Abstract
Background
We investigated mortality in workers of the world’s largest chrysotile mine and enrichment factories located in the town of Asbest, Russian Federation.
Methods
This historical cohort study included all workers employed for at least 1 year between 1975 and 2010 and follow-up until the end of 2015. Cumulative exposure to dust was estimated based on workers’ complete occupational history linked to dust measurements systematically collected from the 1950s. Exposure to chrysotile fibers was estimated using dust-to-fiber conversion factors. Relative risks (RRs) and 95% confidence intervals (CIs) were estimated as mortality rate ratios in Poisson regression models.
Results
A total of 30 445 (32% women) workers accumulated 721 312 person-years at risk and 11 110 (36%) died. Of the workers, 54% had more than 30 years since their first exposure. We found an exposure-response between cumulative dust and lung cancer mortality in men. No clear association with dust exposure but a modest increase in the highest category of fiber exposure was seen for lung cancer in women. Mesothelioma mortality was increased (RR = 7.64, 95% CI = 1.18 to 49.5, to at least 80 fibers per cm3 years and RR = 4.56, 95% CI = 0.94 to 22.1, to at least 150 mg/m3 years [dust]), based on 13 deaths. For colorectal and stomach cancer, there were inconsistent associations. No associations were seen for laryngeal or ovarian cancer.
Conclusion
In this large-scale epidemiological study in the world’s largest active asbestos mine, we confirmed an increased risk of mesothelioma with high fiber exposure and an increasing mortality for lung cancer in men with increasing dust exposure. Less clear-cut increased lung cancer mortality was seen in the women. Continued mortality follow-up is warranted.
The Asbest Chrysotile Cohort Study was set up as a historical cohort study of former and current workers exposed to chrysotile in the mine and enrichment factories of the Public Joint Stock Company (PJSC) Uralasbest in the town of Asbest, Sverdlovsk Region, Russian Federation. All commercially exploited forms of asbestos (amosite, anthophyllite, chrysotile, and crocidolite) and varieties that are not widely used in industry (eg, tremolite and actinolite) are known to cause cancer in humans, with sufficient evidence that asbestos causes cancers of the lung, larynx, and ovary as well as mesothelioma, and limited evidence for some other cancer types (1). Chrysotile has been the most used form of asbestos worldwide and is at present the only type that is commercially mined. The rationale and a detailed description of the cohort has been published elsewhere (2-4).
The study’s main objective was to investigate cancer mortality in workers occupationally exposed to chrysotile, especially to obtain more precise quantification of the site-specific cancer risks, in a large workforce that has not been studied so systematically before. Here we present the main results on cancer mortality and, for a complete overview of results, the findings for the other noncancer disease groups.
Methods
Study setting
Asbest runs the world’s largest open-pit chrysotile mine, which currently produces approximately 20% of the world’s chrysotile and has been in operation for more than 120 years (2). The study was approved by the International Agency for Research on Cancer–World Health Organization (IARC-WHO) ethics committee (No. 12-22, September 2012). The ethics committee and an independent scientific advisory board monitored the progress of the study on a regular basis.
Cohort enrolment
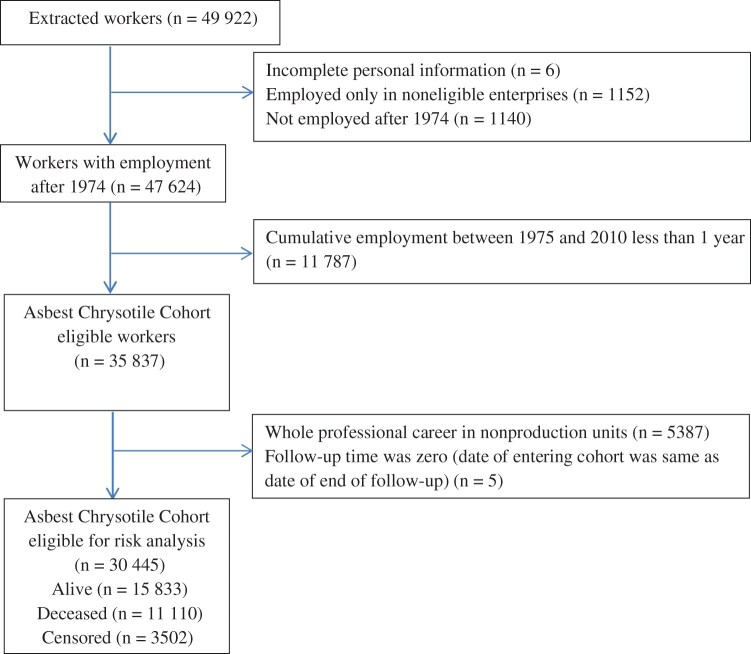
The cohort includes all current and former employees with at least 1 cumulative year of employment between January 1, 1975, and December 31, 2010, of the following enterprises: the mine, all enrichment factories, transport and external rail transportation departments, the central laboratory, and the explosives unit. Consequently, the cohort included workers who were already employed in 1975 and workers who were newly hired in 1975 or afterward. In all, 35 837 workers were eligible and were entered into the cohort study database. Details of how this information was gathered from the company’s archives, and of quality checks to ensure completeness and accuracy, are reported elsewhere (3); a flow diagram is shown in Figure 1. Characteristics of 5387 workers in the excluded group in nonproduction units are shown in Supplementary Table 1 (available online), including the justifications for exclusion.
Figure 1.
Flow diagram from workers extracted from company archives to those eligible for risk analysis.
Exposure assignment
Details on the development of the company-specific job-exposure matrix (5) after the analyses of time trends in concentrations of airborne dust at the site (6) and the calculation of conversion factors between dust exposure and fiber exposure (7) are published elsewhere. A summary is provided in the Supplementary Materials (Summary of the Exposure Assessment, available online). In brief, for each cohort member and each work period, the occupational history was linked with a company-specific job-exposure matrix constructed from more than 90 000 measurements of airborne dust across workplaces in the factories and the mine. Thus, for each individual, cumulative exposure to airborne dust particles (in mg/m3-years) could be estimated for their entire occupational history at PJSC Uralasbest (ie, back to their first exposure, even if this was in the 1950s). Exposure to chrysotile fibers was estimated using dust-to-fiber conversion factors derived from 3 series of parallel measurements of dust and fiber concentrations. Notably, assessment of exposure to airborne dust is based on systematic detailed measurements over more than 4-5 decades, whereas fiber exposure is mainly modeled.
Follow-up and cause-of-death ascertainment
Several procedures were in place to follow up cohort members [see (3)]. The end date for follow-up was December 31, 2015. In brief, first we identified cohort members who were still alive using company records of current workers and using the PJSC Uralasbest Veterans Council’s records for retired workers who still resided in Asbest. The major source for identifying deceased cohort members was the archive of the Civil Act Registration Office (ZAGS) of Sverdlovsk Region, which, in addition to the vital status, provided the date and cause of death of those who died in Sverdlovsk Region from the start of the follow-up, on January 1, 1976, until the end of 2015. Information from ZAGS was complemented by data from the Medical Information and Analytical Center of the Sverdlovsk Region Ministry of Health (8). The Federal Migration Service data were used to identify those who had migrated away from the region. Cohort members for whom the date of migration from the region was not recorded were censored at the last date when they were known to be alive and residing in Sverdlovsk Region (3). This includes cohort members who moved out of Sverdlovsk Region (4.1%) as well as cohort members linked with the Federal Migration Service database, but the date of moving out from Sverdlovsk Region or the date recorded as being alive was not known or ambiguous (7.0%). Only 0.4% of cohort members were censored because they were not found in any source used for follow-up. Therefore, person-years at risk were counted from January 1, 1976, or the date of first employment at PJSC Uralasbest, whichever came last, to the date of death, the date last known to be alive and residing in Sverdlovsk Region, or December 31, 2015, whichever came first.
