nuclease P1 (Yamasa, Choshi, Japan) in a 100 µL solution containing 1 mM ethylenediaminetetracetic acid (EDTA) and 10 mM sodium acetate (pH 4.5), and was then treated with alkaline phosphatase (2 units) in a 250 mM Tris‐HCl (pH 8.0) buffer. This solution was filtered with an Ultrafree‐Probind filter (Millipore, Bedford, MA, USA) and a 70 µL aliquot of the sample was injected onto an HPLC column (Shiseido Fine Chemicals, Tokyo, Japan 5 µM, 4.6 × 250 mm, 27°C, flow rate 1.0 mL/min) equipped with an ECD (Coulochem II, ESA, Chelmsford, MA, USA; electrode 1, 150 mV; electrode 2, 300 mV; guard cell, 350 mV). The mobile phase consisted of 10 mM phosphate buffer (pH 6.7) containing 8% methanol. As the standard samples, 20‐µL aliquots of the deoxyguanosine (dG) (0.5 mg/mL) and 8‐OH‐dG (5 ng/mL) solutions were injected. The concentration of test samples was determined by comparison to the standards. The 8‐OH‐dG level in the DNA was expressed as the number of 8‐OH‐dG per 106 dG.
Efficiency of DNA synthesis in HaCaT cells after UVB irradiation
HaCaT cells were cultured in 96‐well plates (Corning Glass Works, Corning, NY, USA) until semiconfluent. After the culture medium was replaced by PBS, the cells were irradiated with UVB. The irradiated cells were further cultured in medium for 24 h, and 3H‐thymidine (1 µCi/well; Amersham International, Amersham, UK) was added for the last 12 h. Adherent cells were detached with EDTA/trypsin and collected on glass fibers using a cell harvester, and radio‐uptake was measured in a scintillation counter.
Statistical analysis
All analyses were carried out using the StatView‐J® 5.0 program (SAS Institute, Cary, NC, USA). All of the data are expressed as the mean ± SD from four to five independent measurements. Statistical significance was determined by the Student's t‐test, using P < 0.05 as the level of significance.
Results
Quantification of 8‐OH‐dG in HaCaT cells irradiated with broadband or narrowband UVB
HaCaT cells were exposed to broadband or narrowband UVB at various doses, and the 8‐OH‐dG formed in the cells was measured. Figure 1 shows a representative 8‐OH‐dG analysis. The hatched peak in Fig. 1a is derived from authentic 8‐OH‐dG. Untreated cells had a small but discernible amount of 8‐OH‐dG (Fig. 1b). Irradiation of cells with 1000 mJ/cm2 narrowband UVB increased the amount (Fig. 1c).
Figure 1.
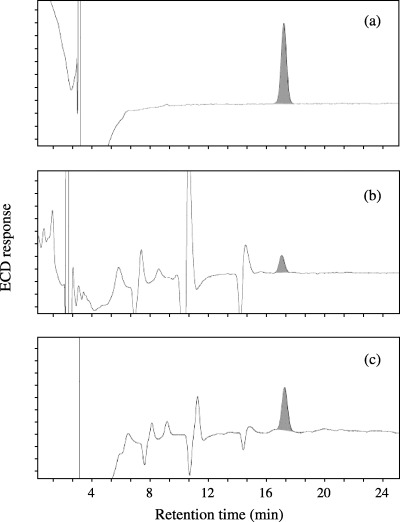
Representative high‐performance liquid chromatography (HPLC)‐electrochemical detector (ECD) analyses of 8‐OH‐dG. DNA isolated from HaCaT cells was treated with the nuclease P1 protein, and a 70‐µL aliquot of each sample was subjected to HPLC‐ECD analysis. (a) Authentic 8‐OH‐dG (100 pg), (b) DNA from unirradiated cells and (c) DNA from 1000 mJ/cm2 narrowband ultraviolet B‐irradiated cells. The amount of DNA in the injected samples (b,c) was adjusted.
As shown in Fig. 2, the level of 8‐OH‐dG in untreated cells was 1.48 ± 0.22 per 106 dG. Irradiation of cells with broadband UVB (50–500 mJ/cm2) induced 8‐OH‐dG formation in a dose‐dependent manner (Fig. 2a), and 0.0113 residues per mJ/cm2 were estimated to be increased by broadband UVB on the per‐dose basis.
