Abstract
Hypoxia is a state of deficiency of available oxygen in the blood and tissues, and it occurs during several pathophysiological processes, including tumorigenesis. Under hypoxia, hypoxia‐inducible factor‐1 (HIF‐1) plays an essential role in cellular oxygen homeostasis. In the present article protein kinase C‐δ (PKC‐δ) is activated by hypoxia, increases the protein stability and transcriptional activity of HIF‐1α in human cervical adenocarcinoma cells. Moreover, the knockdown of PKC‐δ inhibited vascular endothelial growth factor expression and angiogenic activity under hypoxia. These effects were completely reversed by PKC‐δ overexpression following the knockdown of PKC‐δ. Collectively, these findings demonstrate the role of PKC‐δ as a new regulator of hypoxia‐induced angiogenesis. (Cancer Sci 2007; 98: 1476–1481)
Abbreviations:
- ARD1
arrest defective gene 1
- BCA
bicinchoninic acid
- CM
conditioned media
- DAG
diacylglycerol
- DMEM
Dulbecco's modified Eagle's medium
- DOC
deoxycholate
- DTT
dithiothreitol
- EDTA
ethylene diamine tetra‐acetic acid
- EGTA
ethylene glycol bis(βaminoethyl ether)‐N,N,N′,N′‐tetra‐acetic acid
- FBS
fetal bovine serum
- HDAC
histone deacetylase
- HeLa cells
human cervical adenocarcinoma cells
- HEPES
4‐(2‐hydroxyethyl) piperazinc‐1‐ethane sulfonic acid
- HIF‐1α
hypoxia‐inducible factor‐1α
- HRE
hypoxic response elements
- HSF
heat shock transcription factor
- HUVEC
human umbilical vein endothelial cell
- NC
negative control
- NP‐40
Nonidet P40
- p300/CBP
p300/CREB‐binding protein
- PHD
prolyl hydroxylase
- PKC‐δ
protein kinase C‐δ
- pVHL
von Hippel‐Lindau protein
- RIF
radiation‐induced fibrosarcoma
- RLU
relative luciferase unit
- RT‐PCR
reverse transcription–polymerase chain reaction
- SDS
sodium dodecyl sulfate
- SDS‐PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- VEGF
vascular endothelial growth factor.
Hypoxic condition is an important modulator of gene expression in the induction of angiogenesis during tumor development. Most tumor cells are promoted to growth by the activation of HIF‐1, which is an oxygen‐dependent transcriptional activator.( 1 , 2 , 3 , 4 ) As a result, HIF‐1 plays crucial roles in cancer cell growth and survival, tumor development, and angiogenesis.( 5 , 6 , 7 ) HIF‐1 is a heterodimer, composed of α‐ and β‐subunits. HIF‐1α is the hypoxia‐responsive component of the dimer, while HIF‐1β is expressed constitutively.( 8 ) The stability of HIF‐1α is regulated by various post‐translational modifications, hydroxylation, acetylation, and phosphorylation via interaction with several proteins, including PHD, pVHL, ARD‐1, and p300/CBP.( 4 , 9 , 10 , 11 , 12 , 13 ) Under normoxic conditions, HIF‐1α is rapidly degraded via the pVHL‐mediated ubiquitin–proteasome pathway. Whereas, under hypoxic conditions, HIF‐1α becomes stable and interacts with coactivators such as p300/CBP to modulate its transcriptional activity.( 3 , 14 , 15 , 16 )
The PKC isoforms are classified into three subfamilies, based on their structure and allosteric requirements. The classical PKC (cPKC; α, β1, β2, and γ) are regulated by the secondary messengers, both calcium and diacylglycerol (DAG). The novel PKC (nPKC; δ, ɛ, η, θ and µ) are also activated by DAG, but are insensitive to calcium, and the atypical PKC (aPKC: ζ, λ/ι) are calcium‐independent and do not respond to DAG. The serine/threonine kinase PKC‐δ is the most thoroughly studied member of the nPKC subfamily.( 16 ) Previous studies have identified that PKC‐δ was translocated to the cell membrane by hypoxic conditions and that PKC‐δ mediated the transcriptional activation of HSF and HIF‐1 under hypoxic condition in RIF cells.( 17 ) Therefore, a study of the relationship between HIF‐1α and PKC‐δ is worthwhile to gain an understanding of tumor angiogenesis and tumorigenesis. Herein, the authors show that activated PKC‐δ modulates the stability of HIF‐1 and enhances its transcriptional activity under hypoxic conditions in HeLa cells. Furthermore, the knockdown of PKC‐δ reduced angiogenic activity by destabilizing HIF‐1α and decreasing VEGF expression under hypoxia.
Materials and Methods
Reagents and antibodies. Rottlerin was purchased from Sigma‐Aldrich (St Louis, MO, USA). Anti‐PKC‐δ, c‐myc, VEGF, and tubulin antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti‐actin antibody was purchased from Sigma‐Aldrich. Anti‐HIF‐1α antibody was purchased from BD Transduction Laboratories (San Diego, CA, USA). Anti‐PKC‐δ Thr‐505 antibody was purchased from Cell Signaling Technology (Beverly, MA, USA). Anti‐integrin‐β1 antibody was purchased from PharMingen Transduction Laboratories (San Diego, CA, USA).