For each cohort member who died in Sverdlovsk Region, the cause of death was derived from the ZAGS electronic death certificates’ database. ZAGS provided individuals’ causes of death as original text information, and therefore we manually coded the underlying cause of death for deceased cohort members in accordance with the official coding instructions of the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) at IARC-WHO [details published in (8)], that is, death certificates of all 11 110 deaths between 1976 and 2015 were manually coded by IARC-WHO medical staff according to the international ICD-10 coding instructions, preventing underestimation of causes of death that did not have an individual code in earlier versions of the ICD or any Russian edition of ICD.
Statistical analyses
A data analysis plan was developed before linking exposure and outcome data (4). The association with endpoint-specific mortality was investigated using Poisson regression models with 4 exposure categories: workers in the first tertile (reference group) of the exposure distribution, those in the second tertile, and the third tertile of exposed workers split into the 66%-90% and at least 90% percentile (rounded, for integer category boundaries). This algorithm resulted in time-dependent exposure categories of more than 0 to less than 20, 20 to less than 65, 65 to less than 150, and at least 150 mg/m3-years for cumulative dust and of more than 0 to less than 12, 12 to less than 40, 40 to less than 80, and at least 80 fibers/cm3-years (f/cm3-years) for cumulative chrysotile fibers. The same tertiles were used for male and female workers, who were analyzed separately in the Poisson analyses. Supplementary Figure 1 (available online) shows the distribution of cumulative exposure to dust and to fibers within their reference categories.
The main analyses used cumulative dust exposure as the exposure metric and applied a time-dependent 5-year lag of cumulative dust for each individual worker. A 5-year lag time was chosen for the main analysis to discount exposure close in time to the cancer death as not causative of the cancer. When the lag time is applied, there are workers who have no occupational exposure because the observation time at risk is shorter than the lag time (eg, for the 5-year lag, a worker who died less than 5 years after the first occupational exposure). This applied to only a few workers in the main analysis using the 5-year lag time. This group is shown in all tables, for completeness, but all workers in the reference group had at least some occupational exposure. The secondary analyses used modeled cumulative fiber exposure, also applying a 5-year lag.
In both analyses, adjustments were made for attained age, log (attained age), and time since last employment. The adjustment for time since last employment is proposed to be applied when healthy worker survivor effects are observed (or, like in our study, when the unhealthy workers have lower exposures because of shorter survival, as discussed below) (9). We therefore present the adjusted mortality rate ratios in the tables. For lung cancer, we also present the mortality rate ratios without adjustment for time since last employment in the legend of the respective table.
For mortality from mesothelioma, which had small numbers of deaths, male and female workers were combined. Because there were no mesothelioma deaths in the lowest cumulative exposure distribution tertile (used as reference category) for dust exposures and fiber exposures, we combined the 2 lower cumulative exposure distribution tertiles (0%-66%) to form the new reference category.
In sensitivity analyses, the effect of lag time was explored by applying longer lag times of 10 years and 20 years when the number of deaths for the respective outcome was sufficient. As this was a register-based study, no information from workers was available on any lifestyle factors, such as smoking and alcohol consumption.
Results
Table 1 shows employment-related characteristics of the workers eligible for the risk analysis, for male (68%) and female (32%) workers separately and combined. In total, the 30 445 workers accumulated 721 312 person-years at risk. Supplementary Table 2 (available online) shows the exposure distributions for airborne dust and fibers by number of workers and by person-years at risk for men, women, and combined. The correlation between cumulative exposures to dust and cumulative exposures to fibers was high, with a Pearson correlation coefficient of 0.88 for women and 0.70 for men. The mean duration of follow-up for vital status was 24 years, but because this was counted only from January 1, 1976, at the earliest, workers may have had a much longer time since first exposure because 36% of them had their first employment before 1970. In fact, 11% (8% men and 17% women) of workers had their first exposure 50 years or longer before the end of follow-up, 19% (17% men and 21% women) of workers had 40-49 years since their first exposure, and 24% (24% men and 24% women) of workers had 30-39 years since their first exposure. The mean time between first employment and the date of the last observation of the worker was 32 years.
Table 1.
Cohort characteristics, by sex and combined (Asbest Chrysotile Cohort Study)
| Characteristic | Men | Women | All |
|---|---|---|---|
| Workers, No. (%) | 20 662 (68) | 9783 (32) | 30 445 (100) |
| Year of birth | |||
| Mean | 1953 | 1950 | 1952 |
| Min-max | 1901-1992 | 1908-1991 | 1901-1992 |
| Age at start of employment, mean (min-max), y | 24 (12-68) | 24 (13-73) | 24 (12-73) |
| Age at start of employment, No. (%), y | |||
| Younger than 20 | 8171 (40) | 3825 (39) | 11 996 (39) |
| 20-29 | 9292 (45) | 4162 (43) | 13 454 (44) |
| 30 years and older | 3199 (15) | 1796 (18) | 4995 (16) |
| Age at last observation, mean (min-max), y | 54 (18-97) | 59 (16-97) | 55 (16-97) |
| Duration of employment, mean (min-max), y | 15 (1-59) | 17 (1-56) | 15 (1-59) |
| Duration of employment, No. (%), y | |||
| <10 | 9573 (46) | 3459 (35) | 13 032 (43) |
| 10-29 | 7811 (38) | 4951 (51) | 12 762 (42) |
| ≥30 | 3278 (16) | 1373 (14) | 4651 (15) |
| Calendar year of first employment, No. (%) | |||
| Before 1970 | 6827 (33) | 4100 (42) | 10 927 (36) |
| 1970-1999 | 11 970 (58) | 5158 (53) | 17 128 (56) |
| 2000 or later | 1865 (9) | 525 (5) | 2390 (8) |
| Time from first employment until last observation, mean (max), y | 30 (73) | 35 (76) | 32 (76) |
| Duration of follow-up, mean, y | 22 | 27 | 24 |
| Total person-years at risk, No. (%) | 458 883 (64) | 262 429 (36) | 721 312 (100) |
| Vital status, No. (%) | |||
| Alive | 9972 (48) | 5861 (60) | 15 833 (52) |
| Deceased | 8270 (40) | 2840 (29) | 11 110 (36) |
| Censoreda | 2420 (12) | 1082 (11) | 3502 (12) |
| Cumulative exposure, mean (max)b | |||
| Dust, mg/m3-years | 61.2 (1641.5) | 77.8 (1184.1) | 66.5 (1641.5) |
| Fibers, f/cm3-years | 29.2 (392.1) | 42.8 (399.7) | 33.6 (399.7) |
Censored at date last known to be alive in Sverdlovsk Region; this includes cohort members who moved out of Sverdlovsk Region (n = 1244; 4.1%); cohort members linked with the Federal Migration Service database, but the date of moving out from Sverdlovsk Region or the date recorded as being alive was not known or ambiguous (n = 2136; 7.0%); and cohort members who could not be linked to any source used for follow-up (n = 122; 0.4%).
Cumulative exposure lagged 5 years (f/cm3-years = fibers/cm3-years).
Table 2 shows the site-specific mortality for cancer in male and female workers, for cumulative dust and fiber exposure. Increased lung cancer mortality rate ratios were seen in men, somewhat weaker with cumulative fibers. In women, no association was seen between dust exposure and lung cancer mortality, and with fiber exposure, only the mortality rate ratio in the highest exposure category was slightly elevated. No increases in mortality rate ratios were seen for laryngeal cancer or ovarian cancer. For stomach and colorectal cancer, rate ratios were increased in men but decreased or were close to 1.00 in women. When not adjusting for time since last employment, mortality rate ratios for lung cancer were somewhat lower in men (Table 2 footnote d). Table 2 also shows the results for mesothelioma mortality, based on 13 deaths from mesothelioma, for both sexes combined. Mortality rate ratios of the 2 highest exposure categories were elevated compared with the new reference categories. The association was slightly stronger for cumulative exposure to fibers compared with the corresponding relative risk for dust.