Figure 2.
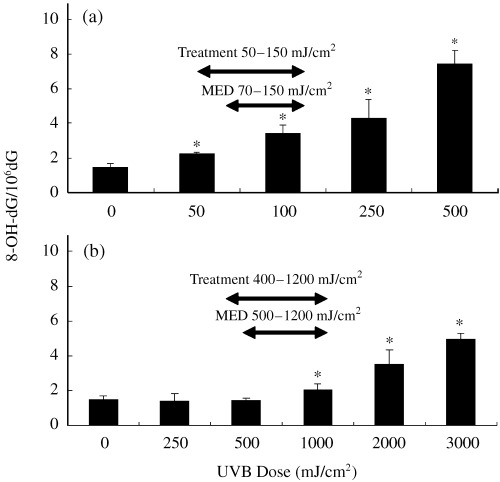
Formation of 8‐OH‐dG in the cellular DNA of HaCaT cells irradiated with (a) broadband and (b) narrowband ultraviolet (UV) B. Data are expressed as the mean ± SD of determinations on four to five independently irradiated dishes of keratinocytes. *P < 0.05, compared with the unirradiated samples (0 mJ/cm2, the background level). MED, minimal erythema doses of Japanese individuals whose skin types were III to IV, defined according to the Fitzpatrick classification. (54) Treatment: broadband UVB and narrowband UVB doses for psoriasis vulgaris in our institution.
At the low doses of 250 and 500 mJ/cm2 of narrowband UVB, the amount of 8‐OH‐dG was not increased compared to that of the non‐irradiated control (Fig. 2b). A significant augmentation of 8‐OH‐dG was found at 1000 mJ/cm2 of narrowband UVB. The amount of 8‐OH‐dG produced by narrowband UVB at 2000 mJ/cm2 (3.51 ± 0.83) was comparable to that generated by 3.42 ± 0.46 of broadband UVB at 100 mJ/cm2. Therefore, broadband UVB seemed to induce approximately 20‐fold higher oxidative DNA stress than narrowband UVB when compared at the same exposure dose.
The minimal erythema doses (MED) of broadband and narrowband UVB were 70–150 and 500–1200 mJ/cm2 in Japanese normal subjects and patients with psoriasis or cutaneous T‐cell lymphoma. Thus, approximately 10‐fold higher doses of narrowband UVB than broadband UVB are used clinically. Given this 10‐fold difference in the biological activities of the two UVB sources, the level of 8‐OH‐dG in 1000 mJ/cm2 narrowband UVB‐treated cells (2.97 ± 0.44), for example, was less than that in 100 mJ/cm2 broadband UVB‐treated cells (3.42 ± 0.46). However, it should be considered that narrowband UVB yields considerable amounts of 8‐OH‐dG in clinical settings.
Quantification of 8‐OH‐dG in HaCaT cells treated with 8‐MOP plus UVA
HaCaT cells were treated with 100 ng/mL of 8‐MOP and various doses of UVA, or UVA alone. As shown in Fig. 3, UVA alone produced low levels of 8‐OH‐dG in a dose‐dependent manner. Endogenous photosensitizers, such as porphyrins and flavins, which have UV absorption in the UVA range (320–400 nm), may be involved in this process. In contrast, the incubation of cells with 8‐MOP before UVA irradiation (2000–10000 mJ/cm2) significantly enhanced 8‐OH‐dG formation. Because PUVA therapy usually starts with 100% of the minimal phototoxic dose (ranging from 500–5000 mJ/cm2 UVA),( 37 ) the amount of 8‐OH‐dG produced by narrowband UVB exposure is considered to be lower than that generated by PUVA therapy.
Figure 3.