Plasmids. PKC‐δ‐WT (wild type of mouse PKC‐δ) and PKC‐δ‐DN (catalytic fragment dominant‐negative mutant [K376R] of mouse PKCδ) plasmids were constructed by subcloning pMTH‐PKC‐δ and pMTH‐PKC‐δ‐DN into pCS2 ± c‐myc plasmid (Clontech, Palo Alto, CA, USA) with XhoI and XbaI. pMTH‐PKC‐δ and pMTH‐PKC‐δ‐DN were provided by Dr Yuspa (Pennsylvania University, USA).( 18 ) pSV40pro‐EpoHRE‐Luc, pCMV‐β‐gal plasmids were prepared as previously described.( 13 )
Cell culture and hypoxic condition. HeLa and human fibrosarcoma (HT1080) cells were maintained in DMEM (Invitrogen, Carlsbad, CA, USA) supplemented with 10% FBS (Invitrogen) and 1% penicillin streptomycin (Invitrogen) in a humidified incubator with 5% CO2 at 37°C. HUVEC cells were maintained in Medium 199 (Invitrogen) supplemented with 20% FBS (Invitrogen) and 1% penicillin/streptomycin (Invitrogen), 3 ng/mL basic fibroblast growth factor (Upstate Biotechnology, New York, NY, USA), and 5 U/mL heparin (Sigma‐Aldrich) in a humidified incubator with 5% CO2 at 37°C. For the hypoxic condition, cells were incubated at 5% CO2 level with 1% O2 balanced with N2 in a hypoxic chamber (Forma Scientific, Marietta, OH, USA) with an interior temperature of 37°C.
Cellular fractionation. The cells were washed and pelleted after treatment, resuspended in 150 µL of hypotonic buffer (20 mM Tris‐HCl, 5 mM EGTA, 2 mM EDTA, 2 mM β‐mercaptoethanol) and left for 30 min at 4°C to swell. Cells were lyzed using sonication for 5 s and centrifuged at 16 000 g at 4°C for 30 min and the supernatant was collected as the cytosol fraction. The pellet was resuspended in the RIPA buffer (50 mM Tris‐HCl, 150 mM NaCl, 0.5 mM EDTA, 1% NP‐40, 0.5% DOC, 0.1% SDS) and left for 30 min at 4°C. The suspension was centrifuged again at 16 000 g at 4°C for 30 min and the supernatant was taken as the membrane fraction.
RT‐PCR analysis. Total RNA from cells was isolated using Trizol reagent (Invitrogen) according to the manufacturer's instructions. First‐stranded cDNA was synthesized with 3 µg of each DNA‐free total RNA and oligo‐(dT)16 primer by Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI, USA). Equal amounts of cDNA were subsequently amplified by PCR in a 50‐µL reaction volume containing 1× PCR buffer, 200 µM dNTPs, 10 µM specific primer for PKC‐δ (5′‐GCAGGGATTAAAGTGTGAAG‐3′ and 5′‐TTCTTCTCGAAACCCTGATA‐3′), HIF‐1α (5′‐CAGAAGATACAAGTAGCCTC‐3′ and 5′‐CTGCTGGAATACTGTAACTG‐3′), VEGF (5′‐GAGAATTCGGCCTCCGAAACCATGAACTTTCTGCT‐3′ and 5′‐GAGCATGCCTCCGAAACCATGAACTTTCTGCT‐3′ and 5′‐GAGCATGCCCTCCTGCCCGGCTCACCGC‐3′), β‐actin (5′‐GACTACCTCATGAAGATC‐3′ and 5′‐GATCCACATCTGCTGGAA‐3′) and 1.25 U Taq DNA polymerase (TaKaRa, Japan). PCR was performed with an initial denaturation step at 94°C for 5 min, followed by 25 (PKC‐δ, HIF‐1α, β‐actin) or 30 (VEGF) cycles of denaturation (94°C for 1 min), annealing (50°C [PKC‐δ, HIF‐1α], 55°C [β‐actin], or 65°C [VEGF] for 30 s), and extension (72°C for 1 min). The PCR products (PKC‐δ, 189 bp; HIF‐1α, 852 bp; β‐actin, 550 bp; VEGF, 450/600 bp) were separated on agarose gels and visualized using ethidium bromide staining under UV transillumination.
siRNA transfection. siRNA duplex (siRNA) targeting human PKC‐δ was chemically synthesized and purified in the 2‐deprotected and desalted form (Dharmacon, Lafayette, CO, USA). The sequences of PKC‐δ siRNA (#1) pair were 5′‐GCUUCAAGGUUCACAACUAUU‐3′ and 5′‐UAGUUGUGAACCUUGAAGCUU‐3′ and the sequences of PKC‐δ siRNA (#2) pair were 5′‐CGCUGCCAUCCACAAGAAAUU‐3′ and 5′‐UUUCUUGUGGAUGGCAGCGUU‐3′. Negative control siRNA (Cat. no.: D‐001210‐02) purchased from Dharmacon. Transfection of siRNA was performed using oligofectamine (Invitrogen), according to the manufacturer's instructions. Briefly, HeLa cells were seeded at 2 × 105 cells in a 6‐well plate 1 day prior to transfection. The cells were transfected at the final concentration of 25 or 50 nM siRNA duplex. The efficacy of knockdown was assessed using western blotting with anti‐PKC‐δ antibody and RT‐PCR using a PKC‐δ‐specific primer.
