Two frameworks inform assessing the complexity of children’s risk and resilience after prematurity. The first is the International Classification of Functioning, Disability and Health for Children and Youth (ICF-CY).1a The ICF-CY is derived from, and compatible with, the International Classification of Functioning, Disability and Health (ICF).1b The components of the ICF in the context of health include: body function and body structure impairments; activity and activity limitations; participation and participation restrictions; and environmental factors. Environment makes up the physical, social and attitudinal environments in which children and adolescent live and conduct their lives. This framework goes behind dichotomous classification of impairments (e.g. Cerebral Palsy (CP), Yes or No; Intellectual Disability, Yes or No) and instead describes a spectrum of functioning at body structure and body function levels. For example: activities in whole-person tasks like running, reading, and dancing, and participation in roles with peers like being on a team, participating in church, temple or mosque, meeting friends for a movie. This model is illustrated in Figure 1 for a child who was born late preterm.
Fig. 1.
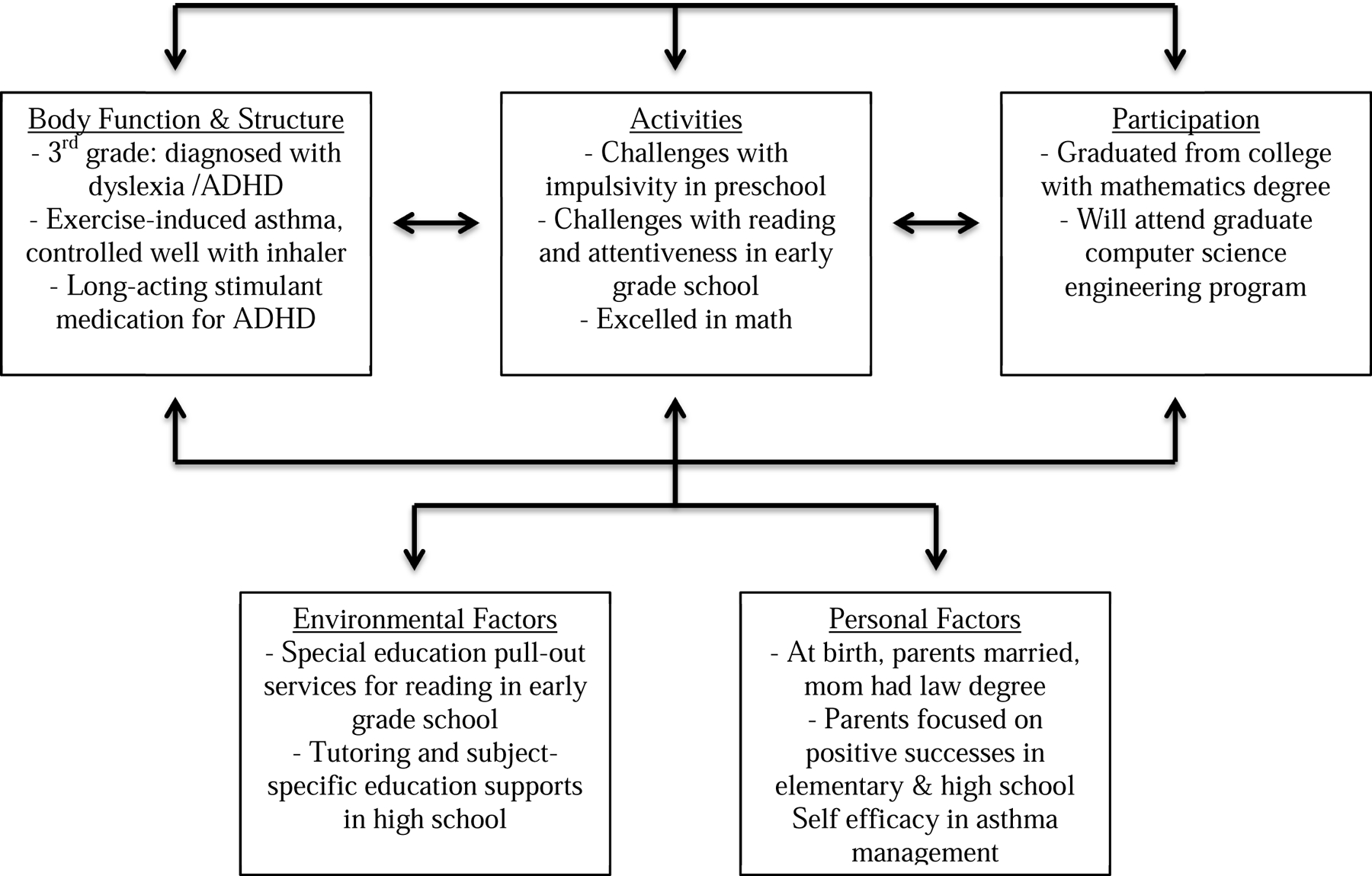
Case 1: 34 weeks gestational age, maternal preeclampsia and stressors: resilience at age 22 years
Historically, functional measures in childhood included basic daily skills of feeding, dressing, toileting, and bathing. However, adaptive behaviors in daily living also include conceptual skills (literacy, numeracy, keyboarding and written language), social skills (self direction, maintaining relationships) and community living skills (household chores, cooking, shopping, using transportation and employment). These composite adaptive outcome skills impact on both becoming an independent adult and participation in community life and are illustrated in Figure 2 for a child who survived extreme prematurity.
Fig. 2.
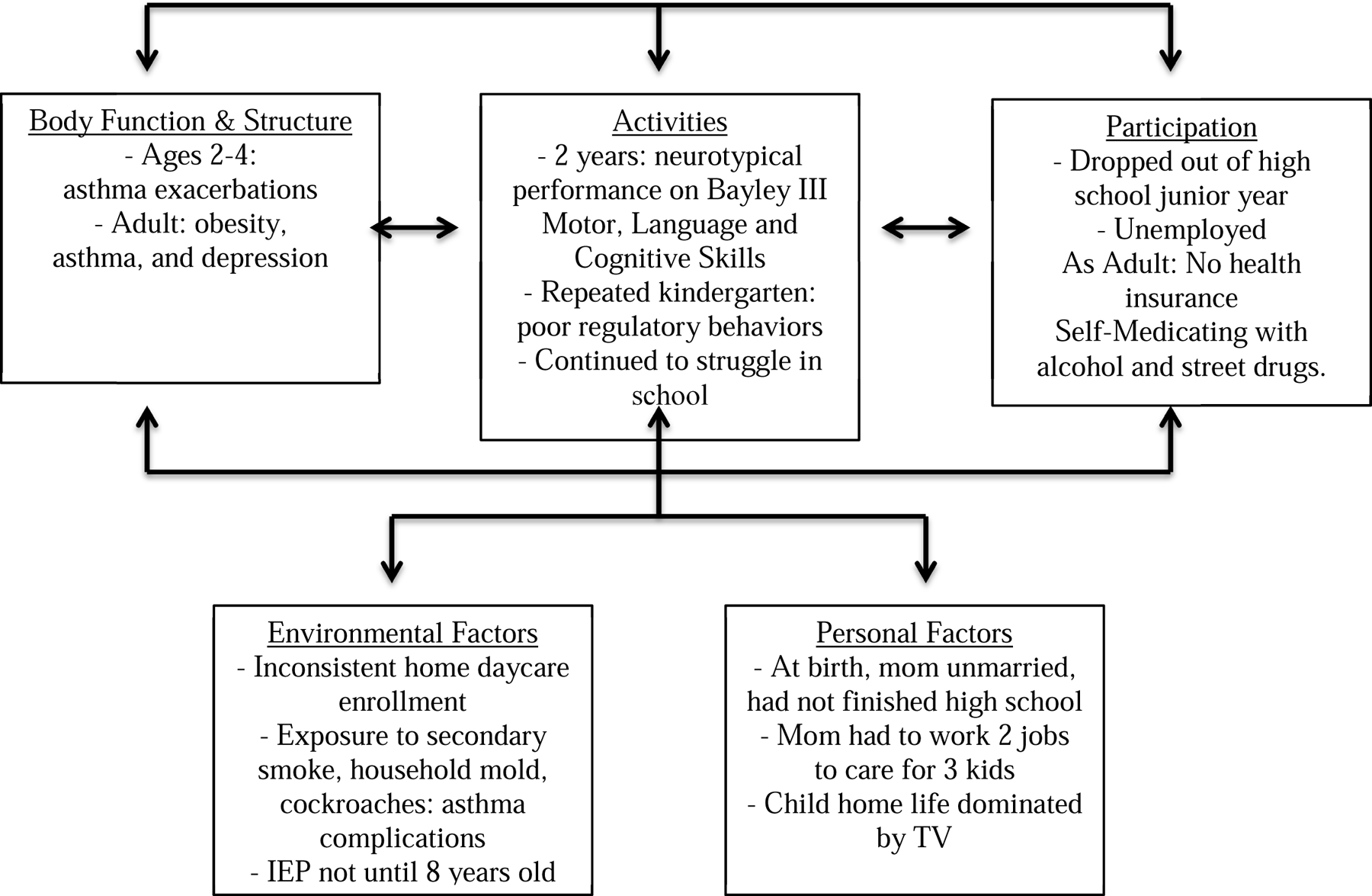
Case 2: 28 weeks gestational age, cumulative adversity, adult jeopardy at 25 years
The second framework, the Life Course Health Development Model (LCHD), holds thftableat the trajectories of children are influenced by the dynamic interactions of multiple risks, protective factors, and promoting factors, especially during sensitive periods of health development.2a From the standpoint of premature infants, due to critical human brain development in the second and third trimesters, this must consider complex maternal, placental, and fetal dynamic interactions. Likewise, infant, toddler, and childhood periods of development are indelibly influenced by multilevel, multidirectional, transactional, and long-lasting interactions, and critically emphasize the importance of timing. Using a LCHD framework to analyze the origins and impact of prematurity and the opportunity to optimize health development outcomes suggests the following considerations for assessing outcomes across the lifecourse2b:
Children who are born prematurely are assumed to be more developmentally vulnerable and are potentially more sensitive to a wide range and nested array of dynamic interacting influences.
