To the Editor:
With advances in neonatal care, survival of very preterm infants has increased over the past several decades, while incidence rates of common chronic pulmonary complications such as bronchopulmonary dysplasia (BPD) have not changed drastically (1). Survivors of prematurity, especially those with BPD, have increased risk of recurrent wheezing, cough, airflow obstruction, and cardiopulmonary exercise limitation, recently defined as post-prematurity respiratory disease (PPRD) (2). The precise mechanisms contributing to increased respiratory morbidity in former preterm infants are poorly understood.
We have previously shown that tracheal aspirates obtained in the first week of life from premature infants mechanically ventilated for respiratory distress syndrome contain mesenchymal stromal cells (MSCs) that demonstrate lung-specific gene signatures; exhibit capacity to differentiate into multiple lineages, including myofibroblasts; and secrete various immunomodulatory factors (3, 4). The presence of MSCs in the first week has a high predictive value for BPD development (5). Therefore, these cells may contribute to the pathogenesis of chronic lung disease of prematurity.
Little is known about MSC involvement in the development of chronic asthma-like symptoms in survivors of prematurity. Through similar mechanisms as proposed for BPD pathogenesis, MSCs may differentiate into myofibroblasts, leading to distal airway remodeling and altered extracellular matrix protein composition (6). Furthermore, MSCs can modulate the immune response and through the secretion of extracellular vesicles may ameliorate neonatal lung injury (7, 8). Thus, MSCs may serve as a biomarker for development of chronic asthma-like symptoms in former preterm infants. As such, we hypothesized that the presence of MSCs in tracheal aspirates predicts the development of PPRD in survivors of prematurity.
In accordance with an institutional review board–approved protocol, we collected tracheal aspirates from infants born at ⩽32 weeks’ gestation who were mechanically ventilated for respiratory distress in the first week of life after the acquisition of informed consent from their guardians. The aspirates were collected during routine endotracheal tube suctioning. After centrifugation, the cell pellet was resuspended in media. Adherent cells were incubated at 37°C. MSC colony-forming units were identified under light microscopy (5). Retrospective chart review was conducted for all patients who had tracheal aspirates obtained in the first week of life and were alive at 5 years of age. Patients with unavailable chart data because of loss to follow-up or unknown MSC status were excluded. Diagnosis of PPRD was defined as at least one episode of bronchial hyperreactivity or wheezing before 5 years of age that was treated with inhaled bronchodilators or inhaled or oral corticosteroids. BPD diagnosis was defined as oxygen requirement at 36 weeks’ postmenstrual age. Demographic and clinical data were compared using the unpaired t test or the Fisher exact test. Diagnosis of PPRD was the primary outcome variable. A logistic regression model was used to estimate the relative odds of PPRD diagnosis as a function of MSCs, controlling for gestational age, birth weight, sex, and race.
