Abstract
Background and Objectives
Multiple sclerosis (MS) is considered a prototypic autoimmune disease of the CNS. It is the leading cause of chronic neurologic disability in young adults. Proinflammatory B cells and autoreactive T cells both play important roles in its pathogenesis. We aimed to study alterations of regulatory T cells (Tregs), which likely also contribute to the disease, but their involvement is less clear.
Methods
By combining multiple experimental approaches, we examined the Treg compartments in 41 patients with relapsing-remitting MS and 17 healthy donors.
Results
Patients with MS showed a reduced frequency of CD4+ T cells and Foxp3+ Tregs and age-dependent alterations of Treg subsets. Treg suppressive function was compromised in patients, who were treated with natalizumab, while it was unaffected in untreated and anti-CD20–treated patients. The changes in natalizumab-treated patients included increased proinflammatory cytokines and an altered transcriptome in thymus-derived (t)-Tregs, but not in peripheral (p)-Tregs.
Discussion
Treg dysfunction in patients with MS might be related to an altered transcriptome of t-Tregs and a proinflammatory environment. Our findings contribute to a better understanding of Tregs and their subtypes in MS.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease of the CNS, characterized by focal inflammatory lesions with demyelination and remyelination and neuronal/axonal damage.1 It affects 2.8 million people worldwide with rising prevalence over the past decades.2 It develops in young adults with a mean age at diagnosis of 32 years,2 and women are affected approximately 3 times as often than men by the most frequent, relapsing-remitting form (RRMS).1 While autoreactive CD4+ T cells, proinflammatory B cells, and activated microglia1 participate in the pathogenesis of MS, regulatory T cells (Tregs) are believed to play protective roles.3 A failure of Treg-dependent immune control has been proposed to contribute to MS pathogenesis. Several studies have assessed Tregs and their characteristics in patients with MS,3-6 however, with sometimes controversial results, partly due to the lack of unique identifying markers for Tregs. In peripheral blood of patients with MS, the number of Tregs has been described to be either normal or low, depending on whether they were defined as CD4+CD25+ cells or CD4+CD25+Foxp3+ cells, respectively.7 Furthermore, Tregs derived from patients with RRMS, but not with secondary progressive MS, showed a reduced suppressive function.6,8 The underlying mechanisms of this dysfunction are incompletely understood. Existing data hint at an impaired thymic function and a proinflammatory environment.9-11 Both can be influenced by genetic susceptibility and environmental factors.12 Reduced thymic output is supported by the lower frequency of CD31+ recent thymic emigrant (RTE) Tregs and contracted TCR repertoire diversity.9 It has been shown that RTE-Tregs decline at a faster rate in pediatric patients with MS.10 The notion that a proinflammatory environment may reduce Treg stability and downregulate Foxp3 is supported by the higher frequency of T helper type 1 (Th1)–like Tregs secreting interferon-γ (IFN-γ) and reduced suppressive function in untreated patients with RRMS.11 The differentiation into this phenotype could be induced in vitro by IL-12. Furthermore, proinflammatory signals, as the ones found during a MS relapse, can shape the expression of molecules involved in Treg migration toward inflamed tissues (CD103 and CD49d), activation (HLA-DR), and inhibitory function (CD39).6,13-15 Impaired thymic Treg development and a proinflammatory environment could preferentially affect either thymus-derived (t) or/and peripheral (p)-Tregs. After release from the thymus, t-Tregs migrate to secondary lymphoid organs, where they can be reactivated. Because they then express activation and memory markers, it is difficult to distinguish them from p-Tregs.16 GPA33 has recently been discovered as marker of stable thymus-derived Tregs.17 It has been identified in naïve Tregs just before exit from the thymus, but its expression remained detectable after Treg activation, thus identifying Tregs with a low propensity of conversion into T effector cells.17
In this study, we used this marker to study the spectrum of Treg alterations in patients with RRMS and to understand their relationship with Treg function. In addition to untreated patients, we examined the effects on Treg homeostasis of 2 highly effective treatments for MS with different mechanisms of action, natalizumab, an antibody that blocks α4 integrin (CD49d) and prevents immune cell entry into the CNS,18 and anti-CD20 therapy, which depletes CD20+ B cells and T cells18 and indirectly also affects the activation and growth of autoreactive CD4+ T cells.19 An important aim of this study was to understand the applicability of Treg-directed therapies of MS.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
Patients and controls were recruited from the NIMS-Neuroimmunology and MS Research Section, Department of Neurology, University Hospital Zurich. Samples were collected in the context of previous research projects that had been approved by the Cantonal Ethical Committee of Zurich, Switzerland (EC-No. 2013-0001 and EC-No. ERC 2014-0699). All study participants had given their written informed consent to use their samples for research before inclusion in the study. The study was conducted according to Declaration of Helsinki principles.
Immunophenotyping
We used 3 flow cytometry panels to stain peripheral blood mononuclear cells isolated from blood samples. In addition to the fundamental Treg markers (CD3, CD4, CD25, CD127, and Foxp3), we analyzed origin and memory markers (GPA33 and CD45RA), functional markers (CD39), and migratory markers (CD103 and CD49d).
Suppression Assay
CD4+CD25− Teffs were stimulated with anti-biotin MACSiBead particles loaded with biotinylated antibodies against human CD2, CD3, and CD28 at a bead-to-cell ratio of 1:2 (Miltenyi Biotec). Then, 5 × 104 Teffs/well were plated alone or in the presence of Tregs at different ratios (from 1:8 to 2:1) in a 96-well U-bottom plate (Greiner Bio-One). In addition, Tregs were also cultured alone. In parallel, Teffs were plated in the presence of the respective ratio of CD4+CD25− cells. At day 5, wells were pulsed with 1 µCi/well [3H] thymidine. Supernatants were harvested and stored at −20°C for subsequent cytokine analysis. After 16 hours, [3H] thymidine uptake was detected using Wallac 1450 Microbeta TriLux scintillation counter (Perkin Elmer). Cell proliferation was measured as counts per minute (cpm). Percent suppression was calculated using the following formula: [1-(cpm Teffs cocultured with Tregs/cpm Teffs cocultured with CD4+CD25− cells)] x 100. The mean suppressive capacity was calculated as the mean of 5 percent suppression values calculated at different Treg:Teff ratios.
RNA Extraction, Sequencing, and Analysis
RNA was purified using QIAzol Lysis Reagent (QIAGEN) and isolated using the PicoPure RNA Isolation Kit (Life Technologies) according to the manufacturer's instructions. The libraries were prepared with the kit SMARTer Stranded Total RNA-Seq Kit v2 (Takara). RNA sequencing (RNAseq) was performed using Illumina's NovaSeq 6000 at the Functional Genomics Center Zurich. Additional methods are described in the eMethods.
Data Availability
RNA sequencing data are deposited in the European Genome-phenome Archive (EGA) and can be accessed upon request.
Results
Phenotypic Treg Alterations in Patients With MS
We used flow cytometry to assess Treg frequency and phenotype in a cohort of 57 individuals. Patients with RRMS included untreated (UNT, n = 19), natalizumab-treated (NAT, n = 12), and anti-CD20–treated (a-CD20, n = 10) patients. The control group was composed of 16 healthy donors (HDs). Untreated patients were either assessed before starting the initial treatment with immunomodulatory drugs or were clinically stable after receiving the base therapy and had not been treated in the last 3 months. Natalizumab-treated and anti-CD20–treated patients had been on treatment for 18.6 and 14.4 months on average, respectively. The sex ratio was not significantly different between patients and HDs (χ2 test of independence). The mean age during blood collection ranged from 35.3 in HDs to 40.7 years in anti-CD20–treated patients and did not differ significantly among groups (one-way ANOVA). The percentage of HLA-DRB1*15:01–positive individuals was 9.1% among HDs, while it was 76% among patients, confirming the known positive association between MS and HLA-DRB1*15:01 (p = 0.002, Chi-square t test of independence). Additional clinical and laboratory information is provided in eTables 1 and 2.