Because alterations in evolutionarily-presumed, developmentally-determined adaptive mechanisms are well documented, lags in developmental processes as well as catch-up and feed-forward processes that are specific to premature infants may influence the nature and dynamics of their health and developmental trajectories.
Understanding how the caregiving environments of premature infants interact with emerging developmental capacities, and how different types of exposures, levels of supports, and adversity influence these emergent developmental trajectories is important if specific and targeted interventions are to be designed to modify developmental pathways based on specific risk profiles. The goal is to shift the health and developmental curves for the entire population of premature infants toward thriving and enablement.
In order to implement a broader approach to improve the health and developmental outcomes of diverse preterm populations, it is important to determine what is known about the special developmental vulnerabilities of premature infants; how these vulnerabilities manifest (timing, context, specific risks); and whether the mechanisms involved are phase or period specific, modifiable or one of cumulative risk.
We illustrate our models with several tables, two cases and two figures.
Case 1 ( Fig 1): James was born late preterm at 34 weeks of gestation due to preeclampsia. His parents were married and mother completed law school. She reported significant job-related stressors during pregnancy. The immediate newborn period was complicated by immature lungs leading to respiratory distress syndrome, however ultimately James was discharged at 38 weeks gestational age without a need for oxygen in the home. He was enrolled in full-day daycare and preschool since the age of 2 years. James was found to have some challenges with impulsivity in preschool, which parents addressed with occupational and behavioral therapies. He entered kindergarten without an Individualized Education Program, and in early grade school was found to struggle with reading and inattentiveness. He received a formal diagnosis of a specific language learning disability (dyslexia) and attention deficit-hyperactivity disorder (ADHD) in 3rd grade. James received special education pull-out services for reading and language arts and was starting on a long-acting stimulant medication for ADHD with the guidance of a Developmental and Behavioral Pediatrician. James received tutoring and subject-specific special education supports throughout high school. His parents focused on his positive successes, such as his strong performance in mathematics. James went to college and pursued a math and engineering program. His adult health is complicated by exercise-induced asthma well controlled on an inhaler , long acting stimulant medications, periodic counseling with a psychologist for management strategies for executive dysfunction and implementing mindfulness practices.
Case 2: ( Fig 2) Michael was born at 28 weeks of gestation due to preterm labor after premature rupture of membranes. His mother was unmarried and did not finish high school. His NICU course was complicated by intubation for the first 4 weeks of life, but he did not experience additional medical complications and was able to be slowly transitioned to room air and oral feedings. At 2 years of age he tested within age appropriate range on the Bayley Scales of Infant and Toddler Development, 3rd Edition. Between ages 2 and 4, he experienced asthma exacerbations complicated by environmental exposures to secondary tobacco smoke, household mold, and cockroach infestations and was hospitalized on average 2–3 times a year. Until the age of 5 years, Michael was enrolled in inconsistent home daycare programs and did not receive early intervention services because he was considered less than 30% delayed and thereby was deemed not eligible for services. He enrolled in kindergarten at age 5 years. However, he had to repeat kindergarten due to poor regulatory behaviors, impulsivity and lack of familiarity with letters and numbers, which interfered with learning. Throughout his early elementary years, he continued to struggle in school. His home life was largely dominated by television watching while his mom worked two jobs to help care for him and his 2 siblings. In school he received an Individual Education Plan at age 8 years, but did not access pharmacotherapies for ADHD. He dropped out of high school in his junior year. His adult health is complicated by obesity, asthma, and depression. Because of unemployment, he does not have health insurance. His state has not expanded Medicaid access.
Changing Survival Rates, Morbidities, and Early Neurodevelopmental Disorders among Extremely Preterm Infants
Tables 1 and 2 explore the overall outcomes by gestational age stratum and early neurodevelopmental disabilities of children who survive extremely preterm birth. These tables both highlight the increased survival of extreme prematurity as well as the spectrum of early and lifecourse challenges impacting on health, education, behavior. Ancel and colleauges examined survival and neonatal morbidities of infants born at 22–34 weeks’ gestation using two waves of the EPIPAGE cohorts born in 1997 and 2011 respectively.3 The major outcomes included survival to discharge and survival without neonatal morbidities associated with high risk for adverse developmental outcomes. The latter included grade 3 or 4 intraventricular hemorrhage, cystic periventricular leukomalacia, severe bronchopulmonary dysplasia, stage 3 or higher retinopathy of prematurity, or stage 2 or stage 3 necrotizing enterocolitis. In the 2011 cohort, <1%(0.7%) of infants born prior to 24 weeks, 31% at 24 weeks, 59% at 25 weeks, 75% at 26 weeks, 94% at 27–31 weeks, and 99% at 32–34 weeks survived to discharge. Between the 1997 and 2011 samples, the portion of infants surviving without severe morbidity increased by 14.4% at 25–29 weeks of gestation and by 6% at 30–31 weeks. The proportion did not significantly change for infants born at less than 25 weeks of gestation. Furthermore, rates of antenatal corticosteroid use, induced preterm deliveries, cesarean deliveries, and surfactant use significantly increased, except for at gestational age 22–23 weeks. Overall, the significant improvement in survival to discharge for infants born at 25–31 weeks of gestation was paired with a decrease in severe morbidities.
Table 1:
| Weeks of Gestation | Children with Special Health Care Needs | Major Neurodevelopmental Disability | Educational Supports | Behavioral Disorders |
|---|---|---|---|---|
| 22 | 80% | 70% (52-100%) | 75% | NK |
| 23-24 | 80% | 50-60% | 60-70% | NK |
| 25-26 | 60% | 56% | 50-67% | NK |
| 27-28 | 50% | 20% | 50% | 20% |
| 29-31 | 40% | 15% | 40% | 15% |
| 32-36 | 30% | 10% | 25% | 10% |
| 37+ | 20% | 5% | 15% | 5% |
Table 2:
Major Neurodevelopmental Disabilities at age 2 years among ELBW survivors in the 1990s and EPT survivors in 2005–2015
| Study | Sample | Cerebral Palsy (%) | Developmental Disability (%) | Hearing Impairment (%) | Vision Impairment (%) |
|---|---|---|---|---|---|
| Adams-Chapanis 2011-2015, USA | Epoch 4 | ||||
| 22-26 wks GA | |||||
| N=2013 | |||||
| 22 wks, n=17 | 41 | 59 | 0 | 0 | |
| 23 wks, n=161 | 22 | 42 | 6 | 2 | |
| 24 wks, n=486 | 14 | 32 | 4 | 1 | |
| 25 wks, n=657 | 8 | 25 | 3 | 2 | |
| 26 wks, n=792 | 5 | 24 | 2 | 0 | |
| Younge et al. 2017, USA | 22-24 wk GA | ||||
| Epoch 1 (2000-2003): N=424 | 15 | 47 | 4 | 2 | |
| Epoch 2 (2004-2007): N=459 | 11 | 45 | 4 | 2 | |
| Epoch 3 (2008-2011): N=487 | 11 | 41 | 3 | 0.4 | |
| Synees 2009-2011, Canada | N=2340 | 6.4 | 21.8 | 2.6 | 1.6 |
| Schmidt et al. 1996-1998, Canada and U.S. | N=944 500-999 g |
12 | 27 | 2 | 2 |
| Vohr et al. 1993-1998, USA | N=2291 22-26 wk |
19 | 30 | 2 | 2 |
| N=1494 27-32 wk GA |
11.6 | 26 | 1 | 0.7 | |
| Moore et al. 2006-2012, U.K. | N=325 | 23 | 38 | 7 | 3 |
| Wood et al. 1995, U.K. | N=235 22-25 wk GA |
25 | 31 | 4 | 6 |
| Doyle et al. 2005, Australia | N=172, 22-27 wk GA (EP) |
9.8 | 47.9 | 4 | 0 |
| N=220, FT | 0 | 20.3 | 0.5 | 0 | |
| Doyle et al. 1997, Australia | N=170 500-999 g |
11 | 22 | 1.8 | 2.4 |
| Pierrat et al. 2017, France | EPIPAGE-2 N=5170 | ||||
| 24-26 wk GA | 6.9 | 34 (V*), 13-25 (NV*) | 1.4 | 0.7 | |
| 27-31 wk GA | 4.3 | 24 (V), 11-16 (NV) | 0.6 | 0.3 | |
| 32-34 wk GA | 1 | 18 (V), 11-13 (NV) | 0.5 | 0.2 | |
| Ishil et al. 2013, Japan | 22 wk GA 23 wk GA 24 wk GA 25 wk GA |
22 18 8 15 |
52 57 37 34 |
0 3.4 1.2 1.3 |
8.7 10.2 3.4 2.2 |
| Serenius 2004-2007, Sweden | N=456; 22-26 6/7 wks GA | 7 | 35.3 | 0.9 | 3.7 |
Developmental disability as defined as Bayley-II Mental Developmental Index<70 or Bayley-III Cog SS <85; GA= Gestational Age; V = Verbal, NV = Nonverbal (problem solving and personal social)
Washburn and colleagues examined infants born between 23 and 27 weeks of gestation with no congenital malformations.4a enrolled from a 13-county region in North Carolina . 4a Outcomes at age one year between the two birth periods 1990–1995 and 1995–2000 were compared. The percentage of live births was 67% in the first epoch and 71% in the second. Major neurodevelopmental impairment—defined as CP, Bayley II Scales Mental Development Index more than 2 standard deviations (SD) below the mean, severe hearing loss or blindness—were present in 20% of survivors in the first period and 14% of survivors in the second period. Amer and colleagues compared the mortality and neurodevelopmental outcomes of infants born at <29 weeks of gestation who were outborn versus those who were inborn in the Canadian Neonatal Network and Canadian Neonatal Follow-up Network databases. 4bCanada has a centralized perinatal care system whose purpose is to improve care of both mothers and neonates during the perinatal period by providing access to regional tertiary care centers for mothers with threatened labor prior to 30–32 weeks of gestation. However, 15–20% of these children are still not born in these specialized perinatal centers. Outborn infants had higher mean birth weight (940 ± 278 g vs 897 ± 237 g), lower rates of antenatal steroid treatment (54% vs 93%), lower rates of small for gestational age (SGA) status (5.3% vs 9.4%), and lower rates of maternal college education (44% vs 54%) than inborn infants. The median 5-minute Apgar score and Score for Neonatal Acute Physiology-II were higher for inborn infants. Furthermore, outborn infants had higher odds of death or severe neurodevelopmental impairments attributed to higher rates of sonographic parenchymal brain injury
Berry and colleagues compared short-term mortality and major morbidities of infants born at 23 or 24 weeks of gestation in New Zealand.5 At the time of discharge, there was parenchymal brain injury in 13% of surviving 23 week infants and in 3% of surviving 24 week infants. The survival rate at 2-year follow-up was 58% for infants born at 23 weeks and 60% for infants born at 24 weeks. There was no difference in rates of disability at 2 years corrected age. The authors argue that, with maximal perinatal care in a tertiary setting, it is possible to have comparable 2-year rates of survival with no significant brain structural injury or severe disability in infants born at 23 or 24 weeks gestation.