Table 2.
Mortality rate ratios and 95% confidence intervals for categories of cumulative dust exposure and cumulative chrysotile fiber exposure, by deaths from different cancer sites,a by sex (except for mesothelioma), applying 5-year lag time, adjusted for age and time since last employment
| Dust category, mg/m3-years | Men |
Women |
Fiber category, fibers/cm3-years | Men |
Women |
||||
|---|---|---|---|---|---|---|---|---|---|
| No. deaths | RR (95% CI) | No. deaths | RR (95% CI) | No. deaths | RR (95% CI) | No. deaths | RR (95% CI) | ||
| Lung cancerd | Lung cancerd | ||||||||
| 0b | 3 | 0.94 (0.30 to 3.01) | 0 | 0 | 3 | 0.82 (0.26 to 2.62) | 0 | ||
| >0 to 20 | 89 | 1.00 | 7 | 1.00 | >0 to 12 | 102 | 1.00 | 7 | 1.00 |
| ≥20 to 65 | 155 | 1.20 (0.92 to 1.57) | 11 | 0.82 (0.32 to 2.12) | ≥12 to 40 | 171 | 1.03 (0.80 to 1.32) | 11 | 0.80 (0.31 to 2.07) |
| ≥65 to 150 | 209 | 1.37 (1.04 to 1.80) | 11 | 0.63 (0.24 to 1.63) | ≥40 to 80 | 208 | 1.13 (0.87 to 1.46) | 10 | 0.67 (0.26 to 1.77) |
| ≥150 | 108 | 1.40 (1.03 to 1.90) | 12 | 1.03 (0.40 to 2.62) | ≥80 | 80 | 1.26 (0.92 to 1.72) | 13 | 1.21 (0.48 to 3.04) |
| Total | 564 | 41 | 564 | 41 | |||||
| P trend c | .02 | 1.00 | .11 | .64 | |||||
| Laryngeal cancere | Ovarian cancer | Laryngeal cancere | Ovarian cancer | ||||||
| 0 | 0 | 1 | 2.27 (0.26 to 20.12) | 0 | 0 | 1 | 2.17 (0.25 to 19.12) | ||
| >0 to 20 | 9 | 1.00 | 8 | 1.00 | >0 to 12 | 12 | 1.00 | 8 | 1.00 |
| ≥20 to 65 | 19 | 1.28 (0.57 to 2.87) | 10 | 0.78 (0.30 to 2.03) | ≥12 to 40 | 17 | 0.78 (0.37 to 1.65) | 11 | 0.80 (0.32 to 2.03) |
| ≥65 to 150 | 8 | 0.42 (0.15 to 1.14) | 11 | 0.96 (0.36 to 2.54) | ≥40 to 80 | 10 | 0.38 (0.16 to 0.92) | 10 | 0.86 (0.33 to 2.28) |
| ≥150 | 10 | 1.03 (0.39 to 2.68) | 5 | 0.72 (0.22 to 2.35) | ≥80 | 7 | 0.76 (0.29 to 2.01) | 5 | 0.62 (0.20 to 2.00) |
| Total | 46 | 35 | 46 | 35 | |||||
| P trend | .40 | .70 | .17 | .48 | |||||
| Stomach cancer | Stomach cancer | ||||||||
| 0 | 2 | 1.23 (0.29 to 5.29) | 3 | 3.10 (0.80 to 11.94) | 0 | 2 | 1.13 (0.26 to 4.84) | 3 | 2.76 (0.72 to 10.52) |
| >0 to 20 | 29 | 1.00 | 16 | 1.00 | >0 to 12 | 32 | 1.00 | 18 | 1.00 |
| ≥20 to 65 | 49 | 1.21 (0.75 to 1.94) | 23 | 0.87 (0.45 to 1.65) | ≥12 to 40 | 55 | 1.09 (0.70 to 1.71) | 21 | 0.66 (0.35 to 1.25) |
| ≥65 to 150 | 72 | 1.55 (0.96 to 2.51) | 15 | 0.54 (0.26 to 1.13) | ≥40 to 80 | 77 | 1.43 (0.91 to 2.27) | 17 | 0.58 (0.29 to 1.14) |
| ≥150 | 35 | 1.49 (0.87 to 2.57) | 14 | 0.78 (0.37 to 1.64) | ≥80 | 21 | 1.16 (0.64 to 2.08) | 12 | 0.57 (0.27 to 1.21) |
| Total | 187 | 71 | 187 | 71 | |||||
| P trend | .08 | .25 | .24 | .12 | |||||
| Colorectal cancer | Colorectal cancer | ||||||||
| 0 | 1 | 2.12 (0.27 to 16.55) | 0 | 0 | 1 | 2.20 (0.28 to 17.16) | 0 | ||
| >0 to 20 | 15 | 1.00 | 13 | 1.00 | >0 to 12 | 15 | 1.00 | 14 | 1.00 |
| ≥20 to 65 | 44 | 1.74 (0.96 to 3.15) | 24 | 1.08 (0.54 to 2.13) | ≥12 to 40 | 47 | 1.70 (0.94 to 3.06) | 26 | 1.03 (0.53 to 1.97) |
| ≥65 to 150 | 44 | 1.20 (0.65 to 2.23) | 21 | 0.80 (0.39 to 1.63) | ≥40 to 80 | 46 | 1.25 (0.68 to 2.31) | 19 | 0.75 (0.37 to 1.51) |
| ≥150 | 30 | 1.52 (0.79 to 2.91) | 19 | 1.05 (0.51 to 2.18) | ≥80 | 25 | 1.96 (1.01 to 3.82) | 18 | 0.97 (0.48 to 1.98) |
| Total | 134 | 77 | 134 | 77 | |||||
| P trend | .66 | .86 | .22 | .69 | |||||
| Mesothelioma, men and women | Mesothelioma, men and women | ||||||||
| No. deaths | RR (95% CI) | No. deaths | RR (95% CI) | ||||||
| ≥0 to 65 | 3 | 1.00 | ≥0 to 40 | 2 | 1.00 | ||||
| ≥65 to 150 | 5 | 2.54 (0.53 to 12.07) | ≥40 to 80 | 7 | 6.27 (1.10 to 35.84) | ||||
| ≥150 | 5 | 4.56 (0.94 to 22.14) | ≥80 | 4 | 7.64 (1.18 to 49.46) | ||||
| Total | 13 | 13 | |||||||
| P trend | .03 | .05 | |||||||
International Statistical Classification of Diseases and Related Health Problems codes for cancer sites are as follows: lung, C33-C34; larynx, C32; ovary, C56; stomach, C16; colon and rectum, C18-C21; mesothelioma, C45 and C38.4. CI = confidence interval; f/cm3-years = fibers/cm3-years; RR = relative risk.
Because the 5-year lag time was applied, some workers had no occupational exposure to dust or fibers; as the counting of risk time started with first exposure, they are kept as a separate group and displayed only for the purpose of completeness (see Materials and Methods).
Two-sided Ptrend across the exposure categories (ie, dust categories >0 to 20, ≥20 to 65, ≥65 to 150, and ≥150).
