Abstract
Preclinical and human studies indicate psilocybin may reduce perseverant maladaptive behaviors, including nicotine and alcohol seeking. Such studies in the opioid field are lacking, though opioids are involved in more >50% of overdose deaths. Psilocybin is an agonist at the serotonin 2A receptor (5-HT2AR), a well-documented target for modulation of drug seeking, and evidence suggests 5-HT2AR agonists may dampen motivation for opioids. We sought to investigate the therapeutic efficacy of psilocybin in mediating cessation of opioid use and maintenance of long-lasting abstinence from opioid seeking behavior in a rat model of heroin self-administration (SA). Psilocybin or 5-HT2AR antagonists ketanserin and volinanserin were administered systemically to rats prior to SA of 0.075 mg/kg/infusion of heroin, or relapse following forced abstinence. Psilocybin did not alter heroin taking, but a single exposure to 3.0 mg/kg psilocybin 4–24 hours prior to a relapse test blunted cue-induced heroin seeking. Conversely, 5-HT2AR antagonists exacerbated heroin relapse. To begin to elucidate mechanisms of psilocybin, drug-naïve rats received psilocybin and/or ketanserin, and tissue was collected from the prefrontal cortex (PFC), a region critical for drug seeking and responsive to psilocybin, 24 hours later for RNA-sequencing. 3.0 mg/kg psilocybin regulated ~2-fold more genes in the PFC than 1.0 mg/kg, including genes involved in the cytoskeleton and cytokine signaling. Ketanserin blocked >90% of psilocybin-regulated genes, including the IL-17a cytokine receptor, Il17ra. Psychedelic compounds have reported anti-inflammatory properties, and therefore we performed a gene expression array to measure chemokine/cytokine molecules in the PFC of animals that displayed psilocybin-mediated inhibition of heroin seeking. Psilocybin regulated 4 genes, including Il17a, and a subset of genes correlated with relapse behavior. Selective inhibition of PFC IL-17a was sufficient to reduce heroin relapse. We conclude that psilocybin reduces heroin relapse and highlight IL-17a signaling as a potential downstream pathway of psilocybin that also reduces heroin seeking.
Keywords: opioid, serotonin, self-administration, relapse, drug-seeking, psychedelic, inflammation, cortex, nucleus accumbens, heroin, psilocybin, transcriptome
Introduction:
Opioid misuse contributed to more than half of the 100,000+ overdose deaths that occurred in the United States in 2021 1. Elucidation of novel mechanisms that may reduce opioid use and sustain abstinence remains an essential and critical goal for the reduction of opioid-related misuse events. Clinical trials investigating the therapeutic efficacy of the psychedelic compound psilocybin for the treatment of neuropsychiatric disorders have shown promise for treating conditions plagued by perseverative maladaptive behaviors, including chronic drug seeking 2–5. Psilocybin and its active metabolite psilocin are serotonin 2A receptor (Htr2a [gene], 5-HT2AR [protein]) 6–9 agonists. It is well-established that pharmacological manipulation of 5-HT2AR can modulate drug self-administration (SA), reinstatement, and incubation of craving in preclinical models of psychostimulant, nicotine or alcohol seeking 10–20. Because psilocybin has demonstrated utility at reducing nicotine and alcohol use in humans 2, 3, it may also reduce seeking for other drugs, including opioids. In human subjects, the HTR2A gene was associated with heroin dependence, suggesting a link between 5-HT2AR and regulation of opioid seeking 21, 22. Several studies have investigated the impact of 5-HT2AR agonists on opioid seeking 23–26, but no studies to date have examined the impact of psilocybin on opioid seeking following forced abstinence. However, the limited data available demonstrates that 5-HT2AR agonists do not enhance opioid seeking and may decrease motivation for opioids 23–26. Thus, pharmacological tools that regulate 5-HT2AR signaling, such as psilocybin, may represent suitable candidates for the modulation of opioid seeking behavior.