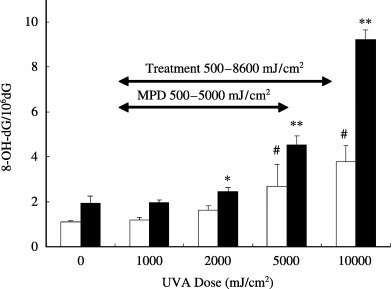
Formation of 8‐OH‐dG in HaCaT cells treated with ultraviolet (UV) A or psoralen plus UVA (PUVA). HaCaT cells were incubated with 100 ng/mL 8‐MOP and irradiated with various doses of UVA. The data represent the mean ± SD of four to five experiments. *P < 0.05, **P < 0.001, compared without UVA and with 8‐MOP. #P < 0.01, compared without UVA and 8‐MOP. MPD, minimal phototoxic doses. Skin was exposed to UVA 2 h after the ingestion of 8‐MOP tablets. The MPD is defined as the dose that induced minimally perceptible erythema 72 h after irradiation. (35) Treatment: oral PUVA therapy doses for psoriasis vulgaris. (35)
In the control experiments without UVA and with 8‐MOP, the 8‐OH‐dG levels were higher than in those without UVA and 8‐MOP (Fig. 3). This may be due to artifactual formation of 8‐OH‐dG during DNA isolation under light.
Removal of 8‐OH‐dG in UVB‐irradiated HaCaT cells
We compared the 8‐OH‐dG levels in HaCaT cells immediately after and 24 h after UVB irradiation. Viability levels of HaCaT cells 24 h after UVB irradiation are shown in Fig. 4a. As UVB exposure induced the detachment of HaCaT cells from the dish, depending on the UVB dose, we quantified the 8‐OH‐dG in the attached cells, so that only living cells were analyzed. As shown in Fig. 4b, the discernibly elevated 8‐OH‐dG amounts in the 100 mJ/cm2 broadband and 2000 mJ/cm2 narrowband UVB‐irradiated HaCaT cells were significantly decreased after 24 h of culture. Therefore, the oxidative DNA damage in living keratinocytes seemed to be repaired within 24 h of UVB irradiation.
Figure 4.
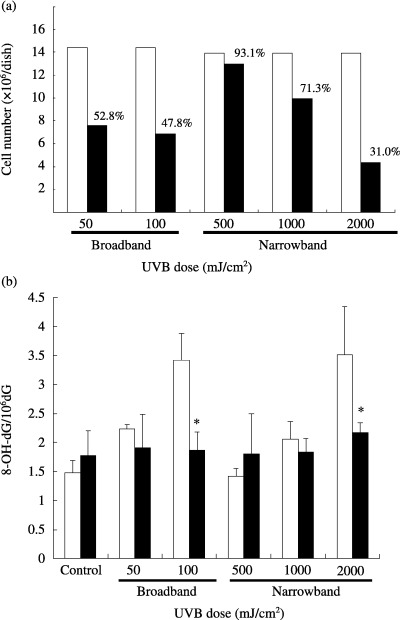
(a) Viability levels of cells 24 h after ultraviolet (UV) B irradiation. □, Non‐irradiated cells; ▪, irradiated cells. (b) 8‐OH‐dG levels immediately (□) and 24 h after (▪) UVB irradiation. HaCaT cells were irradiated with the indicated doses of broadband or narrowband UVB. *P < 0.05, compared with the value immediately after UVB irradiation. The results represent the mean ± SD of four to five experiments.
To further confirm the lack of influence of cell proliferation on the 8‐OH‐dG reduction, the 3H‐thymidine incorporation by UVB‐irradiated HaCaT cells was measured. The DNA synthesis levels in the cells treated with broadband UVB at 100 mJ/cm2 and narrowband UVB at 2000 mJ/cm2 were decreased after 24 h of culture (Fig. 5). Therefore, the 8‐OH‐dG formed in HaCaT cells was probably repaired during the cultivation.
Figure 5.
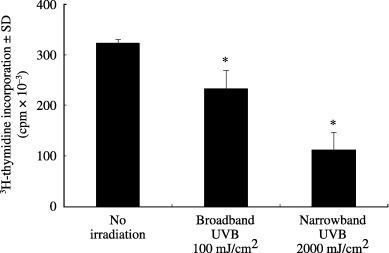
Reduction of 3H‐thymidine incorporation in ultraviolet (UV) B‐irradiated HaCaT cells. After irradiation with broadband UVB at 100 mJ/cm2 and narrowband UVB at 2000 mJ/cm2, HaCaT cells were cultured for 24 h, and were pulsed with 3H‐thymidine for the last 12 h. *P < 0.05, compared with no irradiation.