We initially compared patients and HDs. The frequency of CD3+CD4+ cells was significantly lower in patients with MS when compared with HDs (p = 0.042; Figure 1B). The proportion of CD25+CD127lo cells within this subset was comparable with the percentage observed in HDs, with greater variability in patients (Figure 1C). Nevertheless, the frequency of Foxp3+ cells within CD25+CD127lo subset was significantly lower in patients (p = 0.002; Figure 1D). CD127 expression on CD25+Foxp3+ T cells was not significantly increased in patients (Figure 1E). Similarly, the frequency of CD39+ cells in CD25+CD127lo cells was higher, but not significantly, in patients (Figure 1F). The expression of the adhesion molecules CD103 and CD49d on CD25+CD127lo cells did not differ significantly between patients and HDs (Figure 1, G–H).
Figure 1. Treg Phenotype in Patients With Relapsing-Remitting Multiple Sclerosis and Healthy Donors.
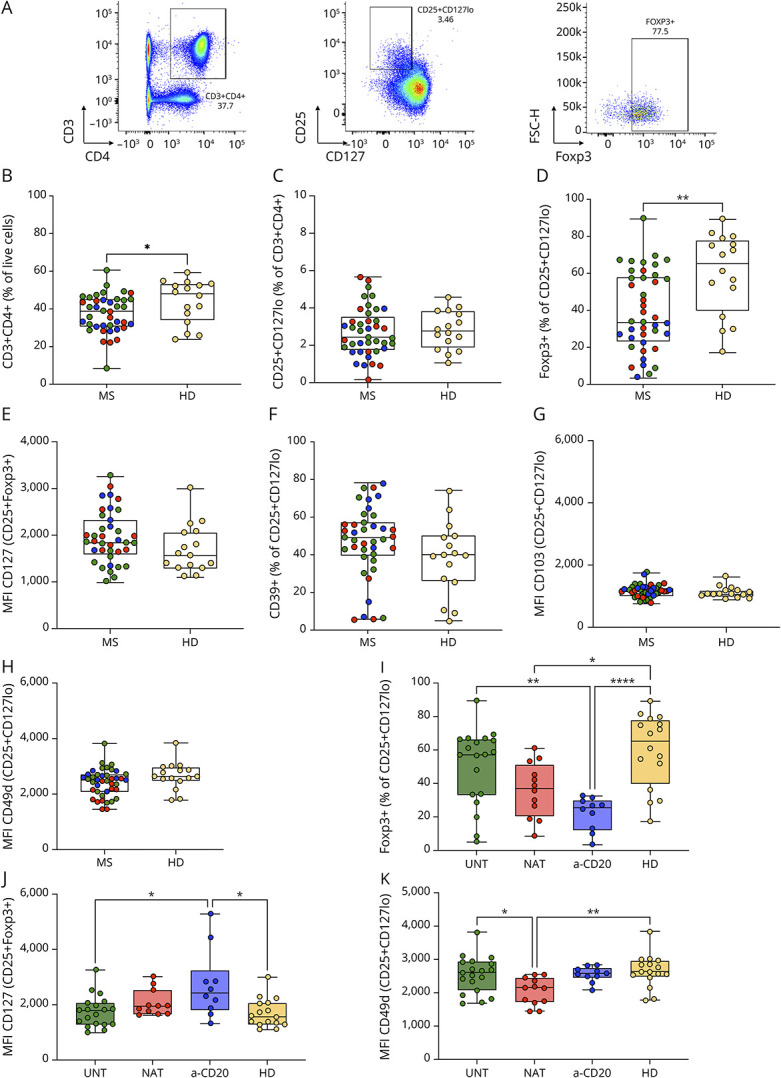
(A) CD25+CD127lo cells were gated on CD3+CD4+ cells. The expression of Foxp3, CD39, CD49d, and CD103 was evaluated on this subset as percentage of positive cells or mean fluorescence intensity (MFI) (only Foxp3 expression is shown). (B–K) Patients with MS (MS) were compared with HDs (HD) and included untreated (green), natalizumab-treated (red), and anti-CD20–treated (blue) patients. (B–C) Frequency of CD3+CD4+ cells and CD25+CD127lo cells. (D) Frequency of Foxp3+ cells in CD25+CD127lo cells. (E) The expression of CD127 was analyzed on CD25+Foxp3+ cells as MFI. (F) Percentage of CD25+CD127lo cells expressing CD39. (G–H) MFI of CD103 and CD49d on CD25+CD127lo cells. (I–K) Multiple comparison between untreated (UNT), natalizumab-treated (NAT), anti-CD20-treated patients (a-CD20), and HDs (HD). Frequency of Foxp3+ cells and MFI of CD127 and CD49d are shown, respectively. (B–K) Dots represent frequency of each donor, boxes extend from the 25th to 75th percentiles, and whiskers from min to max. The line in the middle of the box is the median. Unpaired t test or Mann-Whitney test was used to compare 2 sets of data. One-way ANOVA or Kruskal-Wallis test followed by the Tukey and Dunn statistical hypothesis testing, respectively, were adopted to compare more than 2 sets of data. *p < 0.05; **p < 0.01.
To understand whether MS treatments may lead to differences in Treg phenotype, we performed a multiple comparison analysis. The percentage of Foxp3+CD25+CD127lo cells was significantly reduced in anti-CD20–treated and natalizumab-treated patients (p < 0.0001 and p = 0.013, respectively; Figure 1I), while it was not significantly different in untreated patients in comparison with HDs (Figure 1I). Moreover, anti-CD20–treated patients had an increased expression of CD127 on CD25+Foxp3+ T cells in comparison with untreated patients and HDs (p = 0.046 and p = 0.029, respectively; Figure 1J). Previously, it had been demonstrated that natalizumab induces internalization and degradation of CD49d on circulating lymphocytes, and therefore, anti-CD49d antibody staining is altered.20 Accordingly, we observed reduced CD49d staining of Tregs in natalizumab-treated patients vs untreated patients and HDs (p = 0.024 and p = 0.006, respectively; Figure 1K). We did not find differences in frequencies of CD3+CD4+ cells, CD25+CD127lo cells, CD39+ Tregs, and in CD103 expression within groups of patients (eFigure 1, A–D).
Thus, we observed treatment-dependent alterations of Treg phenotype in patients with MS: treated patients showed a reduced frequency of Foxp3-expressing Tregs, anti-CD20–treated patients showed a higher CD127 expression, and natalizumab-treated patients showed a reduced CD49d expression.