Western blot analysis. Cells were harvested and the pellets were immediately frozen in liquid N2. After thawing, the pellets were lyzed in whole cell extract buffer (10 mM HEPES at pH 7.9, 40 mM NaCl, 0.1 mM EDTA, 5% glycerol, 1 mM DTT, and protease inhibitors) followed by centrifugation for 30 min at 20 000g and protein concentration was measured with BCA assay. Total cell lysates were resolved in SDS‐PAGE gels and transferred onto nitrocellulose membrane (Amersham Pharmacia Bioscience, UK). The membrane was probed with a primary antibody followed by a secondary antibody conjugated with horseradish peroxidase and detected using the ECL Plus system (Amersham Pharmacia Bioscience).
Transient transfection and luciferase assays. Plasmids was transfected to HeLa cells with recombinations of effector plasmids, pSV40 promoter‐EpoHRE‐Luc reporter (1 µg), pCMV‐β‐gal (0.5 µg), and PKC‐δ‐WT (2 µg), PKC‐δ‐DN (2 µg), negative control siRNA (50 nM), or PKC‐δ siRNA (50 nM) using the lipofectamine method (Invitrogen) according to the manufacturer's instructions. After transfection, cells were harvested and extracts were prepared using the reporter lysis buffer (Promega, Madison, WI). Cell lysates were analyzed for the luciferase activity using a luciferase assay kit (Promega) and a luminometer (Turner Design). Extracts were normalized using the β‐galactosidase enzyme assay and the BCA protein assay (Sigma‐Aldrich). The mean RLU was corrected using values obtained from an extract prepared from mock vector or negative control siRNA transfected cells. The relative luciferase activity was calculated as RLU/β‐galactosidase.
Tube formation assay. HeLa cells were transfected with PKC‐δ siRNA (50 nM) or negative control siRNA (NC siRNA; 50 nM) using the oligofectamine method (Invitrogen) according to the manufacturer's instructions. Of necessity, 24 h after the transfection with PKC‐δ siRNA, the HeLa cells were concomitantly transfected with PKC‐δ‐WT (3 µg) to restore PKC‐δ using the lipofectamine method (Invitrogen). After transfection, the cells were grown in serum‐free M199 (Invitrogen) under hypoxic conditions for 24 h. The CM were collected and filtered. HUVEC were diluted with CM and seeded at 5 × 104 in a 48‐well plate on a matrix of previously polymerized Matrigel, and incubated at 37°C. After the incubation time, changes in cell morphology were observed and photographed through a phase‐contrast microscope.
Scratch wound assay. The migratory characteristics of HUVEC were determined using scratch‐wound assay. Confluent monolayers of cells plated on 6‐well dishes were wounded using a micropipette tip, rinsed with PBS to remove detached cells, and incubated at 37°C for 24 h containing CM. The plates were photographed at 24 h and the wound width was calculated according to the Microrulers guidelines (available from http://www.eeob.iastate.edu/faculty/DrewesC/htdocs/microruler‐links.htm).
Statistics. Error bars represent standard error of the mean. Statistical analyses were done using Student's t‐test. Statistical significance was defined as P < 0.05.
Results
PKC‐δ is activated under hypoxic conditions. To determine the relationship between HIF‐1 and PKC‐δ under hypoxia, HeLa cells were treated with PKC inhibitors, GF109203X (a broad inhibitor of PKC) and rottlerin (a specific inhibitor of PKC‐δ) at the indicated concentrations (Fig. 1A). Both inhibitors markedly attenuated HIF‐1α expression under hypoxia, suggesting that PKC‐δ is likely to regulate HIF‐1α expression. Based on this finding, the authors investigated whether PKC‐δ was activated in response to hypoxia by PKC‐δ membrane translocation and phosphorylation. It was found that PKC‐δ was translocated predominantly to the membrane under hypoxia for 1 and 4 h (Fig. 1B) and phosphorylated at Thr 505 of PKC‐δ (Fig. 1C) in HeLa cells. Next, the authors examined whether PKC‐δ activation regulated HIF‐1α expression. Treatment of HeLa cells with 5 µM rottlerin, a specific inhibitor of PKC‐δ, inhibited the phosphorylation of PKC‐δ at Thr 505 residue and the protein level of HIF‐1α, whereas it did not alter the protein level of PKC‐δ (Fig. 1C). These data suggest that rottlerin inhibits PKC‐δ activity and attenuates HIF‐1α expression under hypoxia in HeLa cells. A similar effect was obtained in HT1080, human fibroblastoma cells (Fig. 1D). Exposure of HT1080 cells to either rottlerin or GF203109X dramatically decreased HIF‐1α expression as well as the phosphorylation of PKC‐δ (Fig. 1D). Taken together, these results indicate that PKC‐δ is activated through phosphorylation of Thr 505 under hypoxic conditions. Moreover, PKC‐δ activation is likely to be correlated with HIF‐1α stability.
Figure 1.
