Abstract
Background
Associations between germline alterations in women and cancer risks among their relatives are largely unknown.
Methods
We identified women from 2 Swedish cohorts Karolinska Mammography Project for Risk Prediction of Breast Cancer (KARMA) and prevalent KARMA (pKARMA), including 28 362 women with genotyping data and 13 226 with sequencing data. Using Swedish Multi-Generation Register, we linked these women to 133 389 first-degree relatives. Associations between protein-truncating variants in 8 risk genes and breast cancer polygenic risk score in index women and cancer risks among their relatives were modeled via Cox regression.
Results
Female relatives of index women who were protein-truncating variant carriers in any of the 8 risk genes had an increased breast cancer risk compared with those of noncarriers (hazard ratio [HR] = 1.85, 95% confidence interval [CI] = 1.52 to 2.27), with the strongest association found for protein-truncating variants in BRCA1 and 2. These relatives had a statistically higher risk of early onset than late-onset breast cancer (P = .001). Elevated breast cancer risk was also observed in female relatives of index women with higher polygenic risk score (HR per SD = 1.28, 95% CI = 1.23 to 1.32). The estimated lifetime risk was 22.3% for female relatives of protein-truncating variant carriers and 14.4% for those related to women in the top polygenic risk score quartile. Moreover, relatives of index women with protein-truncating variant presence (HR = 1.30, 95% CI = 1.06 to 1.59) or higher polygenic risk score (HR per SD = 1.04, 95% CI = 1.01 to 1.07) were also at higher risk of nonbreast hereditary breast and ovary cancer syndrome-related cancers.
Conclusions
Protein-truncating variants of risk genes and higher polygenic risk score in index women are associated with an increased risk of breast and other hereditary breast and ovary syndrome–related cancers among relatives.
Female breast cancer became the most diagnosed malignancy worldwide and affected more than 2 million individuals in 2020 (1). Genetic predisposition to breast cancer has been extensively studied. Association analyses using data of more than 113 000 women revealed that protein-truncating variants of CHEK2, BRCA2, ATM, BRCA1, PALB2, BARD1, RAD51C, RAD51D, and TP53 were statistically significantly associated with breast cancer risk with large effect sizes (2). In addition, genome-wide association studies have identified more than 200 common risk variants associated with marginally increased breast cancer risk, most of which are not within known risk genes (3). However, the cumulative effect of the common risk variants, summarized as polygenic risk score, has a substantial impact on breast cancer risk (4,5).
Genetic predisposition to breast cancer has also been shown to be associated with other cancers. For instance, BRCA1 and 2 mutations are causative for hereditary breast and ovary cancers syndrome (6), with BRCA2 further implicated with an increased risk of melanoma, prostate, and pancreatic cancer (7,8). Similarly, associations have been established between non-BRCA breast cancer risk genes and other cancers. For instance, PALB2 has been implicated in pancreatic and prostate cancer (9,10), whereas RAD51C and D have been implicated in ovarian cancer (11,12). Similarly, ATM mutations are associated with an increased risk of melanoma, prostate, and pancreatic cancer (13-15). Furthermore, meta-analyses of genome-wide association studies have identified shared genetic loci between breast cancer and other types of cancers. For example, rs5013329 and rs9375701 are shared with prostate cancer, while rs200182588 and rs8037137 are shared with ovarian cancer (16).
A few studies have investigated other types of cancer risks among relatives of individuals with genetic predisposition to breast cancer (17-24). However, these studies focused on a few specific genes (mainly BRCA1 and 2) and did not consider other risk genes or common risk variants of breast cancer. In addition, the studies were performed using selected populations such as individuals who underwent clinical genetic testing. These limitations may restrict the applicability of these findings to the general population.
With linkage to multiple Swedish national registers, this population-based cohort study aimed to investigate whether the risk of breast cancer and other types of cancer among first-degree relatives of women was associated with a genetic predisposition to breast cancer. Protein-truncating variants of these established risk genes, CHEK2, BRCA2, ATM, BRCA1, PALB2, BARD1, RAD51C, and RAD51D, and breast cancer polygenic risk score were considered in this study.
Methods
Study population
The index women were identified from Karolinska Mammography Project for Risk Prediction of Breast Cancer (KARMA) and prevalent KARMA (pKARMA) cohort. KARMA comprises 70 877 women who attended mammography screening or clinical mammography from 2011 to 2013 at 4 hospitals in Sweden (25). pKARMA is a cohort of 5002 invasive breast cancer patients diagnosed between 2001 and 2008 in Stockholm and 5433 breast cancer-free women from KARMA (26). KARMA and pKARMA participants donated blood samples at study enrollment. All breast cancer patients along with randomly selected breast cancer–free women from KARMA and pKARMA were genotyped. This led to a final sample size of 28 362 genotyped women, 13 226 of whom were also sequenced. All index women provided informed consent, and the research was approved by the regional ethical review board in Stockholm, Sweden.
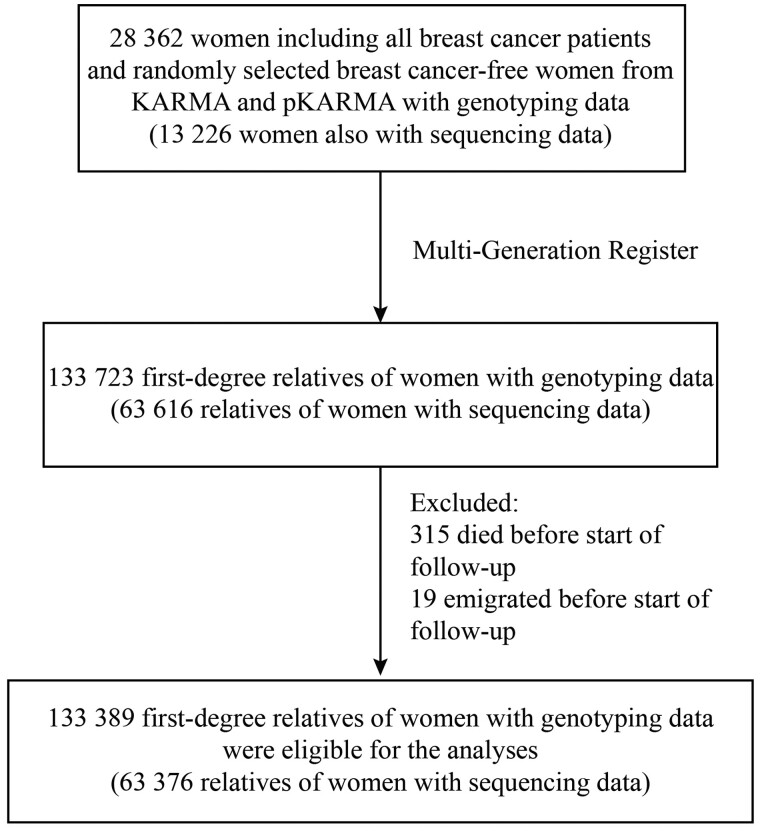
A total of 133 723 first-degree relatives of the index women were first identified from the Swedish Multi-Generation Register (27), which included parents, full siblings, and offspring. After excluding those who died or emigrated before the start of follow-up (age 20 years for the relatives themselves, or January 1, 1958, whichever came later), 133 389 relatives were kept as the study population for further analyses (see Figure 1).