Distinct Transcriptional Profiles of Thymus-Derived Tregs vs Peripheral Tregs
GPA33 has recently been discovered as a cell surface marker predominantly expressed on CD4+CD25+CD45RA+ T cells as compared to their CD45RA- counterpart and to CD4+CD25- T cells.17 In an exemplary experiment, Tregs were isolated from a healthy donor and analyzed by flow cytometry. We confirmed the abovementioned observation, but noted that histograms of GPA33 expression in CD45RA+ and CD45RA- Tregs partly overlapped (Figure 2A). Next, we designed a gating strategy to identify t-Tregs, which expressed both CD45RA and GPA33 (CD45RA+GPA33+), and p-Tregs, which did not express CD45RA nor GPA33 (CD45RA-GPA33-) (Figure 2A). With the aim to compare the transcriptome of these 2 cell types, we selected 12 patients of the cohort, including 9 patients with RRMS (3 untreated, 3 natalizumab-treated and 3 anti-CD20–treated patients) and 3 HDs. From these patients, we isolated t-Tregs and p-Tregs by fluorescence-activated cell sorting, extracted their RNA, and performed bulk RNA sequencing. A total of 802 genes were differentially expressed between these t-Tregs and p-Tregs (false discovery rate <0.05). The 50 most differentially expressed genes are shown in Figure 2B. Differentially upregulated genes in t-Tregs were enriched for genes involved in RNA processing and translation and in p-Tregs for genes playing a role in cell migration, chemokine-mediated and cytokine-mediated signaling pathways, and immune response (Figure 2C, eFigure 2, and eFigure 3). Whereas t-Tregs expressed higher levels of genes identifying naïve Tregs (TCF7, BACH2, LEF1, AFF3, SATB1) and implicated in recirculation through lymphoid tissues (CCR7 and CXCR5), p-Tregs showed a memory signature (upregulation of DUSP4, CDKN1A, and FAS, and downregulation of DNMT3A) (Figure 2D). In addition, p-Tregs expressed higher levels of genes involved in migration and tissue homing (CCR3, CCR4, CCR6, CCR8, CCR10, ITGB1, and CXCR6), HLA-class II (HLADRA, HLADPB1, HLADQB1), T-cell activation (CD80 and TNFRSF9), and negative regulation of T-cell response (CTLA4, LGALS1, LGALS3, and GZMA) (Figure 2D). By contrast, t-Tregs highly expressed AREG, encoding for amphiregulin, an autocrine growth factor essential for Treg function. Of interest, p-Tregs upregulated genes associated with Th17 lineage commitment (RORC, RORA, BATF, CCR6, and IL17RB) (Figure 2D).
Figure 2. Transcriptome Analysis of Thymic Tregs and Peripheral Tregs in 9 Patients With Multiple Sclerosis and 3 Healthy Donors.
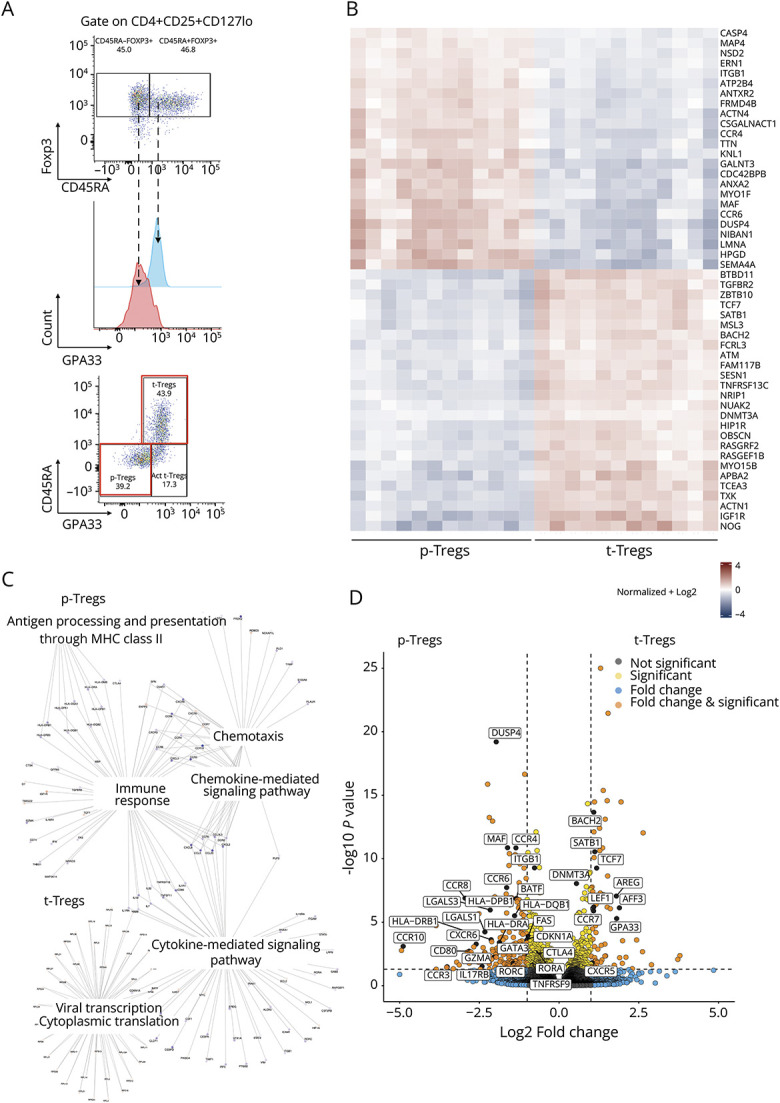
(A) Exemplary dot plots and histogram of Foxp3, CD45RA, and GPA33 expression in Tregs isolated from a HD showing the gating strategy designed to define 2 subsets: p-Tregs (CD45RA-GPA33-) and t-Tregs (CD45RA+GPA33+). A minor cell subset was CD45RA-GPA33+. The first 2 subsets (red squares) were analyzed by RNA sequencing. The third subset was speculated to contain activated (Act.) t-Tregs but was not further investigated by transcriptome analysis. (B) Heatmap showing the expression counts of the top 50 differentially expressed genes across t-Tregs and p-Tregs. Both positive and negative log fold changes are displayed. (C) Genes belonging to different Gene Ontology (GO) Biological Process (BP) terms were upregulated in p-Tregs and t-Tregs, respectively. (D) Volcano plot showing genes involved in Treg homeostasis, homing, activation, and function differentially expressed between t-Tregs and p-Tregs (log2 fold change threshold >1.5; false discovery rate <0.05).
GPA33 was reported to be stably expressed on t-Tregs,17 but was also expressed on a subset of cells among CD45RA-Treg cells (Figure 2A). It has been argued that the CD45RA-GPA33+ Treg subset contains activated t-Tregs,17 which might have occurred due to antigen-specific activation after leaving the thymus. Due to the poor cell viability after sorting, which led to RNA degradation,21 we could not investigate this subset further by transcriptome analysis. Our findings confirm that CD45RA and GPA33 can be used to identify 2 distinct subsets of Tregs, which differentially express genes related to their thymic or peripheral origin.
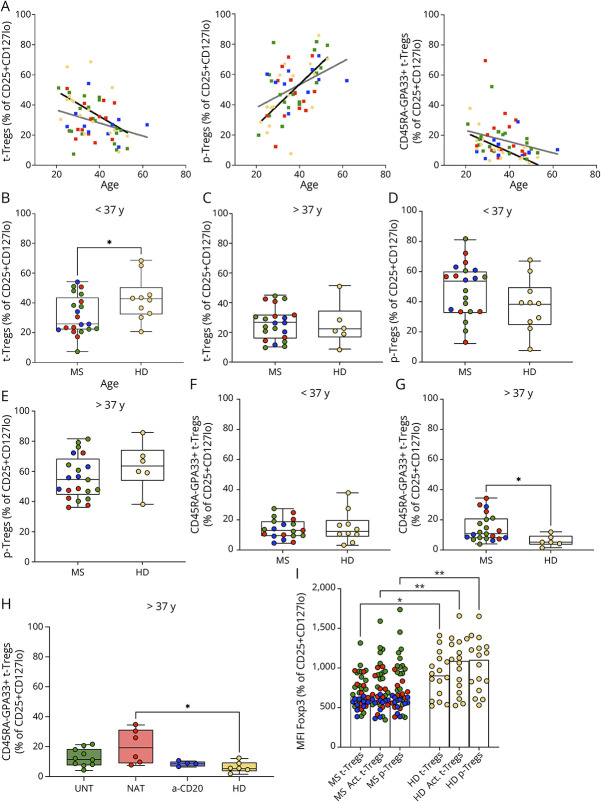
Age-Dependent, Altered Distribution of t-Tregs in Patients With MS
Previous studies have used the expression of CD45RA, CD31, or Helios to discriminate between t-Tregs and p-Tregs.9,14,17 To examine the relative proportion of t-Tregs and p-Tregs in patients with MS when compared with HDs, we used the gating strategy defined earlier (Figure 2A), based on the combination of CD45RA and GPA33. Despite the lack of transcriptome data, we were also interested to analyze the CD45RA-GPA33+ fraction in patients and HDs.