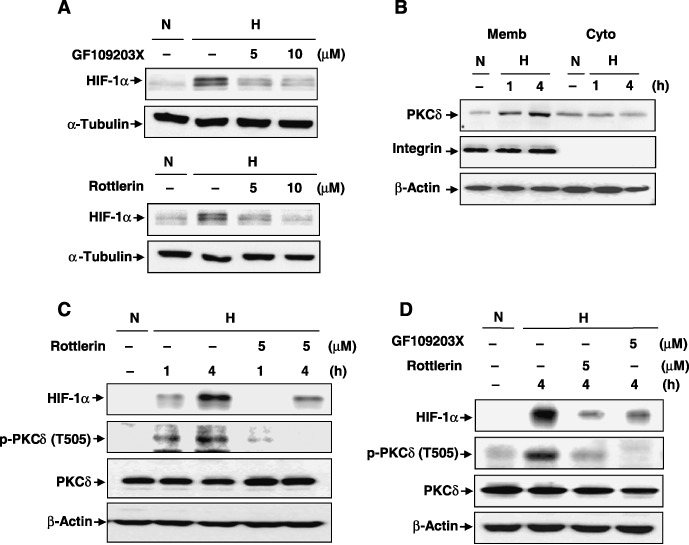
Protein kinase C (PKC)‐δ is activated under hypoxic condition and regulates hypoxia‐inducible factor (HIF)‐1α in human cervical adenocarcinoma (HeLa) cells and human fibrosarcoma (HT1080) cells. (A) HeLa cells were treated with 5 or 10 µM GF109203X and rottlerin, and then exposed immediately to hypoxia for 4 h. HIF‐1α protein expressions were examined using western blot analysis (H hypoxia; N, normoxia). (B) HeLa cells were exposed to hypoxia for 1 h or 4 h and then cytosolic and membrane fractions were extracted as described in ‘Materials and Methods.’ Both fractions were subjected to western blot analysis. (C, D) HeLa and HT1080 cells were treated with (C) 5 µM rottlerin immediately after exposure to hypoxia for 1 h or 4 h in HeLa or (D) with 5 µM GF109203X or rottlerin immediately after exposure to hypoxia for 4 h in HT1080 cells. Western blot analysis showing the expressions of HIF‐1α, phosphorylated PKC‐δ (p‐PKCδ), and endogenous PKC‐δ. A representative experiment is shown. Similar results were observed in two additional experiments.
PKC‐ δ increases the protein stability of HIF‐1α. To determine the role of PKC‐δ in HIF‐1α stability, the authors investigated whether overexpression of wild‐type PKC‐δ (PKC‐δ‐WT) and dominant negative PKC‐δ (PKC‐δ‐DN) regulated HIF‐1α expression. Exposure of PKC‐δ‐WT‐transfected HeLa cells to hypoxia enhanced the HIF‐1α level, compared to mock‐transfected HeLa cells (Fig. 2A). In contrast, overexpression of PKC‐δ‐DN markedly abrogated the HIF‐1α level under hypoxia in a dose‐dependent manner (Fig. 2B), suggesting that PKC‐δ activity is correlated with HIF‐1α stability. To further confirm the role of PKC‐δ in HIF‐1α stability, the effects of siRNA knockdown of PKC‐δ were examined. Two different types of siRNA against human PKC‐δ were used in HeLa cells. Cells were transfected with indicated concentrations of PKC‐δ siRNA for 48 h. As shown in Fig. 2C, endogenous PKC‐δ was dramatically depleted by both siRNA PKC‐δ. The authors concomitantly observed that PKC‐δ‐depleted cells failed to sustain HIF‐1α stability. To investigate whether HIF‐1α stability was regulated in its mRNA level, PKC‐δ was depleted with siRNAs at various indicated concentrations, as shown in Fig. 2D. RT‐PCR analysis showed that PKC‐δ knockdown did not alter the levels of HIF‐1α mRNA (Fig. 2D), in contrast to the level of HIF‐1α protein, as shown in Fig. 2C. These results indicate that PKC‐δ regulates the protein stability of HIF‐1α under hypoxic conditions.
Figure 2.
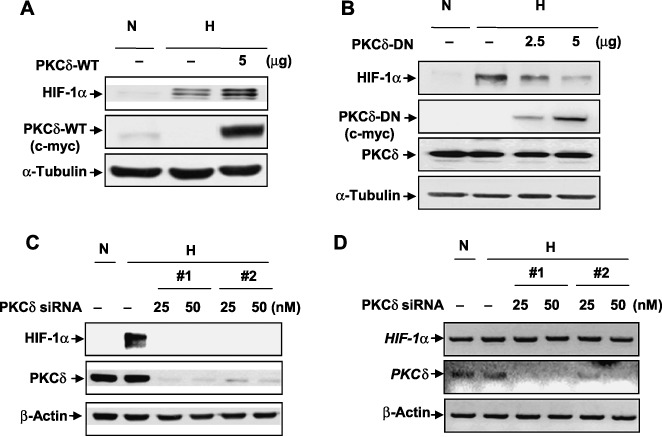
Protein kinase C (PKC)‐δ regulates hypoxia‐inducible factor (HIF)‐1α in the protein level. (A,B) Human cervical adenocarcinoma (HeLa) cells were transfected with (A) PKC‐δ‐WT or (B) PKC‐δ‐DN at the indicated concentration. On the following day, the transfectants were exposed to hypoxia for an additional 4 h. Total cell lysates were subjected to western blot assay using HIF‐1α and c‐myc (for PKC‐δ‐WT and PKC‐δ‐DN) antibodies. (C) HeLa cells were transfected with either carrier alone (negative control siRNA) or siRNA targeting PKC‐δ#1 and #2 (PKC‐δ siRNA) at the indicated concentrations. After 48 h, the transfectants were exposed to hypoxia for 4 h. HIF‐1α and PKC‐δ protein expressions were examined using western blot analysis. (D) HeLa cells were transfected with either carrier alone (negative control siRNA) or PKC‐δ siRNA (#1 and #2) 25 or 50 nM. After 48 h, the transfectants were exposed to hypoxia for 4 h. Total RNA was extracted and transcript levels were quantified using RT–PCR. Mock vector (5 µg), negative control (50 nM). Results are representative of three separate experiments.