Figure 1.
Flowchart of sample attrition and analytical population. First-degree relatives included parents, siblings, and children. Start of follow-up was defined as age 20 years for the relatives or January 1, 1958, whichever came later. KARMA = Karolinska Mammography Project for Risk Prediction of Breast Cancer; pKARMA = prevalent KARMA.
Genetic profile of index women
Blood samples of index women were genotyped using Illumina iCOGS Array or OncoArray as previously described (28,29). Quality control and imputation were performed by the Breast Cancer Association Consortium (https://bcac.ccge.medschl.cam.ac.uk/). In brief, missing genotypes were imputed using the 1000 Genomes Project phase 3 panel in Genome Reference Consortium Human Build 37 (hg19) coordinates using ShapeIt (30) and IMPUTE (31). Polygenic risk score based on the 313 single-nucleotide polymorphisms was computed using PLINK 2.0 with the weights described by Mavaddat et al. (4,32).
A total of 13 226 index women’s blood samples were sequenced using a gene panel that covers coding regions and exon-intron boundaries of known or suspected breast cancer risk genes. The details of sequencing experiments, bioinformatic analyses, and protein-truncating variant definition can be found elsewhere (2,33). Briefly, variants introducing frameshifts, premature stop codons, disruption of transcriptional read frames, or affecting canonical splice sites were regarded as protein-truncating variants, except BRCA2 stop-gain variant c.9976A > T (K3326X) and other variants located 3’ thereof (34). This study focused on protein-truncating variants of the following risk genes: CHEK2, BRCA2, ATM, BRCA1, PALB2, BARD1, RAD51C, and RAD51D (2).
Missense variants were defined as pathogenic based on annotations of “pathogenic” or “likely pathogenic” in ClinVar (35). For BRCA1 and 2, pathogenic missense variants were further defined according to the criteria established by an international panel of the Evidence-Based Network for the Interpretation of Germline Mutant Alleles (ENIGMA) consortium (36).
Exposure definition
Carrier status for index women was defined as the presence of a protein-truncating variant in any of the 8 studied risk genes, while noncarrier status was defined as the absence of protein-truncating variants in these genes. The quartiles of polygenic risk score for all index women were determined based on breast cancer–free index women at the time of study entry.
The exposure of relatives was defined as the genetic profile of the related index women. For relatives linked to multiple index women, relatives were preferentially linked to protein-truncating variant–carrying index women to increase power, otherwise to a randomly selected linked index woman.
Outcome definitions
Information on the first cancer diagnosis was retrieved using the International Classification of Diseases, Seventh Revision codes from the Swedish Cancer Register. Hereditary breast and ovary syndrome–related cancers included breast cancer, ovarian cancer, prostate cancer, pancreatic cancer, and melanoma. Data on death and migration were extracted from the Swedish Cause of Death Register and the Swedish Migration Register, respectively. Each relative of index women was followed from age 20 years, or January 1, 1958 (starting date of the Swedish Cancer Register), whichever came later, until the earliest record of invasive cancer, emigration, or death, alternatively age 80 years or December 31, 2017, whichever came first.
Statistical analyses
Hazard ratios (HRs) were calculated using the Cox regression model to determine cancer risks among relatives using the R package survival (version 3.2.10). Attained age of the relatives was used as the underlying timescale, and models were adjusted for the birth years of the relatives as well as the study cohort and breast cancer case-control status of the related index women at study entry, with robust standard errors to account for within-family correlation. To study associations with early and late-onset cancers separately, we split follow-up time into cancer-specific early and late age periods (defined according to literature) (37-41). Wald tests (simple Wald test for protein-truncating variant carrier status, joint Wald test across quartiles for polygenic risk score) were used to assess the statistical significance of differences in (log-) hazard ratios between early and late-onset cancers using the R package car (version 3.1.2). Adjusted cumulative incidence curves were shown after adjusting for breast cancer case-control status of index women using standardized Cox regression using the R package stdReg (version 3.4.1) and plotted via survminer (version 0.4.9). Associations between diagnoses of cancers at multiple sites among relatives and protein-truncating variant carriership in index women were modeled via logistic regression. All analyses were performed in R (version 4.0.5). All P values were 2-sided and considered statistically significant when the P value was less than .05.
Sensitivity analyses
To examine the robustness of our findings, sensitivity analyses were performed. First, hazard ratios of breast and nonbreast hereditary breast and ovary syndrome–related cancers among relatives by genetic predisposition in index women, stratified by breast cancer case-control status of index women, were calculated. Second, hazard ratios of breast and nonbreast hereditary breast and ovary syndrome–related cancers among relatives were calculated using follow-up during the recent decades (follow-up started from either the age of 20 years or from January 1, 1990, whichever came later). Third, hazard ratios of breast and nonbreast hereditary breast and ovary syndrome–related cancers among cancer-free women at KARMA enrollment were calculated based on these women’s protein-truncating variant status and polygenic risk score. Fourth, pathogenic missense variants were included in the gene-based analyses in addition to protein-truncating variants. Carrier status was defined as the presence of a protein-truncating variant or pathogenic missense variant in any of the 8 studied risk genes, and the noncarrier status was defined as the absence of protein-truncating variants and pathogenic missense variants in these genes.
Results
Supplementary Table 1 (available online) shows baseline characteristics of index women with genetic information. Comparability was observed in age, mammographic density, menopausal status, body mass index, and family structure, regardless of the breast cancer case-control status. Table 1 and Supplementary Figure 1 (available online) present the genetic profile of the index women, as well as a summary of the distribution of cancer patients among their relatives, categorized by the index women’s genetic predisposition. Of the 482 index women who were protein-truncating variant carriers of any of the 8 risk genes, 79.3% had breast cancer. The proportion of breast cancer patients among carriers ranged from 50.0% for RAD51D to 94.9% for BRCA1. Among their female relatives, 11.7% had breast cancer, and 22.2% of all first-degree relatives had any type of cancer; 28 362 women had information on polygenic risk score of whom 25.5% were breast cancer patients. In each quartile, 6073 to 8474 women were defined, with the proportion of breast cancer patients increasing from 12.3% in the bottom quartile to 38.2% in the top quartile. For index women in the bottom polygenic risk score quartile, 4% of their female relatives had breast cancer, and 17% of all first-degree relatives had any type of cancer. In contrast, for index women in the top polygenic risk score quartile, 7.7% of their female relatives had breast cancer, and 18.9% of all first-degree relatives had any type of cancer.