In agreement with previous data (9, 10), we found that t-Tregs tend to decrease during aging in HDs, probably reflecting a progressive thymic involution (r2 = 0.25, p = 0.048; Figure 3A). At the same time, p-Tregs tend to increase (r2 = 0.42, p = 0.007) and CD45RA-GPA33+ t-Tregs tend to decline with age (r2 = 0.45, p = 0.004; Figure 3A). In patients with MS, the trend was less evident for t-Tregs (r2 = 0.1, p = 0.037) and p-Tregs (r2 = 0.18, p = 0.005) in comparison with HDs (Figure 3A). Furthermore, we did not find a significant decline of CD45RA-GPA33+ t-Tregs with aging in patients (Figure 3A).
Figure 3. Balance Between t-Tregs, p-Tregs, and CD45RA-GPA33+ t-Tregs in Patients With Relapsing-Remitting Multiple Sclerosis.
(A–H) Patients with MS (MS) were compared with HDs (HD). Patients included untreated (green), natalizumab-treated (red), and anti-CD20–treated (blue) patients. (A) Age-dependent course of t-Tregs, p-Tregs, and CD45RA-GPA33+ t-Tregs in patients with MS (dark gray line) and HDs (black line) (XY correlation). (B–C) Frequency of t-Tregs in younger and older patients, respectively. (D–E) Frequency of CD45RA-GPA33+ t-Tregs in younger and older patients, respectively. (F–G) Frequency of CD45RA-GPA33+ t-Tregs in younger and older patients, respectively. (B–G) Dots represent the frequency of each donor, boxes extend from the 25th to 75th percentiles, and whiskers from min to max. The line in the middle of the box is the median. Unpaired t test or Mann-Whitney U test. *p < 0.05; **p < 0.01. (H) Multiple comparison of the frequency of CD45RA-GPA33+ t-Tregs between untreated (UNT), natalizumab-treated (NAT), anti-CD20–treated patients (a-CD20), and HDs (HD). (I) Multiple comparison of the median MFI of Foxp3 in t-Tregs, p-Tregs, and CD45RA-GPA33+ t-Tregs (Act. t-Tregs) between patients and HDs. UNT: untreated. NAT: natalizumab-treated. a-CD20: anti-CD20–treated. (H–I) Scatterplot with bar graph. One-way ANOVA or Kruskal-Wallis test. *p < 0.05; **p < 0.01.
Given the influence of age on Treg subset distribution, we used the median age of the cohort (37 years) as cutoff to define 2 age groups. Next, we compared matched age groups of patients and HDs. We found that the frequency of t-Tregs was significantly decreased in younger, but not in older patients with MS (p = 0.039; Figures 3, B and C). The percentage of p-Tregs was not significantly altered both in younger and older patients (Figures 3, D and E). Moreover, in contrast to younger patients, older patients displayed an increased frequency of CD45RA-GPA33+ t-Tregs in comparison with HDs (p = 0.033; Figures 3, F and G). When we performed a multiple comparison analysis, we observed that CD45RA-GPA33+ t-Tregs were mainly increased in older natalizumab-treated patients (p = 0.011; Figure 3H). No significant differences in the proportion of the other Treg subsets between treatment groups were apparent (eFigure 4, A–E).
Furthermore, we observed that Foxp3 expression was lower in all Treg subsets in patients with MS when compared with HDs (p = 0.045, 0.007 and 0.010, respectively; Figure 3H). When we compared treatment groups, we found that the reduction of Foxp3 expression was more pronounced in CD45RA-GPA33+ t-Tregs and p-Tregs and in anti-CD20–treated patients (p = 0.002 and p = 0.003, respectively; eFigure 4F). In summary, we observed age-dependent alterations of t-Treg subsets and a reduced expression of Foxp3 in each subset of Tregs in patients with MS.
Determinants of Treg Function in Patients With MS
To characterize functional aspects of Tregs, we performed suppression assays with Tregs (CD4+CD25+CD127lo) and T effector cells (Teffs, CD4+CD25−) isolated from 30 patients with RRMS and 11 HDs (eTable 1). The suppression assay assesses the effect of different numbers of Tregs on a fixed number of activated bulk effector cells with proliferation as readout (Figure 4A). The mean suppressive capacity at 5 Treg:Teff ratios was significantly reduced in natalizumab-treated patients compared with untreated and anti-CD20–treated patients (p = 0.004 and p = 0.001, respectively; Figure 4A).
Figure 4. Treg Function in Multiple Sclerosis.
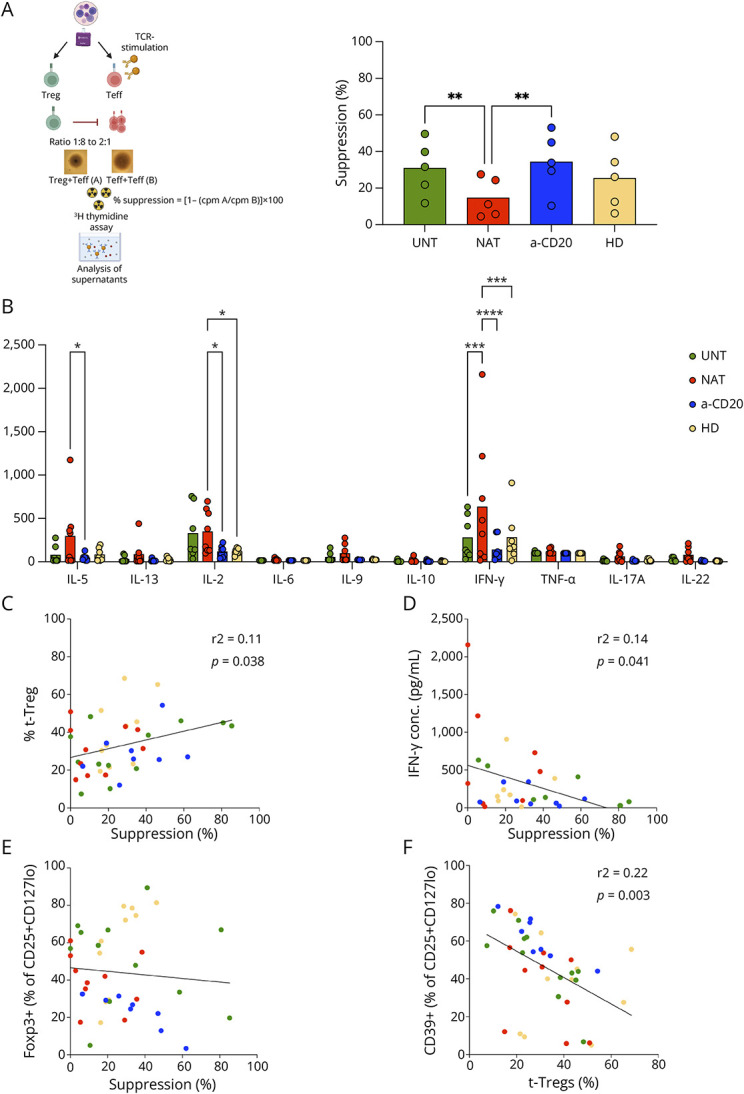
(A) Schematic representation of suppression assay: polyclonally stimulated Teffs were cocultured with different ratio of Tregs, and the proliferation was measured at day 5 (created with Biorender.com). The mean Treg inhibitory capacity calculated at 5 Treg:Teff ratios is shown. (B) Concentrations of cytokines were detected in the supernatants harvested from the suppression assay at ratio Treg:Teff 1:1 by a bead-based immunoassay. (C) XY correlation between Treg suppressive capacity and frequency of t-Tregs within CD25+CD127lo subset. (D) XY correlation between Treg suppressive function and IFN-γ concentration in the supernatants. (E) XY correlation between Treg function and frequency of FOXP3+ cells within CD25+CD127lo fraction. (F) XY correlation between frequency of t-Tregs and CD39+ cells within CD25+CD127lo subset. (A–B) Scatterplot with bar graph. Two-way ANOVA test. *p < 0.05; **p < 0.01; ***p < 0.001; and ****p < 0.0001. (A–F) UNT: untreated (green). NAT: natalizumab-treated (red). a-CD20: anti-CD20–treated (blue). HD: healthy donor (yellow).