The amount of 8‐OH‐dG was monitored in HaCaT cells at 1–24 h after 2000 mJ/cm2 narrowband UVB irradiation and the repair rate was calculated. The viability of the cells attached to the dish was similar to that of the control. As shown in Fig. 6, the level of 8‐OH‐dG was reduced with time, and 65.6% of the 8‐OH‐dG was repaired at 24 h after UVB exposure.
Figure 6.
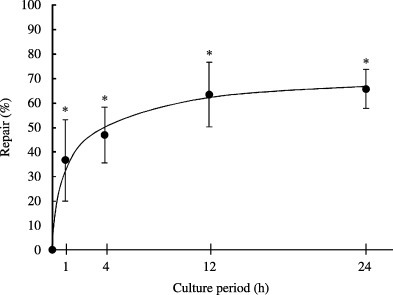
8‐OH‐dG repair rate after irradiation with narrowband ultraviolet (UV) B. HaCaT cells were irradiated with narrowband UVB at 2000 mJ/cm2, cultured for the indicated period, and subjected to the analysis. Repair (%) = (3.51 [8‐OH‐dG level immediately after irradiation] − X [8‐OH‐dG level 1–48 h after irradiation])/(3.51 − 1.48 [8‐OH‐dG level without irradiation]) × 100 = ([3.51–X]/2.03) × 100. *P < 0.01, compared with the value immediately after irradiation.
Discussion
Ultraviolet radiation produces ROS by photodynamic action,( 38 , 39 ) which causes several kinds of DNA damage, such as 8‐OH‐dG, and eventually leads to mutations and abnormal cell proliferation.( 8 , 40 ) Several techniques have been developed to detect 8‐OH‐dG. The measured background levels of 8‐OH‐dG differ, depending on both the DNA isolation technique and the 8‐OH‐dG analysis method.( 41 ) To measure the steady‐state level of DNA oxidation, HPLC‐ECD is particularly useful because of its selectivity, sensitivity and ease of quantification. During the past two decades, improved DNA isolation techniques and enhanced HPLC‐ECD sensitivity have considerably lowered the assayed background levels of 8‐OH‐dG.( 34 ) Reliable data have been obtained mainly by an improved method that uses an iron chelator, desferal, in the lysis step.( 36 ) In the present study, we also analyzed 8‐OH‐dG by HPLC‐ECD, after DNA was isolated by the improved method.
Previous studies revealed that the number of 8‐OH‐dG residues in murine keratinocytes treated with UVB increases in an irradiance‐dependent manner.( 42 , 43 , 44 ) However, those techniques had a limitation derived from the artifactual oxidation of DNA during its extraction. It was recently reported that relatively low doses of UVB (62.5–500 mJ/cm2) cause dose‐dependent increases in 8‐OH‐dG, and DNA from unirradiated normal human epidermal keratinocytes contains 1.49 ± 0.11 8‐OH‐dG residues per 106 dG.( 22 ) This is similar to the background level of 8‐OH‐dG observed in our study using HaCaT cells (1.48 ± 0.22). Furthermore, we report that narrowband UVB at a dose of more than 1000 mJ/cm2 increases the amount of 8‐OH‐dG, but to a lesser degree than broadband UVB with the same clinically effective dose. The maximum recommended dose of narrowband UVB for atopic dermatitis and psoriasis is 1500 mJ/cm2.( 45 , 46 , 47 ) We found that at the highest narrowband UVB dose, such as 1500 mJ/cm2 used in clinical treatment, 8‐OH‐dG increased to 2.82 per 106 dG.
When the biological effects of broadband and narrowband UVB were assessed by the inhibition of macrophage‐derived chemokine production, 10‐fold higher doses of narrowband UVB than broadband UVB exerted a comparable inhibitory effect.( 48 ) This is consistent with the observation that the MED and the therapeutic dose of narrowband UVB are approximately 10‐fold higher than those of broadband UVB. Even when narrowband UVB at 1000 mJ/cm2 was compared with broadband UVB at 100 mJ/cm2, the former induced fewer 8‐OH‐dG residues than broadband UVB.