Table 1.
Summary statistics of cancer distribution among relatives, stratified by index women’s genetic predisposition status
| Genetic predisposition | Index women |
Female relatives |
All relatives |
||||
|---|---|---|---|---|---|---|---|
| No. | Breast cancer patients, No. (%) | No. | Breast cancer patients, No. (%) | No. | Hereditary breast and ovarian cancer syndrome–related cancer patients, No. (%) | Any cancer patients, No. (%) | |
| Protein-truncating variants | |||||||
| All | 13 226 | 7677 (58.0) | 31 754 | 2094 (6.6)d | 63 376 | 4942 (7.8) | 12 013 (19.0) |
| Noncarriersa | 12 744 | 7295 (57.2) | 30 618 | 1962 (6.4) | 61 132 | 4685 (7.7) | 11 517 (18.8) |
| Any risk geneb | 482 | 382 (79.3) | 1140 | 133 (11.7)d | 2253 | 259 (11.5) | 501 (22.2) |
| CHEK2 | 219 | 160 (73.1) | 530 | 53 (10.0) | 1040 | 101 (9.7) | 209 (20.1) |
| BRCA2 | 82 | 72 (87.8) | 189 | 21 (11.1) | 370 | 44 (11.9) | 85 (23.0) |
| ATM | 63 | 51 (81.0) | 149 | 14 (9.4) | 302 | 28 (9.3) | 64 (21.2) |
| BRCA1 | 59 | 56 (94.9) | 132 | 29 (22.0) | 256 | 46 (18.0) | 68 (26.6) |
| PALB2 | 25 | 20 (80.0) | 55 | 8 (14.5) | 113 | 17 (15.0) | 33 (29.2) |
| BARD1 | 20 | 14 (70.0) | 57 | 7 (12.3) | 102 | 11 (10.8) | 22 (21.6) |
| RAD51C | 20 | 16 (80.0) | 45 | 6 (13.3) | 99 | 18 (18.2) | 27 (27.3) |
| RAD51D | 2 | 1 (50.0) | 3 | 0 (0.0) | 8 | 0 (0.0) | 2 (20.0) |
| Polygenic risk scorec | |||||||
| All | 28 362 | 7221 (25.5) | 66 288 | 3897 (5.9)d | 133 389 | 9812 (7.4) | 24 018 (18.0) |
| 0%-25% | 6073 | 750 (12.3) | 14 398 | 571 (4.0) | 28 978 | 1837 (6.3) | 4914 (17.0) |
| 25%-50% | 6594 | 1289 (19.5) | 15 662 | 803 (5.1) | 31 554 | 2165 (6.9) | 5653 (17.9) |
| 50%-75% | 7216 | 1937 (26.8) | 16 941 | 1048 (6.2) | 34 188 | 2600 (7.6) | 6205 (18.1) |
| 75%-100% | 8474 | 3241 (38.2) | 19 902 | 1528 (7.7)d | 39 910 | 3336 (8.4) | 7544 (18.9) |
Noncarrier status was defined as the absence of protein-truncating variants in any studied risk gene, including CHEK2, BRCA2, ATM, BRCA1, PALB2, BARD1, RAD51C, and RAD51D.
Carrier status of any studied risk gene.
Polygenic risk score quartiles were defined according to breast cancer–free index women at study entry.
Of breast cancer patients in female relatives, 6.3% (133 of 2094) were linked to protein-truncating variant carriers of any of the studied risk genes, and 39.2% (1528 of 3897) were linked to index women in the top polygenic risk score quartile.
Table 2 presents the relative breast cancer risk of female relatives of index women with a genetic predisposition to breast cancer. Female relatives of protein-truncating variant carriers of any studied risk genes were at increased breast cancer risk (HR = 1.85, 95% CI = 1.52 to 2.27). Interestingly, the risk was statistically significantly higher for those diagnosed with an early onset breast cancer (HR = 3.00, 95% CI = 2.10 to 4.28) than for those with late-onset breast cancer (HR = 1.49, 95% CI = 1.17 to 1.90; P = .001). The magnitude of the increased risks, diagnosed at any age, were comparable for mothers (HR = 1.99, 95% CI = 1.56 to 2.55) and siblings and offspring (HR = 1.71, 95% CI = 1.25 to 2.36). Increased risk was observed in both relatives of BRCA1 and 2 protein-truncating variant carriers (HR = 2.59, 95% CI = 1.84 to 3.63) and protein-truncating variant carriers of non-BRCA risk genes (HR = 1.59, 95% CI = 1.25 to 2.02). When further stratified by individual gene, increased breast cancer risk was seen in relatives of the protein-truncating variant carriers of CHEK2 (HR = 1.47, 95% CI = 1.10 to 1.98), BRCA2 (HR = 1.64, 95% CI = 1.04 to 2.59), BRCA1 (HR = 4.14, 95% CI = 2.61 to 6.58), and PALB2 (HR = 2.51, 95% CI = 1.13 to 5.57; Supplementary Table 2, available online).
Table 2.