Next, we quantified cytokines in cell culture supernatants of the suppression assays in 24 patients with RRMS and 7 HDs (eTables 1 and 3). The mean concentration of IL-5 was significantly higher in natalizumab-treated compared with that in anti-CD20–treated patients (p = 0.018. Figure 4B). Natalizumab-treated patients had increased concentrations of IL-2 in comparison with HDs and anti-CD20–treated patients (p = 0.044 and p = 0.033, respectively. Figure 4B). Moreover, natalizumab-treated patients had the strongest secretion of IFN-γ compared with HDs, untreated patients and anti-CD20–treated patients (p = 0.0004, p = 0.0004, and p < 0.0001, respectively. Figure 4B), which indicates a functional skew toward Th1.
To understand which factors influence Treg suppressive function, we plotted the mean suppressive capacity against the surface markers and cytokine secretion data using a correlation analysis (eTable 4). The suppressive function of Tregs tended to increase together with the percentages of t-Tregs (r2 = 0.11, p = 0.038; Figure 4C). By contrast, Treg function tended to decrease when IFN-γ concentration in supernatants increased (r2 = 0.14, p = 0.041; Figure 4D). Treg function and frequency of Foxp3+ cells within the CD25+CD127lo fraction did not show a correlation (Figure 4E). Moreover, the frequency of CD39+ cells within CD25+CD127lo subset was not positively correlated to Treg suppressive capacity (eTable 4), but inversely correlated to the frequency of t-Tregs (r2 = 0.22, p = 0.003; Figure 4F), in agreement with a higher expression of CD39 on effector/memory-like Tregs.13 The mean suppressive function was 18.6% in HLA-DRB1*15:01–positive individuals and 20.7% in HLA-DRB1*15:01–negative individuals. Collectively, our results suggest a reduced Treg function in natalizumab-treated patients that might be related to defective t-Tregs and a proinflammatory skew of autoreactive T cells.
Gene Expression Alterations in Thymus-Derived Tregs of Natalizumab-Treated Patients
To further investigate the fact that an impaired Treg function was observed exclusively in natalizumab-treated patients, we compared the gene expression profile between 9 patients (3 untreated, 3 natalizumab-treated and 3 anti-CD20–treated patients) and 3 HDs. Sex ratio, HLA-DR haplotype, mean age during blood collection, and frequency of t-Tregs were not significantly different between patients and HDs (χ2 test of independence and one-way ANOVA, respectively; eTable 1). The mean suppressive capacity was 8% in natalizumab-treated patients and 18.7%, 27%, and 19.3% in untreated patients, anti-CD20–treated patients and HDs, respectively. We isolated both t-Tregs and p-Tregs and performed bulk RNA sequencing. A total of 512 genes were differentially expressed between t-Tregs derived from natalizumab-treated patients and HDs (false discovery rate <0.05; Figure 5A). Most of the differentially expressed genes (DEGs) were downregulated in t-Tregs derived from natalizumab-treated patients (Figures 5, A and B). In contrast and consistent with the similar suppressive activities, we did not find DEGs in t-Tregs from untreated and anti-CD20–treated patients, respectively, in comparison with those from HDs (eFigures 5, A–C). DEGs between patients and HDs in p-Tregs were 0 in untreated, 6 in anti-CD20–treated patients, and 1 in natalizumab-treated patients, respectively (eFigures 5, B, D, and E). We explored the function of the pathways differentially modulated in t-Tregs from natalizumab-treated patients and HDs with reference to the biological process (BP) domain of the Gene Ontology (GO) functional database. We observed that 26 of 512 DEGs showed a significant correlation with Treg function (eTable 5). Genes implicated in RNA splicing and mRNA processing were upregulated in Tregs derived from natalizumab-treated patients in comparison with those in HDs (Figures 6C and eFigure 6; eTable 6). By contrast, genes involved in ion transmembrane transport, signaling, positive regulation of cell proliferation, chemotaxis, and cell adhesion were downregulated in t-Tregs derived from natalizumab-treated patients (Figures 5C and eFigure 7; eTable 7). Among the genes that were negatively correlated to Treg suppressive capacity and overexpressed in natalizumab-treated patients, we found IL7R (CD127), which is among other functions involved in homeostatic proliferation of T cells, genes playing a role in RNA processing, and TXNIP (Figure 5D). Of interest, the latter is induced following sustained T-cell activation and restrains lymphocyte proliferation.22,23 Genes that were positively correlated to Treg inhibitory function and downregulated in natalizumab-treated patients included E2F1, IL13RA1, and MS4A5, which are involved in the regulation of cell proliferation and signal transduction. Therefore, an altered expression of genes implicated in t-Treg homeostasis may determine a functional Treg impairment in natalizumab-treated patients (Figure 5D).
Figure 5. Transcriptomic Analysis of t-Tregs Derived From Natalizumab-Treated Patients and Healthy Donors.
