Abstract
Naturally occurring transmissible spongiform encephalopathy (TSE) diseases such as bovine spongiform encephalopathy in cattle are probably transmitted by oral or other peripheral routes of infection. While prion protein (PrP) is required for susceptibility, the mechanism of spread of infection to the brain is not clear. Two prominent possibilities include hematogenous spread by leukocytes and neural spread by axonal transport. In the present experiments, following oral or intraperitoneal infection of transgenic mice with hamster scrapie strain 263K, hamster PrP expression in peripheral nerves was sufficient for successful infection of the brain, and cells of the spleen were not required either as a site of amplification or as transporters of infectivity. The role of tissue-specific PrP expression of foreign PrP in interference with scrapie infection was also studied in these transgenic mice. Peripheral expression of heterologous PrP completely protected the majority of mice from clinical disease after oral or intraperitoneal scrapie infection. Such extensive protection has not been seen in earlier studies on interference, and these results suggested that gene therapy with mutant PrP may be effective in preventing TSE diseases.
Prion protein (PrP) plays an important role in transmissible spongiform encephalopathy (TSE) diseases (for a review, see reference 10). For example, the presence of both the normal prion protein, PrP-sen, and the abnormal protease-resistant prion protein, PrP-res, is required for the neurodegeneration associated with TSE diseases (4, 8). In addition, after natural infection at oral or other peripheral sites, PrP may play an essential role in the transport of infectivity to the brain. Previous evidence implied that both the nervous system and the lymphoreticular system might be involved in scrapie neuroinvasion. Transport of scrapie to the central nervous system (CNS) by peripheral nerve axons was suggested by experiments showing a delay in disease onset after sectioning the sciatic nerve following scrapie infection in the hindlimb (24). Furthermore, following intraocular inoculation (43), spread of scrapie within the CNS was shown to follow known neuroanatomical pathways, and after oral or intraperitoneal infection the initial sites of detection of abnormal PrP or infectivity in the CNS were consistent with entry via the vagus nerve or other peripheral nerves (1, 9, 27, 28). The role of the lymphoreticular organs in transport to the brain was also suggested by several observations. In mice, hamsters, and sheep, the spleen and lymphoid tissues are early sites of accumulation or replication of the scrapie agent following intraperitoneal (i.p.) inoculation (15, 22, 25, 27, 28). In knockout mice lacking functional B lymphocytes and follicular dendritic cells, a reduced incidence of clinical disease was seen after peripheral scrapie infection (29). However, the required B lymphocytes need not express PrP and appear to function by inducing the development of functional PrP-positive follicular dendritic cells (30), which may be important sites of accumulation of abnormal PrP and agent replication (13, 18, 19). Last, splenectomy prior to or shortly after i.p. infection delays the onset of clinical disease in mice (12, 28). These observations raise the possibility that amplification of infectivity in lymphoid tissues is required for neuroinvasion and even suggested the possibility that circulating lymphoid cells themselves are involved in spreading of TSE infectivity to the brain (6). However, peripheral nerve and lymphoid tissues may both be involved in neuroinvasion depending on the dose or strain of TSE agent involved. For example, in experiments with SCID mice, after i.p. infection with high doses of ME7 mouse scrapie agent, replication in the spleen was not required for CNS infection and disease, whereas after i.p. infection with low doses of ME7, normal lymphoreticular tissues were needed for replication of agent prior to neuroinvasion (17, 31).
Until recently it has been difficult to experimentally separate roles played by the neural and hematogenous routes of infection in live animals. However, new approaches have been facilitated by the development of transgenic (Tg) and knockout animals. By adoptive transfer experiments in PrP knockout mice, PrP-positive bone marrow-derived cells were found to be required for scrapie infection of spleen, but this was not sufficient to mediate neuroinvasion (3). These results indicated that a nonhematopoietic PrP-positive tissue was required for neuroinvasion. The present studies with Tg and knockout mice expressing hamster PrP (HaPrP) under the control of the neuron-specific enolase (NSE) promoter now identify this essential tissue as PrP-positive peripheral nerves, and these results suggest that peripheral nerves may be the final common pathway for neuroinvasion in vivo. These experiments show further that heterologous PrP present on nonneuronal cells can completely prevent clinical CNS disease in certain Tg mice after oral or i.p. scrapie infection, suggesting that interactions between the scrapie agent inoculated and PrP expressed on neural and nonneural cells in the periphery may be an important site on which to focus potential therapeutic strategies for TSE diseases.
MATERIALS AND METHODS
Production of Tg mice.
Derivation of NSE-HaPrP Tg mice has been described previously (37). A 1.0-kb fragment of hamster PrP cDNA including the 762-bp open reading frame was included in the construct used (41). Tg7-HaPrP Tg mice were generated by using a cosmid vector containing the HaPrP gene plus 40 kb of flanking DNA as previously described (37, 44). To obtain NSE-HaPrP and Tg7-HaPrP Tg mice which express HaPrP but not MoPrP, both types of Tg mice were bred to MoPrP-negative mice (32) and the offspring were interbred and selected by PCR typing of DNA (37, 38). (+/+) designates MoPrP-positive mice, and (−/−) denotes MoPrP-negative (knockout) mice.
Scrapie infection.