Without adjustment for time since last employment, the relative risk (95% CI) for lung cancer for the 3 categories of cumulative dust exposure compared with the reference category are as follows: RR = 1.07 (95% CI = 0.82 to 1.39), RR = 1.16 (95% CI = 0.90 to 1.50), and RR = 1.18 (95% CI = 0.88 to 1.58) (men) and RR = 0.83 (95% CI = 0.32 to 2.15), RR = 0.67 (95% CI = 0.26 to 1.75), and RR = 1.05 (95% CI = 0.41 to 2.69) (women); for the 3 categories of cumulative fiber exposure compared with the reference category, they are as follows: RR = 0.93 (95% CI = 0.73 to 1.19), RR = 0.99 (95% CI = 0.78 to 1.27), and RR = 1.13 (95% CI = 0.84 to 1.53) (men) and RR = 0.81 (95% CI = 0.31 to 2.08), RR = 0.70 (95% CI = 0.26 to 1.83), and RR = 1.20 (95% CI = 0.48 to 3.02) (women).
Only 1 case of laryngeal cancer in women; therefore, analysis for women was not carried out.
Table 3 shows the all-cause mortality and mortality from major disease groups for male and female workers, for cumulative exposure to dust and fibers. Given the indication of inverse associations with overall mortality seen especially in men, we also present the mortality from alcohol-related noncancer diseases, which showed a strong inverse association in both men and women. Supplementary Table 3 (available online) shows the average age at death by different causes of death; the average age at death was lower in men for all disease groups, except for all cancers, for which it was equal in men and women.
Table 3.
Mortality rate ratios and 95% confidence intervals for categories, and Ptrend across categories of cumulative dust exposure and of cumulative chrysotile fiber exposure, by all causes of death and by selected disease groups, by sex, applying 5-year lag time, adjusted for age and time since last employmenta
| Dust category, mg/m3-years | Men |
Women |
Fiber category, f/cm3-years | Men |
Women |
||||
|---|---|---|---|---|---|---|---|---|---|
| No. deaths | RR (95% CI) | No. deaths | RR (95% CI) | No. deaths | RR (95% CI) | No. deaths | RR (95% CI) | ||
| All deaths | All deaths | ||||||||
| 0b | 186 | 1.21 (1.03 to 1.42) | 29 | 0.88 (0.58 to 1.33) | 0 | 186 | 1.21 (1.03 to 1.42) | 29 | 0.89 (0.59 to 1.34) |
| >0 to 20 | 2245 | 1.00 | 491 | 1.00 | >0 to 12 | 2266 | 1.00 | 505 | 1.00 |
| ≥20 to 65 | 2318 | 0.93 (0.87 to 0.99) | 835 | 1.06 (0.95 to 1.19) | ≥12 to 40 | 2656 | 0.94 (0.89 to 1.00) | 928 | 1.07 (0.96 to 1.20) |
| ≥65 to 150 | 2278 | 0.88 (0.82 to 0.94) | 858 | 0.98 (0.87 to 1.09) | ≥40 to 80 | 2286 | 0.83 (0.78 to 0.89) | 788 | 0.94 (0.84 to 1.06) |
| ≥150 | 1243 | 0.94 (0.87 to 1.02) | 627 | 1.02 (0.90 to 1.16) | ≥80 | 876 | 0.94 (0.86 to 1.03) | 590 | 0.97 (0.86 to 1.10) |
| Total | 8270 | 2840 | 8270 | 2840 | |||||
| P trend c | .02 | .76 | .00 | .14 | |||||
| All cancers, main ICD group C | All cancers, main ICD group C | ||||||||
| 0 | 11 | 1.02 (0.56 to 1.88) | 7 | 1.41 (0.63 to 3.17) | 0 | 11 | 0.97 (0.53 to 1.78) | 7 | 1.40 (0.62 to 3.15) |
| >0 to 20 | 273 | 1.00 | 87 | 1.00 | >0 to 12 | 293 | 1.00 | 87 | 1.00 |
| ≥20 to 65 | 424 | 1.05 (0.90 to 1.23) | 173 | 1.21 (0.93 to 1.56) | ≥12 to 40 | 475 | 0.99 (0.85 to 1.14) | 175 | 1.15 (0.89 to 1.49) |
| ≥65 to 150 | 541 | 1.10 (0.93 to 1.29) | 149 | 0.98 (0.74 to 1.29) | ≥40 to 80 | 540 | 0.98 (0.84 to 1.15) | 151 | 1.06 (0.81 to 1.38) |
| ≥150 | 277 | 1.09 (0.91 to 1.31) | 119 | 1.18 (0.88 to 1.57) | ≥80 | 207 | 1.10 (0.91 to 1.33) | 115 | 1.12 (0.84 to 1.49) |
| Total | 1526 | 535 | 1526 | 535 | |||||
| P trend | .31 | .78 | .46 | .76 | |||||
| Cardiovascular diseases, main ICD group I | Cardiovascular diseases, main ICD group I | ||||||||
| 0 | 32 | 1.13 (0.79 to 1.62) | 2 | 0.40 (0.10 to 1.65) | 0 | 32 | 1.13 (0.78 to 1.62) | 2 | 0.40 (0.10 to 1.65) |
| >0 to 20 | 754 | 1.00 | 225 | 1.00 | >0 to 12 | 768 | 1.00 | 240 | 1.00 |
| ≥20 to 65 | 996 | 0.98 (0.89 to 1.08) | 416 | 1.06 (0.90 to 1.24) | ≥12 to 40 | 1151 | 1.00 (0.91 to 1.10) | 480 | 1.09 (0.93 to 1.28) |
| ≥65 to 150 | 1153 | 0.94 (0.85 to 1.04) | 501 | 1.05 (0.89 to 1.23) | ≥40 to 80 | 1184 | 0.91 (0.82 to 1.01) | 442 | 0.98 (0.84 to 1.15) |
| ≥150 | 666 | 1.03 (0.92 to 1.16) | 367 | 1.08 (0.91 to 1.27) | ≥80 | 466 | 1.04 (0.92 to 1.17) | 347 | 1.04 (0.88 to 1.23) |
| Total | 3601 | 1511 | 3601 | 1511 | |||||
| P trend | .92 | .47 | .60 | .86 | |||||
| Chronic, noninfectious, respiratory diseasesd | Chronic, noninfectious, respiratory diseases | ||||||||
| 0 | 0 | 0 | 0 | 0 | 0 | ||||
| >0 to 20 | 18 | 1.00 | 7 | 1.00 | >0 to 12 | 23 | 1.00 | 7 | 1.00 |
| ≥20 to 65 | 31 | 1.13 (0.63 to 2.05) | 8 | 0.66 (0.24 to 1.85) | ≥12 to 40 | 34 | 0.85 (0.50 to 1.46) | 11 | 0.87 (0.34 to 2.25) |
| ≥65 to 150 | 42 | 1.15 (0.63 to 2.11) | 17 | 1.22 (0.49 to 3.02) | ≥40 to 80 | 45 | 0.89 (0.52 to 1.54) | 13 | 1.02 (0.40 to 2.60) |
| ≥150 | 35 | 1.79 (0.96 to 3.33) | 9 | 0.94 (0.34 to 2.60) | ≥80 | 24 | 1.37 (0.75 to 2.52) | 10 | 1.09 (0.41 to 2.92) |
| Total | 126 | 41 | 126 | 41 | |||||
| P trend | .06 | .68 | .33 | .73 | |||||
| Infectious respiratory diseasese | Infectious respiratory diseases | ||||||||
| 0 | 3 | 0.90 (0.27 to 2.94) | 0 | 0 | 3 | 0.97 (0.30 to 3.19) | 0 | ||
| >0 to 20 | 84 | 1.00 | 12 | 1.00 | >0 to 12 | 79 | 1.00 | 10 | 1.00 |
| ≥20 to 65 | 65 | 0.73 (0.52 to 1.03) | 4 | 0.22 (0.07 to 0.71) | ≥12 to 40 | 79 | 0.86 (0.62 to 1.20) | 9 | 0.57 (0.23 to 1.43) |
| ≥65 to 150 | 30 | 0.39 (0.24 to 0.62) | 6 | 0.34 (0.12 to 0.99) | ≥40 to 80 | 28 | 0.38 (0.23 to 0.62) | 3 | 0.21 (0.06 to 0.81) |
| ≥150 | 17 | 0.45 (0.25 to 0.81) | 3 | 0.24 (0.06 to 0.91) | ≥80 | 10 | 0.40 (0.20 to 0.80) | 3 | 0.29 (0.08 to 1.10) |
| Total | 199 | 25 | 199 | 25 | |||||
| P trend | .00 | .03 | .00 | .02 | |||||
| External causes, main ICD groups S, T, V, W, X, and Y | External causes, main ICD groups S, T, V, W, X, and Y | ||||||||
| 0 | 118 | 1.32 (1.06 to 1.64) | 16 | 1.16 (0.61 to 2.19) | 0 | 118 | 1.33 (1.07 to 1.66) | 16 | 1.15 (0.61 to 2.17) |
| >0 to 20 | 728 | 1.00 | 68 | 1.00 | >0 to 12 | 709 | 1.00 | 67 | 1.00 |