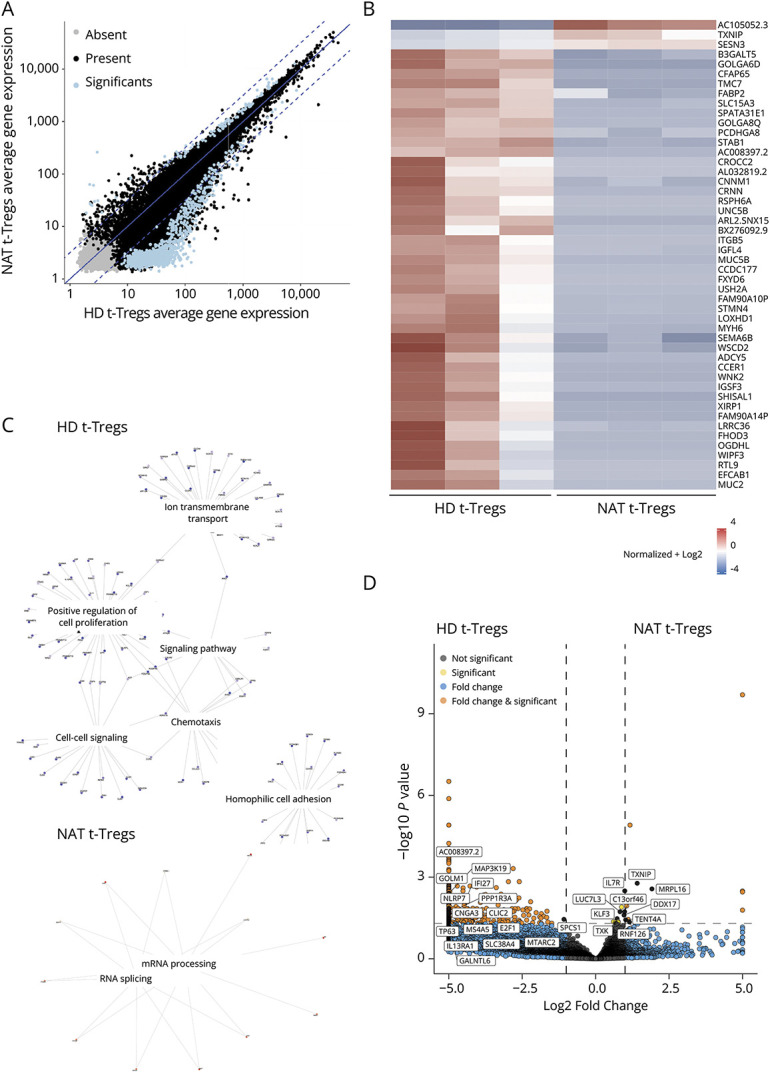
(A) Comparison of average gene expression showing significantly differentially expressed genes between t-Tregs derived from natalizumab-treated patients and HDs. Present (black) indicates genes with read counts ≥10 in more than one sample, absent (gray) indicates all genes that are not flagged as present, and significant (blue) indicates genes with log2 ratio >0.5 and a p value <0.01. Log2 ratio indicates the fold-change in gene expression. (B) Heatmap showing the expression counts of the top 50 differentially expressed genes across t-Tregs derived from natalizumab-treated patients and HDs. Both positive and negative log fold changes are displayed. (C) Genes belonging to different Gene Ontology (GO) Biological Process (BP) terms were upregulated in t-Tregs derived from natalizumab-treated patients and HDs, respectively. (D) Volcano plot showing genes involved in T-cell activation, proliferation, metabolism, and RNA processing differentially expressed between t-Tregs derived from natalizumab-treated patients and HDs (log2 fold change threshold >1.5; false discovery rate <0.05).
Discussion
We analyzed phenotypic and functional aspects of the Treg compartments in a cohort of 41 patients with MS with similar demographics as the 17 HDs with the exception of a strong enrichment of individuals expressing the MS-associated HLA-DR15 haplotype among patients. In agreement with previous reports, we found no evidence for reduced numbers of CD25+CD127lo cells, but observed reduced Foxp3+ CD25+CD127lo cells in patients.7,24 Low Foxp3 expression was found in both thymic and peripheral Tregs and did not seem to influence Treg function. Furthermore, we detected an increased expression of CD127 on Tregs in anti-CD20–treated patients. This molecule is believed to identify activated Tregs25 and has been found to be abnormally upregulated in patients with MS.26 As Tregs constitutively express low surface levels of CD49d,27 one would expect them to be less influenced by natalizumab. By contrast, we confirmed their susceptibility to the treatment.28
To discern possible Treg alterations in MS, we used GPA33 that marks thymus-derived Tregs in a stable way. We observed age-dependent Treg alterations in patients with MS, which may reflect premature thymic involution, which has already been reported.10,29 The relevance of a reduced thymic output in MS pathogenesis is related to the induction of compensatory mechanisms such as homeostatic proliferation, expression of antiapoptotic signals, and conversion of Teffs into Tregs.30 It has been suggested that homeostatic proliferation constricts the TCR repertoire and expands autoreactive T cells.31
The frequency of p-Tregs, which are believed to derive from Teffs, was not significantly changed in patients. However, we found an increased frequency of CD45RA-GPA33+ t-Tregs, which may represent a transitional status between naive t-Tregs and those that had been reactivated, in older patients. Higher numbers of memory-like CD45RAlow Tregs have been detected in older patients with MS.29 Additional studies of CD45RA-GPA33+ t-Tregs, for example by single-cell RNAseq, are required to understand whether they are a distinct entity.
A correlation between the frequency of t-Tregs and Treg inhibitory capacity has already been described,9 but we found that the latter was reduced only in natalizumab-treated patients. Although a functional Treg defect in patients with MS has been reported by numerous studies,4,6,8 Treg function was not always found to be affected.29 Furthermore, some studies assessed Treg function pre- and posttreatment, for instance changes after natalizumab infusion have been excluded.32 The effect of different immunomodulatory therapies has not been examined until now. An enhanced T-cell autoproliferation with production of proinflammatory cytokines after natalizumab treatment, probably sustained by high circulating B cells, has been reported.19 Because brain-homing cells are trapped in the peripheral blood, inflammation is dampened or abrogated in the CNS during the time of treatment. However, when natalizumab is stopped, patients often show rebound of disease activity.20 By contrast, anti-CD20 therapy inhibits T-cell autoproliferation by removing memory B cells, which stimulate proinflammatory CD4+ T cells and proinflammatory cytokine secretion of the latter.20 Consistent with these observations, we found increased inflammatory cytokines in the supernatants harvested from suppression assays performed in natalizumab-treated patients when compared with anti-CD20–treated patients.
The reduced Treg suppressive capacity in natalizumab-treated patients might either be an as yet unknown direct effect on Tregs or be secondary to Teff refractoriness to suppression due to increased autoproliferation and their proinflammatory differentiation state.19 The detection of an altered transcriptome in t-Tregs, but not in p-Tregs, derived from natalizumab-treated patients supports a direct effect on t-Tregs, while the increase in proinflammatory cytokines in the supernatants argues that the increase of autoreactive T cells in the periphery, their activation, and proinflammatory skew also affect t-Tregs and their phenotypes.11 We consider the latter possibility as more likely, but this aspect should be studied in more detail.
Besides a downregulation of genes involved in T-cell activation, proliferation, and migration, we observed an upregulation of IL7R (CD127) that was negatively correlated with Treg function. A sequence variant in the IL7R gene is recognized as susceptibility gene for MS, although the functional consequences of how the IL7R variant influences disease susceptibility and/or pathogenesis are not fully understood.12,33,34 In a mouse model for MS, it has been shown that t-Tregs express IL7R during active disease.35 Thus, the increased expression of CD127 by Tregs in patients with MS may indicate a compensatory mechanism to balance the reduced thymic Treg neogenesis and contribute to the functional Treg impairment. Another gene of interest is TXNIP, which was upregulated in natalizumab-treated patients and was also the most significantly upregulated gene in twins with MS when compared with that in unaffected twins.36 In the case of upregulation of TXNIP during natalizumab treatment, it is not clear, similar to the upregulation of IL7R, whether this is a compensatory mechanism.
Limitations of our study were the cohort size, especially considering the heterogeneity of treatment groups, which requires multiple comparison analyses, and differences in the fraction of HLA-DR15+ individuals, which were much higher in patients compared with those in HDs. We did not investigate the prevalence of known risk factors of MS, such as obesity, smoking, and exposure to vitamin D. These factors influence Treg homeostasis and, if differentially distributed among groups of patients, could influence the results.37-39 Additional studies would be necessary to dissect complex interactions between genetic background, environment, and immune system in MS. Other limitations are that we did not examine in depth the intrinsic Teff proliferative capacity and whether the upregulation of IL7R was related to it. For the latter purpose, the use of a single-cell RNAseq approach might be more sensitive in detecting signaling pathways that could be therapeutically targeted. Finally, regarding the effects of natalizumab on Tregs, it would have been interesting to follow Treg phenotype and function before and on natalizumab treatment, i.e., longitudinally.
While we cannot rule out peripheral mechanisms, we suggest that t-Treg neogenesis might be affected in younger patients with MS. Compensatory mechanisms might ensure a normal Treg function in untreated and anti-CD20–treated patients. However, in natalizumab-treated patients, whose t-Tregs show altered gene expression, these compensatory mechanisms might fail to recover Treg function and/or be impeded by the proinflammatory environment, likely enhanced by anti-VLA treatment.
