Abstract
Herpesvirus glycoproteins play dominant roles in the initiation of infection of target cells in culture and thus may also influence viral tropism in vivo. Whereas the relative contribution of several nonessential glycoproteins to neurovirulence and neurotropism of Pseudorabies virus (PrV), an alphaherpesvirus which causes Aujeszky's disease in pigs, has recently been uncovered in studies using viral deletion mutants, the importance of essential glycoproteins is more difficult to assess. We isolated an infectious PrV mutant, PrV-9112C2, which lacks the gene encoding the essential PrV glycoprotein B (gB) but stably carries in its genome and expresses the homologous gene of bovine herpesvirus 1 (BHV-1) (A. Kopp and T. C. Mettenleiter, J. Virol. 66:2754–2762, 1992). Apart from exhibiting a slight delay in penetration kinetics, PrV-9112C2 was similar in its growth characteristics in cell culture to wild-type PrV. To analyze the effect of the exchange of these homologous glycoproteins in PrV's natural host, swine, 4-week-old piglets were intranasally infected with 106 PFU of either wild-type PrV strain Kaplan (PrV-Ka), PrV-9112C2, or PrV-9112C2R, in which the PrV gB gene was reinserted instead of the BHV-1 gB gene. Animals infected with PrV-Ka and PrV-9112C2R showed a similar course of disease, i.e., high fever, marked respiratory symptoms but minimal neurological disorders, and excretion of high amounts of virus. All animals survived the infection. In contrast, animals infected with PrV-9112C2 showed no respiratory symptoms and developed only mild fever. However, on day 5 after infection, all piglets developed severe central nervous system (CNS) symptoms leading to death within 48 to 72 h. Detailed histological analyses showed that PrV-9112C2R infected all regions of the nasal mucosa and subsequently spread to the CNS preferentially by the trigeminal route. In contrast, PrV-9112C2 primarily infected the olfactory epithelium and spread via the olfactory route. In the CNS, more viral antigen and significantly more pronounced histological changes resulting in more severe encephalitis were found after PrV-9112C2 infection. Thus, our results demonstrate that replacement of PrV gB by the homologous BHV-1 glycoprotein resulted in a dramatic increase in neurovirulence combined with an alteration in the route of neuroinvasion, indicating that the essential gB is involved in determining neurotropism and neurovirulence of PrV.
Pseudorabies virus (PrV) and Bovine herpesvirus 1 (BHV-1) are related alphaherpesviruses of the genus Varicellovirus. PrV, also designated Suid herpesvirus 1, is the causative agent of Aujeszky's disease (AD), a serious illness in domestic and wild animals. PrV has a broad host range and productively infects birds and most mammals, except for horses and higher primates including humans, which are resistant to infection (reviewed in reference 25). Whereas mortality in most infected species regularly amounts to 100%, pigs can survive a productive infection and remain latently infected for life. Thus, pigs are considered the natural host of PrV. Symptoms of AD consist of high fever, respiratory and central nervous system (CNS) disorders, and reproductive failures due to infection in utero. In contrast, BHV-1 has a restricted natural host range, infecting only ruminants and producing relatively minor symptoms related primarily to infection of nasal and genital mucosae (13). However, abortions may also occur. Neurovirulent BHV strains previously classified as BHV-1 are now designated BHV-5 (39). Thus, whereas PrV demonstrates a pronounced neurovirulence, BHV-1 does not. However, both viruses can infect neurons and remain latent in them for the life of the animal.
Natural infection with PrV normally occurs via the oronasopharyngeal route. Primary virus replication occurs in the mucosal surfaces, mostly in nonneuronal cells. Subsequently, the virus infects neurons via nerve endings in the mucosae and ascends toward the CNS, resulting in a nonsuppurative meningoencephalitis (8, 34). The oronasopharyngeal mucosa is innervated by six major brain nerves, from which the nervus olfactorius and nervus trigeminus supply the nasal mucosa. The rostral parts of the nasal cavity (regio cutanea and regio respiratoria) are innervated only by the nervus trigeminus, whereas the olfactory epithelium (regio olfactoria) in the caudal parts of the nasal cavity contains both olfactory and trigeminal nerve endings (32). Characteristically, the olfactory epithelium exhibits high numbers of olfactory receptor cells, which are free-ending nerve cells localized in the epithelial layer. They are part of the olfactory pathway whose axon bundles (fila olfactoria) lead to the cerebrum via the olfactory bulb and the pedunculus olfactorius. In contrast, the nervus trigeminus with all of its branches converges in the trigeminal ganglion leading to the pons and the brain stem. Therefore, after primary replication in the nasal mucosa, these two routes represent the major ways of viral neuroinvasion toward the CNS (20, 21, 30), and the olfactory bulb and the sensory ganglia are major sites of latent virus (40).
Herpesvirus glycoproteins are involved in crucial steps during virus infection, in particular attachment to and penetration into target cells and direct cell-to-cell spread (24, 43). Glycoprotein B (gB) is the most highly conserved herpesvirus glycoprotein, and homologs have been detected throughout members of the Herpesviridae (35). It is essential for penetration, i.e., fusion between virion envelope and cellular cytoplasmic membrane, and transcellular infection by direct viral cell-to-cell spread (43). It is also required for neuroinvasion and transneuronal spread in vivo (1). Functional homology of gB proteins could be demonstrated by heterologous complementation. BHV-1 gB, which exhibits 63% amino acid identity to PrV gB, was able to complement a lethal gB deletion in PrV (37), and PrV gB complemented a gB− herpes simplex virus type 1 (HSV-1) mutant (27). Thus, a PrV recombinant which lacked PrV gB but stably carries in its genome the BHV-1 gB gene could be isolated (19). Expression of BHV-1 gB in gB-deficient PrV (PrV-gB−) restored infectivity, one-step growth kinetics, and in vitro host range to levels similar to wild-type PrV levels. The only difference observed was a slight delay in penetration (19).
gB of herpesviruses has also been implicated in viral pathogenesis. Several syn mutations in the HSV-1 gB directly affected pathogenesis in mice (7, 10, 45). Mutations in gB also altered neuroinvasion in a mouse footpad infection model (48). In human cytomegalovirus, differences in gB genes correlated with viral tropism in vivo and virulence (29). To investigate the role of gB in infection of a natural herpesvirus-host system, wild-type PrV, the BHV-1 gB recombinant PrV-9112C2, and a matching revertant in which the BHV gene was replaced by the parental pseudorabies gene were compared after intranasal infection of pigs.
MATERIALS AND METHODS
Virus strains and cells.
PrV strain Kaplan (PrV-Ka) (15) was used as the wild-type strain. PrV-Ka is a mildly virulent strain which had been used for decades. Construction of the BHV-1 gB-expressing PrV-gB− recombinant PrV-9112C2 has been described elsewhere (19). Viruses were grown in Madin-Darby bovine kidney (MDBK) cells. Infectious titers of virus stocks were assayed on MDBK cells as described elsewhere (11).
Isolation of rescuant PrV-9112C2R.
To restore PrV gB expression in PrV-9112C2, purified PrV-9112C2 DNA was cotransfected into Vero cells with PrV gB expression plasmid gB-CMV (12) by calcium phosphate coprecipitation (14). Resulting virus progeny was titrated on MDBK cells and analyzed by black plaque assay (Vectastain, Camon, Germany) for PrV gB expression, using monoclonal antibody (MAb) 5/14 (23). Positive plaques were picked and propagated on MDBK cells. To further select for PrV gB expression, virus progeny was incubated for 2 h at 37°C in the presence of rabbit complement with anti-BHV-1 gB MAb 42/18/7 (generously provided by G. Keil, Insel Riems, Germany), plated onto MDBK cells, and screened again. After three rounds of plaque purification, one isolate was randomly picked and analyzed by Southern blotting for correct insertion of the PrV gB gene (data not shown). Expression of PrV gB and absence of BHV-1 gB was verified by indirect immunofluorescence and radioimmunoprecipitation using specific MAbs (data not shown).