Tg mice were inoculated intracerebrally (i.c.), i.p., or orally with hamster scrapie strain 263K. Mice inoculated i.c. or i.p. received 1 × 107 i.c. 50% lethal doses (LD50) in 50 μl of physiological buffer. Mice inoculated orally received 2 × 108 i.c. LD50 in 100 μl via a small-diameter flexible polypropylene catheter inserted over the base of the tongue about 1 to 2 cm into the esophagus. Splenectomy was performed under Metafane anesthesia 10 to 14 days prior to scrapie infection in certain experiments as indicated. The mice were observed several times each week for clinical signs of scrapie, which included weight loss, kyphosis, ataxia, and an exaggerated high-stepping gait most noticeable in the hindlimbs. Mice exhibiting short incubation periods (less than 100 days) died within 1 to 4 days after the appearance of clinical symptoms, whereas mice exhibiting longer incubation periods had clinical disease and died after 7 to 10 days. Brain samples from mice in each group with clinical evidence of scrapie were analyzed for HaPrP-res to confirm the clinical diagnosis. HaPrP-res was detected by immunoblotting as described previously (37). Additional brains recovered 600 to 700 days postinoculation from a few clinically normal NSE-HaPrP/MoPrP(+/+) mice were analyzed for HaPrP-res. Absence of HaPrP-res confirmed the scrapie-negative status of these mice.
RT-PCR analysis.
Total RNA was purified from various tissues by using Trizol (Gibco BRL) as specified by the manufacturer. A 1.0-μg portion of RNA was used in a One Step reverse transcription-PCR (RT-PCR) system (Gibco BRL) with upper- and lower-strand HaPrP oligonucleotides described previously (29). Reactions were run for one cycle at 50°C for 30 min and then for 40 cycles consisting of 94°C for 30 s, 50°C for 30 s, and 72°C for 1 min, followed by a 10-min extension at 72°C at the end. The products were then analyzed on 2% acrylamide gels as usual.
PrP analysis by immunoblotting.
A 2-mg portion of tissues from mice were analyzed for the presence of HaPrP by using Western blotting techniques described previously (37). Leukocytes were buffy coat cells obtained after centrifugation of heparinized whole blood. For intestine and Peyer's patches, full-thickness excision biopsies of the intestinal wall were performed with curved scissors at the sites of grossly visible Peyer's patches. Axillary and mesenteric lymph nodes were also tested by Western blotting and were found to be negative (data not shown). HaPrP was detected by using monoclonal antibody 3F4, which reacts with hamster PrP but not with mouse PrP (23). 3F4 ascites fluid was used at a dilution of 1/50,000. To verify the PrP specificity of protein bands, duplicate Western blots were developed with monoclonal antibody preabsorbed by incubation of 50 ml of diluted ascites with 3.4 μg of synthetic HaPrP peptide, p106-126, containing the epitope recognized by 3F4.
RESULTS
Generation of trangenic mice.
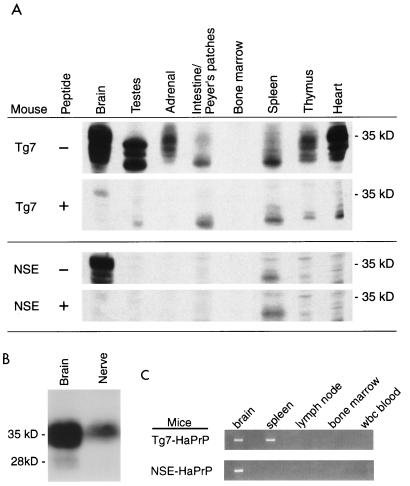
To study the effects of tissue-restricted HaPrP expression on the transport of infectivity from peripheral sites to the brain, NSE-HaPrP/MoPrP(−/−) mice, which express HaPrP by using the NSE promoter (37) but lack a functional mouse PrP (MoPrP) gene (MoPrP knockout), were compared with Tg7-HaPrP/MoPrP(−/−) mice, which express HaPrP in many tissues under control of the endogenous mouse PrP promoter. By Western blotting Tg7-HaPrP/MoPrP(−/−) mice were HaPrP positive in the brain, sciatic nerve, testes, adrenal gland, intestinal Peyer's patches, spleen, thymus, and heart whereas NSE-HaPrP/MoPrP(−/−) mice were HaPrP positive only in the brain and sciatic nerve (Fig. 1A and B). Because of the potential importance of the hematopoietic system in scrapie infection (15), HaPrP mRNA expression was also analyzed in hematopoietic tissues by RT-PCR. NSE-HaPrP/MoPrP(−/−) mice were positive for HaPrP in the brain but negative in the spleen, bone marrow, and circulating leukocytes (Fig. 1C). In contrast, Tg7-HaPrP/ MoPrP(−/−) mice were positive for HaPrP mRNA in both the brain and the spleen. Therefore, the present data confirmed the predominant neuronal specificity of HaPrP expression in NSE-HaPrP/MoPrP(−/−) mice, which was shown in previous experiments by in situ hybridization of brain sections (37), and this was consistent with other transgenes expressed by using this same promoter (39, 40).
FIG. 1.