The adoptive transfer of Tregs has been proposed as a promising strategy to reestablish Treg-mediated tolerance. Tregs might be beneficial in suppressing not only autoreactive T cells but also CNS inflammation because they can enter this compartment different from many of the currently approved MS therapies. This aspect may be particularly relevant in the context of disease progression independent of relapse activity,40 which is attributed to ongoing immune activation in chronically smoldering lesions.41 The use of t-Tregs seems preferable in this situation. Our findings may have implications for the selection of candidates eligible for this therapy and the timing of the adoptive transfer. The effects of the disease course and immunomodulatory treatments on Treg homeostasis should also be considered when defining the therapeutic strategy.
Acknowledgment
The authors thank C.F. Aquino (Functional Genomics Center Zurich) for technical support with RNA sequencing, the members of the laboratory team at NIMS for processing blood samples and technical support, the staff of the Hematology Clinic, University Hospital Zurich, for performing leukaphereses, the members of the clinical team at NIMS for contacting patients and collecting blood samples, the staff of the MS Outpatient Clinic and Day Hospital, Neurology Clinic for patient care–related aspects of the study, and patients for donating blood samples. The authors thank F. Sallusto, M. Kopf, and D.J. Mathis for comments that improved the manuscript.
Glossary
- HDs
healthy donors
- MS
multiple sclerosis
- RNAseq
RNA sequencing
- RRMS
relapsing-remitting MS
- RTE
recent thymic emigrant
Appendix. Authors
| Name | Location | Contribution |
| Tiziana Lorenzini, MD, PhD | Neuroimmunology and MS Research, Neurology Clinic, University Hospital Zurich; Division of Immunology, University Children's Hospital Zurich, University of Zurich; Switzerland | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Wolfgang Faigle, PhD | Neuroimmunology and MS Research, Neurology Clinic, University Hospital Zurich, University of Zurich; Cellerys AG; Schlieren, Switzerland; Immunity and Cancer (U932), Immune Response to Cancer Laboratory, Institut Curie; 26 rue d'Ulm, CEDEX 05, Paris, France | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; and analysis or interpretation of data |
| Josefine Ruder, MD, PhD | Neuroimmunology and MS Research, Neurology Clinic, University Hospital Zurich, University of Zurich, Switzerland | Drafting/revision of the article for content, including medical writing for content |
| María José Docampo, PhD | Neuroimmunology and MS Research, Neurology Clinic, University Hospital Zurich, University of Zurich, Switzerland | Drafting/revision of the article for content, including medical writing for content |
| Lennart Opitz, MS | Functional Genomics Center Zurich, Swiss Federal Institute of Technology and University of Zurich, Switzerland | Analysis or interpretation of data |
| Roland Martin, MS | Neuroimmunology and MS Research, Neurology Clinic, University Hospital Zurich, University of Zurich; Cellerys AG, Schlieren; Institute of Experimental Immunology, University of Zurich, Switzerland; Therapeutic Design Unit, Center for Molecular Medicine, Department of Clinical Neurosciences, Karolinska Institutet; Stockholm, Sweden | Drafting/revision of the article for content, including medical writing for content; study concept or design; and analysis or interpretation of data |
Study Funding
University of Zurich through the Clinical Research Priority Project Precision-MS (RM)Swiss National Science Foundation (SNF), grant 32003B_185003 awarded (RM)Abata Therapeutics, Cambridge, MA, USA (RM, TL).
Disclosure
R. Martin received unrestricted grant support from Biogen, Novartis, Hoffman La Roche, and Third Rock and compensation for advice/lecturing by Biogen, Novartis, Sanofi Genzyme, Merck, Hoffmann La Roche, Neuway, CellProtect, and Abata. R. Martin is employed part-time by Cellerys, a startup company outfounded from the University of Zurich. He is a cofounder and stockholder of Cellerys and currently employed by Cellerys. He is also a cofounder of Abata Therapeutics. R. Martin is listed as an inventor on patents of the University of Zurich about target antigens in multiple sclerosis. R. Martin is further listed as an inventor and received remuneration for an NIH-held patent on the use of daclizumab to treat multiple sclerosis. None of this has affected this work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Go to Neurology.org/NN for full disclosures.
References
- 1.Sospedra M, Martin R. Immunology of multiple sclerosis. Semin Neurol. 2016;36(02):115-127. doi: 10.1055/s-0036-1579739 [DOI] [PubMed] [Google Scholar]
- 2.Walton C, King R, Rechtman L, et al. Rising prevalence of multiple sclerosis worldwide: insights from the Atlas of MS, third edition. Mult Scler. 2020;26(14):1816-1821. doi: 10.1177/1352458520970841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costantino CM, Baecher-Allan C, Hafler DA. Multiple sclerosis and regulatory T cells. J Clin Immunol. 2008;28(6):697-706. doi: 10.1007/s10875-008-9236-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199(7):971-979. doi: 10.1084/jem.20031579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feger U, Luther C, Poeschel S, Melms A, Tolosa E, Wiendl H. Increased frequency of CD4+ CD25+ regulatory T cells in the cerebrospinal fluid but not in the blood of multiple sclerosis patients. Clin Exp Immunol. 2007;147(3):412-418. doi: 10.1111/j.1365-2249.2006.03271.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Venken K, Hellings N, Thewissen M, et al. Compromised CD4+ CD25(high) regulatory T-cell function in patients with relapsing-remitting multiple sclerosis is correlated with a reduced frequency of FOXP3-positive cells and reduced FOXP3 expression at the single-cell level. Immunology. 2008;123(1):79-89. doi: 10.1111/j.1365-2567.2007.02690.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li YF, Zhang SX, Ma XW, et al. The proportion of peripheral regulatory T cells in patients with Multiple Sclerosis: a meta-analysis. Mult Scler Relat Disord. 2019;28:75-80. doi: 10.1016/j.msard.2018.12.019 [DOI] [PubMed] [Google Scholar]
- 8.Haas J, Hug A, Viehöver A, et al. Reduced suppressive effect of CD4+CD25high regulatory T cells on the T cell immune response against myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. Eur J Immunol. 2005;35(11):3343-3352. doi: 10.1002/eji.200526065 [DOI] [PubMed] [Google Scholar]
- 9.Haas J, Fritzsching B, Trübswetter P, et al. Prevalence of newly generated naive regulatory T cells (Treg) is critical for Treg suppressive function and determines Treg dysfunction in multiple sclerosis. J Immunol. 2007;179(2):1322-1330. doi: 10.4049/jimmunol.179.2.1322 [DOI] [PubMed] [Google Scholar]
- 10.Balint B, Haas J, Schwarz A, et al. T-cell homeostasis in pediatric multiple sclerosis: old cells in young patients. Neurology. 2013;81(9):784-792. doi: 10.1212/WNL.0b013e3182a2ce0e [DOI] [PubMed] [Google Scholar]