| ≥20 to 65 | 484 | 0.86 (0.76 to 0.98) | 80 | 0.97 (0.69 to 1.36) | ≥12 to 40 | 544 | 0.90 (0.80 to 1.02) | 88 | 0.96 (0.69 to 1.34) |
| ≥65 to 150 | 243 | 0.70 (0.59 to 0.83) | 54 | 0.77 (0.52 to 1.15) | ≥40 to 80 | 229 | 0.68 (0.57 to 0.81) | 48 | 0.68 (0.46 to 1.01) |
| ≥150 | 101 | 0.67 (0.53 to 0.85) | 29 | 0.69 (0.43 to 1.11) | ≥80 | 74 | 0.70 (0.54 to 0.90) | 28 | 0.60 (0.38 to 0.97) |
| Total | 1674 | 247 | 1674 | 247 | |||||
| P trend | .00 | .07 | .00 | .01 | |||||
| Alcohol-related noncancer diseasesf |
Alcohol-related noncancer diseases |
||||||||
| 0 | 117 | 1.38 (1.12 to 1.72) | 16 | 1.35 (0.74 to 2.47) | 0 | 117 | 1.41 (1.14 to 1.74) | 16 | 1.31 (0.71 to 2.40) |
| >0 to 20 | 845 | 1.00 | 92 | 1.00 | >0 to 12 | 820 | 1.00 | 91 | 1.00 |
| ≥20 to 65 | 561 | 0.83 (0.74 to 0.93) | 108 | 0.93 (0.70 to 1.25) | ≥12 to 40 | 637 | 0.88 (0.79 to 0.99) | 110 | 0.87 (0.65 to 1.16) |
| ≥65 to 150 | 298 | 0.70 (0.60 to 0.82) | 83 | 0.87 (0.62 to 1.20) | ≥40 to 80 | 276 | 0.67 (0.57 to 0.79) | 77 | 0.79 (0.57 to 1.10) |
| ≥150 | 119 | 0.65 (0.52 to 0.80) | 33 | 0.58 (0.38 to 0.89) | ≥80 | 90 | 0.69 (0.54 to 0.87) | 38 | 0.60 (0.40 to 0.89) |
| Total | 1940 | 332 | 1940 | 332 | |||||
| P trend | .00 | .01 | .00 | .01 | |||||
The diseases shown in the table have been selected because of the interest in the association between exposure and outcome, and some of them partly overlap, so the total of the number of deaths of the disease groups does not add up to the number of total deaths. CI = confidence interval; ICD = International Statistical Classification of Diseases and Related Health Problems; f/cm3-years = fibers/cm3-years; RR = relative risk.
Because the 5-year lag time was applied, some workers had no occupational exposure to dust or fibers; as the counting of risk time started with first exposure, they are kept as a separate group and displayed only for the purpose of completeness (see Materials and Methods).
Two-sided Ptrend across the exposure categories (ie, dust categories >0 to 20, ≥20 to 65, ≥65 to 150, and ≥150).
Chronic (noninfectious) respiratory diseases (ICD J30, J31, J33, J34.1-J34.8, J35, J37, J38, J39.2-J39.9, J40-J84, J90-J94, and J95-J99 excluding J98.7).
Infectious respiratory diseases (ICD J00-J06, J09-J18, J20-J22, J32, J34.0, J36, J39.0-J39.1, J85-J86, and J98.7).
Alcohol-related noncancer diseases (ICD E24.4, F10, G31.2, G62.1, I42.6, K29.2, K70, K85.2, K86.0, R78.0, S00-T35, and T51-T78).
Supplementary Table 4 (available online) shows results for all-cause mortality, all-cancer mortality, and mortality from cancer of the lung, larynx, ovary, stomach, and colon and rectum (combined) for lag times of 10 years and 20 years.
Discussion
In this first comprehensive cohort study of chrysotile miners and millers in the world’s largest active chrysotile mine, we observed an increase in risk of dying from mesothelioma with high cumulative exposure to chrysotile. It should be noted that all mesothelioma deaths in the cohort occurred in workers with substantial cumulative exposure; no mesothelioma deaths were found in our reference categories of the lowest tertiles of dust and fibres. The lowest observed cumulative exposures were 12.5 f/cm3-years for fibers and 24.2 mg/m3-years for dust (in the same worker).
Mesothelioma is known to be caused by all forms of asbestos (1). This is also the case for chrysotile as mined in the Russian Federation, as seen in our study, showing a strong association with high cumulative exposure to fibers. We observed 13 deaths from mesothelioma in the cohort of more than 30 000 chrysotile miners and millers. It is well known that, in general, mesothelioma deaths occur in those aged older than 70 years. It is also known that occupationally related mesothelioma typically occurs 20-40 years after the first occupational exposure (10,11). Although the mean time between the first occupational exposure and the end of follow-up in men in our cohort was indeed 30 years, notably the average age at death, as typical for the Russian Federation during this time period, was less than 60 years, so it is the competing causes of premature death that to some extent explain the low observed number of mesothelioma deaths despite the long observation period. It is a notable observation that only 2 of the 13 mesothelioma deaths were observed in women, despite their higher average age at death compared with men and the slightly higher exposure levels in women compared with men.
We also observed an increasing risk of dying from lung cancer with increasing cumulative exposure to airborne dust in male workers and to a lesser extent with increasing cumulative exposure to fibers. In women, based on much smaller numbers of lung cancer deaths, there was no association with cumulative dust exposure, but there was a small elevation in risk in the highest category of cumulative exposure to fibers similar to that in men. When longer lag times were applied, similar results were found in men for the 10-year lag, but with the 20-year lag, the association was considerably weaker, especially for cumulative exposure to fibers. In contrast, in women, stronger associations were seen only with the 20-year lag time.
Lung cancer is known to be caused by all forms of asbestos (1,12), supported by our findings. Lung cancer in general is also caused by inhalation of other workplace air contaminants, and the risk increases with increasing cumulative exposure levels, such as for crystalline silica-containing dust (13), welding fumes (14), or diesel motor engine exhaust (15). All of those exposures have been present either in the Asbest mines, in the enrichment factories, or both, mainly in male workers, but no quantification of the exposures from these air contaminants was available for the present study. This confirms that exposure to chrysotile causes an occupation-related lung cancer burden in this setting, but co-exposures to other lung carcinogens may also have contributed, aligning with the available scientific evidence (12-15).