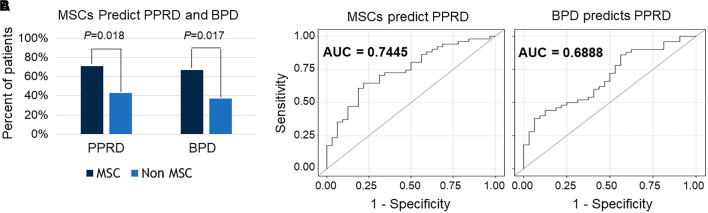
A total of 101 patients were alive at 5 years of age, of whom 86 patients had complete records for review. MSCs were isolated from 58 patients. Demographic and clinical characteristics were compared between patients in whom MSCs were isolated (n = 58) and those in whom MSCs were not isolated (n = 28) (Table 1). There were no significant differences between the groups regarding sex, race, gestational age, delivery mode, and total days of oxygen. Of the 86 patients, 53 (62%) developed PPRD by 5 years of age. In the MSC group, 71% of patients developed PPRD compared with 43% in the non-MSC group, a significant difference with a P value of 0.018 (Figure 1A). As we reported previously, the percentage of patients who developed BPD in the MSC group was significantly higher compared with the non-MSC groups (67% vs. 37%; P = 0.017). In addition, we controlled for gestational age, birth weight, sex, and race, and the presence of MSCs predicted PPRD diagnosis with relative risk (RR) of 1.71 (95% confidence interval [CI], 1.1–2.7). We also controlled for BPD diagnosis, number of days of mechanical ventilation (MV), and days of supplemental oxygen administration (O2 days) and found that the predictive value of MSCs for the diagnosis of PPRD remained, with RR of 1.58 (95% CI, 1.01–2.54). Receiver operating characteristic analysis showed a higher area under the curve for MSCs predicting PPRD compared with BPD predicting PPRD, after controlling for MV days and O2 days (0.74 vs. 0.69, respectively), indicating higher predictive power of MSCs compared with BPD for the future development of PPRD (Figure 1B). Secondary outcomes of hospitalization for respiratory illness and the use of inhaled corticosteroids before 5 years of age were also associated with the isolation of MSCs (RR, 12.0 [95% CI, 2.9–49.8]), independent of BPD diagnosis, MV days, and O2 days.
Table 1.
Patient Characteristics
| MSCs | No MSCs | ||
|---|---|---|---|
| (n = 58) | (n = 28) | P Value | |
| Male sex, n (% of total) | 28 (48.3) | 8 (28.6) | 0.10 |
| Race, n (% of total) | 0.52 | ||
| White | 41 (70.7) | 20 (71.4) | |
| Black | 14 (24.1) | 5 (17.9) | |
| Other/unknown | 3 (5.2) | 3 (10.7) | |
| Delivery mode, n (% of total) | 0.30 | ||
| Vaginal | 10 (17.9) | 4 (14.8) | |
| Cesarean | 41 (73.2) | 23 (85.2) | |
| Unknown | 5 (8.9) | 0 (0) | |
| Total O2 days, mean ± SD | 178 ± 187.5 | 193.4 ± 312.5 | 0.83 |
| Total MV days, mean ± SD | 62 ± 146.6 | 89.8 ± 277.9 | 0.54 |
| Birth weight, g, mean ± SD | 988 ± 293 | 1104 ± 376 | 0.12 |
| Gestational age, wk, mean ± SD | 28 ± 2.0 | 27.9 ± 2.4 | 0.08 |
Definition of abbreviations: MSC = mesenchymal stromal cell; MV = mechanical ventilation.
Figure 1.
(A) Comparisons of the proportions of patients with and without mesenchymal stromal cells (MSCs) in their tracheal aspirates in the first week of life who went on to develop post-prematurity respiratory disease (PPRD) and bronchopulmonary dysplasia (BPD) (Fisher exact test). (B) Receiver operating characteristic curves demonstrate the sensitivity and specificity of accurately identifying premature infants who go on to develop PPRD on the basis of the presence of MSCs in their tracheal aspirates in the first week of life or diagnosis of BPD, after controlling for days on mechanical ventilation, days receiving supplemental O2, and covariates. AUC = area under the curve.
In this study, we have demonstrated that MSCs isolated from tracheal aspirates from mechanically ventilated premature infants in the first week of life strongly predict the development of PPRD in early childhood in both a direct and an indirect manner. The indirect route likely involves the development of BPD, leading to increased risk of PPRD. A proposed mechanism is through MSC differentiation into abnormal alveolar myofibroblasts, leading to changes in extracellular matrix deposition (more collagen) and failed migration to the secondary alveolar crests (9). When controlling for BPD, MV days, and O2 days, the predictive value of MSCs in developing PPRD remained, suggesting a direct effect as well. As the development of chronic respiratory symptoms in survivors of prematurity is linked to inflammation and not associated with atopy (10), it is plausible that MSCs modulate the inflammatory response in the local environment (7). The association between MSCs and hospitalization or inhaled corticosteroid use suggests that MSCs may also predict increased morbidity.