HaPrP detection in various tissues of Tg7-HaPrP/MoPrP(−/−) and NSE-HaPrP/MoPrP(−/−) transgenic mice. (A) Western blots containing 2 mg of tissue extracts were developed with PrP-specific monoclonal antibody 3F4 (23). To verify the PrP specificity of protein bands, duplicate Western blots were developed with monoclonal antibody preabsorbed by incubation of 50 ml of diluted ascites with 3.4 μg of synthetic HaPrP peptide, p106–126, containing the epitope recognized by 3F4. HaPrP bands are specifically eliminated by the peptide competition. These results were consistent with previous findings where other tissues, including lung and muscle, from NSE-HaPrP mice were analyzed and found to be negative (37). (B) Immunoblot detection of HaPrP in the brain and sciatic nerve of NSE-HaPrP/MoPrP(−/−) mice. Brain (0.15 mg) or pooled sciatic nerves (0.5 mg) were loaded per lane, and Western blots were developed as above. (C) RT-PCR detection of HaPrP mRNA from the brain (CNS) and various lymphoreticular tissues of transgenic mice. Insufficient RNA was recovered from the sciatic nerve for testing by RT-PCR. All tissues shown were positive for detection of β-actin mRNA by RT-PCR (data not shown).
Susceptibility to scrapie infection by various routes.
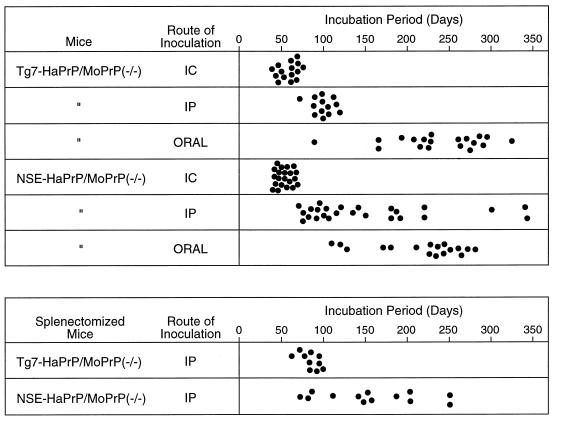
To determine the susceptibility of these transgenic mice to various routes of scrapie infection, mice were infected with hamster scrapie agent, strain 263K, by the i.c., oral, or i.p. route. As expected, Tg7-HaPrP/MoPrP(−/−) mice were 100% susceptible to infection by all three routes, although the incubation periods varied significantly with each route (Fig. 2, top). Because NSE-HaPrP/MoPrP(−/−) mice did not express HaPrP in the lymphoreticular system, we expected that they might be resistant to infection by the oral or i.p. routes. However, in our experiments, all NSE-HaPrP/MoPrP(−/−) mice infected by both these routes developed clinical scrapie (Fig. 2, top), indicating that HaPrP expression controlled by the NSE promoter was sufficient for the transport of scrapie infectivity from the peritoneal cavity or the gastrointestinal tract to the brain. Previous results suggested that PrP-positive tissues such as peripheral nerves might account for such transport from the periphery (3, 27, 28), and our Western blot analysis of sciatic nerves of NSE-HaPrP/MoPrP(−/−) mice was positive (Fig. 1B). Because all the lymphoreticular tissues suspected to be involved in scrapie neuroinvasion following peripheral routes of inoculation were found to be negative for expression of HaPrP, these data support the possibility that peripheral nerves are the only HaPrP-expressing peripheral tissue required for neuroinvasion after oral or i.p. infection with hamster scrapie strain 263K.
FIG. 2.
(Top) Time from inoculation until severe clinical disease in NSE-HaPrP/MoPrP(−/−) and Tg7-HaPrP/MoPrP(−/−) transgenic mice infected with hamster scrapie strain 263K by the i.c., oral, and i.p. routes. The doses used were 1 × 107 i.c. LD50 for the i.c. and i.p. routes and 2 × 108 i.c. LD50 for the oral route. (Bottom) Effect of splenectomy on the susceptibility of transgenic mice to i.p. infection with 107 i.c. LD50 of hamster scrapie strain 263K. Splenectomy was done 10 to 14 days prior to scrapie infection.
Role of the spleen in neuroinvasion.
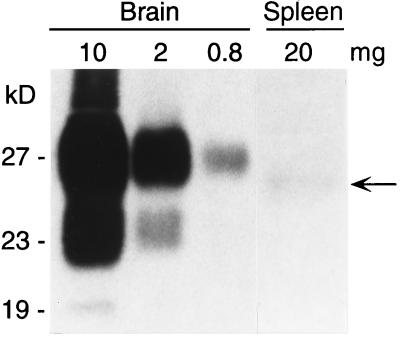
In mice, the spleen is infected after peripheral scrapie infection, and for some scrapie strains, amplification of infectivity in the spleen may be necessary before neuroinvasion can occur. In the present experiments infectivity was analyzed indirectly by testing spleen homogenates for abnormal proteinase K-resistant PrP (PrP-res) by Western blotting. In NSE-HaPrP/MoPrP(−/−) mice infected i.p. with hamster strain 263K, a possible PrP-res band was detected at very low levels in the spleen at the time of clinical disease (Fig. 3). The amount of PrP-res in this spleen-derived band was 100- to 200-fold lower than in the brain. However, it was not clear from this result whether this low-level accumulation of PrP-res in the spleen was required for neuroinvasion. Therefore, the role of the spleen in the present Tg mouse system was analyzed directly by splenectomy. NSE-HaPrP/MoPrP(−/−) mice and Tg7-HaPrP/MoPrP(−/−) mice were splenectomized 10 to 14 days prior to i.p. infection with the 263K strain of hamster scrapie. In these experiments, splenectomy failed to significantly alter the range of incubation periods seen in either line of Tg mice (Fig. 2, bottom), which indicated that in this system spleen cells were not needed either as amplifiers of peripheral scrapie infection or as transporters of the infectivity to the CNS.