Downstream of 5-HT2AR activation, molecular and cellular mechanisms of psilocybin may involve regulation of inflammatory signaling pathways because psychedelic compounds dampen activation of TNF-α processes 27–29. Transcriptome sequencing of postmortem brains following heroin overdose indicated that dysregulation of TNF-α/NF-kB-mediated inflammatory pathways in the nucleus accumbens (NAc) and prefrontal cortex (PFC), brain regions critical for drug reinforcement, is a major neuroadaptation following chronic heroin use 30–32. In this study, we aimed to address critical gaps in the field by investigating the putative efficacy of psilocybin in reducing opioid use and maintenance of abstinence from opioid seeking. We hypothesized that psilocybin would reduce heroin seeking and that antagonism of 5-HT2AR may have the opposite effect. We demonstrated that psilocybin reduces heroin seeking following forced abstinence and report the transcriptional effects of psilocybin on the PFC can be efficiently blocked with ketanserin. To delineate mechanisms of psilocybin on opioid seeking, we show that distinct patterns of inflammatory cytokine and chemokine genes are regulated in the PFC and NAc during psilocybin-mediated inhibition of heroin relapse and are associated with relapse behavior. Furthermore, we highlight the IL-17a pathway as a potential downstream pathway of psilocybin that is sufficient to reduce heroin relapse. Thus, we conclude that psilocybin reduces heroin relapse and regulates brain inflammatory gene expression, including components of the IL-17a pathway. Similarly, inhibition of PFC IL-17a with monoclonal antibodies also reduces heroin relapse. This suggests that diminishing this inflammatory pathway in the PFC may be one of the potential mechanisms through which psilocybin exerts its positive effects in reducing heroin-seeking behaviors.
Methods and Materials:
Subjects
Adult male Sprague Dawley rats (Charles River Laboratories), aged 8 weeks on arrival, were used in this study. Rats for SA were kept on a reverse light/dark cycle (lights on at 9:00 p.m.; off at 9:00 a.m.) with constant room temperature (22 ± 2 °C) and humidity (40%). Rats were pair-housed upon arrival. Following intravenous catheter surgery, rats were single-housed for the remainder of the study. Food was provided ad libitum, except where described. All procedures were compliant with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and approved by Temple University’s Institutional Animal Care and Use Committee.
Drugs
Diamorphine hydrochloride (heroin) was provided by the National Institute on Drug Abuse drug supply program and dissolved in sterile saline (0.9% sodium chloride) for SA. Ketanserin (tartrate) (Cayman Chemical Company, Ann Arbor, MI; catalog # 22058) and volinanserin (Cayman Chemical Company, catalog #15936, also known as MDL 100907) were dissolved in 5% Tween-80, then diluted with sterile saline. Psilocybin (Cayman Chemical Company; catalog # 14041) was dissolved in sterile saline. IL-17a monoclonal antibody (BioCell, Lebanon NH; catalog # BE0173) and IgG1 control (BioCell; catalog # BE0083) were diluted in InVivoPure Dilution Buffer (BioCell; catalog # IP0080).
Heroin self-administration (SA) and relapse
Jugular vein catheter implantation and long-access heroin SA were performed as previously described 33, 34, in 6-hour (hr) daily sessions on a fixed ratio (FR) 1 schedule at a dose of 0.075 mg/kg/infusion of heroin. Following SA, rats underwent forced abstinence in their home cage for 17 or 21 days (D). After abstinence, rats were re-exposed to SA chambers for one or three 30-minute relapse tests, during which all cues associated with active lever pressing were present, but no drug delivery occurred. Only one relapse test occurred per day, in consecutive days. Rats were immediately euthanized at the conclusion of behavioral assessments by rapid decapitation. Brains were removed, frozen by submersion in isopentane on a dry-ice platform and stored at −80°C until dissection.