Ultraviolet A‐induced formation of 8‐OH‐dG has been observed in human skin fibroblasts,( 19 , 20 , 21 ) and has been detected immunohistochemically in human keratinocytes.( 49 ) Our study demonstrated that UVA induced a dose‐dependent increase in 8‐OH‐dG with a fixed concentration of 8‐MOP in keratinocytes. PUVA produced both singlet oxygen and superoxide anions in an in vitro system.( 50 ) PUVA has already been reported to induce 8‐OH‐dG in the human epidermoid carcinoma cell line A431.( 31 ) Upon irradiation of A431 cells with a fixed dose (2500 mJ/cm2) of UVA, the level of 8‐OH‐dG increased, depending on the concentration of 8‐MOP. However, the background 8‐OH‐dG level was as high as 27 per 106 dG,( 31 ) compared with 1.48 per 106 dG in our study.
The amount of 8‐OH‐dG formed by UVB was reduced in living cells during cultivation. As the cell number and the rate of DNA synthesis were decreased after UVB irradiation, the reduction in 8‐OH‐dG does not seem to result from cell proliferation and division. Therefore, it is likely that 8‐OH‐dG is successfully repaired in keratinocytes. The repair rate of 65.6% in 24 h is slightly lower than that of Py–Py dimers( 51 ) and higher than that of 8‐MOP‐DNA photoproducts.( 25 ) The kinetics of 8‐OH‐dG repair in the present study seem to be slower than those determined in the previous study by Osterod et al.( 52 ) This may be explained by the presence of an overwhelming amount of Py–Py dimers in the irradiated DNA.
Clinically, narrowband UVB is as effective as PUVA in patients with psoriasis( 37 , 45 ) and atopic dermatitis when administered in equi‐erythemogenic doses.( 46 , 53 ) The highest final doses of narrowband UVB and PUVA for these treatments were 2450 and 8600 mJ/cm2, respectively.( 37 ) In the present study, narrowband UVB at 2000 mJ/cm2 induced 3.51 8‐OH‐dG per 106 dG, and PUVA at 5000 mJ/cm2 (8‐MOP, 100 ng/mL) induced 4.52 8‐OH‐dG per 106 dG. Thus, narrowband UVB seems to induce less oxidative stress than PUVA at the clinically effective doses. However, this study provides a warning that widely used narrowband UVB and PUVA at the therapeutically used doses induces cellular oxidative DNA damage, which may induce cancer.
References
- 1. Kasai H, Nishimura S. Hydroxylation of deoxyguanosine at the C‐8 position by ascorbic acid and other reducing agents. Nucl Acids Res 1984; 12: 2137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kasai H. Analysis of a form of oxidative DNA damage, 8‐hydroxy‐2′‐deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis. Mutat Res 1997; 387: 147–63. [DOI] [PubMed] [Google Scholar]
- 3. Li J, Stein TD, Johnson JA. Genetic dissection of systemic autoimmune disease in Nrf2‐deficient mice. Physiol Genomics 2004; 18: 261–72. [DOI] [PubMed] [Google Scholar]
- 4. Sander CS, Ali I, Dean D, Thiele JJ, Wojnarowska F. Oxidative stress is implicated in the pathogenesis of lichen sclerosis. Br J Dermatol 2004; 151: 627–35. [DOI] [PubMed] [Google Scholar]
- 5. Pathak MA, Stratton K. Free radicals in human skin before and after exposure to light. Arch Biochem Biophys 1968; 123: 468–76. [DOI] [PubMed] [Google Scholar]
- 6. Yamamoto F, Nishimura S, Kasai H. Photosensitized formation of 8‐hydroxydeoxyguanosine in cellular DNA by riboflavin. Biochem Biopys Res Commun 1992; 187: 809–13. [DOI] [PubMed] [Google Scholar]