Design of animal experiments.
For all experiments, 4-week-old piglets seronegative for PrV were obtained from local farms, housed in isolation, and fed with commercial food and water ad libitum. After 7 days to allow adjustment of the animals to the housing conditions, animals were infected intranasally with 1 ml of virus suspension containing 106 PFU. Animals were observed twice a day for clinical signs, and their rectal temperature was measured. Severity of observed clinical symptoms was assessed as (i) elevated temperature below 41°C; (ii) fever above 41°C correlated with respiratory distress; (iii) ataxia, behavior disorders, and severe respiratory disorders such as rhinitis or pneumonia; (iv) severe convulsions and/or temporary paralyses; and (v) moribund state or death.
A total of three animal experiments were performed. In the first experiment, wild-type PrV-Ka was compared to PrV-9112C2 in four pigs each. In the second experiment, PrV-9112C2R was used for infection of two pigs to assay for a wild-type PrV phenotype. In the third experiment, eight piglets per group were infected with either PrV-9112C2 or PrV-9112C2R to establish kinetics of infection and histopathology.
Sampling of tissues.
Animals were electrically stunned and rendered unconscious, sacrificed, and necropsied. Tissue samples were collected as indicated from the nasal mucosa (regio cutanea, regio respiratoria, and regio olfactoria), nervus olfactorius, ganglion trigeminale, medulla oblongata, pons, mesencephalon, diencephalon, bulbus olfactorius, pedunculus olfactorius, cerebrum, cerebellum, tonsils, and lungs for virus isolation, indirect immunofluorescence on cryosections, and histopathology.
Determination of virus excretion.
To determine the titer of excreted virus, nasal swabs were taken at daily intervals from each animal. After incubation at 4°C overnight in 1 ml of minimal essential medium (MEM) infectivity was titrated on MDBK cells as described elsewhere (11, 12).
Virus isolation.
Tissue samples were weighed, incubated in MEM–5% fetal bovine serum, and ground in a mortar to produce a homogeneous tissue suspension. The final volume was measured, and the suspension was titrated on MDBK cells in 10-fold dilutions. After 3 days at 37°C, cells were fixed with 2% formalin and stained with crystal violet, and plaques were counted. Virus titers were calculated per gram of tissue.
Indirect immunofluorescence.
PrV antigen-positive cells were identified by indirect immunofluorescence in cryosections, using MAb B16-c8 directed against gC (B. G. Klupp and E. Weiland, unpublished results). Tissue samples were snap frozen in n-heptane (−70°C), and cryosections were fixed with acetone at −20°C for 10 to 15 min. Sections were then equilibrated to room temperature and preincubated in phosphate-buffered saline (PBS) containing 5% bovine serum albumin (BSA) for 10 min. Subsequently, they were incubated with MAb B16-c8 diluted in PBS–2% BSA and fluorescein isothiocyanate-labeled secondary goat F(ab)2 anti-mouse immunoglobulins G plus M (heavy plus light chains) (Caltag Laboratories, Medac, Germany). The secondary antibody was diluted in PBS–2% BSA and mixed at a ratio of 3:1 with 0.005% Evans blue solution. Parallel sections were incubated with control MAb 934/H1 (directed against the S protein of mouse hepatitis coronavirus; kindly provided by H. Wege, Insel Riems, Germany). Cryosections from noninfected animals were processed in parallel. All sections were sealed with fluorescence maintenance buffer containing 2.5% DABCO (Sigma, Deisenhofen, Germany) diluted in 90% glycerol–10% PBS (pH 8.6). For evaluation and microphotography, an Optiphot 2 fluorescence microscope (Nikon, Tokyo, Japan) was used. One cryosection per animal was used to semiquantitatively determine the number of positive cells.
Histology.
Tissue samples 2 mm thick were fixed for 20 h in 4% neutral buffered formalin (Merck, Darmstadt Germany) and subsequently embedded in paraffin (Merck), using an automatic tissue processor. Paraffin sections were stained with hematoxylin-eosin and sealed with balsam. For histological evaluation and microphotography, a Jenaval microscope (Carl Zeiss, Jena, Germany) was used. The extent of encephalitis was estimated by counting of encephalitic foci irrespective of their size. Encephalitic foci were defined as vessels with perivascular cellular infiltrations and focal gliosis with more than 10 glial cells per focus. For every section, the average number of encephalitic foci per 25 mm2 was calculated.
RESULTS
Altered pathogenesis in PrV-9112C2-infected pigs.
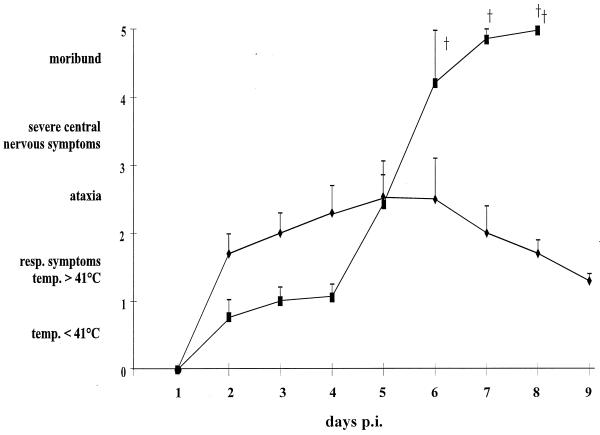
To assay pathogenicity of BHV-1 gB-expressing PrV recombinant PrV-9112C2, four piglets each were infected intranasally with 106 PFU of wild-type PrV-Ka (group I) or PrV-9112C2 (group II). Results are summarized in Fig. 1. Two days postinfection (dpi) animals of group I showed high fever with body temperatures rising to 41.5 to 42°C. On days 2 or 3 postinfection (p.i.) they developed respiratory symptoms correlating with a decrease in food uptake, which resulted in a worsening of their overall condition. At day 5 p.i., all animals in this group exhibited severe respiratory symptoms such as rhinitis with purulent discharge. In addition, one piglet exhibited CNS symptoms including ataxia and convulsions. However, from day 6 all animals steadily improved and appeared normal on day 10 after infection.
FIG. 1.
Clinical symptoms in pigs after infection with PrV-Ka and PrV-9112C2. Four piglets per group were infected intranasally with either PrV-Ka (⧫) or PrV-9112C2 (■). Every animal in each group was observed for symptoms of AD twice a day and assigned a clinical score correlating to five levels of severity as indicated in Materials and Methods. Average values and standard deviations of four pigs from each group are shown. (†) denotes death of animals.
In contrast, group II animals developed only mild symptoms with moderate fever during the first 5 dpi. Their feeding was normal, and no respiratory symptoms were observed in this group at any time. Surprisingly, on day 5 p.i. the condition of all animals within this group rapidly declined; they stopped feeding, showed signs of vomiting, and within hours developed severe CNS symptoms (e.g., disordered behavior, ataxia, opisthotonus, convulsions, and paralysis). Subsequently, all piglets of this group had to be euthanized in moribund state within the next 3 days.
To analyze virus excretion, nasal swabs were taken daily and titrated. As shown in Fig. 2, animals infected with PrV-Ka showed the typical course of virus shedding, with peak titers reaching nearly 107 PFU per ml on days 3 to 4 p.i. and declining thereafter until the end of the experiment. In comparison, animals infected with PrV-9112C2 excreted ca. 100-fold less virus, with titers between 104 and 105 PFU per ml, and there was no indication of an increase in virus excretion before death of the animals.