The different observations by sex could be a result of chance because of small numbers of lung cancer deaths in women but may also be due to the following reasons. Most importantly, whereas the prevalence of smoking was high in men (approximately two-thirds), it was very low (<5%) in women in the past although it gradually increased to almost one-third of female workers being smokers in more recent years, as assessed in our independent smoking survey among alive workers (16). Therefore, the dust-related increase in lung cancer deaths in men might be accelerated by a synergistic effect of simultaneous exposure to various types of dust particles and fibers and smoking. Although we were not able to study effect modification by smoking directly because of the lack of individual smoking histories, the marked differences between the sexes in their smoking behavior and the observation of an increase in lung cancer mortality in men at lower cumulative exposure levels than in women are suggestive of effect modification especially at lower exposure levels. More speculative, the physical demands of job groups may have resulted in a differential uptake of dust to the lungs by sex, although the median overall cumulative exposure was slightly higher in women.
We did not see any increase in laryngeal cancer deaths with increasing exposure to dust or fibers, irrespective of applied lag time. For laryngeal cancer, the potency of different asbestos fiber types is not known; hence, our findings are not in contradiction to the available scientific evidence based on other asbestos-related studies (1,17). Lack of individual information on alcohol consumption is a limitation because this is a risk factor for laryngeal cancer, and the observed healthy worker survivor effect suggests an inverse association between alcohol consumption and cumulative exposure to dust and fibers. Consequently, negative confounding by alcohol consumption may have attenuated the possible association between cumulative exposure and laryngeal cancer mortality. We did not see an association of ovarian cancer deaths with increasing exposure to dust or fibers, and almost all observed mortality rate ratios were less than 1, irrespective of lag time. Our study is the first cohort study of chrysotile miners and enrichment factory workers to include a notable number of women. The associations seen in other studies have been in other industries, particularly textile, gas masks, or insulation material, with exposure also to amphiboles (1,18,19). Consequently, the different exposure circumstances, mix of different fiber types, and study settings may explain the differences in results for laryngeal and ovarian cancer.
For stomach cancer and colorectal cancer, there has been limited evidence in previous epidemiological studies (1). With regard to stomach cancer, our results were inconsistent, as we observed increased mortality in men, but all confidence intervals included 1.00. In women, all mortality rate ratios were decreased. For colorectal cancer, mortality rate ratios were increased for all exposure categories in men but not in women. Given that we had no information on the known nonoccupational risk factors for these 2 cancer sites (eg, dietary habits, alcohol consumption, Helicobacter pylori infections) and that results were not consistent across dust and fiber and sex combinations, these findings are difficult to interpret.
Overall mortality in the cohort was lower in male workers with higher cumulative dust exposure compared with the reference group, driven by inverse associations between exposure and deaths from alcohol-related noncancer diseases, deaths from infectious respiratory diseases, and deaths from external causes, but less so in female workers, with much lower numbers of deaths from alcohol-related noncancer diseases and deaths from external causes than in men. This effect was much stronger when not adjusting for time since last employment (data not shown), confirming the need for adjustment for the healthy worker survivor effect (9). Within our cohort, activities related to higher exposures require good health, so the workers selected for these jobs are healthy individuals, and this advantage of healthiness may have lasted throughout their working life, endorsed through the system of obligatory regular medical examinations enforced in the Russian legislation. All employees must undergo annual periodic medical examinations and, depending on the results of the severity of any health symptoms, either may be recommended to stop working in places with contact with dust or are officially withdrawn from work in such conditions. In addition, according to the pension law of the Russian legislation, the age at which retirement is allowed depends on whether the job is classified as hazardous; therefore, the duration of employment may be shorter in workers with higher exposure levels. Taking all this together, it is therefore possible that early symptoms of poorer health may lead to lower cumulative exposure (more discussion of the healthy worker survivor effect is provided in the Supplementary Materials, Considerations of the Healthy Worker Survivor Bias, available online). Notably, for the cancer sites of most interest (ie, mesothelioma and lung cancer), the healthy worker survivor effect is of less relevance as the first symptoms occur late in life and the time between symptom-based diagnosis and death is short because most patients die within 5 years. Indeed, as shown, adjustment for the healthy worker survivor effect had less effect on the results for lung cancer. Seven workers had died of asbestosis (ICD-10 J61; data not shown), a disease known to occur in chrysotile workers but rarely an immediate or underlying cause of death. Deaths from chronic respiratory disease were increased among workers in the highest 10% of cumulative exposure, but only in men and slightly stronger for dust than for fiber exposure.
Other cohort studies of miners and millers occupationally exposed to chrysotile were carried out in Canada (20,21), China (22,23), and Italy (24-27); notably, all studies included only male workers. In the Italian cohort study, mortality from lung cancer was analyzed by tertiles of cumulative exposure to chrysotile fibers, with mortality rate ratios of 2.1 (95% CI = 0.7 to 6.3; n = 17) for 27 to 345 f/ml-years and of 2.2 (95% CI = 0.6 to 8.0; n = 19) for at least 345 f/ml-years compared with the reference of less than 27 f/ml-years (n = 5). Results for occurrence of mesothelioma for the same cumulative exposure tertiles were 5.6 (95% CI = 0.5 to 57.6; n = 4) and 12.6 (95% CI = 0.9 to 171.0; n = 5) compared with the reference category (n = 1) (24). A more extended comparison with our findings is shown in the Supplementary Materials (Comparison with Other Cohort Studies, available online).
Strengths of our study are its large size, the large proportion of female workers to investigate sex-specific risks, access to the original text of all death certificates, the ability to estimate individuals’ cumulative exposure by using dust measurements systematically carried out over 5-6 decades, and access to various authoritative sources, complementing each other, for vital status follow-up.
The main limitations are inherent in the design of a register-based historical cohort study. Endpoints were deaths, not incidence of disease. Every epidemiological study suffers from some exposure misclassification. However, this is one of the best informed studies in terms of person-years covered by dust measurements. Only few extrapolations were needed, with 76% of person-years of workers in the mine and 86% of person-years of workers in the enrichment factories entirely based on real measurements. Also, the duration component of the cumulative exposure is very precise and is not prone to measurement error. Parallel measurements of dust and chrysotile fibers were available for only a few years, and therefore estimated conversion factors may have uncertainties for earlier years (7). However, given that we assigned exposure to cohort members based on a company-specific job-exposure matrix, this would mostly lead to a Berkson-type error not resulting in biased mortality rate ratios but rather in lesser precision [ie, wider confidence intervals (28)] and lesser so to an attenuation of the association. Point estimates of mortality rate ratios based on cumulative fiber exposure were often slightly lower than those based on cumulative dust exposure, supporting this view; that mesothelioma and female lung cancer were exceptions showing slightly higher point estimates provides some evidence that the fiber exposure modeling and assignment via a job-exposure matrix worked well. Migrants from the region had to be censored at the date last known to be alive. With their low average age at death of approximately 60 years, many male workers died before the ages when cancer becomes more frequent, and the time passed since their last exposure may be short; however, this is a reality in this cohort and not a design limitation.
As already mentioned, because this was a register-based study, we had no information on other disease risk factors, so we cannot exclude confounding bias in some of the observed associations; depending on the combination of risk factor and disease, this can either inflate or attenuate associations. We collected group-level information on smoking, which appeared to have less potential for confounding bias for men as smoking prevalence did not differ much across dust exposure categories, but the possibility of some smoking-related confounding in women remains (16).
In conclusion, in the Asbest Chrysotile Cohort Study, we observed an increased risk of mesothelioma with high exposure to chrysotile fibers; men were more affected than women despite slightly higher cumulative exposure for women. We observed an increased mortality for lung cancer in men with increasing cumulative dust exposure. No increased risk of lung cancer with increasing dust exposure was seen in the female workers, but we observed a modest increase in the highest exposure category for fibers. Future research should aim at disentangling the effects of the exposure to different lung carcinogens in this cohort, especially looking at the risks related to dust and fibers in never smokers, as well as investigating additive and multiplicative interactions between smoking, fibers, and co-exposure to other occupational carcinogens. Less clear-cut evidence was observed for colorectal cancer and stomach cancer, but we observed suggestive increases that merit further attention. No increased mortality was seen for laryngeal cancer or ovarian cancer.