- 7. Hattori‐Nakakuki Y, Nishigori C, Okamoto K, Imamura S, Hiai H, Toyokuni S. Formation of 8‐hydroxy‐2′‐deoxyguanosine in epidermis of hairless mice exposed to near‐UV. Biochem Biopys Res Commun 1994; 201: 1132–9. [DOI] [PubMed] [Google Scholar]
- 8. Hattori Y, Nishigori C, Tanaka T et al. 8‐Hydroxy‐2′‐deoxyguanosine is increased in epidermal cells of hairless mice after chronic ultraviolet B exposure. J Invest Dermatol 1996; 107: 733–7. [DOI] [PubMed] [Google Scholar]
- 9. Parrish JA, Jaenicke KF. Action spectrum for phototherapy of psoriasis. J Invest Dermatol 1981; 76: 359–62. [DOI] [PubMed] [Google Scholar]
- 10. Bilsland D, George SA, Gibbs NK, Aitchison T, Johnson BE, Ferguson J. A comparison of narrow band phototherapy (TL‐01) and photochemotherapy (PUVA) in the management of polymorphic light eruption. Br J Dermatol 1993; 129: 708–12. [DOI] [PubMed] [Google Scholar]
- 11. George SA, Bilsland DJ, Johnson BE, Ferguson J. Narrow‐band (TL‐01) UVB air‐conditioned phototherapy for chronic severe adult atopic dermatitis. Br J Dermatol 1993; 128: 49–56. [DOI] [PubMed] [Google Scholar]
- 12. Green C, Ferguson J, Lakshmipathi T, Johnson BE. 311 nm UVB phototherapy − an effective treatment for psoriasis. Br J Dermatol 1988; 119: 691–6. [DOI] [PubMed] [Google Scholar]
- 13. Scherschun L, Kim JJ, Lim HW. Narrow‐band ultraviolet B is a useful and well‐tolerated treatment for vitiligo. J Am Acad Dermatol 2001; 44: 999–1003. [DOI] [PubMed] [Google Scholar]
- 14. El‐Ghorr AA, Norval M. Biological effects of narrow‐band (311 nm TL01) UVB irradiation: a review. J Photochem Photobiol B 1997; 38: 99–106. [DOI] [PubMed] [Google Scholar]
- 15. British Photodermatology Group. An appraisal of narrowband (TL‐01) UVB phototherapy. Br J Dermatol 1997; 137: 327–30. [PubMed] [Google Scholar]
- 16. Gibbs NK, Traynor NJ, MacKie RM, Campbell I, Johnson BE, Ferguson J. The phototumorigenic potential of broad‐band (270–350 nm) and narrow‐band (311–313 nm) phototherapy source cannot be predicted by their edematogenic potential in hairless mouse skin. J Invest Dermatol 1995; 104: 359–63. [DOI] [PubMed] [Google Scholar]
- 17. Young AR. Carcinogenicity of UVB phototherapy assessed. Lancet 1995; 345: 1431–2. [Google Scholar]
- 18. Tzung TY, Rünger TM. Assessment of DNA damage induced by broadband and narrowband UVB in cultured lymphoblasts and keratinocytes using the comet assay. Photochem Photobiol 1998; 67: 647–50. [PubMed] [Google Scholar]
- 19. Kvam E, Tyrrell RM. Induction of oxidative DNA base damage in human skin cells by UV and near visible radiation. Carcinogenesis 1997; 18: 2379–84. [DOI] [PubMed] [Google Scholar]
- 20. Warmer WG, Wei RR. In vitro photooxidation of nucleic acids by ultraviolet A radiation. Photochem Photobiol 1997; 65: 560–3. [DOI] [PubMed] [Google Scholar]
- 21. Oikawa S, Tada‐Oikawa S, Kawanishi S. Site‐specific DNA damage at the GGG sequence by UVA involves acceleration of telomere shortening. Biochemistry 2001; 40: 4763–8. [DOI] [PubMed] [Google Scholar]
- 22. Pelle E, Huang X, Mammone T, Marenus K, Maes D, Frenkel K. Ultraviolet‐B‐induced oxidative DNA base damage in primary normal human epidermal keratinocytes and inhibition by a hydroxyl radical scavenger. J Invest Dermatol 2003; 121: 177–83. [DOI] [PubMed] [Google Scholar]
- 23. Budiyanto A, Ueda M, Ueda T, Ichihashi M. Formation of cyclobutane pyrimidine dimers and 8‐oxo‐7,8‐dihydro‐2′‐deoxyguanosine in mouse and organ cultured human skin by irradiation with broadband or with narrowband UVB. Photochem Photobiol 2002; 76: 397–400. [DOI] [PubMed] [Google Scholar]