Hazard ratio (95% CI) for breast cancer among female relatives by genetic predisposition in index womena
| Genetic predisposition in index women and No. cancer patients among relatives | All female relatives |
Mothers |
Siblings and offspring |
||||||
|---|---|---|---|---|---|---|---|---|---|
| By age at breast cancer diagnosis among relatives |
By age at breast cancer diagnosis among relatives |
By age at breast cancer diagnosis among relatives |
|||||||
| Any age | Younger than 50 y | 50 y and older | Any age | Younger than 50 y | Any age | Younger than 50 y | |||
| Protein-truncating variant status by the related index women | |||||||||
| No. breast cancer patients among relatives | 2094 | 456 | 1638 | 1173 | 142 | 1031 | 922 | 314 | 608 |
| Noncarriersb | Referent | Referent | Referent | Referent | Referent | Referent | Referent | Referent | Referent |
| Any risk genec | 1.85 (1.52 to 2.27) | 3.00 (2.10 to 4.28) | 1.49 (1.17 to 1.90) | 1.99 (1.56 to 2.55) | 3.79 (2.35 to 6.11) | 1.68 (1.26 to 2.23) | 1.71 (1.25 to 2.36) | 2.56 (1.63 to 4.01) | 1.24 (0.80 to 1.92) |
| Non-BRCA risk genes | 1.59 (1.25 to 2.02) | 2.08 (1.29 to 3.37) | 1.44 (1.09 to 1.91) | 1.78 (1.33 to 2.40) | 2.56 (1.32 to 4.97) | 1.65 (1.19 to 2.30) | 1.38 (0.92 to 2.07) | 1.84 (0.98 to 3.43) | 1.15 (0.68 to 1.94) |
| BRCA1 and 2 | 2.59 (1.84 to 3.63) | 5.05 (3.03 to 8.41) | 1.69 (1.10 to 2.59) | 2.62 (1.76 to 3.91) | 6.32 (3.37 to 11.86) | 1.88 (1.14 to 3.10) | 2.64 (1.60 to 4.38) | 4.28 (2.26 to 8.12) | 1.44 (0.67 to 3.11) |
| Polygenic risk score quartiles by the related index womend | |||||||||
| No. breast cancer patients among relatives | 3897 | 878 | 3019 | 2467 | 348 | 2119 | 1430 | 530 | 900 |
| 0%-25% | Referent | Referent | Referent | Referent | Referent | Referent | Referent | Referent | Referent |
| 25%-50% | 1.26 (1.12 to 1.41) | 1.02 (0.81 to 1.29) | 1.35 (1.18 to 1.54) | 1.25 (1.08 to 1.45) | 0.90 (0.63 to 1.29) | 1.34 (1.14 to 1.57) | 1.26 (1.05 to 1.51) | 1.10 (0.82 to 1.49) | 1.36 (1.08 to 1.72) |
| 50%-75% | 1.54 (1.37 to 1.72) | 1.40 (1.13 to 1.74) | 1.58 (1.39 to 1.80) | 1.55 (1.35 to 1.78) | 1.35 (0.98 to 1.86) | 1.60 (1.37 to 1.86) | 1.50 (1.26 to 1.80) | 1.43 (1.08 to 1.90) | 1.55 (1.24 to 1.95) |
| 75%-100% | 1.85 (1.66 to 2.06) | 1.58 (1.28 to 1.95) | 1.95 (1.73 to 2.21) | 1.91 (1.67 to 2.18) | 1.62 (1.18 to 2.21) | 1.98 (1.71 to 2.29) | 1.76 (1.48 to 2.09) | 1.55 (1.18 to 2.03) | 1.90 (1.53 to 2.36) |
| Per SD increase | 1.28 (1.23 to 1.32) | 1.25 (1.16 to 1.34) | 1.29 (1.24 to 1.34) | 1.30 (1.24 to 1.35) | 1.26 (1.13 to 1.42) | 1.30 (1.24 to 1.36) | 1.25 (1.18 to 1.32) | 1.23 (1.12 to 1.35) | 1.26 (1.18 to 1.35) |
Hazard ratios were estimated via Cox regression, adjusted for birth years of relatives, cohort, and breast cancer case-control status of index women at study entry. Hazard ratios for any age and early and late-onset age are shown respectively. Statistically significant associations (P < .05) are marked in bold. CI = confidence interval.
Noncarrier status was defined as the absence of protein-truncating variants in any studied risk gene, including CHEK2, BRCA2, ATM, BRCA1, PALB2, BARD1, RAD51C, and RAD51D.
Carrier status of any studied risk gene.
Polygenic risk score quartiles were defined according to breast cancer-free index women at study entry.
Female relatives of index women with a higher polygenic risk score were at an elevated risk for breast cancer, with a hazard ratio per standard deviation of 1.28 (95% CI = 1.23 to 1.32; Table 2). The effect sizes varied, ranging from 1.26 (95% CI = 1.12 to 1.41) for the second polygenic risk score quartile to 1.85 (95% CI = 1.66 to 2.06) for the top polygenic risk score quartile when compared with the bottom quartile (Table 2). The magnitude of these increased risks was also comparable for mothers and siblings and offspring. No statistically significant difference between risks for early and late-onset breast cancer was observed among relatives of index women in higher polygenic risk score quartiles (P = .18).
Table 3 presents the relative risk of different types of cancers among relatives of index women with a genetic predisposition to breast cancer. Relatives of index women with higher polygenic risk score were at a slightly increased risk of nonbreast hereditary breast and ovary syndrome–related cancers (HR per SD = 1.04, 95% CI = 1.01 to 1.07). For all cancers, relatives of index women with a higher polygenic risk score had a slightly increased risk (HR per SD = 1.05, 95% CI = 1.04 to 1.07), with the effect size ranging from 1.08 (95% CI = 1.03 to 1.12) for the bottom quartile to 1.13 (95% CI = 1.08 to 1.17) for the top quartile. Relatives of protein-truncating variant carriers of any studied risk genes were at increased risk of nonbreast hereditary breast and ovary syndrome–related cancers (HR = 1.30, 95% CI = 1.06 to 1.59), which may be largely attributed to the association between BRCA1 and 2 mutation of women and ovarian cancer among women’s relatives (HR = 7.05, 95% CI = 4.15 to 11.98). When further stratified by individual gene, statistically significantly higher risks of early onset cancer among relatives of protein-truncating variant carriers of these risk genes were observed: ATM-melanoma (HR = 4.97, 95% CI = 1.26 to 19.61), PALB2-prostate (HR = 7.77, 95% CI = 1.05 to 57.51), PALB2-pancreas (HR = 34.23, 95% CI = 4.74 to 246.94), and RAD51C-ovary (HR = 7.81, 95% CI = 1.11 to 55.05) (Supplementary Table 2, available online). Furthermore, we examined the association between carriers of individual genes and the occurrence of multiple types of cancers within families. Among relatives, diagnoses of prostate and pancreatic cancer (odd ratio [OR] = 37.89, 95% CI = 4.79 to 299.62), breast and pancreatic cancer (OR = 20.38, 95% CI = 2.66 to 156.28), and breast and prostate cancer (OR = 8.91, 95% CI = 2.65 to 30.04) were more likely to relate to PALB2 protein-truncating variant carriers (Supplementary Table 3, available online).
Table 3.