Thus, these series of international studies highlight a changing picture of survival at the extremes of viability. Survival and outcomes after survival are strongly influenced by gestational age at birth, inborn versus outborn status, and era in which the child is born.
Academic performance and learning disorders
It has been known for two decades that children born very preterm (VPT; < 32 weeks of gestation) or with very low birth weight (VLBW; <1500 grams) have higher rates of neurosensory abnormalities as well as behavioral and socialization difficulties, and lower performance on cognitive, language, and motor skill assessments than their normal birth weight term (NBWT) peers.2b Furthermore, over the past decade there is evidence that these problems tend to be more severe and more common in children born extremely preterm (EPT; <28 weeks of gestation) or with extremely low birth weight (ELBW; <1000 grams) and that executive function, attention, and specific learning disorders are part of these difficulties. Past studies of educational outcomes in high risk neonates predominantly focused on VLBW/VPT in the surfactant era. However, investigators in Australia, the United Kingdom and Scandinavia have followed regional cohorts of extremely preterm cohorts in the past decade. These cohorts have benefitted from full term controls and a commitment to collaborate with educational, disability, and community systems of care. The following portion of this review explores several important studies that have provided insight into the cognitive, academic, and behavioral outcomes of children, adolescents, and adults who were born EPT or with ELBW.
Academic achievement and school performance problems tend to become clear very soon after starting school. They often manifest as grade repetition, a need for special education assistance, parent and teacher reports of poor school performance, and low scoring on tests of reading, spelling, writing, and mathematics skills. There is evidence that difficulties related to academic performance are more common among preterm or low birth weight survivors than health disorders like asthma or epilepsy or more global development problems such as significant intellectual disability (defined as IQ <55 with concurrent adaptive behavior challenges). This is possibly due to a large number of children with mild cognitive impairments (standard scores 1–2 standard deviations (SD) below the mean) and specific neuropsychological weaknesses. These early childhood school performance difficulties are significant because they are often predictive of later adverse educational outcomes, including long-term learning, skills competing with peers academically, and pursuing higher education after high school completion. Behavioral problems including impulsivity, inattention, anxiety, and depression too often compromise extracurricular, social, vocational and community success.
When compared to NBWT children, VLBW/VPT children are more likely to repeat grades, to require special education assistance, and to be rated by their teachers as having school performance weaknesses. One multinational review based on cohorts from New Jersey, Ontario, Bavaria, and Holland found that these suboptimal educational outcomes were consistent for ELBW children across sites despite differences in education policy.6 Another study found that rates of qualification for special education programs were 38% and 11% for ELBW and NBWT children, respectively, and another found that their rates of grade retention differed as well (20% vs. 7%).7,8 These and similar studies have revealed a “gradient” between degree of low birth weight/preterm status and educational outcomes. Klebanov et al. examined the rates of grade failure and/or special education placements among 9 year olds distributed across birth weights.9 There was a clear graded relationship with birth weight status categorized as <1000 grams, 1001–1500 grams, 1501–2500 grams, and >2500 grams (normal birth weight). Rates of grade failure were 37%, 26%, 27%, and 14%, and rates of special education enrollment were 12%, 6%, 6%, and 4% across these birth weight categories. A Cleveland study found rates of grade retention were 30% for a group with birth weight <750 grams, 13% for 750–1499 grams, and 8% for term controls, and that rates of special education were 50%, 27%, and 8% for the same groups, respectively.10 One study also found that VLBW children had higher rates of placement in nearly all special education programs than children with birth weight 3000–4749 grams, with the exception of programs for the “emotionally handicapped,” and that this difference was most pronounced in programming for more severe and multiple disabling conditions and less pronounced in programming for conditions that allowed for academic competitiveness.11
Many studies have found that VLBW/VPT and ELBW/EPT children perform worse than NBWT children on reading, spelling, writing, mathematics, and handwriting tests. In addition, ELBW/EPT children perform less well than VLBW/VPT children, and the sizes of these group differences range from moderate to large across studies. This pattern of results also holds when excluding children with global intellectual or neurosensory disability.
Among VLBW/VPT-born young adults, educational outcomes vary, with some studies reporting that there are no differences between groups on attained education levels, and other studies reporting that high school graduation rates, college enrollment rates, and performance on word recognition and mathematics tests are higher among NBWT individuals than among VLBW/VPT individuals. This wide variability in educational outcomes, both within and between samples, which can be attributed to biological risks (e.g., neonatal medical complications, postnatal neurosensory impairments, and subnormal head circumference) and to social risk factors (e.g., low socio-economic status, disadvantaged family environments, low parental educational achievement). Modifying factors that can impact the effect of these risks include quality early developmental interventions, family and teacher supports, prenatal drugs or alcohol exposure, and family history of learning difficulties.
Garfield and colleagues used state academic testing from 1.3 million Florida children to examine how gestational age and kindergarten readiness impact on academic achievement trajectories.12 Children were born between 23 and 41 weeks of gestation and attended public school. Overall, kindergarten readiness performance, standardized academic achievement test scores, and gifted status were positively related to higher gestational ages, while low kindergarten and academic performance was inversely related to gestational age. It should be noted that standardized test data was unavailable for 16% of the sample, and the kindergarten readiness measure was unavailable in 56% of the sample. Among children surviving 23–24 weeks gestation, 2 in 3 were kindergarten ready, compared to 85% of children born at term. Children born at 23–24 weeks of gestation scored 0.66 SD lower than those born at full term. While 9.5% of all students were considered gifted, this included only 1.8% of those born at 23–24 weeks of gestation. 5.8% of students were low performing, and 33.5% of these children had been born at 23–24 weeks of gestation. The Florida population data indicate that a large majority of children (65%) born near the limits of viability performed within expected school norms and that further investigation is needed to examine how and why some children are able to demonstrate resiliency after preterm birth.
Serenius and colleagues examined the neurodevelopmental outcomes of a national cohort of Swedish children born extremely preterm (<27 weeks of gestation) and compared them to matched term-born controls.13 Neurodevelopmental outcomes at age 6.5 years included intellectual disability measured by Weschler intelligence batteries, the diagnoses of CP, hearing impairment requiring amplification and visual disability impairment. Overall 441 extremely preterm children and 371 controls were assessed. The EPT children’s adjusted mean IQ was 14.2 points lower than that of the term controls. 18.8% and 11.1% of extremely preterm children had moderate and severe cognitive disability, respectively, compared to only 2.2% and 0.3% of controls. CP, blindness, and hearing impairment were observed in 9.5%, 2.0%, and 2.1% of extremely preterm children, respectively, and in 0.0%, 0.0%, and 0.5% of controls. Overall, for extremely preterm children, 30% experience mild, 20% moderate and 13% severe disability. Among extremely preterm children, moderate or severe disability decreased with increasing gestational age. Importantly, among children assessed at both 2.5 and 6.5 years, rates of disability increased from 1 in 4 to 1 in 3.
Heeren and colleagues examined the nature and prevalence of cognitive functional limitations in US children born extremely preterm using latent profile analysis. This statistical technique allows for the identification of subgroups of extremely preterm children with similar IQ and executive functioning profiles.14 Four different neurocognitive profiles emerged in these ELGAN survivors: normal (34%), low-normal (41%), moderately impaired (17%), and severely impaired (8%). Children in the “low-normal” group showed impaired inhibition compared to their reasoning and working memory functional abilities, while children in “impaired” groups demonstrated global limitations across cognitive and executive function domains. The most preterm (23–24 weeks of gestation) survivors were the least likely to have a normal profile and the most likely to have a severely impaired profile; children in the moderately or severely impaired groups tended to perform worse on academic achievement measures of math and literacy; and children who had poorer cognitive function scores (based on IQ and executive functioning) tended to require more special education resources. Importantly, after categorizing the extremely preterm children by IQ, there were still significant variations in executive functioning within each category. These results highlight that among extremely preterm survivors in the modern era of neonatology, both IQ and executive functioning assessments are necessary for describing outcomes at school age. It is for this reason that we highlight middle childhood behavioral health impairments that impact on academic and social outcomes in Table 3 and cognitive, executive function and academic achievement outcomes in adolescence and adulthood in Table 4.