- 24. Douki T, Perdiz D, Grof P et al. Oxidation of guanine in cellular DNA by solar UV radiation: biological role. Photochem Photobiol 1999; 70: 184–90. [PubMed] [Google Scholar]
- 25. Tokura Y, Edelson RL, Gasparro FP. Formation and removal of 8‐MOP‐DNA photoadducts in keratinocytes: effects of calcium concentration and retinoids. J Invest Dermatol 1991; 96: 942–9. [DOI] [PubMed] [Google Scholar]
- 26. Matsui M, Nishigori C, Toyokuni S et al. The role of oxidative DNA damage in human arsenic carcinogenesis: detection of 8‐hydroxy‐2′‐deoxyguanosine in arsenic‐related Bowen's disease. J Invest Dermatol 1999; 113: 26–31. [DOI] [PubMed] [Google Scholar]
- 27. Nishigori C, Hattori Y, Toyokuni S. Role of reactive oxygen species in skin carcinogenesis. Antioxid Redox Signal 2004; 6: 561–70. [DOI] [PubMed] [Google Scholar]
- 28. Budiyanto A, Ahmed NU, Wu A et al. Protective effect of topically applied olive oil against photocarcinogenesis following UVB exposure of mice. Carcinogenesis 2000; 21: 2085–90. [DOI] [PubMed] [Google Scholar]
- 29. Ichihashi M, Ahmed NU, Budiyanto A et al. Preventive effect of antioxidant on ultraviolet‐induced skin cancer in mice. J Dermatol Sci 2000; 23: 45–50. [DOI] [PubMed] [Google Scholar]
- 30. Kunisada M, Sakumi K, Tominaga Y et al. 8‐Oxoguanine formation induced by chronic UVB exposure makes Ogg1 knockout mice susceptible to skin carcinogenesis. Cancer Res 2005; 65: 6006–10. [DOI] [PubMed] [Google Scholar]
- 31. Liu Z, Lu Y, Lebwohl M, Wei H. PUVA (8‐methoxy‐psoralen plus ultraviolet A) induces the formation of 8‐hydroxy‐2′‐deoxyguanosine and DNA fragmentation in calf thymus DNA and human epidermoid carcinoma cells. Free Radic Biol Med 1999; 27: 127–33. [DOI] [PubMed] [Google Scholar]
- 32. Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol 1988; 106: 761–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gasparro FP, Battista J, Song J, Edelson RL. Rapid and sensitive analysis of 8‐methoxypsoralen in plasma. J Invest Dermatol 1988; 90: 234–6. [DOI] [PubMed] [Google Scholar]
- 34. Nakae D, Mizumoto Y, Kobayashi E, Noguchi O, Konishi Y. Improved genomic/nuclear DNA extraction for 8‐hydroxydeoxyguanosine analysis of small amounts of rat liver tissue. Cancer Lett 1995; 97: 233–9. [DOI] [PubMed] [Google Scholar]
- 35. Yamaguchi R, Hirano T, Asami S, Chung MH, Sugita A, Kasai H. Increased 8‐hydroxyguanine levels in DNA and its repair activity in rat kidney after administration of a renal carcinogen, ferric nitrilotriacetate. Carcinogenesis 1996; 17: 2419–22. [DOI] [PubMed] [Google Scholar]
- 36. Helbock HJ, Beckman KB, Shigenaga MK et al. DNA oxidation matters: the HPLC‐electrochemical detection assay of 8‐oxo‐deoxyguanosine and 8‐oxo‐guanine. Proc Natl Acad Sci USA 1998; 95: 288–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tanew A, Radakovic‐Fijan S, Schemper M, Honigsmann H. Narrowband UV‐B phototherapy vs photochemotherapy in the treatment of chronic plaque‐type psoriasis. Arch Dermatol 1999; 135: 519–24. [DOI] [PubMed] [Google Scholar]
- 38. Yamamoto F, Nishimura S, Kasai H. Photosensitized formation of 8‐hydroxydeoxyguanosine in cellular DNA by riboflavin. Biochem Biophys Res Commun 1992; 187: 809–13. [DOI] [PubMed] [Google Scholar]
- 39. Cadet J, Berger M, Buchko G, Ravanat JL, Kasai H. Photooxidation reactions of nucleic acids. In: Shima A, Ichihashi M, Fujiwara Y, Takebe H, eds. Frontiers of Photobiology. Amsterdam: Elsevier Science Publishers, 1993; 49–54. [Google Scholar]