- 11.Dominguez-Villar M, Baecher-Allan CM, Hafler DA. Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat Med. 2011;17(6):673-675. doi: 10.1038/nm.2389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.International Multiple Sclerosis Genetics Consortium. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science. 2019;365(6460):eaav7188. doi: 10.1126/science.aav7188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verma ND, Lam AD, Chiu C, Tran GT, Hall BM, Hodgkinson SJ. Multiple sclerosis patients have reduced resting and increased activated CD4+CD25+FOXP3+T regulatory cells. Sci Rep. 2021;11(1):10476. doi: 10.1038/s41598-021-88448-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Álvarez-Sánchez N, Cruz-Chamorro I, Díaz-Sánchez M, Lardone PJ, Guerrero JM, Carrillo-Vico A. Peripheral CD39-expressing T regulatory cells are increased and associated with relapsing-remitting multiple sclerosis in relapsing patients. Sci Rep. 2019;9(1):2302. doi: 10.1038/s41598-019-38897-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borsellino G, Kleinewietfeld M, Di Mitri D, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110(4):1225-1232. doi: 10.1182/blood-2006-12-064527 [DOI] [PubMed] [Google Scholar]
- 16.Opstelten R, Amsen D. Separating the wheat from the chaff: making sense of Treg heterogeneity for better adoptive cellular therapy. Immunol Lett. 2021;239:96-112. doi: 10.1016/j.imlet.2021.03.002 [DOI] [PubMed] [Google Scholar]
- 17.Opstelten R, de Kivit S, Slot MC, et al. GPA33: a marker to identify stable human regulatory T cells. J Immunol. 2020;204(12):3139-3148. doi: 10.4049/jimmunol.1901250 [DOI] [PubMed] [Google Scholar]
- 18.Martin R, Sospedra M, Rosito M, Engelhardt B. Current multiple sclerosis treatments have improved our understanding of MS autoimmune pathogenesis. Eur J Immunol. 2016;46(9):2078-2090. doi: 10.1002/eji.201646485 [DOI] [PubMed] [Google Scholar]
- 19.Jelcic I, Al Nimer F, Wang J, et al. Memory B cells activate brain-homing, autoreactive CD4+ T cells in multiple sclerosis. Cell. 2018;175(1):85-100.e23. doi: 10.1016/j.cell.2018.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benkert TF, Dietz L, Hartmann EM, et al. Natalizumab exerts direct signaling capacity and supports a pro-inflammatory phenotype in some patients with multiple sclerosis. PLoS One. 2012;7(12):e52208. doi: 10.1371/journal.pone.0052208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishimoto KP, Newkirk D, Hou S, Fruehauf J, Nelson EL. Fluorescence activated cell sorting (FACS) using RNAlater to minimize RNA degradation and perturbation of mRNA expression from cells involved in initial host microbe interactions. J Microbiol Methods. 2007;70(1):205-208. doi: 10.1016/j.mimet.2007.03.022 [DOI] [PubMed] [Google Scholar]
- 22.Muri J, Heer S, Matsushita M, et al. The thioredoxin-1 system is essential for fueling DNA synthesis during T-cell metabolic reprogramming and proliferation. Nat Commun. 2018;9(1):1851. doi: 10.1038/s41467-018-04274-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muri J, Thut H, Kopf M. The thioredoxin-1 inhibitor Txnip restrains effector T-cell and germinal center B-cell expansion. Eur J Immunol. 2021;51(1):115-124. doi: 10.1002/eji.202048851 [DOI] [PubMed] [Google Scholar]
- 24.Huan J, Culbertson N, Spencer L, et al. Decreased FOXP3 levels in multiple sclerosis patients. J Neurosci Res. 2005;81(1):45-52. doi: 10.1002/jnr.20522 [DOI] [PubMed] [Google Scholar]
- 25.Simonetta F, Chiali A, Cordier C, et al. Increased CD127 expression on activated FOXP3+CD4+ regulatory T cells. Eur J Immunol. 2010;40(9):2528-2538. doi: 10.1002/eji.201040531 [DOI] [PubMed] [Google Scholar]
- 26.Michel L, Berthelot L, Pettré S, et al. Patients with relapsing-remitting multiple sclerosis have normal Treg function when cells expressing IL-7 receptor alpha-chain are excluded from the analysis. J Clin Invest. 2008;118(10):3411-3419. doi: 10.1172/JCI35365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kleinewietfeld M, Starke M, Di Mitri D, et al. CD49d provides access to "untouched" human Foxp3+ Treg free of contaminating effector cells. Blood. 2009;113(4):827-836. doi: 10.1182/blood-2008-04-150524 [DOI] [PubMed] [Google Scholar]
- 28.Kimura K, Nakamura M, Sato W, et al. Disrupted balance of T cells under natalizumab treatment in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2016;3(2):e210. doi: 10.1212/NXI.0000000000000210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venken K, Hellings N, Broekmans T, Hensen K, Rummens JL, Stinissen P. Natural naive CD4+CD25+CD127low regulatory T cell (Treg) development and function are disturbed in multiple sclerosis patients: recovery of memory Treg homeostasis during disease progression. J Immunol. 2008;180(9):6411-6420. doi: 10.4049/jimmunol.180.9.6411 [DOI] [PubMed] [Google Scholar]
- 30.Fessler J, Ficjan A, Duftner C, Dejaco C. The impact of aging on regulatory T-cells. Front Immunol 2013;4:231. doi: 10.3389/fimmu.2013.00231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haegert DG. Multiple sclerosis: a disorder of altered T-cell homeostasis. Mult Scler Int 2011;2011:461304. doi: 10.1155/2011/461304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stenner MP, Waschbisch A, Buck D, et al. Effects of natalizumab treatment on Foxp3+ T regulatory cells. PLoS One. 2008;3(10):e3319. doi: 10.1371/journal.pone.0003319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tavakolpour S. Interleukin 7 receptor polymorphisms and the risk of multiple sclerosis: a meta-analysis. Mult Scler Relat Disord. 2016;8:66-73. doi: 10.1016/j.msard.2016.05.001 [DOI] [PubMed] [Google Scholar]
- 34.Jäger J, Schulze C, Rösner S, Martin R. IL7RA haplotype-associated alterations in cellular immune function and gene expression patterns in multiple sclerosis. Genes Immun. 2013;14(7):453-461. doi: 10.1038/gene.2013.40 [DOI] [PubMed] [Google Scholar]
- 35.Chen X, Fang L, Song S, Guo TB, Liu A, Zhang JZ. Thymic regulation of autoimmune disease by accelerated differentiation of Foxp3+ regulatory T cells through IL-7 signaling pathway. J Immunol. 2009;183(10):6135-6144. doi: 10.4049/jimmunol.0901576 [DOI] [PubMed] [Google Scholar]
- 36.Ingelfinger F, Gerdes LA, Kavaka V, et al. Twin study reveals non-heritable immune perturbations in multiple sclerosis. Nature. 2022;603(7899):152-158. doi: 10.1038/s41586-022-04419-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marrodan M, Farez MF, Balbuena Aguirre ME, Correale J. Obesity and the risk of multiple sclerosis. The role of Leptin. Ann Clin Transl Neurol. 2021;8(2):406-424. doi: 10.1002/acn3.51291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Correale J, Farez MF. Smoking worsens multiple sclerosis prognosis: two different pathways are involved. J Neuroimmunol. 2015;281:23-34. doi: 10.1016/j.jneuroim.2015.03.006 [DOI] [PubMed] [Google Scholar]
- 39.da Costa DS, Hygino J, Ferreira TB, et al. Vitamin D modulates different IL-17-secreting T cell subsets in multiple sclerosis patients. J Neuroimmunol. 2016;299:8-18. doi: 10.1016/j.jneuroim.2016.08.005 [DOI] [PubMed] [Google Scholar]
- 40.University of California San Francisco MS-EPIC Team, Cree BAC, Hollenbach JA, et al. Silent progression in disease activity-free relapsing multiple sclerosis. Ann Neurol. 2019;85(5):653-666. doi: 10.1002/ana.25463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Absinta M, Maric D, Gharagozloo M, et al. A lymphocyte-microglia-astrocyte axis in chronic active multiple sclerosis. Nature. 2021;597(7878):709-714. doi: 10.1038/s41586-021-03892-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
RNA sequencing data are deposited in the European Genome-phenome Archive (EGA) and can be accessed upon request.