FIG. 3.
Western blot detection of PrP-res in the brains and spleens of NSE-HaPrP/MoPrP(−/−) mice 195 days after i.p. inoculation with 107 i.c. LD50 of hamster scrapie strain 263K. The milligram equivalents loaded in each lane are shown above the lane itself. The blot was developed with monoclonal antibody 3F4 (23). The faint band in lane Spleen (arrow), which was more intense after longer exposures, had a lower molecular mass than brain-derived PrP-res; however, it is well documented that the PrP-res apparent molecular mass can vary in different organs depending on differences in glycosylation (36).
Prevention of disease by peripheral expression of foreign PrP.
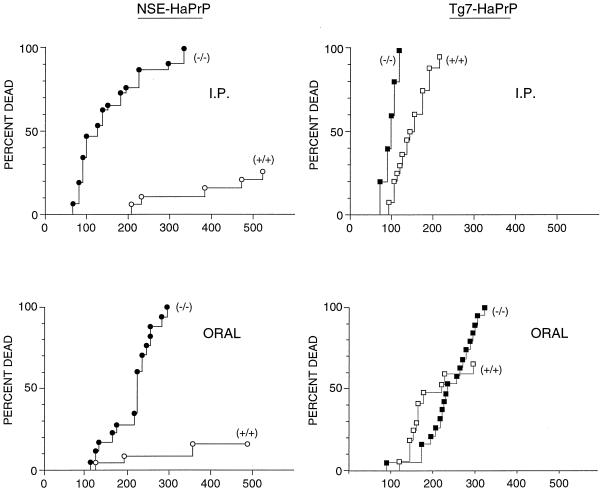
Resistance to TSE disease has been seen when two different PrP molecules are expressed in the same individual (21, 34, 37, 44). Such resistance might be due to competition between differing PrP molecules for agent binding or during replication. To investigate the possible role of differences in tissue sites of PrP expression in this process, NSE-HaPrP and Tg7-HaPrP Tg mice with (+/+) or without (−/−) intact mouse PrP genes were infected with hamster scrapie strain 263K. Since natural TSE infections in animals and humans are believed to occur by peripheral routes, in the present experiments we used not only i.c. but also i.p. and oral routes of infection to study the effect of expression of “heterologous” mouse PrP-sen molecules on susceptibility to hamster scrapie. Consistent with previous reports (37), following i.c. infection a 40-day delay in the time of death was seen in NSE-HaPrP mice expressing MoPrP (data not shown). More notably, a marked increase in resistance to disease was seen after both i.p. and oral inoculation of these mice. At 600 days postinfection, 75 to 80% of NSE-HaPrP mice expressing mouse PrP showed no symptoms and appeared to have completely escaped clinical disease following either oral or i.p. administration of scrapie (Fig. 4). The ability to totally prevent clinical disease in susceptible animals by expression of heterologous PrP has not been observed previously (37, 44). Partial interference was also seen after oral or i.p. infection of Tg7-HaPrP mice, but the level of protection was much lower (Fig. 4). Because Tg7-HaPrP mice and NSE-HaPrP mice have similar PrP expression levels in neurons but differ extensively in expression in nonneural tissues (Fig. 1), it is likely that the higher ratio of mouse PrP to hamster PrP in peripheral lymphoid tissues of NSE-HaPrP mice than of Tg7-HaPrP mice might be important to the increased protection observed in NSE-HaPrP mice.
FIG. 4.
Effect of MoPrP expression [(+/+) versus (−/−)] on the incidence of hamster scrapie in NSE-HaPrP and Tg7-HaPrP mice inoculated i.p. or orally with hamster scrapie agent, strain 263K. Mice inoculated i.p. received 1 × 107 i.c. LD50, and those inoculated orally received 2 × 108 i.c. LD50. The vertical axis shows cumulative deaths due to scrapie. The horizontal axis indicates days postinoculation. ●, NSE-HaPrP/MoPrP(−/−) mice (n = 21 for i.p., n = 17 for oral); ○, NSE-HaPrP/MoPrP(+/+) mice (n = 20 for i.p., n = 19 for oral); ■, Tg7-HaPrP/MoPrP(−/−) mice (n = 13 for i.p., n = 19 for oral); □, Tg7-HaPrP/MoPrP(+/+) mice (n = 16 for i.p., n = 17 for oral).
DISCUSSION
Our data obtained by using Tg mice with tissue-specific HaPrP expression provide a new approach demonstrating the crucial role of PrP-positive peripheral nerves in the process of neuroinvasion following peripheral infection. In the present experiments with the hamster scrapie agent, HaPrP expression in neurons was sufficient for infection of the brain not only after i.c. infection but also after oral or i.p. infection. The oral route is probably the most common in many natural situations, and these results suggest that even with this route some strains of TSE agent may be capable of bypassing the lymphoreticular system and may proceed directly to the brain via peripheral nerves. If so, these strains may be less amenable to treatment by drugs which have difficulty entering the CNS.