FIG. 2.
Virus excretion of pigs infected with PrV-Ka and PrV-9112C2. After intranasal infection of animals with PrV-Ka (⧫) or PrV-9112C2 (■), nasal swabs were taken daily and titrated on MDBK cells. Indicated are average titers and standard deviations in PFU per milliliter.
Thus, although PrV-9112C2 appeared to replicate to a significantly lesser extent in the nasal tissues compared to PrV-Ka, which correlated with absence of respiratory symptoms, it proved to be highly neurovirulent.
Altered neurovirulence in PrV-9112C2 is due to expression of BHV-1 gB.
Since this phenotype was unexpected and very striking, we wanted to verify that the observed alteration in virulence was caused by expression of the heterologous gB. Therefore, we constructed a rescuant from PrV-9112C2, designated as PrV-9112C2R, in which the BHV-1 gB gene was substituted by the PrV gB gene, thus reconstituting the wild-type genotype. After infection of cultured cells with PrV-9112C2, no difference was observed compared to PrV-Ka (data not shown). To analyze the phenotype in vivo, two pigs were infected intranasally with 106 PFU of PrV-9112C2R. Both animals developed high fever with temperatures above 41°C and severe respiratory symptoms. At days 5 and 6 p.i., one animal exhibited neurological symptoms; from day 6 p.i. onward, however, the condition of both animals improved, and they survived the infection. Thus, PrV-9112C2R infection produced a clinical picture similar to that observed after infection with PrV-Ka (summarized in Table 1). This was also true for excretion of infectious virus, since virus titers in nasal swabs rose to ca. 107 PFU per ml. We conclude that the alteration in virulence of PrV-9112C2 was caused by the insertion of the BHV-1 gB gene into PrV-gB−.
TABLE 1.
Summary of clinical symptoms in pigs after intranasal infection with PrV-Ka, PrV-9112C2R, or PrV-9112C2a
| Virus | Fever | Respiratory symptoms | CNS symptoms | Death |
|---|---|---|---|---|
| PRV-Ka | +++ | +++ | + | − |
| PrV-9112C2R | +++ | +++ | + | − |
| PrV-9112C2 | + | − | +++ | +++ |
+++, high fever or severe symptoms; +, elevated temperature or mild symptoms; −, no symptoms.
Alteration of neuroinvasion and neurovirulence by expression of heterologous gB.
To determine whether the change in neurovirulence was correlated with an alteration in cell tropism and/or neuroinvasiveness, two groups of eight piglets each were infected with PrV-9112C2R (group I) or PrV-9112C2 (group II). Four animals were inoculated with MEM only as negative controls (group III). At each of the following days after infection one animal from groups I and II, and on every second day one animal of group III, were euthanized, necropsied, and analyzed.
Clinical course and virus excretion.
Starting at day 2 p.i., the group I animals showed high fever with temperatures increasing up to 41.5°C, a worsening in overall condition, and severe respiratory symptoms. In contrast, animals of group II did not develop any respiratory symptoms and displayed only low fever. However, as previously observed (see above), at day 5 p.i., within hours all animals developed severe CNS symptoms and had to be euthanized within the following 48 h. None of the control animals exhibited any signs of disease. In nasal secretions of PrV-9112C2R-infected animals, titers of up to 107 PFU per ml were reached, whereas titers in PrV-9112C2-infected animals amounted to only ca. 105 PFU per ml, which was again as observed before (see above). Thus, there was an excellent reproducibility of these parameters given the fact that outbred animals were used for the experiments.
Virus isolation.
Virus titers in organs were determined by titration of homogenized tissue suspensions on MDBK cells. As shown in Table 2, high amounts of infectious virus were recovered from all regions of the nasal mucosa of PrV-9112C2R-infected animals, whereas from PrV-9112C2-infected pigs generally lower amounts of virus were isolated, in particular from the regio cutanea. However, at early stages p.i. virus was detected in the regio olfactoria of PrV-9112C2-infected animals but was still undetectable in this region of PrV-9112C2R-infected pigs. Correlating with this finding, high amounts of PrV-9112C2 were found in the olfactorial bulb at 2 dpi. These data indicated that the preferred route of neuroinvasion of PrV-9112C2 is the olfactory pathway, which is in contrast to the situation in PrV-9112C2R. In the CNS, PrV-9112C2 could be detected in higher amounts later after infection compared to PrV-9112C2R, particularly in the cerebellum. These results explain the severe CNS symptoms seen in PrV-9112C2-infected animals. To ascertain correct infection, reisolated viruses were genotyped by Southern blotting of BamHI-digested viral DNA. All analyzed samples showed the expected genotype (data not shown).
TABLE 2.
Virus isolation from different tissuesa
| Tissue | PrV-9112C2R
|
PrV-9112C2
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1b | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Regio cutanea | ++++ | ++++++ | ++++++ | +++++ | ++++++ | +++ | ++++ | ++ | + | ++++ | ++++ | +++++ | +++ | ++++ | ||
| Regio respiratoria | ++++++ | +++++ | +++++ | ++++++ | +++ | ++ | +++ | ++++ | +++++ | ++++ | ++++ | +++ | ||||
| Regio olfactoria | +++++ | +++++ | +++++ | +++ | +++ | ++++ | +++ | ++++++ | ++ | |||||||
| Ganglion trigeminale | ++++ | ++++ | ++++ | ++++ | ++ | +++ | ++++ | ++ | ++++ | + | ||||||
| Bulbus olfactorius | + | +++ | +++ | +++++ | ++++ | +++++ | ++ | ++ | ||||||||
| Pedunculus olfactorius | +++ | ++ | ++++ | |||||||||||||
| Pons | +++ | ++ | ||||||||||||||
| Medulla oblongata | +++ | ++ | + | |||||||||||||
| Mesencephalon | + | ++ | ++ | ++ | ||||||||||||
| Diencephalon | ++ | ++ | +++ | ++ | ||||||||||||
| Cerebrum | + | |||||||||||||||
| Cerebellum | ++ | ++ | +++ | +++ | ||||||||||||
| Lung | ++ | ++ | ++ | ++ | ||||||||||||
| Tonsil | ++ | ++ | +++ | ++++ | ++ | ++ | + | +++ | ||||||||
Indicated as + (100 to 101 PFU/g of tissue) to ++++++ (106 to 107 PFU/g of tissue); no entry, no PRV isolated.
Day p.i.
Demonstration of PrV antigen by immunofluorescence in cryosections.
PrV antigen-positive cells were identified by using anti-gC MAb B16-c8. As summarized in Table 3, a good correlation between virus isolation and semiquantitative evaluation of immunofluorescence was observed. After inoculation with PrV-9112C2R, infected cells could be found in all examined parts of the nasal mucosa. In contrast, no infection by PrV-9112C2 of the regio cutanea and only moderate infection of the regio respiratoria were observed, correlating with the lack of detectable respiratory symptoms, the lower amount of virus shedding, and the decreased amounts of isolated virus. However, a severe infection of the olfactory mucosa was observed. Viral antigen was detected mainly in cells of the covering mucosal epithelium, less often in the glandular epithelium and in desquamated epithelial cells, and more rarely in macrophages of the lamina propria. PrV antigen was present in the olfactory mucosa in single nerve fibers and in axon bundles of the fila olfactoria (Fig. 3A, D, and E).
TABLE 3.