This is the first comprehensive study of the workforce of the worlds’ largest active chrysotile mine. Even without extensive industrial use, chrysotile is in the environment and will remain there for many more decades. Therefore, our results are informative for public health at both the local and global scales.
Supplementary Material
Acknowledgements
The study team mourns the loss of their dear colleague Dr Sergey Kashanskiy (SK) from the Yekaterinburg Medical Research Center for Prophylaxis and Health Protection in Industrial Workers, Yekaterinburg, Russian Federation, who was instrumental in the conduct of the study but passed away on February 9, 2023, before the draft of this manuscript was completed.
We also acknowledge the work conducted by the extended study team. The late Dr Nikolai Izmerov, director (1971-2012) and scientific supervisor (2012-2016) of the Federal State Budgetary Scientific Institution, Scientific Research Institute of Occupational Health, made a large contribution to the organization and implementation of the study at its initial stages. The data entry team in Asbest town rigorously entered written information into electronic format: the late Isolina Smirnova, Zoya Lapuhina, and Yulia Nurdinova. Dr Valerie McCormack of the International Agency for Research on Cancer–World Health Organization (IARC-WHO) helped tremendously in setting up the study and writing the study protocol. Dr Madar Talibov and Dr Dana Kristjansson supported specific activities of the study during their stay at IARC-WHO. Dr Veronika Fedirko, while at IARC-WHO, was involved in pilot activities preparing for the full-scale study, and Dr Paolo Boffetta was IARC-WHO section head when the initial contact about the study was made. Ms Christine Bassier and Ms Catherine Chassin, IARC-WHO secretaries, were involved in organizing study-related travels and meetings. Dr Kirsten Frederiksen, a senior statistician at the Danish Cancer Institute, Copenhagen, Denmark, conducted selected independent analyses of the data as an additional quality assurance measure.
The study is monitored by an independent scientific advisory board, which oversees the progress of the study. The scientific advisory board members are Professor Franco Merletti (chair), Professor Mads Melbye (until 2017), Professor Julian Peto, Professor Martin Röösli (from 2017), and Dr Antti Tossavainen. Scientific advisory board members provided their reviews of this manuscript, but the integration of their comments remained at the discretion of the authors. The authors like to express their gratitude to the scientific advisory board for many years of advice and their helpful comments on the manuscript.
Where authors are identified as personnel of the IARC-WHO, the authors alone are responsible for the views expressed in this article, and they do not necessarily represent the decisions, policy, or views of the IARC-WHO. The study sponsors had no role in the design of the study; the collection, analysis, and interpretation of the data; or the writing of the manuscript.
Contributor Information
Joachim Schüz, International Agency for Research on Cancer-World Health Organization, Lyon, France.
Evgeny Kovalevskiy, Federal State Budgetary Scientific Institution, Izmerov Research Institute of Occupational Health, Moscow, Russian Federation; I.M. Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russian Federation.
Ann Olsson, International Agency for Research on Cancer-World Health Organization, Lyon, France.
Monika Moissonnier, International Agency for Research on Cancer-World Health Organization, Lyon, France.
Evgenia Ostroumova, International Agency for Research on Cancer-World Health Organization, Lyon, France.
Gilles Ferro, International Agency for Research on Cancer-World Health Organization, Lyon, France.
Eleonora Feletto, International Agency for Research on Cancer-World Health Organization, Lyon, France; The Daffodil Centre, The University of Sydney, A Joint Venture with Cancer Council New South Wales, Sydney, Australia.
Sara J Schonfeld, International Agency for Research on Cancer-World Health Organization, Lyon, France; Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Graham Byrnes, International Agency for Research on Cancer-World Health Organization, Lyon, France.
Iraklii Tskhomariia, Federal State Budgetary Scientific Institution, Izmerov Research Institute of Occupational Health, Moscow, Russian Federation.
Kurt Straif, International Agency for Research on Cancer-World Health Organization, Lyon, France.
Tatiana Morozova, I.M. Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russian Federation.
Hans Kromhout, Institute for Risk Assessment Sciences, Utrecht University, Utrecht, the Netherlands.
Igor Bukhtiyarov, Federal State Budgetary Scientific Institution, Izmerov Research Institute of Occupational Health, Moscow, Russian Federation; I.M. Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russian Federation.
Data availability
Raw data cannot be made publicly available according to the data confidentiality legislation of the Russian Federation.
Author contributions
Joachim Schüz, PhD (Conceptualization; Formal analysis; Investigation; Methodology; Project administration; Resources; Supervision; Writing—original draft; Writing—review & editing), Evgeny Kovalevskiy, PhD, MD (Conceptualization; Data curation; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Writing—original draft; Writing—review & editing), Ann Olsson, PhD (Data curation; Investigation; Methodology; Project administration; Supervision; Writing—original draft; Writing—review & editing), Monika Moissonnier, MSc (Data curation; Formal analysis; Methodology; Software; Validation; Visualization; Writing—review & editing), Evgenia Ostroumova, PhD, MD (Data curation; Investigation; Methodology; Supervision; Validation; Writing—review & editing), Gilles Ferro, Msc (Data curation; Formal analysis; Methodology; Software; Validation; Visualization; Writing—review & editing), Eleonora Feletto, PhD (Data curation; Formal analysis; Methodology; Visualization; Writing—review & editing), Sarah Schonfeld, PhD (Conceptualization; Data curation; Investigation; Methodology; Project administration; Writing—review & editing), Graham Byrnes, PhD (Formal analysis; Methodology; Writing—review & editing), Iraklii Tskhomariia (Data curation; Investigation; Project administration; Writing—review & editing), Kurt Straif, PhD, MPH, MD (Conceptualization; Methodology; Writing—review & editing), Tatiana Morozova, PhD (Investigation; Project administration; Resources; Validation; Writing—review & editing), Hans Kromhout, PhD (Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Supervision; Validation; Writing—original draft; Writing—review & editing), and Igor Bukhtiyarov, PhD, MD (Conceptualization; Funding acquisition; Investigation; Project administration; Resources; Supervision; Validation; Writing—original draft; Writing—review & editing).
Funding
The work was supported by the Ministry of Health of the Russian Federation in the framework of the Federal target programme, National System of Chemical and Biological Safety of the Russian Federation of 2009-2014 and of 2015-2020 under a general framework of action between the Federal State Budgetary Scientific Institution, Izmerov Research Institute of Occupational Health (IRIOH) and the International Agency for Research on Cancer–World Health Organization (IARC-WHO) (2015-2023). The work by Dr Schonfeld on this manuscript was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Conflicts of interest
Dr Kovalevskiy reported receiving, on behalf of his institute and personally through consulting firms, payments from companies to evaluate exposure to asbestos and risk of asbestos-related disease in those workplaces. All other authors have no competing interests to declare.
For full transparency, Dr Kovalevskiy reported participation as an occupational and environmental health expert as part of the delegation of the Russian Ministry of Health at multiple World Health Assembly meetings as well as at the Conference of the Parties to the Basel and Rotterdam Conventions. Dr Kovalevskiy reported attending meetings organized by the International Chrysotile Association and reported that all expenses for attendance were paid by his institute.
Dr Schüz, who is a JNCI Associate Editor and co-author on this paper, was not involved in the editorial review or decision to publish the manuscript.