Hazard ratio (95% CI) of any cancers among relatives by genetic predisposition in index womena
| Genetic predisposition in index women and No. cancer patients among relatives | Prostate | Melanoma | Ovarian | Pancreatic | Nonbreast HBOC–relatede | Any HBOC–related | Any nonbreast cancer | Any cancer |
|---|---|---|---|---|---|---|---|---|
| Protein-truncating variant status by the related index women | ||||||||
| No. cancer patients among relatives | 1757 | 539 | 284 | 255 | 2836 | 4942 | 9909 | 12 013 |
| Noncarriersb | Referent | Referent | Referent | Referent | Referent | Referent | Referent | Referent |
| Any risk genec | 1.10 (0.85 to 1.44) | 0.99 (0.57 to 1.73) | 2.75 (1.76 to 4.31) | 1.36 (0.72 to 2.55) | 1.30 (1.06 to 1.59) | 1.51 (1.31 to 1.75) | 1.09 (0.97 to 1.23) | 1.22 (1.11 to 1.35) |
| Non-BRCA risk genes | 1.10 (0.81 to 1.49) | 1.07 (0.57 to 1.99) | 1.42 (0.68 to 2.97) | 1.28 (0.60 to 2.71) | 1.16 (0.90 to 1.49) | 1.33 (1.11 to 1.59) | 1.05 (0.92 to 1.21) | 1.15 (1.02 to 1.29) |
| BRCA1 and 2 | 1.06 (0.65 to 1.73) | 0.71 (0.23 to 2.21) | 7.05 (4.15 to 11.98) | 1.51 (0.49 to 4.72) | 1.69 (1.23 to 2.32) | 2.02 (1.60 to 2.54) | 1.20 (0.97 to 1.48) | 1.44 (1.21 to 1.72) |
| Polygenic risk score quartiles by the related index womend | ||||||||
| No. cancer patients among relatives | 3736 | 1122 | 520 | 517 | 5896 | 9812 | 20 106 | 24 018 |
| 0%-25% | Referent | Referent | Referent | Referent | Referent | Referent | Referent | Referent |
| 25%-50% | 1.02 (0.92 to 1.14) | 0.90 (0.75 to 1.08) | 0.92 (0.69 to 1.22) | 1.21 (0.91 to 1.62) | 1.00 (0.92 to 1.09) | 1.08 (1.01 to 1.16) | 1.05 (1.00 to 1.10) | 1.08 (1.03 to 1.12) |
| 50%-75% | 1.11 (1.00 to 1.23) | 0.90 (0.75 to 1.08) | 1.00 (0.76 to 1.31) | 1.29 (0.98 to 1.70) | 1.08 (0.99 to 1.17) | 1.22 (1.14 to 1.30) | 1.03 (0.99 to 1.08) | 1.09 (1.05 to 1.14) |
| 75%-100% | 1.09 (0.98 to 1.21) | 0.95 (0.80 to 1.14) | 1.07 (0.82 to 1.39) | 1.14 (0.86 to 1.50) | 1.07 (0.99 to 1.16) | 1.32 (1.24 to 1.41) | 1.02 (0.98 to 1.07) | 1.13 (1.08 to 1.17) |
| Per SD increase | 1.04 (1.00 to 1.08) | 1.00 (0.93 to 1.06) | 1.03 (0.94 to 1.13) | 1.07 (0.97 to 1.17) | 1.04 (1.01 to 1.07) | 1.13 (1.10 to 1.15) | 1.01 (1.00 to 1.03) | 1.05 (1.04 to 1.07) |
Hazard ratios were estimated via Cox regression, adjusted for birth years of relatives, cohort, and breast cancer case-control status of index women at study entry. Statistically significant associations (P < .05) are marked in bold. CI = confidence interval; HBOC = hereditary breast and ovarian cancer syndrome.
Noncarrier status was defined as the absence of protein-truncating variants in any studied risk gene, including CHEK2, BRCA2, ATM, BRCA1, PALB2, BARD1, RAD51C, and RAD51D.
Carrier status of any studied risk gene.
Polygenic risk score quartiles were defined according to breast cancer–free index women at study entry.
Nonbreast HBOC-related sites were defined as prostate, skin (melanoma), ovary, and pancreas.
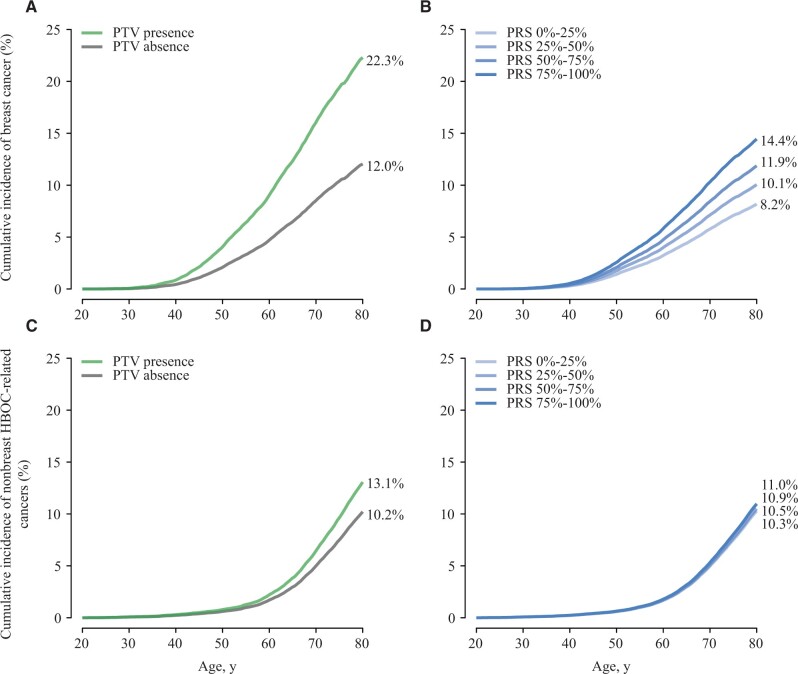
Female relatives of protein-truncating variant carriers of any of the studied risk genes had a breast cancer risk of 22.3% (95% CI = 18.4% to 26.2%) by age 80 years in contrast with 12.0% (95% CI = 10.7% to 13.4%) for relatives of noncarriers (Figure 2, A). Similarly, female relatives of index women in higher polygenic risk score quartiles were at an increased risk of breast cancer. Specifically, relatives of index women in the top polygenic risk score quartile had the lifetime risk of 14.4% (95% CI = 10.9% to 18.0%), which was higher than 8.2% (95% CI = 5.6% to 10.8%) for relatives of index women in the bottom polygenic risk score quartile (Figure 2, B). For nonbreast hereditary breast and ovary syndrome–related cancers, relatives of protein-truncating variant carriers of any of the studied risk genes had a risk of 13.1% (95% CI = 10.5% to 15.7%) by age 80 years compared with 10.2% (95% CI = 8.9% to 11.6%) among relatives of noncarriers (Figure 2, C). However, relatives from different polygenic risk score groups had similar risks of hereditary breast and ovary syndrome–related cancers by age 80 years, ranging from 10.3% (95% CI = 7.2% to 13.3%) for the bottom quartile to 11.0% (95% CI = 8.2% to 13.7%) for the top quartile (Figure 2, D).
Figure 2.
Adjusted cumulative incidence of breast (A, B) and nonbreast HBOC-related cancers (C, D) among relatives stratified by the germline genetic profile of index women. The adjusted cumulative incidence was estimated using standardized Cox regression, adjusting for the breast cancer case-control status of index women at study entry. PTV presence was defined based on the presence of PTVs in any of the studied risk genes including CHEK2, BRCA2, ATM, BRCA1, PALB2, BARD1, RAD51C, and RAD51D, while PTV absence was defined as absence of PTVs in these genes. The PRS quartiles were defined according to index women without breast cancer at study entry. HBOC = hereditary breast and ovarian cancer syndrome; PRS = polygenic risk score; PTV = protein-truncating variant.