Table 3.
Middle Childhood Behavioral Health Outcomes after Extreme Prematurity
| Study | Years of inception of birth cohort | ELBW/EP NBW (n tested) | Age at assessment (years) | Mental health outcomes eligible for meta-analysis | Estimates of effect SMD (g) and SE (g) |
|---|---|---|---|---|---|
| Szatmari et al., 1990, Canada | 1980-1982 | 82 ELBW 208 NBW |
5.0 5.0 |
P: Attention problems P: Emotion problems P: Conduct disorder T: Attention problems T: Emotion problems T: Conduct disorder |
0.94 (0.73) 0.01 (0.60) 0.87 (1.22) 0.41 (0.52) 0.06 (0.60) −0.39 (1.07) |
| Szatmari et al., 1993, Canada | 1977-1981 | 129 ELBW 145 NBW |
7.8 (0.4) 8.1 (0.5) |
P: Attention problems P: Emotion problems P: Conduct disorder T: Attention problems T: Emotion problems T: Conduct disorder |
1.50 (1.07) 0.69 (0.66) 0.69 (1.19) 0.37 (0.48) −0.36 (0.61) −0.78 (0.83) |
| Taylor et al., 2000, U.S. | 1982-1986 | 60 ELBW 49 NBW |
6.7 (0.9) 7.0 (1.0) |
C: Self-esteem P: Attention problems P: Behavior competence P: Hyperactivity T: Attention problems T: Social skills rating T: Hyperactivity |
−0.19 (0.13) 0.76 (0.20) −0.47 (0.20) 0.58 (0.20) 48 (0.20) −0.14 (0.28) 0.25 (0.20) |
| Akshoomoff et al., 2017, U.S. | 2002-2004 | 668 <28 wks GA | 10 |
Working Memory |
Mean (SD) RD: 91.4 (10.2) MD: 88.2 (8.9) MD/RD:84.5(11.5) NoLD: 98.1 (12.3) |
| Attention | RD: 8.4 (3.0) MD: 8.0 (2.7) MD/RD: 7.4 (2.8) No LD: 9.3 (2.5) |
||||
| Inhibition | RD: 6.6 (2.8) MD: 6.8 (2.7) MD/RD:5.7 (2.5) No LD: 8.3 (2.9) |
||||
| Visual Perception | RD: 8.0 (2.4) MD: 7.1 (2.3) MD/RD:7.0 (2.5) No LD: 9.1 (2.5) |
||||
| Visuomotor Precision | RD: 7.5 (3.3) MD: 7.6 (3.1) MD/RD:6.8 (3.7) No LD: 8.5 (3.4) |
||||
| Hirschberger et al., 2018, U.S. | 2002-2004 | 873 <28 wks GA | 10 | Cognitive/Executive Function Class (CFC 1-4) Cerebral Palsy;ASD;Epilepsy |
#impairments (CFC3-4,CP, ASD, Epilepsy) 23-24 wksGA: 0: 48% 1: 26% 2: 21% 3: 5% 4: 0% 25-26 wksGA: 0: 68% 1: 21% 2: 10% 3: 2% 4: 0.25% 27 wks GA: 0: 79% 1: 13% 2: 4% 3: 3% 4: 0.3% |
| Burnett et al., 2017, Australia | 1991-1992, 1997, 2005 | 613 EP/ELBW 564 controls |
7-8 | P: Disability status(blindness, deafness, CP, delayed development) at 2 Yand 8Y BRI (inhibit, shift, emotional control) MI (initiate, working memory, plan/organize, organization of materials, monitor) GEC (BRI and MI);WM (working memory) |
% Elevated Scores 1991-1992 EP/ELBW vs FT GEC: 13 vs 8 BRI: 13 vs 8 MI: 15 vs 7 WM: 20 vs 7 1997 EP/ELBW vs C GEC: 96 vs 6 BRI: 13 vs 7 MI: 10 vs 5 WM: 15 vs 7 2005 EP/ELBW vs C GEC: 27 vs 11 BRI: 22 vs 9 MI: 29 vs 8 WM: 37 vs 9 |
| Leviton et al., 2018, U.S. | 2002-2004 | 716 <28 wks GA | 10 | C: IQ (verbal, nonverbal) Working Memory Inhibition Inhibition in Switching Executive Dysfunction(ED) |
% Z-scores ≤-1 WM: 24% Inhibition: 49% Switching: 50% All 3: 15% ED Risk Profiles 1. Low SES = 2. Prematurity = 3. inflammatory biomarkers Cog Limitation risks: FGR |
LD: learning disability (score <16th percentile), RD: reading-only LD, MD: mathematics-only LD, MD/RD: combined mathematics/reading LD; C=child, P=parent, T=teacher FGR= fetal growth restiction
Table 4.
| Authors | Cohort | Assessment Age | Developmental Outcome |
|---|---|---|---|
| Botting, et al. 1997 | 1980-1983 Liverpool, England 138 VLBW and 108 matched controls |
12 years | 1. Any psychiatric disorder: 28% VLBW vs. 9% controls had any psychiatric disorder 2. ADHD: 23% VLBW vs. 6% controls 3. VLBW lower IQ 4. |
| Saigal. et al. 2000 | 1977-1982 Ontario, Canada 141 ELBW 124 matched controls |
12-16 years | 1. 28% reported neurosensory impairments 2. 25% of ELBW vs. 6% repeated a grade 3. 49% of ELBW vs. 10% required special education services 4. 22% ELBW required full-time educational assistance (vs. 0%) 5. Lower mean IQ 6. Lower mean math, reading, and spelling 7. At age 22-25, 1.3% ELBW had ASD |
| Levy-Shiff et al. 1994 | Israel 90 VLBW and 90 NBW |
13-14 years | Significantly increased hyperactive behavior among VLBW, however paternal involvement was as predictive as birth weight for hyperactivity in childhood |
| Dahl, et al. 2006 | 1978-1989 Norway 99 VLBW |
13-18 years | 1. VLBW adolescents report less externalizing behaviors than NBW adolescents. 2. Parents of VLBW adolescents report more externalizing behaviors and emotional problems than NBW adolescents. |
| Rushe, et al. 2001 | 1979-1980 London, England <33 weeks; 75 premature and 53 FT |
14-15 years | 1. No differences for tests of executive function, verbal memory, attention 2. Preterm group had impaired verbal fluency |
| Grunau, et al. 2004 | 1981-1986 British Columbia 79 < 800g vs. 31 term |
17 years | 1. No differences focus and attention 2. Significantly more parental reported internalizing, externalizing, and problem behaviors |
| Lefebvre et al. 2005 | 1976-1981 Montreal, Canada 57 ELBW and 44 NBW |
18 years | 1. 56.1% ELBW vs. 84.6% controls completed HS 2. 33% vs. 9% required special education 3. Significant differences in low IQ (<85) |
| Linsell, et al. 2017 | UK & Ireland Prospective, population-based cohort study 315 <26 wk GA, 160 term controls |
19 years | 1. EP IQ: 85.7, term IQ: 103.9 2. If moderate/severe brain injury, IQ: 78.4 3. IF GA <25 weeks, IQ: 83.1 |
| Hack et al. 2002 | 1977-1979 Cleveland, Ohio 242 VLBW and 233 controls |
20 years | 1. 74% VLBW graduated HS vs. 83% NBW 2. 30% pursued secondary education vs. 53% NBW 3. 40% repeated grade vs. 27% NBW 4. Scored 1/3 SD lower on WAIS-R |
| Lindstrom, et al. 2009 | 1973-1979 Sweden 24-28 weeks (EP) vs. FT |
1987-2002 national registry | 71% EP vs. 78.6% FT completed 12 or more years of school |
| Moster, et al. 2008 | 1976-1983 Norway 325 preterm (23-27 weeks) vs. 828,227 FT |
20-36 years | 1. 67.7% preterm vs. 75.4% completed HS 2. 4.4% preterm vs. 0.1% fullterm with ID |
| Nomura, et al. 2009 Johns Hopkins Collaborative Perinatal Study | 1960-1965 Baltimore 226 near-term and 1393 FT |
27-33 years | 1. Near-term birth associated with lower adult educational attainment only for those living below poverty line 2. SGA had no association with educational attainment |
| Saigal, et al. 2006 | 1977-1982 Ontario, Canada 166 ELBW and 145 controls |
22-25 years | No significant difference in: 1. % graduation from high school 2. Pursuit of post-secondary education 1.3% ELBW had ASD |
| Breeman, et al. 2015 | Bavarian Longitudinal study 260 VP/VLBW |
26 years | 1. VP IQ: 86.2, term IQ: 102.6 2. IQ <70: VP 27%, term 3.9% |
| Laerum, et al. 2016 | 44 VLBW, 63 SGA, and 81 controls | 26 years | 1. Mood disorders: 18% VLBW, 14% SGA, 0% controls 2. Anxiety disorders: 27% VLBW, 20% SGA, 9% controls 3. Employed: 66% VLBW, 64% SGA, 67% controls 4. Receiving disability benefits: 14% VLBW, 3% SGA, 0% controls 5. Completed Bachelors’ degree or higher: 25% VLBW, 40% SGA, 55% controls |
HS= high school; SD= standard deviation; WAIS-R = Wechsler Adult Intelligence Scale-Revised; WISC-R= Wechsler Intelligence Scale for Children-Revised; DQ= deviation quotient; WRAT-R= Wide Range Achievement Test-Revised; FT = full term; VLBW= very low birth weight (<1500 grams); ELBW = extremely low birth weight (<1000 grams); NBW= normal birth Data
Hirschberger and colleagues examined the prevalence of neurodevelopmental impairments in the US ELGAN cohort at age 10 years, in order to create a categorization framework for neurological limitations.15 889 10-year-old children(<28 weeks gestation) were recruited, and cognitive impairment prevalence was assessed using multidomain cognitive assessments as well as observations of executive function, CP, autism spectrum disorder, and epilepsy. Three categories of neurodevelopmental impairment severity were assessed: I: no major neurodevelopmental impairment; II: normal cognitive ability with CP, ASD, and/or epilepsy; III: cognitive impairment(IQ and Executive Functioning). Category I included 68% of children, category II included 8%, and category III included 24%. 1 in 4 children had cognitive disability, 11% had CP, 7% had ASD, and 6% had epilepsy. 19% of participants had one neurodevelopmental disability, 10% had two, and 3% had three. Furthermore, gestational age was inversely associated with number of neurodevelopmental disabilities. Approximately half of children with cognitive disability and one third of children with either CP, ASD, or epilepsy had only one impairment. In terms of resilience, approximately 75% of children were considered to have normal intellect at 10 years of age, and nearly 70% had no neurodevelopmental disability. According to the existing literature, there are certain neuropsychological functions that seem to be especially influenced by preterm birth. There is evidence that very preterm children who have learning difficulties tend to have both decreased IQ and selective cognitive impairments. There is also evidence that, among VPT children, academic achievement is associated with a variety of factors, including low socioeconomic status. Akshoomoff and colleagues examined academic achievement, rates of learning disability (LD), and neuropsychological outcomes at 10 years of age of children born between 23 and 27 weeks of gestation from the US ELGAN study cohort.16 Both grade-based and age-based academic achievement measures were used . Children with IQ ≥2 SD below the mean were excluded. The authors examined the rates of LD in reading and math (defined as standard scores <16th percentile) as well as the neuropsychological test correlates of reading and math LD. Socioeconomic status (as indicated by maternal education) was correlated with academic achievement, IQ, and neuropsychological measures. However, after controlling for socioeconomic status, the sample still had higher than expected rates of LD, especially in mathematics. The risk of low math scores was 27%, 1.5 times greater than the risk of low reading scores (17%). 6.4% of the sample was classified as having low reading achievement only, 16.2% was classified as having low math achievement only, and 8.3% was classified as having low math and reading achievement. All three of these groups exhibited multiple neuropsychological weaknesses when compared with the 69% of the sample with neither low math nor low reading scores. However, the low math group and low reading group differed in their neuropsychological profiles. These data suggest that there are specific cognitive weaknesses that differ between preterm children with low math achievement and low reading achievement.