- 40. Ahmed NU, Ueda M, Nikaido O, Osawa T, Ichihashi M. High levels of 8‐hydroxy‐2′‐deoxyguanosine appear in normal human epidermis after a single dose of ultraviolet radiation. Br J Dermatol 1999; 140: 226–31. [DOI] [PubMed] [Google Scholar]
- 41. European Standards Committee on Oxidative DNA Damage (ESCODD). Measurement of DNA oxidation in human cells by chromatographic and enzymic methods. Free Radic Biol Med 2003; 34: 1089–99. [DOI] [PubMed] [Google Scholar]
- 42. Beehler BC, Przybyszewski J, Box HB, Kulesz‐Martin MF. Formation of 8‐hydroxydeoxyguanosine within DNA of mouse keratinocytes exposed in culture to UVB and H2O2 . Carcinogenesis 1992; 13: 2003–7. [DOI] [PubMed] [Google Scholar]
- 43. Maccubbin AE, Przybyszewski J, Evans MS et al. DNA damage in UVB‐irradiated keratinocytes. Carcinogenesis 1995; 16: 1659–60. [DOI] [PubMed] [Google Scholar]
- 44. Stewart MS, Cameron GS, Pence BC. Antioxidant nutrients protect against UVB‐induced oxidative damage to DNA of mouse keratinocytes in culture. J Invest Dermatol 1996; 106: 1086–9. [DOI] [PubMed] [Google Scholar]
- 45. Gordon PM, Diffey BL, Matthews JN, Farr PM. A randomized comparison of narrow‐band TL‐01 phototherapy and PUVA photochemotherapy for psoriasis. J Am Acad Dermatol 1999; 41: 728–32. [DOI] [PubMed] [Google Scholar]
- 46. Reynolds NJ, Franklin V, Gray JC, Diffey BL, Farr PM. Narrow‐band ultraviolet B and broad‐band ultraviolet A phototherapy in adult atopic eczema: a randomised controlled trial. Lancet 2001; 357: 2012–16. [DOI] [PubMed] [Google Scholar]
- 47. Youn JI, Park JY, Jo SJ, Rim JH, Choe YB. Assessment of the usefulness of skin phototype and skin color as the parameter of cutaneous narrow band UVB sensitivity in psoriasis patients. Photodermatol Photoimmunol Photomed 2003; 19: 261–3. [DOI] [PubMed] [Google Scholar]
- 48. Hino R, Shimauchi T, Tokura Y. Treatment with IFN‐γ increases serum levels of Th‐1 chemokines and decreases those of Th2 chemokines in patients with mycosis fungoides. J Dermatol Sci 2003; 31: 37–42. [DOI] [PubMed] [Google Scholar]
- 49. Cooke MS, Mistry N, Ladapo A, Herbert KE, Lunec J. Immunochemical quantitation of UV‐induced oxidative and dimeric DNA damage to human keratinocytes. Free Radic Res 2000; 33: 369–81. [DOI] [PubMed] [Google Scholar]
- 50. Carraro C, Pathak MA. Studies on the nature of in vitro and in vivo photosensitization reactions by psoralens and porphyrins. J Invest Dermatol 1988; 90: 267–75. [DOI] [PubMed] [Google Scholar]
- 51. Rafferty TS, Green MH, Lowe JE et al. Effects of selenium compounds on induction of DNA damage by broadband ultraviolet radiation in human keratinocytes. Br J Dermatol 2003; 148: 1001–9. [DOI] [PubMed] [Google Scholar]
- 52. Osterod M, Hollenbach S, Hengstler JG, Barnes DE, Lindahl T, Epe B. Age‐related and tissue‐specific accumulation of oxidative DNA base damage in 7,8‐dihydro‐8‐oxoguanine‐DNA glycosylase (Ogg1) deficient mice. Carcinogenesis 2001; 22: 1459–63. [DOI] [PubMed] [Google Scholar]
- 53. Der‐Petrossian M, Seeber A, Hönigsmann H, Tanew A. Half‐side comparison study on the efficacy of 8‐methoxypsoralen bath‐PUVA versus narrow‐band ultraviolet B phototherapy in patients with severe chronic atopic dermatitis. Br J Dermatol 2000; 142: 39–43. [DOI] [PubMed] [Google Scholar]
- 54. Fitzpatrick TB. The validity and practicality of sun‐reactive skin types I through VI. Arch Dermatol 1988; 124: 869–71. [DOI] [PubMed] [Google Scholar]