In the sensitivity analyses, an increased breast cancer risk was seen in relatives of protein-truncating variant carriers in both analyses among relatives of women with breast cancer (HR = 1.69, 95% CI = 1.31 to 2.17) and breast cancer–free women (HR = 2.31, 95% CI = 1.68 to 3.18) (Supplementary Table 4, available online). Among relatives of protein-truncating variant carriers, relatives of women with breast cancer had a lifetime risk of 21.5% in contrast to 20.6% for relatives of breast cancer–free women (Supplementary Figure 2, A and B, available online). An increased breast cancer risk was also observed in relatives of women with a higher polygenic risk score in both analyses among relatives of women with breast cancer (HR = 1.19, 95% CI = 1.12 to 1.27) and of breast cancer–free women (HR = 1.32, 95% CI = 1.26 to 1.37). When stratified by breast cancer case-control status of index women, relatives of women with breast cancer had a lifetime breast cancer risk ranging from 11.6% to 16.6% across polygenic risk score quartiles in contrast to 7.2% to 13.9% for relatives of breast cancer–free women (Supplementary Figure 2, C and D, available online). In contrast, less pronounced increased risks of nonbreast hereditary breast and ovary syndrome–related cancers were observed among relatives of index women with a genetic predisposition, regardless of breast cancer case-control status (Supplementary Table 4; Supplementary Figure 3, available online).
In the sensitivity analyses of cancer risks in recent decades (relatives were followed from 1990) (Supplementary Table 5, available online), a similarly increased breast cancer risk was observed among female relatives of both protein-truncating variant carriers (HR = 1.76, 95% CI = 1.38 to 2.25) and women with a higher polygenic risk score (HR per SD = 1.28, 95% CI = 1.23 to 1.33) in comparison with those reported in Table 2.
In the sensitivity analyses among cancer-free women at KARMA enrollment, an increased breast cancer risk was observed among women carrying protein-truncating variants (HR = 3.24, 95% CI = 2.31 to 4.54) and women with a higher polygenic risk score (HR per SD = 1.59, 95% CI = 1.50 to 1.68) (Supplementary Table 6, available online), substantially higher than those reported among relatives in Table 2.
In the sensitivity analyses including pathogenic missense variants, similar risk estimates were observed for breast cancer (HR = 1.81, 95% CI = 1.48 to 2.22) and nonbreast hereditary breast and ovary syndrome–related cancers (HR = 1.24, 95% CI = 1.01 to 1.52) among relatives of risk gene variant carriers (Supplementary Table 7, available online) as compared with those reported in Tables 2 and 3.
Discussion
In this population-based cohort study, leveraging data from 133 389 first-degree relatives of 28 362 index women, we found female relatives of index women who had protein-truncating variants in any of the risk genes or a higher polygenic risk score were at increased breast cancer risk. Additionally, we found that relatives of protein-truncating variant carriers of any of the risk genes were at a modestly increased risk of nonbreast hereditary breast and ovary syndrome–related cancers, whereas relatives of index women with a higher polygenic risk score were at a slightly increased risk of nonhereditary breast and ovary syndrome–related cancers. The increased cancer risk in the relatives of KARMA women with germline alterations was substantially lower than the risk among KARMA women, which reflects that first-degree relatives share on average 50% genetics.
For the protein-truncating variant analyses, we focused on risk genes whose protein-truncating variants were statistically significantly associated with breast cancer risk among carriers, as determined by a study using data from more than 113 000 women (2). As expected, breast cancer risk was highest among female relatives of BRCA1 and 2 protein-truncating variant carriers. We also observed a notably increased risk among relatives of protein-truncating variant carriers of other risk genes, which aligns with the effect sizes reported in carriers (2). The association of BRCA1 and 2 protein-truncating variants was statistically more significant in the analysis for early onset breast cancer and weaker, but still significant, in the analysis for late-onset breast cancer. This finding suggests that the age-specific effects of protein-truncating variants on breast cancer risk observed in index women also apply to their female relatives (2). When studying the protein-truncating variant of BRCA1 and BRCA2 separately, we found that presence of BRCA1 protein-truncating variant in index women was statistically significantly associated with early and late-onset breast cancer risks among relatives. No statistically significant associations with early or late-onset breast cancer risks were found for protein-truncating variants of BRCA2, which agrees with the observed larger impact of BRCA1 mutations on breast cancer risk (2,42). Moreover, we also found that female relatives of protein-truncating variant carriers of other risk genes were at a higher breast cancer risk, a finding that, to our knowledge, has not been revealed previously.
In addition, this study is the first to investigate the lifetime risk of breast and other cancers among the relatives of women with breast cancer polygenic risk score. Our results demonstrated that polygenic risk score of index women was positively associated with increased breast cancer risk among female relatives, without any evidence of a statistically significant age-specific effect.
We also provided breast cancer risk estimates for relatives based on the protein-truncating variant status and polygenic risk score quartile of index women. Female relatives of protein-truncating variant carriers of any of the studied genes had a cumulative breast cancer incidence of 22.3% by age 80 years. In addition, relatives of index women in the top polygenic risk score quartiles had a cumulative breast cancer incidence of 14.4%. Both estimates are higher than the 10.1% reported for the general Swedish population (43). In addition, given that 39.2% of breast cancer patients in female relatives were related to index women in the top polygenic risk score quartile, while only 6.3% were related to protein-truncating variant carriers, our results suggest that considering the polygenic risk score of index women may be more effective for identifying relatives at increased risk for breast cancer than considering only variants of risk genes in a population setting.
Germline mutations in BRCA1 and 2 predispose carriers to hereditary breast and ovary syndrome–related cancers, including melanoma, pancreatic, ovarian, and prostate cancer among high-risk groups (7,44,45). In our population-based study, relatives of BRCA1 and 2 protein-truncating variant carriers were at an increased risk of ovarian cancer. This finding aligns with those previously reported among pedigrees of BRCA1 and 2 mutation carriers (18). In contrast, we did not find an elevated risk of prostate cancer among male relatives of BRCA2 protein-truncating variant carriers, despite BRCA2 having been implicated in prostate cancer (7). This discrepancy might be attributed to the protein-truncating variants located within the ovarian cancer cluster region of BRCA2 covered by our sequencing regions, which confers a limited increased risk of prostate cancer among carriers (46). In addition, we found several statistically significant associations between protein-truncating variants and early onset cancer risks among relatives, such as ATM-melanoma, PALB2-pancreas, and PALB2-prostate, corroborating gene-cancer associations previously reported in case-control or family-based studies (47-49).
When we examined the association of protein-truncating variant carriers and cancer aggregation within families (multiple types of cancers in 1 family), we found that carriership of PALB2 protein-truncating variant in index women was associated with multiple diagnoses of hereditary breast and ovary syndrome–related cancers. These results were consistent with previous studies identifying PALB2 as a breast and pancreatic cancer susceptibility gene (2,9,49). Taken together, PALB2 might be another key gene that facilitates the clustering of these cancers within families and warrants further research.