PKC‐δ increases the transcriptional activity of HIF‐1. HIF‐1 protein is stabilized under hypoxia and then activates a specific set of genes by binding to HRE in the promoter region of these genes, including VEGF. To determine the effect of PKC‐δ in HIF‐1 transcriptional activity, a luciferase reporter system, pSV40 promoter‐Epo‐HRE‐Luc, was used. Hypoxia induced the transcriptional activity of HIF‐1 compared with normoxia (Fig. 3A, first and second columns). The hypoxia‐induced activity was further increased by transfection with PKC‐δ‐WT, compared with mock transfection (Fig. 3A, second and fourth columns). In contrast, transfection of PKC‐δ‐DN and PKC‐δ siRNA suppressed the transcriptional activity of HIF‐1 compared with mock transfection and negative control siRNA (Fig. 3A, second and sixth, eighth and tenth columns), respectively. The effect of PKC‐δ on the HIF‐1‐mediated transcriptional activity was further confirmed. Hypoxic‐induced VEGF expression, a target gene of HIF‐1, was inhibited by administration of PKC‐δ siRNA in HeLa cells (Fig. 3B). Therefore, these data suggest that PKC‐δ stabilizes the HIF‐1α protein level and increases the transcriptional activity of HIF‐1 under hypoxic conditions.
Figure 3.
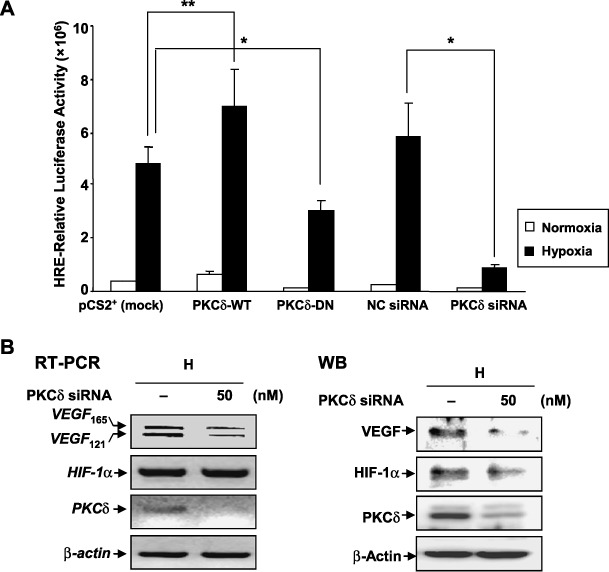
Protein kinase C (PKC)‐δ enhances the transcriptional activity of hypoxia‐inducible factor (HIF)‐1. (A) Human cervical adenocarcinoma (HeLa) cells were transfected with mock vector (2 µg), PKC‐δ‐WT (2 µg), PKC‐δ‐DN (2 µg), negative control siRNA (50 nM), PKC‐δ siRNA #1 (50 nM) in the presence of pSV40pro‐Epo‐HRE‐Luc (1 µg) and pCMV‐β‐gal (0.5 µg). 24 h after transfection, the cells were exposed under either normoxia or hypoxia for an additional 24 h. Results are the mean ± SE of three independent experiments. *P < 0.01; **P < 0.05. (B) HeLa cells were transfected with negative control siRNA (50 nM) or PKC‐δ siRNA #1 (50 nM) and, after 24 h, the transfectants were exposed to hypoxia for 24 h. Left, RT‐PCR analysis was carried out using specific primers for VEGF, PKC‐δ, HIF‐1α and β‐actin. Right, HIF‐1α, VEGF, PKC‐δ, and β‐actin were performed using western blot analysis (WB). Negative control. The immunoblots are representative of three separate experiments.
PKC‐δ induces angiogenic activity in vitro. The role of PKC‐δ in hypoxia‐induced angiogenesis in vitro was evaluated next. CM were obtained under the condition without alteration of growth/survival rates of HeLa cells, compared with the control. The CM from PKC‐δ siRNA‐transfected cells under hypoxia showed a negative effect on the tube formation of HUVEC, compared with the NC, siRNA‐transfected cells. The decreased effect on the tube formation was reversed by the CM from concomitant transfection with PKC‐δ‐WT and PKC‐δ siRNA, implying that PKC‐δ‐mediated signaling affects the activity of endothelial tube formation (Fig. 4A). As another assay to evaluate the angiogenic activity of PKC‐δ, an in vitro scratch–wound assay in HUVEC was performed (Fig. 4B). CM taken from PKC‐δ siRNA‐transfected cells markedly inhibited cell migration compared with the NC siRNA. In contrast, CM taken from cotransfected cells with PKC‐δ siRNA and PKC‐δ‐WT recovered the cell migration inhibitory effect of HUVEC by CM of PKC‐δ siRNA. Taken together, these results suggest that PKC‐δ may play an important role in angiogenesis by mediating the generation of angiogenic factors such as VEGF from cells exposed to hypoxia.