In our experiments, we cannot exclude the possibility that very low levels of HaPrP are produced in the lymphoreticular tissues, even though we found them to be negative by RT-PCR. However, such low levels of PrP expression would be unlikely to influence scrapie susceptibility since no such influence was observed in previous low-expressor HaPrP transgenic mice (37, 44).
Furthermore, in our experiments the spleen was not required for successful neuroinvasion, since splenectomy had no influence on the timing of neuroinvasion (Fig. 2, bottom). Nevertheless, we did detect small amounts of a possible PrP-res band in the spleens of i.p.-infected NSE-HaPrP/MoPrP(−/−), mice which could be an indication of associated infectivity in the spleen. However, the source of this PrP is not obvious. Since the spleens of such mice were negative for PrP mRNA by RT-PCR, it seems possible that this PrP-res is derived from PrP-positive nerves ennervating the spleen. This was suggested by the observation that the axons of the sciatic nerve were found to be positive for PrP protein by Western blotting and negative for PrP mRNA, presumably because the PrP mRNA in nerve cells is located primarily in the cell bodies nearer to the nuclei rather than in the axons. Alternatively, it is possible that splenic PrP-res was generated elsewhere and subsequently sequestered in the spleen.
The tight clustering of the incubation periods in i.c.-inoculated Tg mice of both strains was in contrast to the wide range of incubation periods seen in orally infected mice (Fig. 2). This broadening of the incubation period suggested that the infectious dose used might have been close to the end-point titer in mice infected orally. A similar broad range of values was also seen in i.p.-inoculated NSE-HaPrP/MoPrP(−/−) mice but not in Tg7-HaPrP/MoPrP(−/−) mice, suggesting that at limiting infectivity doses, amplification of infectivity in the lymphoreticular organs of these latter mice might make them more sensitive to the dose of scrapie agent used here.
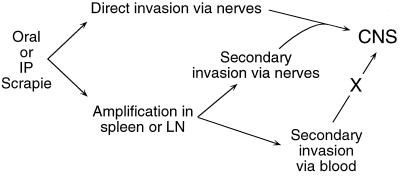
Our results support the earlier findings of others using nerve transection to show that infectivity could be transported from the periphery via axons (24). They are also similar to previous experiments with SCID mice infected i.p. with a high dose of mouse scrapie strain ME7, where neuroinvasion also appeared to proceed directly via peripheral nerves without a susceptible lymphoreticular system or evidence of replication in the spleen (17). Together with these results, our data suggest a unifying concept for neuroinvasion by TSE agents. After high-dose peripheral infection with many TSE strains including 263K and ME7, direct neuroinvasion via nerves might occur, whereas after lower-dose infection or infection with less neuroinvasive isolates, amplification in follicular dendritic cells in lymphoid tissues might be necessary prior to neuroinvasion via peripheral nerves (Fig. 5). A similar interpretation has been proposed previously following experiments suggesting that TSE agents could vary considerably in their neuroinvasiveness (20, 26).
FIG. 5.
Possible pathways of neuroinvasion after peripheral scrapie infection. Direct invasion of the CNS by nerves may occur after peripheral infection with high doses of agent or exposure to highly neuroinvasive agent strains. Alternatively, after infection with lower doses of agent or with less neuroinvasive agent strains, amplification of infectivity in lymphoreticular organs such as spleen or lymph nodes (LN) might be necessary prior to neuroinvasion via PrP-positive peripheral nerves. Additional experiments with lower doses and different strains of hamster scrapie will be required to confirm this possibility. Neuroinvasion by the hematogenous route may occur only rarely (3). IP, intraperitoneal.
The present results do not exclude the possibility of hematogenous spread of infectivity to the brain via circulating lymphocytes. The best evidence against this possibility comes from earlier studies where replication of scrapie infectivity in the spleen was not by itself sufficient to mediate brain infection in the absence of PrP expression in other peripheral tissues (3). This conclusion is consistent with recent studies showing barely detectable levels of infectivity in the blood, which were only rarely transmissible by blood transfusion (7).
In a previous study we showed that MoPrP expression was required for long-term sequestration of the hamster scrapie agent in mouse brain and spleen tissue (35). These results suggested that MoPrP could act as a receptor for the hamster scrapie agent without necessarily providing the ability to support replication and CNS disease (35). Competition between different PrP molecules as receptors for the agent might form the basis for the resistance to TSE disease seen in our present experiments. This resistance is probably a result of competition between HaPrP and MoPrP as a potential receptor for the inoculated agent (35). In this case, the relative amounts of HaPrP and MoPrP expressed in peripheral tissues would be important determinants of protection. For example, after i.p. or oral infection of NSE-HaPrP mice with the hamster scrapie agent, HaPrP expressed in peripheral nerves (Fig. 1) might not be effective at competing with endogenous MoPrP expressed in lymphoid tissues (2) for interactions with the inoculated agent, resulting in inhibition of the process of infection. In contrast, in Tg7-HaPrP mice, HaPrP and MoPrP genes are driven by the same endogenous PrP promoter and HaPrP (Fig. 1) and MoPrP (2) are probably expressed on similar cells. This might result in less protection against hamster scrapie infection by MoPrP expression in these mice. In the brain, HaPrP is expressed at high levels in both NSE-HaPrP and Tg7-HaPrP mice; therefore MoPrP expressed at similar high levels in the brains of these mice might be less effective at inhibiting i.c. infection with the hamster scrapie agent.