References
- 1. Straif K, Benbrahim-Tallaa L, Baan R, et al. ; WHO International Agency for Research on Cancer Monograph Working Group. A review of human carcinogens—part C: metals, arsenic, dusts, and fibres. Lancet Oncol. 2009;10(5):453-454. doi: 10.1016/s1470-2045(09)70134-2. [DOI] [PubMed] [Google Scholar]
- 2. Schüz J, Schonfeld SJ, Kromhout H, et al. A retrospective cohort study of cancer mortality in employees of a Russian chrysotile asbestos mine and mills: study rationale and key features. Cancer Epidemiol. 2013;37(4):440-445. doi: 10.1016/j.canep.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 3. Schüz J, Bukhtiyarov I, Olsson A, et al. Occupational cohort study of current and former workers exposed to chrysotile in mine and processing facilities in Asbest, the Russian Federation: Cohort profile of the Asbest Chrysotile Cohort study. PLoS One 2020;15(7):e0236475. doi: 10.1371/journal.pone.0236475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. International Agency for Research on Cancer. Asbest Chrysotile Cohort Study. https://asbest-study.iarc.fr. Accessed January 2, 2024.
- 5. Feletto E, Kovalevskiy EV, Schonfeld SJ, et al. Developing a company-specific job exposure matrix for the Asbest Chrysotile Cohort Study. Occup Environ Med. 2021;79(5):339-346. doi: 10.1136/oemed-2021-107438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schonfeld SJ, Kovalevskiy EV, Feletto E, et al. Temporal trends in airborne dust concentrations at a large chrysotile mine and its asbestos-enrichment factories in the Russian Federation during 1951-2001. Ann Work Expo Health. 2017;61(7):797-808. doi: 10.1093/annweh/wxx051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feletto E, Schonfeld SJ, Kovalevskiy EV, et al. A comparison of parallel dust and fibre measurements of airborne chrysotile asbestos in a large mine and processing factories in the Russian Federation. Int J Hyg Environ Health. 2017;220(5):857-868. doi: 10.1016/j.ijheh.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schüz J, Kovalevskiy E, Moissonnier M, et al. Comparison of two information sources for cause-of-death follow up in the Russian Federation: the Asbest Chrysotile Cohort Study. Methods Inform Med. 2020;59(1):9-17. doi: 10.1055/s-0040-1710381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Richardson D, Wing S, Steenland K, McKelvey W.. Time-related aspects of the healthy worker survivor effect. Ann Epidemiol. 2004;14(9):633-639. doi: 10.1016/j.annepidem.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 10. Schonfeld SJ, McCormack V, Rutherford MJ, Schüz J.. Regional variations in German mesothelioma mortality rates: 2000–2010. Cancer Causes Control. 2014;25(5):615-624. doi: 10.1007/s10552-014-0368-4. [DOI] [PubMed] [Google Scholar]
- 11. Oddone E, Bollon J, Nava CR, et al. Effect of asbestos consumption on malignant pleural mesothelioma in Italy: forecasts of mortality up to 2040. Cancers. 2021;13(13):3338. doi: 10.3390/cancers13133338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Olsson AC, Vermeulen R, Schüz J, et al. Exposure-response analyses of asbestos and lung cancer subtypes in a pooled analysis of case-control studies. Epidemiology. 2017;28(2):288-299. doi: 10.1097/EDE.0000000000000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ge C, Peters S, Olsson A, et al. Respirable crystalline silica exposure, smoking, and lung cancer subtype risks: a pooled analysis of case-control studies. Am J Respir Crit Care Med. 2020;202(3):412-421. doi: 10.1164/rccm.201910-1926OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kendzia B, Behrens T, Jöckel K-H, et al. Welding and lung cancer in a pooled analysis of case-control studies. Am J Epidemiol. 2013;178(10):1513-1525. doi: 10.1093/aje/kwt201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ge C, Peters S, Olsson A, et al. Diesel engine exhaust exposure, smoking, and lung cancer subtype risks: a pooled exposure-response analysis of 14 case-control studies. Am J Respir Crit Care Med. 2020;202(3):402-411. doi: 10.1164/rccm.201911-2101OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Olsson A, Kovalevskiy E, Talibov M, et al. Tobacco smoking among chrysotile asbestos workers at JSC Uralasbest in the Russian Federation. Occup Environ Med. 2020;77(9):623-627. doi: 10.1136/oemed-2019-106263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hall AL, Kromhout H, Schüz J, et al. Laryngeal cancer risks in workers exposed to lung carcinogens: exposure-effect analyses using a quantitative job exposure matrix. Epidemiology. 2020;31(1):145-154. doi: 10.1097/EDE.0000000000001120. [DOI] [PubMed] [Google Scholar]
- 18. Camargo MC, Stayner LT, Straif K, et al. Occupational exposure to asbestos and ovarian cancer: a meta-analysis. Environ Health Perspect. 2011;119(9):1211-1217. doi: 10.1289/ehp.1003283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Slomovitz B, de Haydu C, Taub M, Coleman RL, Monk BJ.. Asbestos and ovarian cancer: examining the historical evidence. Int J Gynecol Cancer. 2021;31(1):122-128. doi: 10.1136/ijgc-2020-001672. [DOI] [PubMed] [Google Scholar]
- 20. McDonald JC, McDonald AD.. Chrysotile, tremolite and carcinogenicity. Ann Occup Hyg. 1997;41(6):699-705. doi: 10.1016/S0003-4878(97)89350-7. [DOI] [PubMed] [Google Scholar]
- 21. Liddell FD, McDonald AD, McDonald JC.. The 1891-1920 birth cohort of Quebec chrysotile miners and millers: development from 1904 and mortality to 1992. Ann Occup Hyg. 1997;41(1):13-36. doi: 10.1016/S0003-4878(96)00044-0. [DOI] [PubMed] [Google Scholar]
- 22. Wang X, Yano E, Lin S, et al. Cancer mortality in Chinese chrysotile asbestos miners: exposure-response relationships. PLoS One 2013;8(8):e71899. doi: 10.1371/journal.pone.0071899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin S, Wang X, Yano E, et al. Exposure to chrysotile mining dust and digestive cancer mortality in a Chinese miner/miller cohort. Occup Environ Med. 2014;71(5):323-328. doi: 10.1136/oemed-2013-101360. [DOI] [PubMed] [Google Scholar]
- 24. Ferrante D, Mirabelli D, Silvestri S, et al. Mortality and mesothelioma incidence among chrysotile asbestos miners in Balangero, Italy: a cohort study. Am J Ind Med. 2020;63(2):135-145. doi: 10.1002/ajim.23071. [DOI] [PubMed] [Google Scholar]
- 25. Pira E, Pelucchi C, Piolatto PG, Negri E, Bilei T, La Vecchia C.. Mortality from cancer and other causes in the Balangero cohort of chrysotile asbestos miners. Occup Environ Med. 2009;66(12):805-809. doi: 10.1136/oem.2008.044693. [DOI] [PubMed] [Google Scholar]
- 26. Schüz J, Kromhout H, Re Ferrante. et al. Mortality and mesothelioma incidence among chrysotile asbestos miners in Balangero, Italy: a cohort study. Am J Ind Med. 2020;63(9):834-835. doi: 10.1002/ajim.23154. [DOI] [PubMed] [Google Scholar]
- 27. Ferrante D, Mirabelli D, Silvestri S, et al. Ferrante et al respond. Am J Ind Med. 2020;63(9):836-837. doi: 10.1002/ajim.23153. [DOI] [PubMed] [Google Scholar]
- 28. Armstrong BG. Effect of measurement error on epidemiological studies of environmental and occupational exposures. Occup Environ Med. 1998;55(10):651-656. doi: 10.1136/oem.55.10.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data cannot be made publicly available according to the data confidentiality legislation of the Russian Federation.