Demonstration of PrV-infected cells by immunofluorescence in different tissuesa
| Tissue | PrV-9112C2R
|
PrV-9112C2
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1b | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Regio cutanea | + | ++++ | +++ | ++++ | + | + | ||||||||||
| Regio respiratoria | ++ | +++ | ++ | +++ | ++ | +++ | + | +++ | ||||||||
| Regio olfactoria | ++++ | + | + | ++++ | + | + | ++++ | ++++ | +++ | +++ | ++ | |||||
| Ganglion trigeminale | ++++ | +++ | ++ | + | + | + | + | + | ||||||||
| Bulbus olfactorius | ++ | +++ | ++ | +++ | +++ | ++++ | ++ | + | ||||||||
| Pedunculus olfactorius | + | ++ | + | +++ | ++++ | ++ | + | |||||||||
| Pons | + | ++ | ++ | + | ||||||||||||
| Medulla oblongata | + | + | ++ | + | ++ | + | ||||||||||
| Mesencephalon | + | + | + | |||||||||||||
| Diencephalon | + | ++ | + | |||||||||||||
| Cerebrum | ++ | |||||||||||||||
| Cerebellum | + | ++ | +++ | |||||||||||||
| Tonsil | + | ++ | + | |||||||||||||
Evaluation: +, <50 cells in nasal mucosa and tonsils, <10 nerve fibers and/or 5 perikarya in trigeminal ganglion, <25 large perikarya and/or <5 plaques with <30 small perikarya in brain; ++, <150 cells in nasal mucosa and tonsils, <50 nerve fibers and/or 10 perikarya in trigeminal ganglion, <200 large perikarya and/or <20 plaques with <30 small perikarya in brain; +++, <300 cells in nasal mucosa and tonsils, <100 nerve fibers and/or 20 perikarya in trigeminal ganglion, <500 large perikarya and/or <50 plaques with <30 small perikarya in brain; ++++, >300 cells in nasal mucosa and tonsils, >100 nerve fibers and/or 50 perikarya in trigeminal ganglion, >500 large perikarya and/or >50 plaques with <30 small perikarya in brain.
Day p.i.
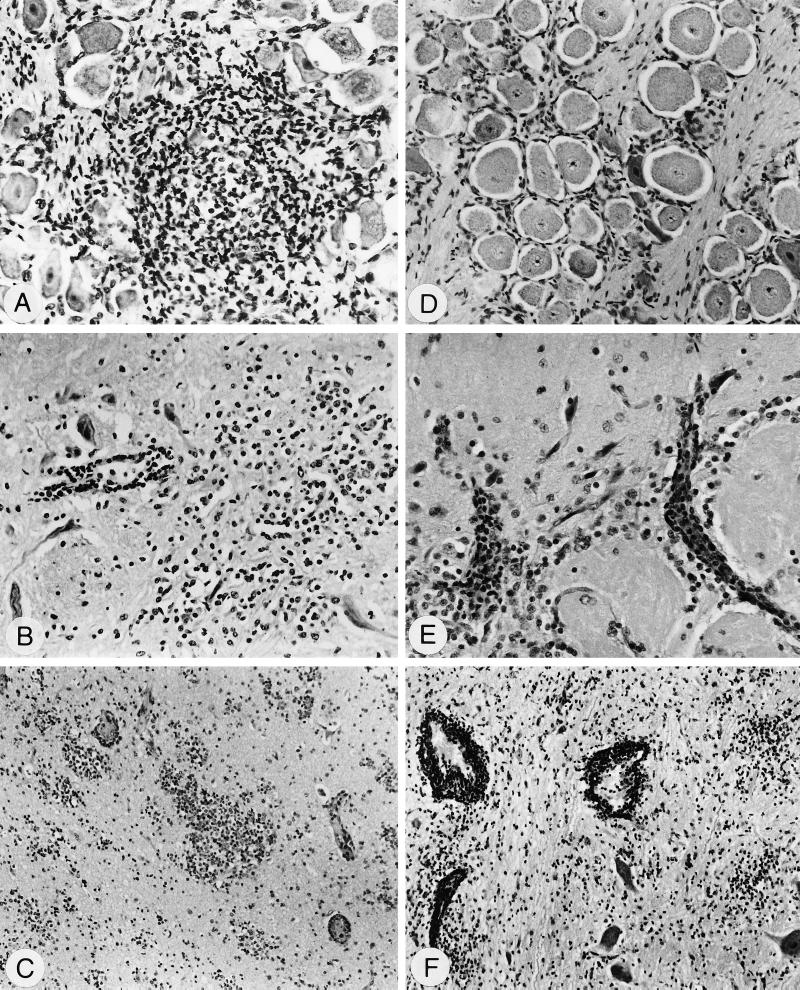
FIG. 3.
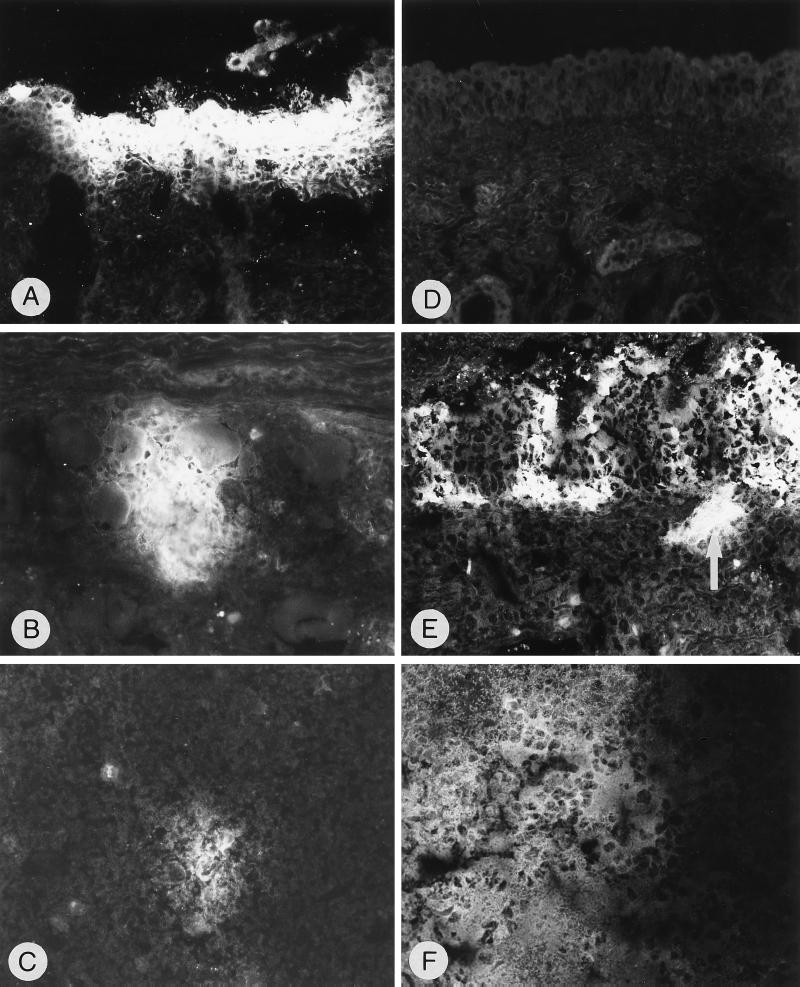
Detection of PrV-infected cells in cryosections by indirect immunofluorescence with anti-gC MAb after infection with PrV-9112C2R (A to C) or PrV-9112C2 (D to F). (A) Regio cutanea, 3 dpi, PrV antigen-positive epithelial cells; magnification, ×240. (B) Ganglion trigeminale, 3 dpi, PrV antigen-positive perikarya, nerve fibers, and infiltrating cells; ×250. (C) Medulla oblongata, 2 dpi, small focus with PrV-positive perikarya and surrounding neuropil; ×375. (D) Regio cutanea, no PrV antigen detectable, 3 dpi; ×240. (E) Regio olfactoria, 3 dpi, PrV antigen-positive foci in the olfactory epithelium and logitudinal section of PrV-positive nerve fibers (arrow); ×250. (F) Bulbus olfactorius, 5 dpi, section of a prominent large focus within the granular layer containing PrV-positive perikarya, neuropil, and glial cells; ×240.