In earlier experiments involving sequential inoculation of two scrapie or Creutzfeld-Jacob disease strains with different rates of pathogenesis, competition or interference between strains was demonstrated (14, 33). The interpretation of these studies was that the two strains were competing for a limited supply of agent-specific receptors. It seems likely now that normal endogenous PrP itself could be the receptor involved. If so, competition between strains would be somewhat analogous to competition between heterologous PrP molecules, as suggested in the present experiments.
Our results indicate that manipulation of the expression of heterologous PrP molecules may be a feasible approach to increase resistance to certain TSE diseases. Although the most obvious plan would be to produce TSE-resistant animals by simply eliminating the PrP gene entirely (8, 32), it is possible that such animals will have serious neurodevelopmental abnormalities (42). In this case, one might be able to prevent such effects by introduction of a homologous PrP gene driven by the NSE promoter. TSE susceptibility might be prevented at the same time by introducing a foreign or truncated PrP gene, which might block both PrP-res formation and disease (11) without providing susceptibility to other TSE strains (16). In addition, lack of PrP expression in lymphoid tissues of such animals might provide additional resistance to infection by TSE agents from foreign species, as suggested by bovine spongiform encephalopathy infection experiments in SCID mice (5). However, before such an approach could be considered, studies would have to determine whether altered PrP molecules themselves induced any deleterious effects (45).
ACKNOWLEDGMENT
This work was supported in part by NIH grant AG04342 to M.O.
Footnotes
Publication 11526-NP from the Division of Virology, Department of Neuropharmacology, Scripps Research Institute.
REFERENCES
- 1.Beekes M, McBride P A, Baldauf E. Cerebral targeting indicates vagal spread of infection in hamsters fed with scrapie. J Gen Virol. 1998;79:601–607. doi: 10.1099/0022-1317-79-3-601. [DOI] [PubMed] [Google Scholar]
- 2.Bendheim P E, Brown H R, Rudelli R D, Scala L J, Goller N L, Wen G Y, Kascsak R J, Cashman N R, Bolton D C. Nearly ubiquitous tissue distribution of the scrapie agent precursor protein. Neurology. 1992;42:149–156. doi: 10.1212/wnl.42.1.149. [DOI] [PubMed] [Google Scholar]
- 3.Blättler T, Brandner S, Raeber A J, Klein M A, Voigtlander T, Weissmann C, Aguzzi A. PrP-expressing tissue required for transfer of scrapie infectivity from spleen to brain. Nature. 1997;389:69–73. doi: 10.1038/37981. [DOI] [PubMed] [Google Scholar]
- 4.Brandner S, Isenmann S, Raeber A, Fischer M, Sailer A, Kobayashi Y, Marino S, Weissmann C, Aguzzi A. Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature. 1996;379:339–343. doi: 10.1038/379339a0. [DOI] [PubMed] [Google Scholar]
- 5.Brown K L, Stewart K, Bruce M E, Fraser H. Severely combined immunodeficient (SCID) mice resist infection with bovine spongiform encephalopathy. J Gen Virol. 1997;78:2707–2710. doi: 10.1099/0022-1317-78-10-2707. [DOI] [PubMed] [Google Scholar]
- 6.Brown P. B lymphocytes and neuroinvasion. Nature. 1997;390:662–663. doi: 10.1038/37733. [DOI] [PubMed] [Google Scholar]
- 7.Brown P, Rohwer R G, Dunstan B C, MacAuley C, Gajdusek D C, Drohan W N. The distribution of infectivity in blood components and plasma derivatives in experimental models of transmissible spongiform encephalopathy. Transfusion. 1998;38:810–816. doi: 10.1046/j.1537-2995.1998.38998408999.x. [DOI] [PubMed] [Google Scholar]
- 8.Bueler H, Aguzzi A, Sailer A, Greiner R A, Autenried P, Aguet M, Weissmann C. Mice devoid of PrP are resistant to scrapie. Cell. 1993;73:1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- 9.Carp R I, Callahan S M, Patrick B A, Mehta P D. Interaction of scrapie agent and cells of the lymphoreticular system. Arch Virol. 1994;136:255–268. doi: 10.1007/BF01321056. [DOI] [PubMed] [Google Scholar]
- 10.Caughey B, Chesebro B. Prion protein and the transmissible spongiform encephalopathies. Trends Cell Biology. 1997;7:56–62. doi: 10.1016/S0962-8924(96)10054-4. [DOI] [PubMed] [Google Scholar]
- 11.Chabry J, Caughey B, Chesebro B. Specific inhibition of in vitro formation of protease-resistant prion protein by synthetic peptides. J Biol Chem. 1998;273:13203–13207. doi: 10.1074/jbc.273.21.13203. [DOI] [PubMed] [Google Scholar]