Figure 4.
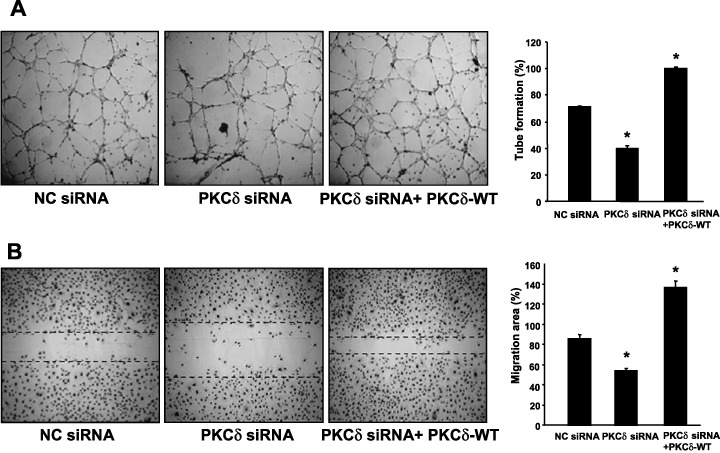
Protein kinase C (PKC)‐δ enhances angiogenic activities of endothelial cells. (A, B) Human cervical adenocarcinoma (HeLa) cells were transfected with either negative control siRNA or PKC‐δ siRNA #1 (50 nM). 24 h after transfection, the HeLa cells were additionally transfected with either PKC‐δ‐WT (3 µg) or mock vector (3 µg) and incubated for another 24 h. After that, the transfected cells were replaced to fresh serum‐free M199 and incubated for a further 24 h under hypoxia. Conditioned media (CM) were collected from HeLa cells transfected with negative control siRNA (NC siRNA) and mock vector, with PKC‐δ siRNA#1 (PKC‐δ siRNA) and mock vector, and with PKC‐δ siRNA#1 and PKC‐δ‐WT (PKC‐δ siRNA + PKC‐δ‐WT). Each CM was treated to human umbilical vein endothelial cells (HUVEC) for (A) 8 h in tube formation assay or for (B) 24 h in scratch–wound assay. (A) Tube formation (%) was quantified by the number of three‐way branching point (right). (B) Cell migration was determined by scratch wounding of HUVEC monolayers and wound closure was monitored by photography at 24 h (right). Cell migration (%) was quantified by calculating the wound width as described in ‘Materials and Methods’ (left). Bar = 200 µm. Dotted lines indicate the margin of migrating cells. Values are means ± SE of three independent experiments. *Statistical significance (P < 0.05) compared with NC siRNA.
Discussion
HIF‐1 acts as a master regulator of various oxygen‐dependent genes that are particularly related to tumors expressing angiogenic and survival factors.( 2 , 3 , 4 , 5 ) The activation of HIF‐1 is closely associated with tumorigenesis.( 19 , 20 , 21 ) Therefore, blockage of HIF‐1 activity may be advantageous for inhibiting tumor progression.( 22 , 23 , 24 ) Meanwhile, PKC‐δ plays an important role in the regulation of several functional events, including proliferation, differentiation, tumor promotion, apoptosis, and angiogenesis.( 25 , 26 , 27 , 28 ) Recently PKC‐δ has been reported to be activated under hypoxic conditions.( 17 , 29 ) However, because the function of PKC‐δ has not been fully characterized under hypoxic conditions, whether PKC‐δ activation regulated HIF‐1α stability and angiogenic activity under hypoxia was investigated.
The authors here demonstrate that under hypoxic conditions PKC‐δ is redistributed to the membrane and phosphorylated at Thr 505 residue, which is located at the activation loop of its catalytic domain.( 30 , 31 ) Based on the result, the authors examined whether the hypoxic activation of PKC‐δ was involved in the regulation of HIF‐1α protein level. PKC inhibitors, GF109203X and rottlerin, seemed to inhibit HIF‐1α accumulation by the reduction of the phosphorylated PKC‐δ in HeLa and HT1080 cells. In addition, overexpression of the kinase inactive form of PKC‐δ (PKC‐δ‐DN) attenuates the expression of HIF‐1α under hypoxia, while overexpression of the full length of PKC‐δ (PKC‐δ‐WT) potentiates it in HeLa cells. Accordingly, the authors suggest that PKC‐δ activation regulates HIF‐1α stability. Interestingly, under normoxic conditions, the authors could not detect PKC‐δ phosphorylation and HIF‐1α expression in cells overexpressing either the full length or the catalytic kinase fragment of PKC‐δ (data not shown). Therefore, the effect of PKC‐δ on HIF‐1α stability may be related to its phosphorylation state under hypoxia, even though the present studies have not directly addressed the reason.