In the trigeminal ganglion, more PrV antigen-positive nerve fibers and perikarya were found after inoculation with PrV-9112C2R, especially between days 3 and 5 p.i., when more than 100 PrV-positive nerve fibers and numerous positive perikarya per section could be found (Fig. 3B). In PrV-9112C2-infected pigs, considerably lower numbers of PrV-positive neurons (fewer than 10 positive nerve fibers or 5 positive perikarya per section) were observed. Conversely, in the bulbus olfactorius and pedunculus olfactorius, high numbers of PrV-9112C2-infected neurons were present between days 3 and 6 p.i., with sometimes more than 500 PrV-antigen positive perikarya or more than 50 plaques per section. In contrast, in PrV-9112C2R-infected pigs, fewer positive cells were detected beginning on day 4 p.i. In the other brain regions, considerably more PrV antigen-positive cells were found in PrV-9112C2-infected animals, with a maximum at days 5 and 6 p.i. (Table 3; Fig. 3F). However, as early as 2 dpi, positive cells could be found in the medulla oblongata of PrV-9112C2R-infected animals (Fig. 3C), which is suggestive of the trigeminal pathway. In the tonsils, PrV-positive crypt cells of the epithelium and the lining lymphoid tissue were detectable only after infection with PrV-9112C2R. Neither mutant could be detected in lung samples. All control sections were clearly negative.
Histological alterations.
After infection with either virus mutant, we observed desquamation, degeneration, and focal necrosis of the mucosal epithelium in the nasal cavity which was rapidly followed by an inflammatory response. As observed by virus isolation and immunofluorescence, PrV-9112C2R affected all areas to similar extents, resulting in focal desquamation and degeneration of the epithelium at 2 dpi and larger epithelial erosions, focal necrosis of the mucosa and lining of the lamina propria, and infiltration of macrophages together with a lower number of lymphocytes and polymorphonuclear neutrophilic granulocytes at 4 dpi. In contrast, PrV-9112C2 produced no lesions in the regio cutanea, only moderate alterations in the regio respiratoria, but severe inflammation in the regio olfactoria (data not shown).
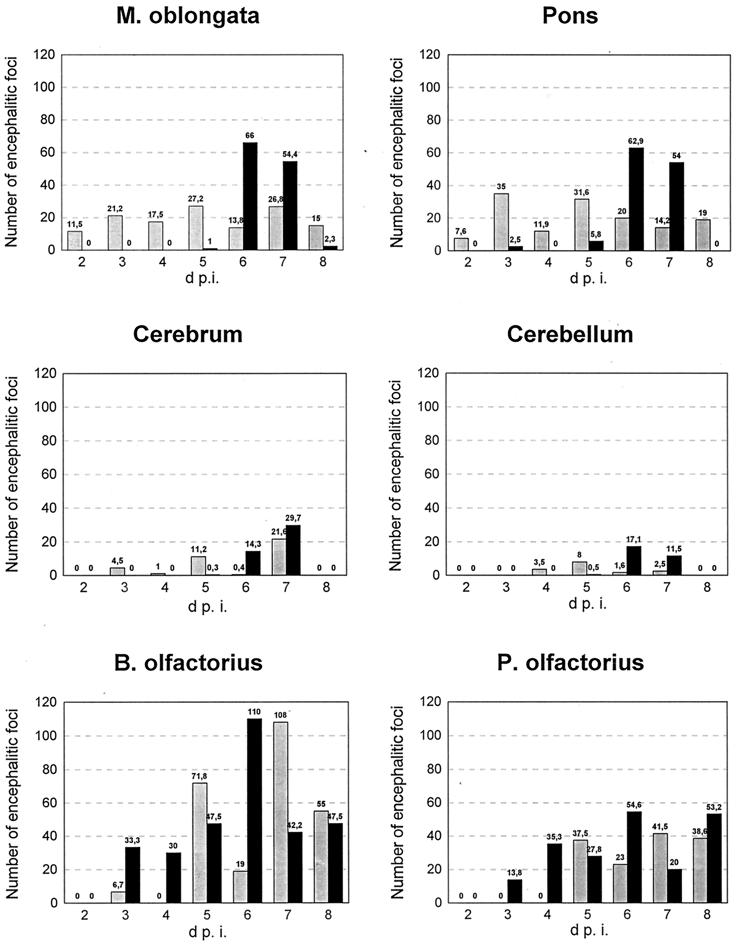
In the trigeminal ganglion, both mutants caused a ganglioneuritis characterized by degeneration of perikarya and infiltration of macrophages and lymphocytes. However, the extent of inflammation differed between the two groups. Two days after infection with PrV-9112C2R, we observed a ganglioneuritis which increased in severity until 6 dpi (see Fig. 5). After infection with PrV-9112C2, ganglioneuritis was first observed at 3 dpi and was significantly less severe. Conversely, in the olfactory bulb and pedunculi, infection with PrV-9112C2 resulted in a severe nonsuppurative encephalitis starting at day 3 p.i. (Fig. 4 and 5), whereas infection with PrV-9112C2R led to only a mild encephalitis at day 2 and a delayed severe encephalitis starting only on day 5 p.i. (Fig. 4 and 5). In the other areas of the brain, both mutants caused also a nonsuppurative encephalitis characterized by perivascular infiltration with lymphocytes and macrophages, multifocal gliosis, and neuronal degeneration. However, the time of onset and the extent of the encephalitic foci were different for the two mutants (Fig. 4 and 5). In the medulla oblongata and pons, moderate inflammation was caused by PrV-9112C2R between 2 and 8 dpi. In contrast, PrV-9112C2 infection resulted in only minimal encephalitic lesions between 2 and 5 dpi but induced severe inflammation on days 6 and 7 p.i. (Fig. 4 and 5). Similar differences were found in the mesencephalon, diencephalon, cerebrum, and cerebellum, although the severity of encephalitis was less than in the medulla and pons. Interestingly, the onset and extent of encephalitic changes correlated well with the onset and severity of neurological symptoms in PrV-9112C2-infected animals.
FIG. 5.
Histopathological alterations after infection with PrV-9112C2R (A to C) or PrV-9112C2 (D to F) visualized by hematoxylin-eosin staining. (A) Ganglion trigeminale, 2 dpi, ganglioneuritis, degeneration of perikarya, and focal infiltrations with macrophages and lymphocytes; magnification, ×200. (B) Medulla oblongata, 2 dpi, encephalitis, blood vessel with perivascular infiltrations (left) and prominent glia herds (right); ×200. (C) Bulbus olfactorius, 5 dpi, encephalitis, small vessels with perivascular infiltrates, and severe focal gliosis within the granular layer; ×100. (D) Ganglion trigeminale, 2 dpi, no inflammatory lesions at this time point; ×150. (E) B. olfactorius, 3 dpi, encephalitis, perivascular infiltrations within the glomerula olfactoria (bottom) and the molecular layer (top); ×240. (F) Pons, 6 dpi, encephalitis, severe perivascular infiltrates, and focal gliosis; ×100.
FIG. 4.
Comparison of the number of encephalitic foci detected after infection with PrV-9112C2R (shaded columns) or PrV-9112C2 (black columns) between days 2 to 8 p.i. in different regions of the brain. Each column represents the total number of encephalitic lesions within 25 mm2 per section and animal. M, medulla; B, bulbus; P, pedunculus.