Our study has several limitations, including a limited definition of PPRD and absent information about virus triggers, documented bronchodilator response, maternal smoking, and other comorbidities. In addition, the number of samples per patient varied (1.55 in the MSC group vs. 1.18 in the no-MSC group; P < 0.05), and 82% of the aspirates in the MSC group cultured MSCs. Therefore, some patients without MSCs may have been misclassified. Future work is needed to validate these results in a larger, prospective cohort with longer follow-up and deep phenotyping of clinical symptoms, lung function, and biomolecular signatures.
Footnotes
Supported by National Institutes of Health grant R01HL140572.
Author Contributions: D.R.V. and A.P.P. designed research. D.R.V. and T.X.C. collected the data. D.R.V., N.K., and A.P.P. analyzed the data. D.R.V. and A.P.P. wrote the manuscript. All authors have read and approved the manuscript.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Abman SH, Collaco JM, Shepherd EG, Keszler M, Cuevas-Guaman M, Welty SE, et al. Bronchopulmonary Dysplasia Collaborative Interdisciplinary care of children with severe bronchopulmonary dysplasia. J Pediatr . 2017;181:12–28.e1. doi: 10.1016/j.jpeds.2016.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cristea AI, Ren CL, Amin R, Eldredge LC, Levin JC, Majmudar PP, et al. Outpatient respiratory management of infants, children, and adolescents with post-prematurity respiratory disease: an official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med . 2021;204:e115–e133. doi: 10.1164/rccm.202110-2269ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Popova AP, Bozyk PD, Goldsmith AM, Linn MJ, Lei J, Bentley JK, et al. Autocrine production of TGF-beta1 promotes myofibroblastic differentiation of neonatal lung mesenchymal stem cells. Am J Physiol Lung Cell Mol Physiol . 2010;298:L735–L743. doi: 10.1152/ajplung.00347.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bozyk PD, Popova AP, Bentley JK, Goldsmith AM, Linn MJ, Weiss DJ, et al. Mesenchymal stromal cells from neonatal tracheal aspirates demonstrate a pattern of lung-specific gene expression. Stem Cells Dev . 2011;20:1995–2007. doi: 10.1089/scd.2010.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Popova AP, Bozyk PD, Bentley JK, Linn MJ, Goldsmith AM, Schumacher RE, et al. Isolation of tracheal aspirate mesenchymal stromal cells predicts bronchopulmonary dysplasia. Pediatrics . 2010;126:e1127–e1133. doi: 10.1542/peds.2009-3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roche WR, Beasley R, Williams JH, Holgate ST. Subepithelial fibrosis in the bronchi of asthmatics. Lancet . 1989;1:520–524. doi: 10.1016/s0140-6736(89)90067-6. [DOI] [PubMed] [Google Scholar]
- 7. Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an immunosuppressive MSC2 phenotype. PLoS One . 2010;5:e10088. doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Willis GR, Reis M, Gheinani AH, Fernandez-Gonzalez A, Taglauer ES, Yeung V, et al. Extracellular vesicles protect the neonatal lung from hyperoxic injury through the epigenetic and transcriptomic reprogramming of myeloid cells. Am J Respir Crit Care Med . 2021;204:1418–1432. doi: 10.1164/rccm.202102-0329OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Popova AP, Bentley JK, Anyanwu AC, Richardson MN, Linn MJ, Lei J, et al. Glycogen synthase kinase-3β/β-catenin signaling regulates neonatal lung mesenchymal stromal cell myofibroblastic differentiation. Am J Physiol Lung Cell Mol Physiol . 2012;303:L439–L448. doi: 10.1152/ajplung.00408.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lum S, Kirkby J, Welsh L, Marlow N, Hennessy E, Stocks J. Nature and severity of lung function abnormalities in extremely pre-term children at 11 years of age. Eur Respir J . 2011;37:1199–1207. doi: 10.1183/09031936.00071110. [DOI] [PubMed] [Google Scholar]