Pharmacological manipulations
Psilocybin, ketanserin and volinanserin were administered prior to heroin SA on days 9–11, and prior to relapse tests following forced abstinence. For manipulations during SA and relapse, rats were balanced for drug intake after day 8 of SA and divided into experimental groups that were maintained throughout the duration of the study. For examination of the effects of a single dose of psilocybin on relapse, rats were divided into experimental groups based on heroin intake after day 10 of SA. 0.1, 1, or 3.0 mg/kg psilocybin, 0.1, 1, or 3.0 mg/kg ketanserin, or 0.04 or 0.2 mg/kg volinanserin were administered intraperitoneally (IP) either 15 minutes (ketanserin, volinanserin) or 4 hr (psilocybin) prior to the beginning of each SA or relapse session. One rat in the 1.0 mg/kg psilocybin group was excluded from the relapse test due to death during the abstinence period. For transcriptome profiling, drug-naïve rats were injected IP once with 1.0 mg/kg psilocybin, 3.0 mg/kg psilocybin, 3.0 mg/kg ketanserin, or 3.0 mg/kg of psilocybin and ketanserin simultaneously and euthanized 24 hr later. Sample sizes were as follows: psilocybin administration during SA and relapse-vehicle n=12; 0.1 mg/kg psilocybin n=5; 1.0 mg/kg psilocybin n=9; 3 mg/kg psilocybin n= 5; psilocybin administration once, prior to relapse-vehicle n=15; 3 mg/kg psilocybin, 4 hr n=8; 3 mg/kg psilocybin, 24 hr n=8; ketanserin administration during SA and relapse-vehicle n=12; 0.1 mg/kg ketanserin n=5; 1 mg/kg ketanserin n=8; 3 mg/kg ketanserin n=8; volinanserin administration during SA and relapse-vehicle n=6; 0.04 mg/kg volinanserin n=9; 0.2 mg/kg volinanserin n=10; For PFC infusion of IL-17a antibody prior to relapse-IgG n=8; IL-17a antibody n=7.
Intra-PFC infusion of IL-17a antibody
Rats underwent stereotaxic surgery for the bilateral implantation of cannulas (Protech International, Inc, Boerne, TX), as we have described35, into the medial PFC, 5 days following the end of heroin SA, into the following coordinates from Bregma: anterior-posterior (AP) +2.7 mm, medial-lateral (ML) ±0.5 mm, and dorsal-ventral (DV) −3.5 mm. Rats received bilateral infusions of either 50 ng/ul IL-17a monoclonal antibody, or an IgG control, in a total volume of 1ul per hemisphere, delivered at a rate of 0.2 μL/min. The delivery of the correct volume was controlled using an infusion pump connected to two 10-microliter Hamilton syringes, which were linked to an infuser (Protech International, Inc,) via a tubing system. The first infusion was administered approximately 48 hours before the relapse test, and the second infusion occurred on the day of the relapse test, four hours prior. At the conclusion of the relapse test, rats were euthanized within one hour, their brains were rapidly removed and snap frozen by immersion in isopentane on a dry ice platform. Verification of cannula placements was performed through visual analysis during the tissue punching for the extraction of the PFC, utilizing the same coordinates as those used for cannulation.
RNA extraction and gene expression analyses
PFC or NAc (core and shell) tissue from rat brains was dissected on a cold plate at −20°C. Total RNA was extracted using the miRNeasy Mini Kit (Qiagen, Hilden, Germany), following manufacturer’s instructions. The RT2 Profiler™ Rat Inflammatory Cytokines & Receptors PCR Array (Qiagen) was used to measure inflammatory cytokines and chemokines in PFC and NAc tissue of rats that underwent heroin SA, forced abstinence and a single injection of 3.0 mg/kg psilocybin 4 or 24 hr prior to one 30-minute relapse test. For qPCR measurement of Fos in the PFC of rats that received IL-17a antibody prior to a heroin relapse test, RNA was reverse transcribed into cDNA using the Maxima Reverse Transcriptase kit (Thermo Fisher Scientific) and qPCR was performed using the PrimeTime Gene Expression Master Mix and gene assays (Integrated DNA Technologies, Coralville, IA). Primer sequences are available in the supplement. The RNA sequencing of PFC tissue from drug-naïve animals 24 hr following administration of psilocybin and/or ketanserin was performed by Novogene. Raw sequencing data are available in the Gene Expression Omnibus (Accession # GSE235175). For RNA sequencing, n=4 for all groups.