- 12.Clarke M C, Haig D A. Multiplication of scrapie agent in mouse spleen. Res Vet Sci. 1971;12:195–197. [PubMed] [Google Scholar]
- 13.Clarke M C, Kimberlin R H. Pathogenesis of mouse scrapie: distribution of agent in the pulp and stroma of infected spleens. Vet Microbiol. 1984;9:215–225. doi: 10.1016/0378-1135(84)90039-7. [DOI] [PubMed] [Google Scholar]
- 14.Dickinson A G, Fraser H, McConnell I, Outram G W, Sales D I, Taylor D M. Extraneural competition between different scrapie agents leading to loss of infectivity. Nature. 1975;253:556. doi: 10.1038/253556a0. [DOI] [PubMed] [Google Scholar]
- 15.Eklund C M, Kennedy R C, Hadlow W J. Pathogenesis of scrapie virus infection in the mouse. J Infect Dis. 1967;117:15–22. doi: 10.1093/infdis/117.1.15. [DOI] [PubMed] [Google Scholar]
- 16.Fischer M, Rulicke T, Raeber A, Sailer A, Moser M, Oesch B, Brandner S, Aguzzi A, Weissmann C. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J. 1996;15:1255–1264. [PMC free article] [PubMed] [Google Scholar]
- 17.Fraser H, Brown K L, Stewart K, McConnell I, McBride P, Williams A. Replication of scrapie in spleens of SCID mice follows reconstitution with wild-type mouse bone marrow. J Gen Virol. 1996;77:1935–1940. doi: 10.1099/0022-1317-77-8-1935. [DOI] [PubMed] [Google Scholar]
- 18.Fraser H, Bruce M E, Davies D, Farquhar C F, McBride P A. The lymphoreticular system in the pathogenesis of scrapie. In: Prusiner S B, Collinge J, Powell J, Anderton B, editors. Prion diseases of humans and animals. New York, N.Y: Ellis Horwood; 1992. pp. 308–317. [Google Scholar]
- 19.Fraser H, Farquhar C F. Ionising radiation has no influence on scrapie incubation period in mice. Vet Microbiol. 1987;13:211–223. doi: 10.1016/0378-1135(87)90084-8. [DOI] [PubMed] [Google Scholar]
- 20.Fraser J R. Infectivity in extraneural tissues following intraocular scrapie infection. J Gen Virol. 1996;77:2663–2668. doi: 10.1099/0022-1317-77-10-2663. [DOI] [PubMed] [Google Scholar]
- 21.Goldmann W, Hunter N, Smith G, Foster J, Hope J. PrP genotype and agent effects in scrapie: change in allelic interaction with different isolates of agent in sheep, a natural host of scrapie. J Gen Virol. 1994;75:989–995. doi: 10.1099/0022-1317-75-5-989. [DOI] [PubMed] [Google Scholar]
- 22.Hadlow W J, Kennedy R C, Race R E. Natural infection of Suffolk sheep with scrapie virus. J Infect Dis. 1982;146:657–664. doi: 10.1093/infdis/146.5.657. [DOI] [PubMed] [Google Scholar]
- 23.Kascsak R J, Rubenstein R, Merz P A, Tonna-DeMasi M, Fersko R, Carp R I, Wisniewski H M, Diringer H. Mouse polyclonal and monoclonal antibody to scrapie-associated fibril proteins. J Virol. 1987;61:3688–3693. doi: 10.1128/jvi.61.12.3688-3693.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimberlin R H, Walker C A. Pathogenesis of mouse scrapie: evidence for neural spread of infection to the CNS. J Gen Virol. 1980;51:183–187. doi: 10.1099/0022-1317-51-1-183. [DOI] [PubMed] [Google Scholar]
- 25.Kimberlin R H, Walker C A. Pathogenesis of scrapie (strain 263K) in hamsters infected intracerebrally, intraperitoneally or intraocularly. J Gen Virol. 1986;67:255–263. doi: 10.1099/0022-1317-67-2-255. [DOI] [PubMed] [Google Scholar]
- 26.Kimberlin R H, Walker C A. Incubation periods in six models of intraperitoneally injected scrapie depend mainly on the dynamics of agent replication within the nervous system and not the lymphoreticular system. J Gen Virol. 1988;69:2953–2960. doi: 10.1099/0022-1317-69-12-2953. [DOI] [PubMed] [Google Scholar]
- 27.Kimberlin R H, Walker C A. Pathogenesis of scrapie in mice after intragastric infection. Virus Res. 1989;12:213–220. doi: 10.1016/0168-1702(89)90040-3. [DOI] [PubMed] [Google Scholar]
- 28.Kimberlin R H, Walker C A. The role of the spleen in the neuroinvasion of scrapie in mice. Virus Res. 1989;12:201–211. doi: 10.1016/0168-1702(89)90039-7. [DOI] [PubMed] [Google Scholar]
- 29.Klein M A, Frigg R, Flechsig E, Raeber A J, Kalinke U, Bluethmann H, Bootz F, Suter M, Zinkernagel R M, Aguzzi A. A crucial role for B cells in neuroinvasive scrapie. Nature. 1997;390:687–690. doi: 10.1038/37789. [DOI] [PubMed] [Google Scholar]
- 30.Klein M A, Frigg R, Raeber A J, Flechsig E, Hegyi I, Zinkernagel R M, Weissman C, Aguzzi A. PrP expression in B lymphocytes is not required for prion neuroinvasion. Nat Med. 1998;4:1429–1433. doi: 10.1038/4022. [DOI] [PubMed] [Google Scholar]