DISCUSSION
One of the hallmarks of alphaherpesviruses is their ability to invade the host nervous system and productively replicate or establish latency in neuronal cells (8, 9). Natural infection by PrV normally occurs via the oronasopharyngeal route, with primary replication taking place in the oronasopharyngeal mucosa, mainly in nonneuronal cells, which results in severe respiratory disorder. Subsequently, neuroinvasion occurs by infection of nerve endings in the nasal mucosa and spread via infected nerves toward the CNS, resulting in a nonsuppurative meningoencephalitis (5, 8). In this stage, virus can be isolated from the olfactory bulb, sensory ganglia, tonsils, and lymphoid tissues (3, 34). Pathogenicity and virulence depend on the infecting virus strain, infectious dose, age and constitution of the animal, route of virus uptake, and environmental conditions.
In this study, we demonstrated that infection with the BHV-1 gB-expressing recombinant PrV-9112C2 (19) resulted in a drastic alteration in virulence. When outbred animals housed in parallel were infected with PrV-9112C2, no respiratory symptoms were observed and virus titers in nasal secretions were 100- to 1,000-fold lower than after wild-type virus infection. Absence of respiratory symptoms and decreased virus excretion correlated with inability of the mutant virus to infect the nasal epithelium to the same extent as wild-type or revertant virus. Immunohistological analyses showed absence of infected cells and viral lesions in the regio cutanea and less efficient infection in the respiratory epithelium. In contrast, the olfactory epithelium was infected to a greater extent than after infection with revertant PrV-9112C2R. Thus, PrV-9112C2 appears to replicate preferentially in the olfactory epithelium. Although no PrV-9112C2-infected cells were observed in the regio cutanea, infectious virus could be recovered from this part of the nasal mucosa, possibly due to contamination by nasal secretions derived from the upper parts of the nasal mucosa.
After intranasal inoculation, PrV-9112C2 replicated efficiently in the olfactory epithelium and to a lesser extent also in the respiratory epithelium, whereas infected cells were not detected in the regio cutanea. This finding indicates that the virus has lost the ability to infect these epithelial cells directly. However, in the regio olfactoria and bordering areas of the regio respiratoria there are olfactory receptor cells which represent free-ending bipolar nerve cells. Their cell body is part of the epithelial layer and surrounded by sustentacular and basal cells. The dendrite extends from the cell body to the periphery and ends as a small swelling with extremely long cilia (47). We speculate that PrV-9112C2 may directly infect olfactory cells via their dendrites and spread to neighboring nonneuronal cells by direct viral cell-to-cell spread.
Neuroinvasion of PrV occurs by ascending within the axon via anterograde and retrograde transport (2, 4, 6, 20, 21, 30). Innervation of the nasal mucosa is provided by the olfactory and trigeminal nerves. Whereas the mucocutaneous and respiratory epithelial layers of the nasal mucosa are supplied by branches of the trigeminal nerve, the olfactory epithelium is supplied by both the olfactory and trigeminal nerves, with olfactory receptor cells themselves being part of the olfactory nerve. Correlating with its increased ability to replicate in the olfactory epithelium, PrV-9112C2 efficiently replicated in the olfactory bulb already at early stages after infection, as demonstrated by virus isolation and immunofluorescence, whereas the trigeminal ganglion appeared to be less infected. In contrast, PrV-9112C2R was detected primarily in the trigeminal pathway, with a more severe ganglioneuritis in the trigeminal ganglion during the first 5 days after infection. However, extensive spread in the CNS was not observed, which explains the only sporadic neurological symptoms. PrV-9112C2 infection produced severe neurological disorders starting at day 5 p.i., which correlates well with viral invasion of the brain which was detected at the same time.
PrV infects a wide range of neurons in the peripheral and CNS, which makes it a preferred candidate for tracing neural circuits in the brain (9, 26, 41). Infection directs a focused, temporally organized response of microglia and brain macrophages to areas of pathological alterations (4, 36, 38). It was speculated that infiltrating lymphocytes may be directed by signals from infected neurons or surrounding glial cells, e.g., astrocytes, by expressing adhesion molecules and chemoattractants. In this study, we counted histological alterations including focal lesions, perivascular infiltrations, and gliosis and found significantly more alterations in the CNS of PrV-9112C2-infected animals. This observation explains the more severe clinical symptoms and confirms that expression of BHV-1 gB in a PrV-gB− strain results in a dramatic increase in neurovirulence.
Encephalitic lesions in the CNS were particularly pronounced after infection with PrV-9112C2 in the medulla oblongata and pons at days 6 and 7 p.i.; in comparison, PrV-9112C2R infection produced encephalitic foci from 2 dpi onward at lower levels. Since both areas are directly accessible via the trigeminal pathway, both viruses could reach these target tissues via the ganglion trigeminale. Virus detection and inflammation in the ganglion trigeminale, the extent of encephalitis in the medulla oblongata and pons, and the significant decrease of intensity of encephalitis in the mesencephalon and diencephalon argue against a preferential accession to these areas via the olfactory pathway after PrV-9112C2 infection. However, we cannot exclude infection of the brain stem by PrV-9112C2 via the olfactory pathway through the brain. Unfortunately, localization of encephalitic lesions in medulla oblongata and pons did not allow any conclusion as to the preferred route of invasion. The late onset of neurological symptoms after PrV-9112C2 infection may be due to a less efficient use of the trigeminal pathway, as indicated by the limited presence of PrV-infected cells in the trigeminal ganglion (Table 3). Surprisingly, virus could be isolated at relatively high titers (Table 2). The reason for this discrepancy is unclear. However, once it reaches the brain, PrV-9112C2 is able to rapidly spread to different parts of the CNS, which parallels the development of severe neurological disorders. In contrast, PrV-9112C2R did not spread to a significant extent in the CNS, and symptoms were only sporadically observed. Thus, the preferential infection of the olfactory pathway by PrV-9112C2 may be unrelated to the increase in neurovirulence in the CNS, which could constitute two separate manifestations of the gB exchange.
The molecular basis for neurovirulence of alphaherpesviruses is still largely unknown. Characterization of attenuated strains or genetically engineered virus mutants allowed the identification of several gene products, such as viral glycoproteins or enzymes which affect neurovirulence (reviewed in reference 26). The glycoprotein E homologues are probably the best-analyzed envelope glycoproteins with an effect on viral neuroinvasion. Deletion of gE from the PrV genome has been shown to decrease virulence for pigs (28) by a block in infection of secondary neurons (20, 21, 30). Replication in the nasal mucosa and infection of primary neurons by gE-deleted virus strains probably is not or only slightly affected by deletion of gE (16, 20, 21). gE is also important for infection of certain neuronal circuits in the rat brain (6). It is generally believed that the noncovalently linked complex of gE and gI (49) is responsible for this effect since gI deletion mutants are also attenuated in neurovirulence (12, 21, 46). A defect in neuronal spread in the absence of the PrV gE/I proteins could partially be complemented by the homologous BHV-1 proteins in a rodent model (18). However, virulence and CNS invasiveness may be separate phenotypic manifestations of the role of gE in neuronal infection, since in the rat model, mutations in the carboxy terminus of gE significantly increase the survival time of the animals despite a more extensive spread in the CNS as compared to wild-type virus (44).