Statistics
Statistical analyses were performed using GraphPad software (Prism version 10; GraphPad, San Diego, CA) and R. Error bars represent the mean ± the standard error of the mean (SEM). No randomization was performed in this study, due to the necessity to balance heroin intake between treatment groups. Sample sizes for experiments were determined based on similar studies in the neuroscience field. For behavioral data, repeated measures analysis of variance (RM-ANOVA) was used to analyze differences in lever pressing or infusions during SA. Outlier analysis was performed using the ROUT method in GraphPad. Shapiro-Wilk normality tests were performed to evaluate the distribution of data and similar levels of variation were observed between groups. For analysis of behavioral data at a single SA day or relapse test with a normal distribution, one-way ANOVAs were performed with post hoc Tukey tests to determine differences between more than two groups. For analysis of behavioral data at a single SA day or relapse test without a normal distribution, Kruskal-Wallis tests with post hoc Dunn’s tests were used to compare 3 or more groups. Paired t-tests were used to compare drug SA within individual rats before and after administration of psilocybin or ketanserin. Pearson correlations were used to compare the relationship between gene expression and relapse responses. For qPCR, differential expression analyses were performed using the ΔΔct method of analysis 36, followed by unpaired t-test between treatment groups. Replicates were excluded from qPCR analysis if a Ct value ≥ 35. For RNA-seq, DESeq2 R package (1.20.0) 37 was used to determine differential expression between two groups, and the GeneOverlap package in R Bioconductor, version 1.36.0 was used to determine statistical significance of two transcriptome datasets using the Fisher’s exact test. RNA sequencing was performed on biological replicate samples. Results from pharmacological manipulations were obtained from 2–3 cohorts of animals per manipulation. A p value of <0.05 was considered statistically significant.
Additional methodological details are available in the supplemental material.
Results:
Psilocybin reduced heroin seeking, but not taking.