Joseph and colleagues examined whether or not maternal education, which is widely regarded to be an important marker of socioeconomic status, is associated with neurocognitive and academic outcomes for extremely preterm children.17 Using 873 preterm children from the ELGAN cohort with gestational ages between 23 and 27 weeks, researchers compared the outcomes for children whose mothers had fewer years of education at time of delivery and children whose mothers advanced in education during the 10 years after birth. Adjustments were made for gestational age and potential confounding variables. It was found that children whose mothers were in the lowest educational bracket at their birth were significantly more likely to score at least two SD worse than expected on 17 of the 18 neurocognitive and academic achievement tests given at age 10 years. Children whose mothers advanced in education were at a reduced risk of scoring at least two SD worse than expectation on 15 of the 18 tests. However, this reduced risk was only statistically significant for two tests (inhibitory control and processing speed).
Leviton and colleagues examined the antecedents of learning functional limitations at 10 years in 874 extremely preterm children using multinomial logistic regression analyses in the US ELGAN cohort.18 Variables were entered in a chronological, temporal order with earlier predictors and covariates entered first but not displaced by later covariates. Reading and math functional limitations were defined as scores of one or more SD below the expected average on a reading or math examination. Of the 874 subjects, 56 were classified as reading limited, 132 as math limited, and 89 as having both. The risk profiles included indicators of socioeconomic status (maternal racial identity or eligibility for government-provided health insurance), medical vulnerability (such as a high illness severity score or receiving hydrocortisone for severe bpd), fetal growth restriction and inflammation (such as urinary tract infection during pregnancy) or late ventilator dependence. Overall fetal growth restriction and inflammation were antecedent of reading functional limitations and limitations in both reading and math functioning. Socioeconomic disadvantage and medical vulnerability were antecedents of educational underachievement in math as well as in both reading and math.
Kuban and colleagues reported on 889 children in the Extremely Low Gestational Age Newborns (ELGAN) Cohort who underwent a comprehensive neuropsychological and autism assessment battery at age 10 years.19 Parent reported on health, behavior, development, and seizures . 28% of the males and 21% of the females had moderate/severe cognitive impairment. Furthermore, boys had a higher prevalence of impairment in nearly all measures of cognition, were more likely to have microcephaly (15% versus 8%), and more frequently required assistive devices to walk (6% versus 4%). However, boys and girls were at similar risk for current epilepsy (7% of cohort overall, though 10% had had seizures in the past). The prevalence of autism spectrum disorder was 9% in boys and 5% in girls, reflecting substantially higher risks in ELGAN than the 2% risk in term control boys and 1 % risk in term control girls.
Caffeine citrate therapy for apnea of prematurity is safe and has been known to reduce rates of bronchopulmonary dysplasia, severe retinopathy, and neurodevelopmental disability at 18 months and improve motor function at 5 years. Schmidt and colleagues20 investigated if neonatal caffeine therapy is associated with improved functional outcomes at 11 years. 920 children from the multicenter international randomized, placebo-controlled Caffeine for Apnea of Prematurity trial were assessed in follow-up, 457 VPT/EPT (birth weight 500–1249 grams) had received caffeine, and 463 placebo. The functional outcome was a composite of academic performance (at least 1 score of less than 2 SD below the mean on the Wide Range Achievement Test-4), motor impairment (percentile rank ≤5 on the Movement Assessment Battery for Children-Second Edition), and behavior problems (Total Problem T score ≥2 SD above the mean on the Child Behavior Checklist). The composite rate of functional limitations was not significantly different between the treatment (32%) and placebo (38%) groups. However, the treatment group did have a lower risk of motor impairment (20%) compared to the placebo group (28%). This study demonstrated that neonatal caffeine improved motor functioning, did not adversely impact on long term cognitive and behavioral performance .and was safe.
Luu and colleagues examined 275 children born between 1989 and 1992 with birthweight 600–1250 grams who were enrolled in the New England Indomethacin Intraventricular Hemorrhage (IVH) Prevention Trial.21 Concurrently at 12 years there were an additional 111 term controls. All participants were assessed with neuropsychometric testing and received a neurological exam. Parents provided information about educational needs. The preterm group’s scores were 6–14 points lower than those of the term group on all psychometric tests, after adjustment for socio-demographic factors. On a test of basic language skills, 22–24% of preterm children scored in the abnormal range (<70), compared to 2–4% of term children. Furthermore, preterm children both with and without sonographic brain injury required more school services than full term children (76% and 44% versus 16%) and supports in reading (44% and 28% versus 9%), writing (44% and 20% versus 4%), and mathematics (47% and 30% versus 6%). The preterm group also had more internalizing and externalizing behavioral challenges. The strongest predictors of lower cognitive scores were severe neonatal brain injury and minority status also was associated with worse cognition. Predictors of higher cognitive performance scores included antenatal steroids as well as the social capital of higher maternal education, and 2-parent family status.
Roze and colleagues conducted a prospective cohort study of preterm infants <37 weeks of gestation with periventricular hemorrhagic infarction (PVHI) in order to examine motor, cognitive, and behavioral outcomes at school age.22 Motor outcomes at 4 and 12 years of age were classified using the Gross Motor Function Classification System (GMFCS) and the Manual Ability Classification System (MACS). Participants were assessed for cognition, visual-motor integration, visual perception, and verbal memory and behavior was assessed using the Child Behavior Checklist and the Behavior Rating Inventory of Executive Function. 15 of the original 38 infants died, and 21 of the survivors were included in the follow-up. In this small study, characteristics of the PVHI were not related to functional motor outcomes or intelligence. Post-hemorrhagic ventricular dilatation was a risk factor for lower full scale IQ, performance IQ and fine motor dysfunction. Most of the surviving participants with PVHI had mild CP with the ability to walk (GMFCS<3) or perform manipulative tasks (MACS<3) across home and school activities. . Importantly, intelligence was within 1 SD of the norm of preterm children without lesions in 60–80% of the children. However verbal memory was often impaired, and behavioral and executive functional challenges occurred with high frequencies in those preterm infants with and without lesions.
Yu and colleagues investigated cognitive and educational outcomes of adults born SGA, including the potential impact of family attitudes toward education on the effects of SGA birth on educational outcomes.23 9598 subjects of the Stockholm Birth Cohort were followed from infancy through age fifty years, with educational measures at age 13 years and 48 years. The verbal, spatial, and numerical test scores were lower for individuals born SGA (n=798) than for individuals born appropriate for gestational age (AGA, n=7364) or large for gestational age (LGA, n=1436). The differences between the SGA and AGA groups were statistically significant, although small, and the effects of being born SGA were mediated by family attitudes toward education. Importantly attainment of higher education after high school was largely (although not entirely) explained by family attitudes toward education.