So far, the essential glycoprotein gB has been implicated in viral pathogenesis and virulence by several groups. Absence of gB results in loss of infectivity, as observed in cell culture, and after infection of primary target cells in vivo by phenotypically complemented mutant virus, viral spread does not occur (1, 31, 33, 37). Mutations in the cytoplasmic domain of gB resulting in a syncytial phenotype profoundly influence pathogenesis in vivo (7, 10, 42, 45). We show here that exchange of PrV gB by the homologous BHV-1 gB resulted in a dramatic increase in neurovirulence. This was unexpected since analyses in cell culture did not reveal any particular phenotype of recombinant PrV-9112C2 compared to wild-type PrV besides a slight delay in penetration. Also, virus excretion from intranasally infected animals did not indicate any growth advantage of the virus in vivo. However, the efficient replication of PrV-9112C2 in the olfactory epithelium and its successful invasion of the CNS resulted in a dramatic phenotype which we never observed before. Thus, our results demonstrate an important role for alphaherpesvirus gB in neurovirulence.
The reason for this effect of BHV-1 gB expression is unclear. BHV-1 strain Schönböken, from which the gB gene was derived, is not particularly neurovirulent although it can enter neurons and establish latency. Thus, other factors may play a more prominent role than gB in neurotropism of this virus. However, when placed in a different background, in this case PrV, the heterologous gB exerts a profound influence on the biological properties of the recipient virus. BHV-1 gB has been postulated to have receptor-binding properties (22) besides interaction with heparan sulfate, which does apparently not occur in a PrV background (17). Thus, the observed phenomena may involve alterations in receptor recognition. Whatever the reason, in the light of our results, construction and evaluation of herpesvirus vectors expressing foreign proteins should be carried out with proper caution.
ACKNOWLEDGMENTS
This work was supported by the Deutsche Forschungsgemeinschaft (DFG Me 854/3-3).
We thank G. Keil for MAb 42/18/7 and BHV-1 strain Schönböken, H. Wege for MAb 934/H1, H.-J. Rziha for MAb 5/14, B. Nolting and G. Czerwinski for expert technical assistance, J. Teifke for valuable comments on and help with the manuscript, and the animal caretakers R. Brill, K. Franz, D. Bresemann, and J. Spaller for invaluable help.
REFERENCES
- 1.Babic N, Mettenleiter T C, Flamand A, Ugolini G. Role of essential glycoproteins gII and gp50 in transneuronal transfer of pseudorabies virus from the hypoglossal nerve of mice. J Virol. 1993;67:4421–4426. doi: 10.1128/jvi.67.7.4421-4426.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babic N, Mettenleiter T C, Ugolini G, Flamand A, Coulon P. Propagation of pseudorabies virus in the nervous system of the mouse after intranasal inoculation. Virology. 1994;204:616–625. doi: 10.1006/viro.1994.1576. [DOI] [PubMed] [Google Scholar]
- 3.Balasch M, Pujols J, Plana-Duran J, Pumarola M. Study of the persistence of Aujeszky's disease (pseudorabies) virus in peripheral blood mononuclear cells and tissues of experimentally infected pigs. Vet Microbiol. 1998;62:171–183. doi: 10.1016/s0378-1135(98)00208-9. [DOI] [PubMed] [Google Scholar]
- 4.Card J P, Rinaman L, Lynn R B, Lee B H, Meade R P, Miselis R R, Enquist L W. Pseudorabies virus infection of the rat central nervous system: ultrastructural characterization of viral replication, transport and pathogenesis. J Neurosci. 1993;13:2515–2539. doi: 10.1523/JNEUROSCI.13-06-02515.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Card J P, Enquist L W. Neurovirulence of pseudorabies virus. Crit Rev Neurobiol. 1995;9:137–162. [PubMed] [Google Scholar]
- 6.Card J P, Leavitt P, Enquist L W. Different patterns of neuronal infection after intracerebral injection of two strains of pseudorabies virus. J Virol. 1998;72:4434–4441. doi: 10.1128/jvi.72.5.4434-4441.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engel J P, Boyer E P, Goodman J L. Two novel single amino acid syncytial mutations in the carboxy terminus of glycoprotein B of herpes simplex virus type 1 confer a unique pathogenic phenotype. Virology. 1993;192:112–120. doi: 10.1006/viro.1993.1013. [DOI] [PubMed] [Google Scholar]
- 8.Enquist L W. Infection of the mammalian nervous system by pseudorabies virus (PRV) Semin Virol. 1994;5:221–231. [Google Scholar]
- 9.Enquist L W, Husak P J, Banfield B W, Smith G A. Infection and spread of alphaherpesviruses in the nervous system. Adv Virus Res. 1998;51:237–347. doi: 10.1016/s0065-3527(08)60787-3. [DOI] [PubMed] [Google Scholar]
- 10.Gage P J, Levine M, Glorioso J C. Syncytium-inducing mutations localize to two discrete regions within the cytoplasmic domain of herpes simplex virus type 1 glycoprotein gB. J Virol. 1993;67:2191–2201. doi: 10.1128/jvi.67.4.2191-2201.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerdts V, Jöns A, Makoschey B, Visser N, Mettenleiter T C. Protection of pigs against Aujeszky's disease by DNA vaccination. J Gen Virol. 1997;78:2139–2146. doi: 10.1099/0022-1317-78-9-2139. [DOI] [PubMed] [Google Scholar]
- 12.Gerdts V, Jöns A, Mettenleiter T C. Potency of an experimental DNA vaccine against Aujeszky's disease in pigs. Vet Microbiol. 1999;66:1–13. doi: 10.1016/s0378-1135(98)00300-9. [DOI] [PubMed] [Google Scholar]
- 13.Gibbs E, Rweyemamu M. Bovine herpesviruses. Part I. Bovine herpesvirus 1. Vet Bull. 1977;47:317–343. [Google Scholar]
- 14.Graham F L, van der Eb A J. A new technique for the assay of infectivity of human adenovirus. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan A S, Vatter A E. A comparison of herpes simplex and pseudorabies viruses. Virology. 1959;7:394–407. doi: 10.1016/0042-6822(59)90068-6. [DOI] [PubMed] [Google Scholar]
- 16.Kimman T G, de Wind N, Oie-Lie N, Pol J M A, Berns A J M, Gielkens A L J. Contribution of single genes within the unique short region of Aujeszky's disease virus (suid herpesvirus type 1) to virulence, pathogenesis, and immunogenicity. J Gen Virol. 1992;73:243–251. doi: 10.1099/0022-1317-73-2-243. [DOI] [PubMed] [Google Scholar]