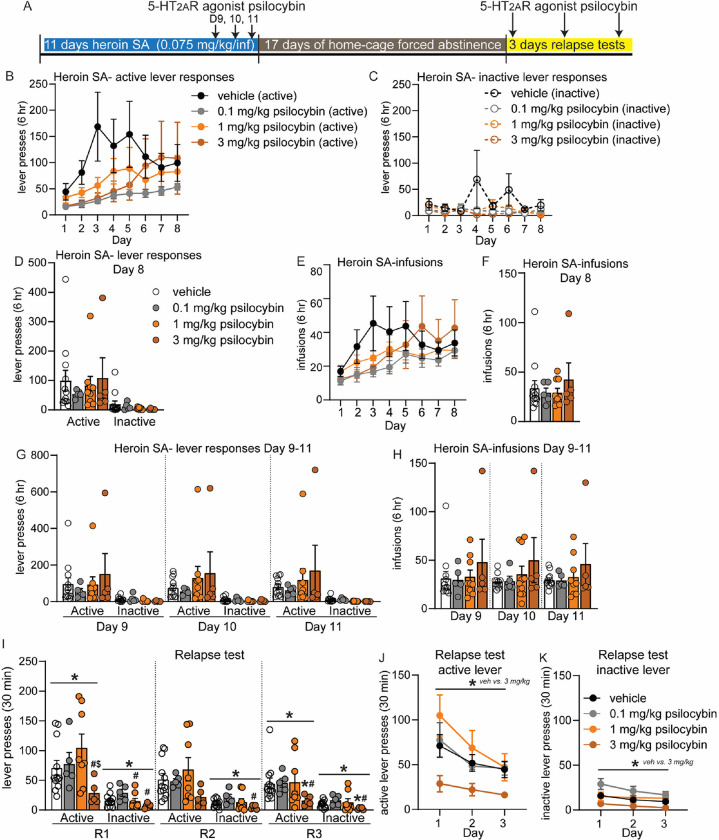
To investigate the potential therapeutic efficacy of psilocybin in mediating cessation of opioid use and maintenance of long-lasting abstinence from opioid seeking behavior, we first tested the consequences of psilocybin on heroin SA (Figure 1A). Based on previous observations that 5-HT2AR agonists may decrease motivation for opioids23, 24, we hypothesized that psilocybin may reduce heroin taking. Adult male rats were trained to self-administer heroin in 6 hr daily sessions at a dose of 0.075mg/kg/infusion for 8 days (Figure 1B–F). Rats were separated into 4 treatment groups, with active lever responses and infusions during SA balanced: 0.1, 1, or 3.0 mg/kg psilocybin and saline vehicle (Figure 1D, F). On D9, 10 and 11 of heroin SA, rats received a single IP injection of psilocybin or vehicle 4 hr prior to SA. The 4 hr timepoint was chosen because at this timepoint, the psilocybin-induced head twitch response, thought to represent a hallucinogenic effect, has subsided and psilocybin will have been cleared from the brain and blood, thus eliminating any motor impairment confounds 38–43. Doses were chosen based on prior research involving psilocybin in rats 18, 19, 41. The 0.1 mg/kg dose is categorized as a microdose, given that it is one-tenth of the minimum dose required for eliciting behavioral and cellular effects in rats 18, 44. The 1 mg/kg dose is among the most extensively studied doses in rodents for both behavioral and molecular effects44–46, while 3 mg/kg falls within the range of therapeutic doses for humans 18. RM-ANOVA analysis revealed no main effect of psilocybin treatment or psilocybin by time interactions for any dose of psilocybin compared to vehicle for either lever presses or infusions (Figure 1G, H). Likewise, lever responses and infusions during D9–11 did not differ for SA of heroin when rats were pretreated with psilocybin. Analysis of individual rats’ heroin intake determined that rats do not significantly alter SA of heroin at the 0.075 mg/kg/infusion dose behavior following psilocybin (Supplemental Figure 1).
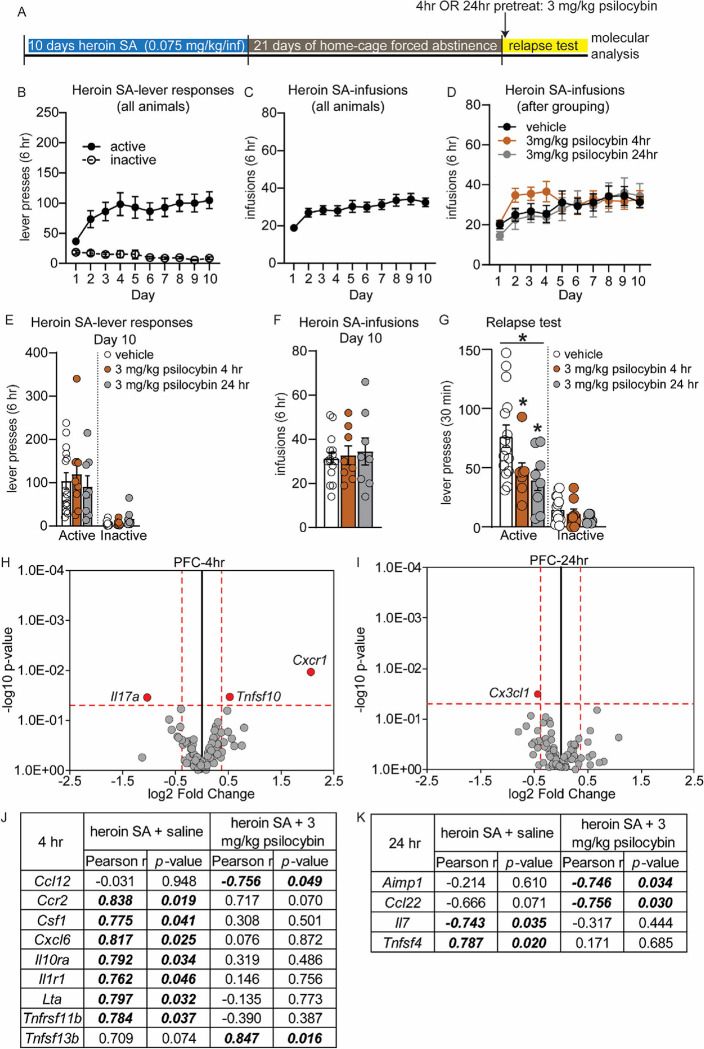
Figure 1: Psilocybin reduces heroin relapse after forced abstinence but not heroin intake.