The major strength of this study lies in the large number of women for whom we had genotype and sequence data, as well as the linkage to multiple Swedish national registers. Through this linkage, we were able to identify first-degree relatives with a long and virtually complete follow-up, which represents a substantial advantage over family-based studies. Our findings are generalizable to countries where the populations have a similar genetic background to the Swedish population and where similar mammographic screening programs are in place. The main limitation is the restricted statistical power for protein-truncating variant analyses of individual gene as well as the pathogenic missense variant analyses due to the low frequency of protein-truncating variant carriers, particularly for less common cancers like melanoma and pancreatic cancer.
In summary, our study demonstrated that relatives of women with protein-truncating variants in any of the 8 studied risk genes, or those with a higher breast cancer polygenic risk score, are at elevated risk for both breast and other hereditary breast and ovary syndrome–related cancers at the population level. Our results suggest that rare genetic predispositions to breast cancer, beyond BRCA1 and 2—such as PALB2—have the potential to be informative for cancer aggregation in families. Moreover, our findings indicate that relatives of women who either carry these rare mutations or have a high breast cancer polygenic risk score could benefit from increased awareness because of their elevated cancer risk.
Supplementary Material
Acknowledgement
The funding sources had no role in study design; data collection, analysis, or interpretation; or the decision to approve publication of the finished manuscript.
Contributor Information
Qingyang Xiao, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden.
Xinhe Mao, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden.
Alexander Ploner, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden.
Felix Grassmann, Institute for Clinical Research and Systems Medicine, Health and Medical University, Potsdam, Germany.
Juan Rodriguez, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden.
Mikael Eriksson, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden.
Per Hall, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden; Department of Oncology, Södersjukhuset, Stockholm, Sweden.
Kamila Czene, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden.
Data availability
Access to phenotypes, biospecimens, and genotypes from the KARMA study can be requested from https://karmastudy.org/data-access/. Access to the pKARMA phenotypes and genotypes is restricted because of institutional review board requirements, but data can be shared upon reasonable request to the principal investigator of pKARMA (Kamila Czene).
Author contributions
Qingyang Xiao, PhD (Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Software; Validation; Visualization; Writing—original draft; Writing—review & editing), Xinhe Mao, MD, MSc (Data curation; Investigation; Methodology; Writing—review & editing), Alexander Ploner, PhD (Investigation; Methodology; Software; Visualization; Writing—review & editing), Felix Grassmann, PhD (Data curation; Investigation; Software; Writing—review & editing), Juan Rodriguez, PhD (Data curation; Investigation; Writing—review & editing), Mikael Eriksson, PhD (Investigation; Writing—review & editing), Per Hall, MD, PhD (Funding acquisition; Investigation; Project administration; Resources; Writing—review & editing), and Kamila Czene, PhD (Conceptualization; Data curation; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Writing—review & editing).
Funding
This work was supported by the Swedish Research Council (Grant 2022-00584), the Swedish Cancer Society (Grants 22 2207 and 19 0267), and the Stockholm County Council (Grant 20200102). XM was supported by the China Scholarship Council (grant number: 201806210002).
Conflicts of interest
The authors declare no conflict of interest.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. [DOI] [PubMed] [Google Scholar]
- 2. Dorling L, Carvalho S, Allen J, et al. Breast Cancer Association Consortium. Breast cancer risk genes—association analysis in more than 113,000 women. N Engl J Med. 2021;384(5):428-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang H, Ahearn TU, Lecarpentier J, et al. ; GEMO Study Collaborators. Genome-wide association study identifies 32 novel breast cancer susceptibility loci from overall and subtype-specific analyses. Nat Genet. 2020;52(6):572-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mavaddat N, Michailidou K, Dennis J, et al. ; NBCS Collaborators. Polygenic risk scores for prediction of breast cancer and breast cancer subtypes. Am J Hum Genet. 2019;104(1):21-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mavaddat N, Pharoah PD, Michailidou K, et al. Prediction of breast cancer risk based on profiling with common genetic variants. J Natl Cancer Inst. 2015;107(5):djv036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yoshida R. Hereditary breast and ovarian cancer (HBOC): review of its molecular characteristics, screening, treatment, and prognosis. Breast Cancer. 2021;28(6):1167-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Consortium BCL. Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst. 1999;91(15):1310-1316. [DOI] [PubMed] [Google Scholar]
- 8. Li S, Silvestri V, Leslie G, et al. Cancer risks associated with BRCA1 and BRCA2 pathogenic variants. J Clin Oncol. 2022;40(14):1529-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang X, Leslie G, Doroszuk A, et al. Cancer risks associated with germline PALB2 pathogenic variants: an international study of 524 families. J Clin Oncol. 2020;38(7):674-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wokołorczyk D, Kluźniak W, Stempa K, et al. ; Polish Hereditary Prostate Cancer Consortium. PALB2 mutations and prostate cancer risk and survival. Br J Cancer. 2021;125(4):569-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Loveday C, Turnbull C, Ruark E, et al. ; Breast Cancer Susceptibility Collaboration (UK). Germline RAD51C mutations confer susceptibility to ovarian cancer. Nat Genet. 2012;44(5):475-476; author reply 476. [DOI] [PubMed] [Google Scholar]
- 12. Loveday C, Turnbull C, Ramsay E, et al. ; Breast Cancer Susceptibility Collaboration (UK). Germline mutations in RAD51D confer susceptibility to ovarian cancer. Nat Genet. 2011;43(9):879-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karlsson Q, Brook MN, Dadaev T, et al. ; PRACTICAL Consortium. Rare germline variants in ATM predispose to prostate cancer: A PRACTICAL Consortium Study. Eur Urol Oncol. 2021;4(4):570-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dalmasso B, Pastorino L, Nathan V, et al. ; MelaNostrum consortia. Germline ATM variants predispose to melanoma: a joint analysis across the GenoMEL and MelaNostrum consortia. Genet Med. 2021;23(11):2087-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hsu F-C, Roberts NJ, Childs E, et al. Risk of pancreatic cancer among individuals with pathogenic variants in the ATM gene. JAMA Oncol. 2021;7(11):1664-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kar SP, Beesley J, Amin Al Olama A, et al. ; PRACTICAL Consortium. Genome-wide meta-analyses of breast, ovarian, and prostate cancer association studies identify multiple new susceptibility loci shared by at least two cancer types. Cancer Discov. 2016;6(9):1052-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gronwald J, Huzarski T, Byrski B, et al. Cancer risks in first degree relatives of BRCA1 mutation carriers: effects of mutation and proband disease status. J Med Genet. 2006;43(5):424-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Streff H, Profato J, Ye Y, et al. Cancer incidence in first- and second-degree relatives of BRCA1 and BRCA2 mutation carriers. Oncologist. 2016;21(7):869-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johannsson O, Loman N, Möller T, et al. Incidence of malignant tumours in relatives of BRCA1 and BRCA2 germline mutation carriers. Eur J Cancer. 1999;35(8):1248-1257. [DOI] [PubMed] [Google Scholar]