The authors also revealed that PKC‐δ inhibition using PKC‐δ‐DN, PKC‐δ siRNA, or rottlerin remarkably decreased both HIF‐1α and VEGF expression in HeLa cells. In addition, the authors checked that HIF‐1α stability was also down‐regulated by the treatment with rottlerin or transfection of PKC‐δ siRNA in HT1080 and HepG2 cells under hypoxia. Moreover, PKC‐δ inhibition using PKC‐δ siRNA or rottlerin remarkably decreased VEGF expression in HT1080 cells under hypoxia (data not shown). Considering these findings, the impact of PKC‐δ on HIF‐1α and VEGF is unlikely to be restricted in specific cell types. However, depletion of PKC‐δ by siRNA did not alter the mRNA levels of HIF‐1α, implying that PKC‐δ activation seems to regulate the expression of HIF‐1α in the protein levels and its stability in HeLa cells. This result is consistent with previous reports that the expression level of HIF‐1α is mainly regulated by post‐transcriptional levels.( 9 , 10 , 11 , 12 , 13 )
Next, it was determined whether hypoxic activation of PKC‐δ was linked to the biological function of HIF‐1 using endothelial tube formation and scratch–wound assays. Conditioned media from PKC‐δ siRNA attenuated the angiogenic activities of HUVEC. Moreover, the authors observed that HIF‐1α down‐regulation by PKC‐δ‐depletion was restored by PKC‐δ overexpression even under hypoxia. Therefore, the present data strongly suggest that PKC‐δ enhances the angiogenic activities of endothelial cells by mediating the generation of angiogenic factors such as VEGF from cells exposed to hypoxia.
In summary, the authors have shown that PKC‐δ plays a role in the enhancement of HIF‐1α stability under hypoxia. Moreover, the present findings demonstrate a novel function of PKC‐δ that acts as a possible regulator of hypoxia‐induced angiogenesis. Additionally, further study to elucidate the mechanisms by which PKC‐δ promotes HIF‐1 stability and angiogenesis is currently underway.
Acknowledgments
This work was supported by the Creative Research Initiatives (Neurovascular Coordination Research Center) of MOST/KOSEF, Korea. We thank Dr Y. Fujii‐Kuriyama for the gift of pSV40pro‐EpoHRE‐Luc vector and Dr Yuspa for the gifts of pMTH‐PKC‐δ and pMTH‐PKC‐δ‐DN vectors.
References
- 1. Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia‐inducible factor 1. Annu Rev Cell Dev Biol 1999; 15: 551–78. [DOI] [PubMed] [Google Scholar]
- 2. Jaakkola P, Mole DR, Tian YM et al . Targeting of HIF‐alpha to the von Hippel‐Lindau ubiquitylation complex by O2‐regulated prolyl hydroxylation. Science 2001; 292: 468–72. [DOI] [PubMed] [Google Scholar]
- 3. Ivan M, Kondo K, Yang H et al . HIFalpha targeted for VHL‐mediated destruction by proline hydroxylation: implications for O2 sensing. Science 2001; 292: 464–8. [DOI] [PubMed] [Google Scholar]
- 4. Masson N, Willam C, Maxwell PH, Pugh CW, Ratcliffe PJ. Independent function of two destruction domains in hypoxia‐inducible factor‐alpha chains activated by prolyl hydroxylation. Embo J 2001; 20: 5197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maxwell PH, Dachs GU, Gleadle JM et al . Hypoxia‐inducible factor‐1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth. Proc Natl Acad Sci USA 1997; 94: 8104–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Semenza GL. HIF‐1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med 2002; 8: S62–7. [DOI] [PubMed] [Google Scholar]
- 7. Kim KR, Moon HE, Kim KW. Hypoxia‐induced angiogenesis in human hepatocellular carcinoma. J Mol Med 2002; 80: 703–14. [DOI] [PubMed] [Google Scholar]
- 8. Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia‐inducible factor 1 is a basic‐helix‐loop‐helix‐PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 1995; 92: 5510–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berra E, Benizri E, Ginouves A, Volmat V, Roux D, Pouyssegur J. HIF prolyl‐hydroxylase 2 is the key oxygen sensor setting low steady‐state levels of HIF‐1alpha in normoxia. Embo J 2003; 22: 4082–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Min JH, Yang H, Ivan M, Gertler F, Kaelin WG Jr, Pavletich NP. Structure of an HIF‐1alpha ‐pVHL complex: hydroxyproline recognition in signaling. Science 2002; 296: 1886–9. [DOI] [PubMed] [Google Scholar]
- 11. Minet E, Mottet D, Michel G et al . Hypoxia‐induced activation of HIF‐1: role of HIF‐1alpha‐Hsp90 interaction. FEBS Lett 1999; 460: 251–6. [DOI] [PubMed] [Google Scholar]