Vohr and colleagues assessed neurocognitive functioning at age 16 years in 338 preterm (birthweight 600–1250 grams) born in New England. . The cohort consisted of 11 preterm participants with grades 3–4 IVH, 44 with grade 2 IVH, 31 with grade 1 IVH, and 251 without IVH. Regression models were used to identify associations between low-grade hemorrhage and cognitive, executive function, and memory deficits. Preterm adolescents with grade 2 hemorrhage were at increased risk for learning challenges, including cognitive and executive function linitations, as well as higher rates of functional challenges on activities of verbal intelligence, receptive vocabulary, phonemic fluency, cognitive flexibility, and phonological fluency. The comparison groups included preterm adolescents with grade 1 hemorrhage or no hemorrhage, or term controls(n=102). Preterm adolescents with grade 2 hemorrhage and no cystic periventricular leukomalacia were at an increased risk of cognitive and executive functional limitations when compared to term controls, and at a higher risk of cognitive challenges than preterm adolescents with no hemorrhage.
Brydges and colleagues performed a systematic review to evaluate the association between VP preterm birth(<32 weeks of gestation) and intelligence, executive function, and processing speed through childhood and adolescence.25 Inclusion criteria were English speaking subjects who had an age-matched control group born at term, and were tested with standardized measures between ages 4 and 16 years. 6163 VPT children and 5471 term-born controls from 60 different studies were included. The authors found that VPT children tended to score 0.82 SD lower on intelligence measures, 0.51 SD lower on executive functioning measures, and 0.49 SD lower on processing speed measures compared to their age-matched term-born controls. The investigators concluded that there may be a cascade effect: preterm birth predicts processing speed, which predicts executive functioning (including working memory), and working memory predicts math and reading abilities.
Doyle and colleagues examined the relative importance of biological versus social factors on long-term outcomes of extremely preterm using cognitive ability and academic achievement assessments in a regional Australian cohort.26 298 EP survivors (gestational age <28 weeks or birthweight <1000 grams) and 262 NBWT controls were evaluated at ages 2, 5, 8, and 18 years. Intraventricular hemorrhage and postnatal corticosteroid therapy were the biological variables most associated with cognitive and educational functioning. Among social variables, being reared in a multilingual household was disadvantageous early on, while social class and maternal education became important for later outcomes. Though the strengths of the biological associations equaled or exceeded the strengths of the social associations throughout all age points, both factors contributed to long-term cognitive and educational outcomes. It is important to note that settings of social disadvantage, access to health services, and quality of education in public schools may be more adverse in the United States.
Linsell and colleagues followed the EPICure cohort of surviving individuals born <26 weeks of gestation until age 19 years.27 The investigators compared cognitive development trajectories between EPT and term children using the Bayley Scales of Infant Development-Second Edition (2.5 years), the Kaufman Assessment Battery for Children (6 and 11 years), and the Wechsler Abbreviated Scale of Intelligence-Second Edition (19 years). At 19 years, researchers were able to assess 129 of the original 315 EPT subjects (41%) and 65 term-born matched controls. They found significantly lower cognitive performance in EPT survivors with an IQ of 85.7 compared to 103.9 in terms (mean difference 18.2, effect size 1.2 Z). Importantly, the cognitive test scores of EPT participants with moderate or severe neonatal brain injury were 10.9 points below participants with no or mild brain injury. On average, males and those who experienced brain injury early in life were at highest risk. These data suggest that among preterm children there are ongoing vulnerabilities that limit brain function and plasticity and that these cumulative challenges impact on long term physical, cognitive, and social health. Table 5 highlights several studies of adult outcomes focusing on employment, educational attainment, employment, and health related quality of life.
Table 5.
Adult Outcomes after Very and Extremely Preterm Birth
| Authors | Cohort | Age at Assessment | Developmental Outcome |
|---|---|---|---|
| Baumgardt, et al. 2012 | 1983-1985 Zurich, Switzerland 52 VLBW (<1250g) 75 controls |
23 years | No difference in overall self-reported quality of life |
| Hack, et al. 2007 | 1977-1979 Cleveland, Ohio 241 VLBW 232 NBW |
20 years | 1. No difference on self-reported health satisfaction 2. No difference on self-reported comfort (physical or emotional) 3. Decreased self-reported resiliency 4. Increased self-reported risk avoidance |
| Hille, et al. 2008 | 1983 Netherlands 959 adult survivors of prematurity (<32 weeks or VLBW) |
19 years | 1. 11.4% had moderate/severe problem with profession 2. ½ of individuals with moderate/severe problems in education had full-time employment |
| Moster, et al. 2008 | 1976-1983; Norway 325- 23-27 weeks 1,608- 28-30 weeks 6,363- 31-33 weeks 31,169- 34-36 weeks 828,227- full term |
20-36 years | 1. 10.6% vs. 1.7% receiving disability pension 2. Lower gestation less likely to have found life partner 3. Lower gestation less likely to have children |
| Saigal, et al. 2006 | 1977-1982 Ontario, Canada 166 ELBW and 145 controls |
22-25 years | No significant difference in rates of: ● Employment ● Independent living ● Married/cohabitation ● Parenthood |
VLBW= very low birth weight (<1500 G); ELBW = extremely low birth weight (<1000 G); NBW= normal birth weight (>2500G).
Severe retinopathy of prematurity (ROP) is associated with increased risk for visual disability, but the long-term impact on other neurodevelopmental domains is not as well understood. Molloy and colleagues examined the relationship between severe ROP and cognitive, educational, and visual outcomes in 180 EPT individuals between the ages of 17 and 18 years.28 Rates of CP were 11%, intellectual disability 7%; and severe ROP (grade 3–5) 15%. The participants were sequentially assessed at ages 2, 5, 8, and 17–18 years using a wide range of neurodevelopmental measures, including academic achievement, cognition, visual processing, and visual-motor integration. Severe ROP was associated with an almost 9.8 IQ point difference (effect size 0.67 Z) and 6–7 standard score achievement point differences for reading, spelling, and mathematics (effect size 0.4–0.47 Z). In multiple logistic regression severe ROP significantly increased the odds for IQ<85 (OR 2.85). Thus, even without sequelae of blindness, EPT children with severe ROP are at an increased risk for difficulties with higher cortical visual processing, cognition, and educational achievement. This study demonstrates the critical role that ROP has on both visual and neurodevelopmental functioning.
Breeman and colleagues sought to determine the stability of cognitive functions from childhood to adulthood for preterm (less than 32 weeks gestational age) and VLBW individuals when compared to individuals born at term, as well as how early adult cognitive functioning can be predicted.29 They used the cohort of the Bavarian Longitudinal Study, which is a prospective cohort study following 260 VPT/VLBW individuals and 229 controls born at term from birth through adulthood. Developmental and IQ tests were administered at 5 and 20 months as well as at 4, 6, 8, and 26 years of age. For all assessments, VPT/VLBW individuals had significantly lower IQs than the controls born at term. This finding held even when individuals with severe cognitive impairment were excluded. IQ scores tended to be more stable over time for VPT/VLBW individuals compared to term-born individuals, although this effect went away when the subjects with cognitive impairment were excluded from analysis. Adult IQ scores could be predicted with a fair amount of certainty starting as early as 20 months of age for the VPT/VLBW sample, and as early as 6 years of age for the term-born control sample. These persistent deficits emphasize that proactive strategies are required for vulnerable preterm children to support their information, reading, and math vulnerabilities.
Mathewson and colleagues performed a systematic review and meta-analysis to examine the risk for mental health problems in ELBW survivors compared to NPWT controls in childhood, adolescence, and adulthood.30 Previous evidence indicated that there may be gradient effects within preterm groups, with earlier gestational birth associated with higher rates of cognitive problems, attentional difficulties, hyperactivity, internalizing problems, and total psychological problems. In total, 41 studies with 2,712 ELBW children, adolescents, and adults, and 11,127 NBW peers were included. Researchers analyzed the impacts of birthplace, birth era, and neurosensory impairment on outcomes. In particular, they chose to examine the effects of birth era in order to compare outcomes in cohorts before and after the widespread availability of surfactant and steroid therapies. They also compared rates and types of mental health problems in participants with and without neurosensory impairment. The standardized mean difference from every study was used as the effect estimate, because difference measurement scales were used across studies. According to parent and teacher reports, ELBW children were at significantly greater risk for inattention and hyperactivity, internalizing symptoms, and externalizing symptoms when compared to NBWT controls. However, self-reports of inattention, hyperactivity, and oppositional behavior were lower for ELBW teens compared to NBWT controls. ELBW young adults had higher self-reported levels of internalizing problems and shyness than their NBWT peers. ELBW adults showed elevated levels of depression, anxiety, and social difficulties. Group differences were found to be robust for region of birth, birth era, and neurosensory impairments.
In conclusion:
If we are to understand the trajectories of risk and resilience in the vulnerable preterm and neonatal brain, we must go beyond survival and critically examine on a population basis the functional outcomes of children, adolescents and adults across their lifecourse. Our evaluations must go well beyond Bayley assessments and counts of neonatal morbidities such as bronchopulmonary dysplasia, ROP, sonographic brain injury, sepsis and necrotizing enterocolitis. We must proactively provide supports to families and developmental and educational supports to children, in order to we optimize academic functioning and participation in adult learning, physical and behavioral health activities, community living, relationships, and employment . We must better understand what underlies resiliency and how cumulative missed opportunities influence trajectories of physical, developmental and social health. In this way, we can truly develop prenatal, perinatal, and postnatal neuroprotective interventions.
Key Points:
To understand the trajectories of risk and resilience in the vulnerable preterm and neonatal brain, clinicians must go beyond survival and critically examine on a population basis the functional outcomes of children, adolescents and adults across their lifecourse.
Evaluations must go well beyond Bayley assessments and counts of neonatal morbidities such as bronchopulmonary dysplasia, ROP, sonographic brain injury, sepsis and necrotizing enterocolitis.