- 20. Kim H, Choi DH, Park W, et al. The association between non-breast and ovary cancers and BRCA mutation in first- and second-degree relatives of high-risk breast cancer patients: a large-scale study of Koreans. Hered Cancer Clin Pract. 2019;17:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gronwald J, Cybulski C, Piesiak W, et al. Cancer risks in first-degree relatives of CHEK2 mutation carriers: effects of mutation type and cancer site in proband. Br J Cancer. 2009;100(9):1508-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dite G, Whittemore A, Knight J, et al. Increased cancer risks for relatives of very early-onset breast cancer cases with and without BRCA1 and BRCA2 mutations. Br J Cancer. 2010;103(7):1103-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Loman N, Bladström A, Johannsson O, et al. Cancer incidence in relatives of a population-based set of cases of early-onset breast cancer with a known BRCA1 and BRCA2mutation status. Breast Cancer Res. 2003;5(6):R175-R186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Horton C, Hoang L, Zimmermann H, et al. Risk of syndrome-associated cancers among first-degree relatives of patients with pancreatic ductal adenocarcinoma with pathogenic or likely pathogenic germline variants. JAMA Oncol. 2023;9(7):955–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gabrielson M, Eriksson M, Hammarstrom M, et al. Cohort Profile: The Karolinska Mammography Project for Risk Prediction of Breast Cancer (KARMA). Int J Epidemiol. 2017;46(6):1740-1741g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ugalde-Morales E, Li J, Humphreys K, et al. Common shared genetic variation behind decreased risk of breast cancer in celiac disease. Sci Rep. 2017;7(1):5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ekbom A. The Swedish multi-generation register. In: Dillner J, ed. Methods in Biobanking. Totowa, NJ: Humana Press; 2011:215-220. [DOI] [PubMed] [Google Scholar]
- 28. Michailidou K, Lindstrom S, Dennis J, et al. ; ConFab/AOCS Investigators. Association analysis identifies 65 new breast cancer risk loci. Nature. 2017;551(7678):92-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Michailidou K, Hall P, Gonzalez-Neira A, et al. GENICA (Gene Environment Interaction and Breast Cancer in Germany) Network. Large-scale genotyping identifies 41 new loci associated with breast cancer risk. Nat Genet. 2013;45(4):353-361, 361e1-361e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. O’Connell J, Gurdasani D, Delaneau O, et al. A general approach for haplotype phasing across the full spectrum of relatedness. PLoS Genet. 2014;10(4):e1004234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Williams AL, Patterson N, Glessner J, et al. Phasing of many thousands of genotyped samples. Am J Hum Genet. 2012;91(2):238-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chang CC, Chow CC, Tellier LC, et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li J, Ugalde-Morales E, Wen WX, et al. Differential Burden of Rare and Common Variants on Tumor Characteristics, Survival, and Mode of Detection in Breast Cancer. Cancer Res. 2018;78(21):6329-6338. [DOI] [PubMed] [Google Scholar]
- 34. Borg Å, Haile RW, Malone KE, et al. Characterization of BRCA1 and BRCA2 deleterious mutations and variants of unknown clinical significance in unilateral and bilateral breast cancer: The WECARE study. Hum Mutat. 2010;31(3):E1200-E1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Landrum MJ, Chitipiralla S, Brown GR, et al. ClinVar: improvements to accessing data. Nucleic Acids Res. 2020;48(D1):D835-D844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cline MS, Liao RG, Parsons MT, et al. ; BRCA Challenge Authors. BRCA Challenge: BRCA exchange as a global resource for variants in BRCA1 and BRCA2. PLoS Genetics. 2018;14(12):e1007752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cust AE, Armstrong BK, Goumas C, et al. Sunbed use during adolescence and early adulthood is associated with increased risk of early-onset melanoma. Int J Cancer. 2011;128(10):2425-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Neidhardt G, Hauke J, Ramser J, et al. Association between loss-of-function mutations within the FANCM gene and early-onset familial breast cancer. JAMA Oncol. 2017;3(9):1245-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Salinas CA, Tsodikov A, Ishak-Howard M, et al. Prostate cancer in young men: An important clinical entity. Nat Rev Urol. 2014;11(6):317-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schildkraut JM, Halabi S, Bastos E, et al. Prognostic factors in early-onset epithelial ovarian cancer: a population-based study11The authors thank Judy Fine, Dr Linda K. Weiss, Dr Charles F. Lynch, Sara Kelley, Dr Jonathan Liff, Betsey Bridgeman, Dr Sally Glaizer, and Alice Dantsuka for assistance in tissue retrieval. Obstetr Gynecol. 2000;95(1):119-127. [DOI] [PubMed] [Google Scholar]
- 41. Varghese AM, Singh I, Singh R, et al. Early-onset pancreas cancer: clinical descriptors, genomics, and outcomes. JNCI J Natl Cancer Inst. 2021;113(9):1194-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72(5):1117-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Engholm G, Ferlay J, Christensen N, et al. NORDCAN—a Nordic tool for cancer information, planning, quality control and research. Acta Oncol. 2010;49(5):725-736. [DOI] [PubMed] [Google Scholar]
- 44. Thompson D, Easton DF; Breast Cancer Linkage Consortium. Cancer incidence in BRCA1 mutation carriers. J Natl Cancer Inst. 2002;94(18):1358-1365., [DOI] [PubMed] [Google Scholar]
- 45. Mersch J, Jackson MA, Park M, et al. Cancers associated with BRCA1 and BRCA2 mutations other than breast and ovarian. Cancer. 2015;121(2):269-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nyberg T, Frost D, Barrowdale D, et al. Prostate cancer risks for male BRCA1 and BRCA2 mutation carriers: a prospective cohort study. Eur Urol. 2020;77(1):24-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Karlsson Q, Brook MN, Dadaev T, et al. ; PRACTICAL Consortium. Rare germline variants in ATM predispose to prostate cancer: a PRACTICAL consortium study. Eur Urol Oncol. 2021;4(4):570-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Erkko H, Xia B, Nikkila J, et al. A recurrent mutation in PALB2 in Finnish cancer families. Nature. 2007;446(7133):316-319. [DOI] [PubMed] [Google Scholar]
- 49. Jones S, Hruban RH, Kamiyama M, et al. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science. 2009;324(5924):217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Access to phenotypes, biospecimens, and genotypes from the KARMA study can be requested from https://karmastudy.org/data-access/. Access to the pKARMA phenotypes and genotypes is restricted because of institutional review board requirements, but data can be shared upon reasonable request to the principal investigator of pKARMA (Kamila Czene).