- 12. Hon WC, Wilson MI, Harlos K et al . Structural basis for the recognition of hydroxyproline in HIF‐1 alpha by pVHL. Nature 2002; 417: 975–8. [DOI] [PubMed] [Google Scholar]
- 13. Jeong JW, Bae MK, Ahn MY et al . Regulation and destabilization of HIF‐1alpha by ARD1‐mediated acetylation. Cell 2002; 111: 709–20. [DOI] [PubMed] [Google Scholar]
- 14. Ohh M, Park CW, Ivan M et al . Ubiquitination of hypoxia‐inducible factor requires direct binding to the beta‐domain of the von Hippel‐Lindau protein. Nat Cell Biol 2000; 2: 423–7. [DOI] [PubMed] [Google Scholar]
- 15. Lando D, Gorman JJ, Whitelaw ML, Peet DJ. Oxygen‐dependent regulation of hypoxia‐inducible factors by prolyl and asparaginyl hydroxylation. Eur J Biochem 2003; 270: 781–90. [DOI] [PubMed] [Google Scholar]
- 16. Poole AW, Pula G, Hers I, Crosby D, Jones ML. PKC‐interacting proteins: from function to pharmacology. Trends Pharmacol Sci 2004; 25: 528–35. [DOI] [PubMed] [Google Scholar]
- 17. Baek SH, Lee UY, Park EM, Han MY, Lee YS, Park YM. Role of protein kinase Cdelta in transmitting hypoxia signal to HSF and HIF‐1. J Cell Physiol 2001; 188: 223–35. [DOI] [PubMed] [Google Scholar]
- 18. Li L, Lorenzo PS, Bogi K, Blumberg PM, Yuspa SH. Protein kinase Cdelta targets mitochondria, alters mitochondrial membrane potential, and induces apoptosis in normal and neoplastic keratinocytes when overexpressed by an adenoviral vector. Mol Cell Biol 1999; 19: 8547–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schindl M, Schoppmann SF, Samonigg H et al . Overexpression of hypoxia‐inducible factor 1alpha is associated with an unfavorable prognosis in lymph node‐positive breast cancer. Clin Cancer Res 2002; 8: 1831–7. [PubMed] [Google Scholar]
- 20. Burri P, Djonov V, Aebersold DM et al . Significant correlation of hypoxia‐inducible factor‐1alpha with treatment outcome in cervical cancer treated with radical radiotherapy. Int J Radiat Oncol Biol Phys 2003; 56: 494–501. [DOI] [PubMed] [Google Scholar]
- 21. Takahashi R, Tanaka S, Hiyama T et al . Hypoxia‐inducible factor‐1alpha expression and angiogenesis in gastrointestinal stromal tumor of the stomach. Oncol Rep 2003; 10: 797–802. [PubMed] [Google Scholar]
- 22. Mazure NM, Chen EY, Laderoute KR, Giaccia AJ. Induction of vascular endothelial growth factor by hypoxia is modulated by a phosphatidylinositol 3‐kinase/Akt signaling pathway in Ha‐ras‐transformed cells through a hypoxia inducible factor‐1 transcriptional element. Blood 1997; 90: 3322–31. [PubMed] [Google Scholar]
- 23. Unruh A, Ressel A, Mohamed HG et al . The hypoxia‐inducible factor‐1 alpha is a negative factor for tumor therapy. Oncogene 2003; 22: 3213–20. [DOI] [PubMed] [Google Scholar]
- 24. Aebersold DM, Burri P, Beer KT et al . Expression of hypoxia‐inducible factor‐1alpha: a novel predictive and prognostic parameter in the radiotherapy of oropharyngeal cancer. Cancer Res 2001; 61: 2911–16. [PubMed] [Google Scholar]
- 25. Gschwendt M. Protein kinase C delta. Eur J Biochem 1999; 259: 555–64. [DOI] [PubMed] [Google Scholar]
- 26. Shirai Y, Saito N. Activation mechanisms of protein kinase C: maturation, catalytic activation, and targeting. J Biochem (Tokyo) 2002; 132: 663–8. [DOI] [PubMed] [Google Scholar]
- 27. Gliki G, Wheeler‐Jones C, Zachary I. Vascular endothelial growth factor induces protein kinase C (PKC) ‐dependent Akt/PKB activation and phosphatidylinositol 3′‐kinase‐mediates PKC delta phosphorylation: role of PKC in angiogenesis. Cell Biol Int 2002; 26: 751–9. [DOI] [PubMed] [Google Scholar]
- 28. Wellner M, Maasch C, Kupprion C, Lindschau C, Luft FC, Haller H. The proliferative effect of vascular endothelial growth factor requires protein kinase C‐alpha and protein kinase C‐zeta. Arterioscler Thromb Vasc Biol 1999; 19: 178–85. [DOI] [PubMed] [Google Scholar]
- 29. Kim MJ, Moon CH, Kim MY et al . Role of PKC‐delta during hypoxia in heart‐derived H9c2 cells. Jpn J Physiol 2004; 54: 405–14. [DOI] [PubMed] [Google Scholar]
- 30. Rybin VO, Sabri A, Short J, Braz JC, Molkentin JD, Steinberg SF. Cross‐regulation of novel protein kinase C (PKC) isoform function in cardiomyocytes. Role of PKC epsilon in activation loop phosphorylations and PKC delta in hydrophobic motif phosphorylations. J Biol Chem 2003; 278: 14555–64. [DOI] [PubMed] [Google Scholar]
- 31. Rahman A, Anwar KN, Uddin S et al . Protein kinase C‐delta regulates thrombin‐induced ICAM‐1 gene expression in endothelial cells via activation of p38 mitogen‐activated protein kinase. Mol Cell Biol 2001; 21: 5554–65. [DOI] [PMC free article] [PubMed] [Google Scholar]