Proactively providing support to families and developmental and educational supports to children, can optimize academic functioning and participation in adult learning, physical and behavioral health activities, community living , relationships, and employment .
Acknowledgements:
Dr. Msall was supported in part by T73 MC11047 HRSA/DHSS Leadership Education in Neurodevelopmental and Related Disorders Training Program (LEND.) UG3 OD023348-01 NIH/NICHD_ELGAN-III: Environment, Epigenetics, Neurodevelopment & Health of Extremely Preterm Children and UG3 OD023281-01 NIH/NICHD_The Microbiome as a Potential Mediator of Socio-economic Disparities in Preterm Infant Neurodevelopmental Trajectories from NICU Discharge to School Age Both of these NIH grants are is part of the NICHD Environmental Influences on Child Health Outcomes(ECHO) Consortium. There were no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Frances A. Carter, Department of Psychology, The Center for Early Childhood Research, University of Chicago.
Michael E. Msall, Kennedy Research Center on Intellectual and Neurodevelopmental Disabilities University of Chicago Comer Children’s Hospital.
References:
- 1a.World Health Organization(WHO). International Classification of Functioning, Disability and Health -Children and Youth Version. ICF-CY; Geneva: Switzerland, 2009. [Google Scholar]; 1b. World Health Organization. International Classification of Functioning, Disability and Health (ICF). Geneva, Switzerland: 2001. [Google Scholar]
- 2a.Halfon N, Hochstein M. Life Course Health Development: An Integrated Framework for Developing Health, Policy, and Research. The Milbank Quarterly. 2002;80(3):422–479. [DOI] [PMC free article] [PubMed] [Google Scholar]; 2b. Msall ME, Sobotka SA, Dmowska A, Hogan D, Sullivan MC. Life-Course Health Development Outcomes after Prematurity: Developing a Community, Clinical and Translational Research Agenda to Optimize Health, Behavior and Functioning in Handbook of Life Course Health Development Science (Editors: Halfon N, Forrest C, Lerner R, Faustman EM). Springer Publishers. 2017 [PubMed] [Google Scholar]
- 3.Ancel P-Y, Goffinet F, Group E-W. Survival and morbidity ofpreterm children born at 22 through 34 weeks’ gestation in France in 2011:Results of the EPIPAGE-2 cohort study. JAMA Pediatrics 2015;169:230–238. [DOI] [PubMed] [Google Scholar]
- 4a.Washburn LK, Dillard RG, Goldstein DJ, Klinepeter KL, deRegnier R-A, O’Shea T. Survival and major neurodevelopmental impairment in extremely low gestational age newborns born 1990–2000: a retrospective cohort study. BMC Pediatrics 2007;7:20–8 [DOI] [PMC free article] [PubMed] [Google Scholar]; 4b. Amer R, Moddemann D, Seshia M et al. Neurodevelopmental Outcomes of Infants Born at <29 weeks of gestation admitted to Canadian Neonatal Intensive Care Units Based on Location of birth J Pediatr. 2018; 196(5): Pages 31–37.e1 [DOI] [PubMed] [Google Scholar]
- 5.Berry MJ, Saito-Benz M, Gray C, et al. Outcomes of 23- and 24-weeks gestation infants in Wellington, New Zealand: A single centre experience. Scientific Reports 2017,7:12769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saigal S, den Ouden L, Wolke D, et al. School-Age Outcomes in Children Who Were Extremely Low Birth Weight From Four International Population-Based Cohorts. Pediatrics. 2003;111:943–950. [DOI] [PubMed] [Google Scholar]
- 7.Taylor H, Klein N, Drotar D, Schluchter M, Hack M. Consequences and risks of <1000-g birth weight for neuropsychological skills, achievement, and adaptive functioning. Journal of Developmental & Behavioral Pediatrics. 2006;27(6):459–469. [DOI] [PubMed] [Google Scholar]
- 8.Anderson P, Doyle L, Group VICS. Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. JAMA. 2003;289(24):3264–3272. [DOI] [PubMed] [Google Scholar]
- 9.Klebanov P, Brooks-Gunn J, McCormick M. School achievement and failure in very low birth weight children. Journal of Developmental & Behavioral Pediatrics. 1994;15(4):248–256. [PubMed] [Google Scholar]
- 10.Taylor H, Klein N, Minich N, Hack M. Middle-School-Age Outcomes in Children with Very Low Birthweight. Child Development. 2000;71(6):1495–1511. [DOI] [PubMed] [Google Scholar]
- 11.Resnick M, Gueorguieva R, Carter R, et al. The Impact of Low Birth Weight, Perinatal Conditions, and Sociodemographic Factors on Educational Outcome in Kindergarten. Pediatrics. 1999;104(6):e74. [DOI] [PubMed] [Google Scholar]
- 12.Garfield CF, Karbownik K, Murthy K, et al. Educational performance of children born prematurely. JAMA Pediatrics2017;171:764–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serenius F, Ewald U, Farooqi A, et al. Neurodevelopmental outcomes among extremely preterm infants 6.5 years after active perinatal care in Sweden. JAMA Pediatrics 2016,170:954–963. [DOI] [PubMed] [Google Scholar]
- 14.Heeren T, Joseph RM, Allred EN, O’Shea TM, Leviton A, Kuban KCK. Cognitive functioning at the age of 10 years among children born extremely preterm: a latent profile approach. Pediatric Research 2017;82(4):614–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirschberger RG, Kuban KCK, O’Shea TM, et al. Co-occurrence and severity of neurodevelopmental burden (cognitive impairment, cerebral palsy, autism spectrum disorder, and epilepsy) at age ten years in children born extremely preterm. Pediatric Neurology 2018;79:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akshoomoff N, Joseph RM, Taylor HG, et al. Academic achievement deficits and their neuropsychological correlates in children born extremely preterm. Journal of Developmental and Behavioral Pediatrics 2017;38:627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joseph RM, O’Shea TM, Allred EN, Heeren T, Kuban KCK. Maternal educational status at birth, maternal educational advancement, and neurocognitive outcomes at age 10 years among children born extremely preterm. Pediatr Res. 2017. Nov 22. doi: 10.1038/pr.2017.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leviton A, Joseph RM, Allred EN, O’Shea TM, Kuban KCK. Antenatal and neonatal antecedents of learning limitations in 10-year old children born extremely preterm. Early Human Development 2018;118(4):8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuban KCK, Joseph RM, O’Shea TM, et al. Girls and boys born before 28 weeks gestation: Risks of cognitive, behavioral, and neurologic outcomes at age 10 years. Journal of Pediatrics 2016;173(6):69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt B, Roberts RS, Anderson PJ, et al. Academic performance, motor function, and behavior 11 years after neonatal caffeine citrate therapy for apnea of prematurity: An 11-year follow-up of the CAP randomized clinical trial. JAMA Pediatrics 2017; 171:564–572. [DOI] [PubMed] [Google Scholar]
- 21.Luu TM, Ment LR, Schneider KC, Katz KH, Allan WC, Vohr BR. Lasting effects of preterm birth and neonatal brain hemorrhage at 12 years of age. Pediatrics 2009; 12:1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roze E, Van Braeckel KNJA, van der Veere CN, Maathuis CGB, Martijn A, Bos AF. Functional Outcome at School Age of Preterm Infants with Periventricular Hemorrhagic Infarction. Pediatrics 2009; 123:1493–1500. [DOI] [PubMed] [Google Scholar]
- 23.Yu B, Garcy AM. A longitudinal study of cognitive and educational outcomes of those born small for gestational age. Acta Paediatrica 2017; 107:86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vohr BR, Allan W, Katz KH, Schneider K, Tucker R, Ment LR. Adolescents born prematurely with isolated grade 2 haemorrhage in the early 1990s face increased risks of learning challenges. Vol 2014;103:1066–1071. [DOI] [PubMed] [Google Scholar]
- 25.Brydges CR, Landes JK, Reid CL, Campbell C, French N, Anderson M. Cognitive outcomes in children and adolescents born very preterm: a meta-analysis. Dev Med Child Neurol. 2018;60(5):452–468. [DOI] [PubMed] [Google Scholar]
- 26.Doyle LW, Cheong JLY, Burnett A, Roberts G, Lee KJ, Anderson PJ. Biological and Social Influences on Outcomes of Extreme-Preterm/Low-Birth Weight Adolescents. Pediatrics 2015;136:e1513–e1152. [DOI] [PubMed] [Google Scholar]
- 27.Linsell L, Johnson S, Wolke D, et al. Cognitive trajectories from infancy to early adulthood following birth before 26 weeks of gestation: aprospective, population-based cohort study. Vol 0. Arch Dis Child 2017:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molloy CS, Anderson PJ, Anderson VA, Doyle LW. The long-term outcome of extremely preterm (<28 weeks’ gestational age) infants with and without severe retinopathy of prematurity. Journal of Neuropsychology 2016; 10:276–294. [DOI] [PubMed] [Google Scholar]
- 29.Breeman LD, Jaekel J, Baumann N, Bartmann P, Wolke D. Preterm cognitive function into adulthood. Pediatrics 2015; 136:415–423. [DOI] [PubMed] [Google Scholar]