- 31.Lasmezas C I, Cesbron J Y, Deslys J P, Demaimay R, Adjou K T, Rioux R, Lemaire C, Locht C, Dormont D. Immune system-dependent and -independent replication of the scrapie agent. J Virol. 1996;70:1292–1295. doi: 10.1128/jvi.70.2.1292-1295.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manson J C, Clarke A R, Hooper M L, Aitchison L, McConnell I, Hope J. 129/Ola mice carrying a null mutation in PrP that abolishes mRNA production are developmentally normal. Mol Neurobiol. 1994;8:121–127. doi: 10.1007/BF02780662. [DOI] [PubMed] [Google Scholar]
- 33.Manuelidis L. Vaccination with an attenuated Creutzfeldt-Jakob disease strain prevents expression of a virulent agent. Proc Natl Acad Sci USA. 1998;95:2520–2525. doi: 10.1073/pnas.95.5.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palmer M S, Dryden A J, Hughes J T, Collinge J. Homozygous prion protein genotype predisposes to sporadic Creutzfeldt-Jakob disease. Nature. 1991;352:340–342. doi: 10.1038/352340a0. [DOI] [PubMed] [Google Scholar]
- 35.Race R, Chesebro B. Scrapie infectivity found in resistant species. Nature. 1998;392:770. doi: 10.1038/33834. [DOI] [PubMed] [Google Scholar]
- 36.Race R, Jenny A, Sutton D. Scrapie infectivity and proteinase K-resistant prion protein in sheep placenta, brain, spleen, and lymph node: implications for transmission and antemortem diagnosis. J Infect Dis. 1998;178:949–953. doi: 10.1086/515669. [DOI] [PubMed] [Google Scholar]
- 37.Race R E, Priola S A, Bessen R A, Ernst D R, Dockter J, Rall G F, Mucke L, Chesebro B, Oldstone M B A. Neuron-specific expression of a hamster prion protein minigene in transgenic mice induces susceptibility to hamster scrapie agent. Neuron. 1995;15:1183–1191. doi: 10.1016/0896-6273(95)90105-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raeber A J, Race R E, Brandner S, Priola S A, Sailer A, Bessen R A, Mucke L, Manson J, Aguzzi A, Oldstone M B A, Weissman C, Chesebro B. Astrocyte-specific expression of hamster prion protein (PrP) renders PrP knockout mice susceptible to hamster scrapie. EMBO J. 1997;16:6057–6065. doi: 10.1093/emboj/16.20.6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rall G F, Manchester M, Daniels L R, Callahan E M, Belman A R, Oldstone M B. A transgenic mouse model for measles virus infection of the brain. Proc Natl Acad Sci USA. 1997;94:4659–4663. doi: 10.1073/pnas.94.9.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rall G F, Mucke L, Oldstone M B. Consequences of cytotoxic T lymphocyte interaction with major histocompatibility complex class I-expressing neurons in vivo. J Exp Med. 1995;182:1201–1212. doi: 10.1084/jem.182.5.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robakis N K, Sawh P R, Wolfe G C, Rubenstein R, Carp R I, Innis M A. Isolation of a cDNA clone encoding the leader peptide of prion protein and expression of the homologous gene in various tissues. Proc Natl Acad Sci USA. 1986;83:6377–6381. doi: 10.1073/pnas.83.17.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakaguchi S, Katamine S, Nishida N, Moriuchi R, Shigematsu K, Sugimoto T, Nakatani A, Kataoka Y, Houtani T, Shirabe S, Okada H, Hasegawa S, Miyamoto T, Noda T. Loss of cerebellar Purkinje cells in aged mice homozygous for a disrupted PrP gene. Nature. 1996;380:528–531. doi: 10.1038/380528a0. [DOI] [PubMed] [Google Scholar]
- 43.Scott J R, Davies D, Fraser H. Scrapie in the central nervous system: neuroanatomical spread of infection and Sinc control of pathogenesis. J Gen Virol. 1992;73:1637–1644. doi: 10.1099/0022-1317-73-7-1637. [DOI] [PubMed] [Google Scholar]
- 44.Scott M, Foster D, Mirenda C, Serban D, Coufal F, Walchli M, Torchia M, Groth D, Carlson G, DeArmond S J, Westaway D, Prusiner S B. Transgenic mice expressing hamster prion protein produce species-specific scrapie infectivity and amyloid plaques. Cell. 1989;59:847–857. doi: 10.1016/0092-8674(89)90608-9. [DOI] [PubMed] [Google Scholar]
- 45.Shmerling D, Hegyi I, Fischer M, Blättler T, Brandner S, Gotz J, Rulicke T, Flechsig E, Cozzio A, von Mering C, Hangartner C, Aguzzi A, Weissmann C. Expression of amino-terminally truncated PrP in the mouse leading to ataxia and specific cerebellar lesions. Cell. 1998;93:203–214. doi: 10.1016/s0092-8674(00)81572-x. [DOI] [PubMed] [Google Scholar]