(A) Overview of experiment. Animals underwent heroin SA for 8 days. On days 9–11, animals were pretreated with 5-HT2AR agonist psilocybin 4 hr prior to SA. After 17D abstinence, animals underwent 3 relapse tests on consecutive days and were pretreated with psilocybin 4 hr prior to the test. (B-H) Shown are drug-paired active or inactive lever presses (B-D, G) and heroin infusions during SA (E, F, H). (B-F) SA behavior for each treatment group, prior to psilocybin treatment for days 1–8. Animals were split into treatment groups on day 8, with no significant differences observed in lever responses (D) or heroin infusions (F). (G-H) Heroin SA lever responses (G) and infusions (H) on days 9–11, following pretreatment with psilocybin. (I-K) Active and inactive lever presses during relapse tests on subsequent days (R1, R2, R3) for animals that were pretreated with psilocybin prior to the relapse test. (J-K) Shown are lever presses from panel I combined. Error bars indicate mean +/− standard error of the mean (S.E.M). n=5–12/group. Symbols above line indicate ANOVA: * p<0.05; Post hoc test indicated directly above individual histograms, * p<0.05 vs vehicle; # p<0.05 vs 0.1 mg/kg psilocybin; $ p<0.05 vs 1 mg/kg psilocybin.
We next examined the effect of psilocybin on heroin seeking and relapse after homecage forced abstinence (Figure 1A). Following 17D abstinence, rats were again injected with 0.1, 1 or 3 mg/kg psilocybin 4 hr prior to a 30-minute cue-induced relapse test in the SA chamber on three consecutive days (Figure 1A). Psilocybin pretreatment altered the response to heroin cues during each relapse test, with a significant reduction in active lever responses observed for rats treated with 3 mg/kg psilocybin on days 1 and 3 (Kruskal-Wallis test, R1: KW statistic = 8.33, p=0.04; R2: KW statistic = 5.65, p=0.130; R3: KW statistic = 7.85, p=0.049; Figure I). At the first relapse test, rats treated with 3.0 mg/kg psilocybin made significantly less active lever presses than 0.1 mg/kg and 1 mg/kg groups, with a trend for a reduction versus (vs) the vehicle group (Dunn’s tests, 3 mg/kg psilocybin vs vehicle: p=0.069; vs 0.1 mg/kg psilocybin: p=0.034; vs 1 mg/kg: p=0.005; Figure I). At the third relapse test, rats treated with 3 mg/kg psilocybin made significantly less active lever responses than vehicle and 0.1 mg/kg treated rats (Dunn’s tests, 3 mg/kg vs vehicle: p=0.016; vs 0.1 mg/kg psilocybin: p=0.010). Rats also differed in inactive lever responses when pretreated with psilocybin on all relapse test days (Kruskal-Wallis test, R1: KW statistic = 9.34, p=0.025; Dunn’s tests: 1 mg/kg vs 0.1 mg/kg psilocybin, p=0.034; 0.1 mg/kg vs 3 mg/kg psilocybin, p=0.003; R2: KW statistic = 7.96, p=0.047; Dunn’s tests: 0.1 mg/kg vs 3 mg/kg psilocybin, p=0.006; R3: KW statistic = 8.77, p=0.032; Dunn’s tests: 3 mg/kg psilocybin vs vehicle, p=0.031; 0.1 mg/kg vs 3 mg/kg psilocybin, p=0.004; Figure 2A). Rats treated with 0.1 mg/kg or 1.0 mg/kg psilocybin did not differ from vehicle. RM-ANOVA of lever responses for relapse days 1, 2, and 3 revealed a significant main effect of 3.0 mg/kg psilocybin treatment on both active and inactive lever presses, with psilocybin resulting in consistently lower lever responses (RM-ANOVA-vehicle vs 3 mg/kg psilocybin active lever: F(1, 15) = 5.60, p=0.032; inactive lever: F(1,15) = 9.373, p=0.008; Figure 1J, K). Thus, pretreatment with 3.0 mg/kg psilocybin diminishes heroin relapse after forced abstinence. This data suggests that activation of 5-HT2AR by 3.0 mg/kg psilocybin may be beneficial for reducing future heroin seeking behavior following abstinence.