- 30.Mathewson KJ, Chow CHT, Dobson KG, Pope EI, Schmidt LA, Van Leishout RJ. Mental health of extremely low birth weight survivors: A systematic review and meta-analysis. Psychological Bulletin 2017;143:347–383. [DOI] [PubMed] [Google Scholar]
- 31.Morse SB, Zheng H, Tang Y, Roth J. Early school-age outcomes of late preterm infants. Pediatrics. 2009;123(4):e622–629. [DOI] [PubMed] [Google Scholar]
- 32.Allen MC, Cristofalo EA, Kim C. Outcomes of preterm infants: morbidity replaces mortality. Clin Perinatol. 2011;38(3):441–454. [DOI] [PubMed] [Google Scholar]
- 33.Stephens BE, Vohr BR. Neurodevelopmental outcome of the premature infant. Pediatr Clin North Am. 2009;56(3):631–646, Table of Contents. [DOI] [PubMed] [Google Scholar]
- 34.Vohr B Long-term outcomes of moderately preterm, late preterm, and early term infants. Clin Perinatol. 2013;40(4):739–751. [DOI] [PubMed] [Google Scholar]
- 35.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371(9608):261–269. [DOI] [PubMed] [Google Scholar]
- 36.Laerum A, Reitan S, Evensen K, et al. Psychiatric Disorders and General Functioning in Low Birth Weight Adults: A Longitudinal Study. Pediatrics. 2017;139(2):e20162135. [DOI] [PubMed] [Google Scholar]
- 37.Breeman L, Jaekel J, Baumann N, Bartmann P, Wolke D. Preterm Cognitive Function Into Adulthood. Pediatrics. 2015;138(3):415–423. [DOI] [PubMed] [Google Scholar]
- 1.Morse SB, et al. Early school-age outcomes of late preterm infants. Pediatrics, 2009. 123(4): p. e622–9. [DOI] [PubMed] [Google Scholar]
- 2.Allen MC, Cristofalo EA, and Kim C, Outcomes of preterm infants: morbidity replaces mortality. Clin Perinatol, 2011. 38(3): p. 441–54. [DOI] [PubMed] [Google Scholar]
- 3.Stephens BE and Vohr BR, Neurodevelopmental outcome of the premature infant. Pediatr Clin North Am, 2009. 56(3): p. 631–46, Table of Contents. [DOI] [PubMed] [Google Scholar]
- 4.Vohr B, Long-term outcomes of moderately preterm, late preterm, and early term infants. Clin Perinatol, 2013. 40(4): p. 739–51. [DOI] [PubMed] [Google Scholar]
- 5.Saigal S and Doyle LW, An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet, 2008. 371(9608): p. 261–9. [DOI] [PubMed] [Google Scholar]
- 6.Serenius F , et al. Neurodevelopmental Outcomes Among Extremely Preterm Infants 6.5 Years After Active Perinatal Care in Sweden. JAMA Pediatrics, 2016. 170(10): p. 954–963. [DOI] [PubMed] [Google Scholar]
- 7.Herber-Jonat S, et al. Long-term outcome at age 7-10 years after extreme prematurity – a prospective, two centre cohort study of children born before 25 completed weeks of gestation (1999-2003). The Journal of Maternal-Fetal & Neonatal Medicine, 2014. 27(16): 1620–1626. [DOI] [PubMed] [Google Scholar]
- 8.Berry MJ, et al. Outcomes of 23- and 24-weeks gestation infants in Wellington, New Zealand: A single centre experience. Nature: Scientific Reports, 2017. 7: 12769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharp M, et al. Survival and neurodevelopmental outcomes in extremely preterm infants 22-24 weeks of gestation born in Western Australia. Journal of Paediatrics and Child Health, 2018. 54: 188–193. [DOI] [PubMed] [Google Scholar]
- 10.Vohr BR, et al. Neurodevelopmental Outcomes of Extremely Preterm Infants. Clinical Perinatology, 2014. 14: 241–255. [DOI] [PubMed] [Google Scholar]
- 11.Washburn LK, et al. Survival and major neurodevelopmental impairment in extremely low gestational age newborns born 1990-2000: a retrospective cohort study. BMC Pediatrics, 2007. 7(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1.Moore T, et al. Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: the EPICure studies. BMJ, 2012. 345: e7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Younge N, et al. Survival and Neurodevelopmental Outcomes among Periviable Infants. New England Journal of Medicine, 2017. 376(7): 617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ancel P-Y, et al. Survival and Morbidity of Preterm Children Born at 22 Through 34 Weeks’ Gestation in France in 2011: Results of the EPIPAGE-2 Cohort Study. JAMA Pediatrics, 2015. 169(3): 230–238. [DOI] [PubMed] [Google Scholar]
- 4.Pierrat V, Neurodevelopmental outcome at 2 years for preterm children born at 22 to 34 weeks’ gestation in France in 2011: EPIPAGE-2 cohort study. BMJ Open Access, 2017. 358:j3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vohr BR, et al. Neurodevelopmental Outcomes of Extremely Preterm Infants. Clinical Perinatology, 2014. 14: 241–255. [DOI] [PubMed] [Google Scholar]
- 6.Doyle LW, et al. Outcomes at Age 2 Years of Infants < 28 Weeks’ Gestational Age Born in Victoria in 2005. The Journal of Pediatrics, 2010. 156: 49–53. [DOI] [PubMed] [Google Scholar]
- 7.Synnes A, et al. Determinants of developmental outcomes in a very preterm Canadian cohort. Archives of Disease in Childhood: Fetal and Neonatal Edition, 2017. 102: 235–243. [DOI] [PubMed] [Google Scholar]
- 8.Serenius F, et al. Neurodevelopmental Outcome in Extremely Preterm Infants at 2.5 Years After Active Perinatal Care in Sweden. JAMA, 2013. 309(17): 1810–1820. [DOI] [PubMed] [Google Scholar]
- 9.Adams-Chapman I, et al. Neurodevelopmental Impairment Among Extremely Preterm Infants in the Neonatal Research Network. Pediatrics, 2018. 141(5): e20173091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Msall ME, Neurodevelopmental surveillance in the first 2 years after extremely preterm birth: Evidence, challenges, and guidelines. Early Human Development, 2006. 82: 157–166. [DOI] [PubMed] [Google Scholar]
- 1.Akshoomoff N, et al. Academic Achievement Deficits and Their Neuropsychological Correlates in Children Born Extremely Preterm. Journal of Developmental & Behavioral Pediatrics, 2017. 38(8):627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirschberger RG, et al. Co-occurrence and Severity of Neurodevelopmental Burden (Cognitive Impairment, Cerebral Palsy, Autism Spectrum Disorder, and Epilepsy) at Age Ten Years in Children Born Extremely Preterm. Pediatric Neurology, 2018. 79: 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burnett AC, et al. Trends in Executive Functioning in Extremely Preterm Children Across 3 Birth Eras. Pediatrics, 2018. 141(1): e20171958. [DOI] [PubMed] [Google Scholar]
- 4.Leviton A, et al. Antenatal and Neonatal Antecedents of Executive Dysfunctions in Extremely Preterm Children. Journal of Child Neurology, 2018. 33(3): 198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saigal S, Functional outcomes of very premature infants into adulthood. Seminars in Fetal & Neonatal Medicine, 2014. 19: 125–130. [DOI] [PubMed] [Google Scholar]
- 6.Szatmari P, Saigal S, Rosenbaum P, Campbell D, King S. Psychiatric disorders at five years among children with birthweights less than 1000g: a regional perspective. Dev Med Child Neurol. 1990. Nov;32(11):954–62. [PubMed] [Google Scholar]
- 7.Saigal S, Rosenbaum P, Szatmari P, Campbell D. Learning disabilities and school problems in a regional cohort of extremely low birth weight (less than 1000 G) children: a comparison with term controls. J Dev Behav Pediatr. 1991. Oct;12(5):294–300. [PubMed] [Google Scholar]
- 8.Taylor HG, Klein N, Hack M. School-age consequences of birth weight less than 750 g: a review and update . Dev Neuropsychol. 2000;17(3):289–321. DOI: 10.1207/S15326942DN1703_2 [DOI] [PubMed] [Google Scholar]
- 1.Laerum A, Reitan S, Evensen K, et al. Psychiatric Disorders and General Functioning in Low Birth Weight Adults: A Longitudinal Study. Pediatrics. 2017;139(2):e20162135. [DOI] [PubMed] [Google Scholar]
- 2.Breeman L, Jaekel J, Baumann N, Bartmann P, Wolke D. Preterm Cognitive Function Into Adulthood. Pediatrics. 2015;138(3):415–423 [DOI] [PubMed] [Google Scholar]
- 1.Baumgardt M, Bucher HU, Mieth RA, Fauchère JC. Health-related quality of life of former very preterm infants in adulthood Acta Paediatr. 2012. Feb;101(2):e59–63. doi: 10.1111/j.1651-2227.2011.02422.x. Epub 2011 Sep 7. [DOI] [PubMed] [Google Scholar]
- 2.Hack M, Cartar L, Schluchter M, Klein N, Forrest CB.Self-perceived health, functioning and well-being of very low birth weight infants at age 20 years.J Pediatr. 2007. Dec;151(6):635–41, 641.e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hille ET, Weisglas-Kuperus N, van Goudoever JB,et al. for ; Dutch Collaborative POPS 19 Study Group. Functional outcomes and participation in young adulthood for very preterm and very low birth weight infants: the Dutch Project on Preterm and Small for Gestational Age Infants at 19 years of age. Pediatrics 2007. Sep;120(3):e587–95. [DOI] [PubMed] [Google Scholar]
- 4.Moster D, Lie RT, Markestad T. Long-term medical and social consequences of preterm birth N Engl J Med. 2008. Jul 17;359(3):262–73. doi: 10.1056/NEJMoa0706475. [DOI] [PubMed] [Google Scholar]
- 5.Saigal S, Stoskopf B, Streiner D, Boyle M, Pinelli J, Paneth N, Goddeeris J. Transition of extremely low-birth-weight infants from adolescence to young adulthood: comparison with normal birth-weight controls JAMA. 2006. Feb 8;295(6):667–75. [DOI] [PubMed] [Google Scholar]