- 17.Klupp B G, Karger A, Mettenleiter T C. Bovine herpesvirus 1 glycoprotein gB does not productively interact with cell surface heparan sulfate in a pseudorabies virion background. J Virol. 1997;71:4838–4841. doi: 10.1128/jvi.71.6.4838-4841.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knapp A C, Hussak P J, Enquist L W. The gE and gI homologs from two alphaherpesviruses have conserved and divergent neuroinvasive properties. J Virol. 1998;71:5820–5827. doi: 10.1128/jvi.71.8.5820-5827.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kopp A, Mettenleiter T C. Stable rescue of a glycoprotein gII deletion mutant of pseudorabies virus by glycoprotein gI of bovine herpesvirus 1. J Virol. 1992;66:2754–2762. doi: 10.1128/jvi.66.5.2754-2762.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kritas S K, Pensaert M B, Mettenleiter T C. Invasion and spread of single glycoprotein deleted mutants of Aujeszky's disease virus (ADV) in the trigeminal nervous pathway of pigs after intranasal inoculation. Vet Microbiol. 1994;40:323–334. doi: 10.1016/0378-1135(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 21.Kritas S K, Pensaert M B, Mettenleiter T C. Role of gI, gp63 and gIII in the invasion and spread of Aujeszky's disease virus (ADV) in the olfactory nervous pathway of pigs. J Gen Virol. 1994;75:2319–2327. doi: 10.1099/0022-1317-75-9-2319. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, van Drunen Littel-van den Hurk S, Babiuk L A, Liang X. Characterization of cell-binding properties of bovine herpesvirus 1 glycoproteins B, C, and D: identification of a dual cell-binding function of gB. J Virol. 1995;69:4758–4768. doi: 10.1128/jvi.69.8.4758-4768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lukács N, Thiel H-J, Mettenleiter T C, Rziha H-J. Demonstration of three major species of pseudorabies virus glycoproteins and identification of a disulfide-linked glycoprotein complex. J Virol. 1985;53:155–173. doi: 10.1128/jvi.53.1.166-173.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mettenleiter T C. Initiation and spread of α-herpesvirus infections. Trends Microbiol. 1994;2:2–4. doi: 10.1016/0966-842x(94)90335-2. [DOI] [PubMed] [Google Scholar]
- 25.Mettenleiter T C. Pseudorabies (Aujeszky's disease) virus: state of the art. Acta Vet Hung. 1994;42:153–177. [PubMed] [Google Scholar]
- 26.Mettenleiter T C. Molecular properties of alphaherpesviruses used in transneuronal pathway tracing. In: Kaplitt M G, Loewy A D, editors. Viral vectors. San Diego, Calif: Academic Press; 1995. pp. 367–393. [Google Scholar]
- 27.Mettenleiter T C, Spear P G. Glycoprotein gB (gII) of pseudorabies virus can functionally substitute for glycoprotein gB in herpes simplex virus type 1. J Virol. 1994;68:500–504. doi: 10.1128/jvi.68.1.500-504.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mettenleiter T C, Zsak L, Kaplan A, Ben-Porat T, Lomniczi B. Role of a structural glycoprotein of pseudorabies in virus virulence. J Virol. 1987;61:4030–4032. doi: 10.1128/jvi.61.12.4030-4032.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer-Koenig U, Vogelberg C, Bongarts A, Kampa D, Delbruck R, Wolff-Vorbeck G, Kirste G, Haberland M, Hufert F T, von Laer D. Glycoprotein gB genotype correlates with cell tropism in vivo of human cytomegalovirus infection. J Med Virol. 1998;55:75–81. [PubMed] [Google Scholar]
- 30.Mulder W, Jacobs L, Priem J, Kok G, Wagenaar F, Kimman T, Pol J. Glycoprotein E-negative pseudorabies virus has a reduced capability to infect second and third order neurons of the olfactory and trigeminal routes in the porcine central nervous system. J Gen Virol. 1994;75:3095–3106. doi: 10.1099/0022-1317-75-11-3095. [DOI] [PubMed] [Google Scholar]
- 31.Neubauer A, Beer M, Brandmuller C, Kaaden O R, Osterrieder N. Equine herpesvirus 1 mutants devoid of glycoprotein B or M are apathogenic for mice but induce protection against challenge infection. Virology. 1997;239:36–45. doi: 10.1006/viro.1997.8857. [DOI] [PubMed] [Google Scholar]
- 32.Nickel R, Schummer A, Seiferle E. Geruchsorgan. 1984. Lehrbuch der Anatomie der Haustiere, Band IV: Nervensystem, Sinnesorgane, Endokrine Drüsen; Gehirnnerven; pp. 250–290. p. 344–346. Paul Parey, Berlin, Germany. [Google Scholar]
- 33.Peeters B, de Wind N, Hooisma M, Wagenaar F, Gielkens A, Moormann R. Pseudorabies virus envelope glycoproteins gp50 and gII are essential for virus penetration, but only gII is involved in membrane fusion. J Virol. 1992;66:894–905. doi: 10.1128/jvi.66.2.894-905.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pensaert M, Kluge J. Pseudorabies virus (Aujeszky's disease) In: Pensaert M, editor. Virus infections of porcines. Amsterdam, The Netherlands: Elsevier Science Publishers; 1989. pp. 39–64. [Google Scholar]
- 35.Pereira L. Function of glyoprotein B homologues of the family herpesviridae. Infect Agents Dis. 1994;3:9–28. [PubMed] [Google Scholar]
- 36.Rassnick S, Enquist L W, Sved A F, Card P. Pseudorabies virus-induced leukocyte trafficking into the rat central nervous system. J Virol. 1998;72:9181–9191. doi: 10.1128/jvi.72.11.9181-9191.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rauh I, Weiland F, Fehler F, Keil G M, Mettenleiter T C. Pseudorabies virus mutants lacking the essential glycoprotein gII can be complemented by glycoprotein gI of bovine herpesvirus 1. J Virol. 1991;65:621–631. doi: 10.1128/jvi.65.2.621-631.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rinaman L, Card J P, Enquist L W. Spatiotemporal responses of astrocytes, ramified microglia, and brain macrophages to central neuronal infection with pseudorabies virus. J Neurosci. 1993;13:685–702. doi: 10.1523/JNEUROSCI.13-02-00685.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roizman B, Desrosiers R C, Fleckenstein B, Lopez C, Minson A C, Studdert M J. The family of herpesviridae: an update. Arch Virol. 1992;123:425–449. doi: 10.1007/BF01317276. [DOI] [PubMed] [Google Scholar]
- 40.Rziha H-J, Mettenleiter T C, Ohlinger V, Wittmann G. Herpesvirus (pseudorabies virus) latency in swine: occurrence and physical state of viral DNA in neural tissues. Virology. 1986;155:600–613. doi: 10.1016/0042-6822(86)90220-5. [DOI] [PubMed] [Google Scholar]
- 41.Sams J M, Jansen A S, Mettenleiter T C, Loewy A D. Pseudorabies virus mutants as transneuronal markers. Brain Res. 1995;687:182–190. doi: 10.1016/0006-8993(95)00484-8. [DOI] [PubMed] [Google Scholar]
- 42.Sivadon V, Lebon P, Rozenberg F. Variations of HSV-1 glycoprotein B in human herpes simplex encephalitis. J Neurovirol. 1998;4:106–114. doi: 10.3109/13550289809113488. [DOI] [PubMed] [Google Scholar]
- 43.Spear P. Entry of alphaherpesviruses into cells. Semin Virol. 1993;4:167–180. [Google Scholar]
- 44.Tirabassi R S, Townley R A, Eldrige M G, Enquist L W. Characterization of pseudorabies virus mutants expressing carboxy-terminal truncations of gE: evidence for envelope incorporation, virulence, and neurotropism domains. J Virol. 1997;71:6455–6464. doi: 10.1128/jvi.71.9.6455-6464.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tugizov S, Wang Y, Qadri I, Navarro D, Maidji E, Pereira L. Mutated forms of human cytomegalovirus glycoprotein B are impaired in inducing syncytium formation. Virology. 1995;209:580–591. doi: 10.1006/viro.1995.1290. [DOI] [PubMed] [Google Scholar]
- 46.Whealy M E, Card J P, Robbins A K, Dublin J R, Rziha H J, Enquist L W. Specific pseudorabies infection of the rat visual system requires both gI and gp63 glycoproteins. J Virol. 1993;67:3786–3797. doi: 10.1128/jvi.67.7.3786-3797.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wheater P R, Burkitt H G, Daniels V G. Functional histology, a text and colour atlas. 2nd ed. 1987. , revised by P. R. Wheater and H. G. Burkitt. Churchill Livingstone, Edinburgh, Scotland. [Google Scholar]
- 48.Yuhasz S A, Stevens J G. Glycoprotein gB is a specific determinant of herpes simplex virus type 1 neuroinvasiveness. J Virol. 1993;67:5948–5954. doi: 10.1128/jvi.67.10.5948-5954.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zuckermann F, Mettenleiter T C, Schreurs C, Sugg N, Ben-Porat T. Complex between glycoproteins gI and gp63 of pseudorabies virus: its effect on virus replication. J Virol. 1988;62:4622–4626. doi: 10.1128/jvi.62.12.4622-4626.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]