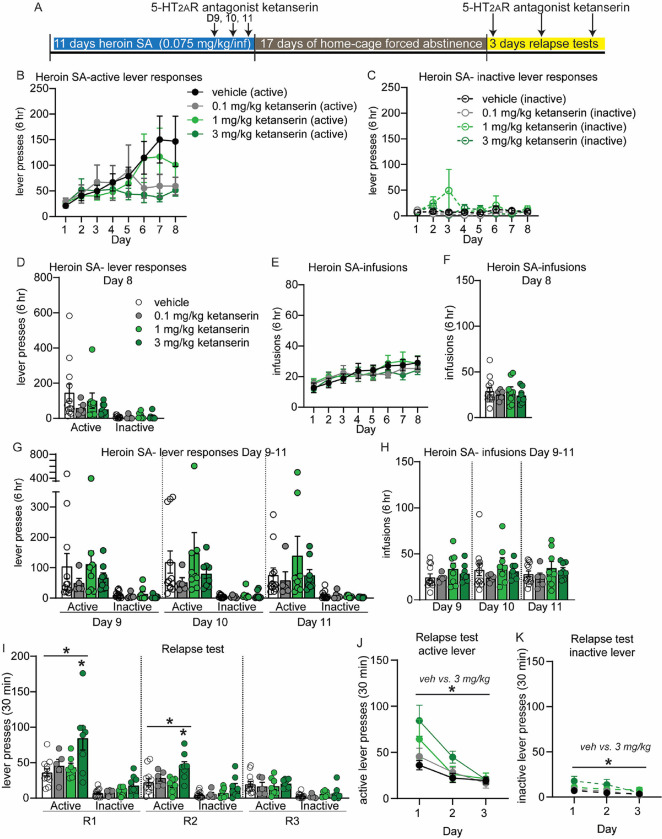
Figure 2: 5-HT2AR antagonist ketanserin increases heroin self-administration and may exacerbate heroin relapse after forced abstinence at a dose of 3 mg/kg.
(A) Overview of experiment. Animals underwent heroin SA for 8 days. On days 9–11, animals were pretreated with 5-HT2AR antagonist ketanserin IP 15 min prior to SA. After 17D abstinence, animals underwent 3 relapse tests on consecutive days and were pretreated with ketanserin 15 min prior to the test. (B-H) Shown are drug-paired active or inactive lever presses (B-D, G) and heroin infusions during SA (E, F, H). (B-F) SA behavior for each treatment group, prior to ketanserin treatment for days 1–8. Animals were split into treatment groups on day 8, with no significant differences observed in lever responses (D) or heroin infusions (F). (G-H) Heroin SA lever responses (G) and infusions (H) on days 9–11, following pretreatment with ketanserin. (I-K) Active and inactive lever presses during relapse tests on subsequent days (R1, R2, R3) for animals that were pretreated with ketanserin prior to the relapse test. (J, K) Shown are active lever presses from panel I combined. Error bars indicate mean +/− S.E.M. Post hoc test indicated directly above individual histograms, * p<0.05; n=5–12/group.
5-HT2AR antagonists may increase heroin intake and seeking
To provide more insight into the impact of 5-HT2AR signaling on heroin SA and relapse, we performed the same experiments with the widely-used 5-HT2AR antagonist ketanserin and the more selective antagonist volinanserin. Rats self-administered heroin for 8 days, then received 0.1, 1 or 3 mg/kg ketanserin 15 min prior to SA on D9–11 (Figure 2A). This timepoint was chosen to allow for comparisons to prior studies that have tested 5-HT2AR antagonists on drug seeking 15, 17. No differences in lever responses or heroin infusions were present prior to (Figure 2B–F) or following ketanserin, vs vehicle controls rats (Figure 3G, H). However, analysis of individual rats’ behavior during SA revealed a significant increase in heroin SA for rats in the 3.0 mg/kg ketanserin group (Supplemental Figure 2). Each rat pretreated with 3.0 mg/kg ketanserin prior to SA made more heroin infusions in the three days post treatment (9–11) compared to the three days preceding treatment (6–8) (Paired t-test 3.0 mg/kg ketanserin: t (7) =3.980, p=0.005; Supplemental Figure 2). Drug infusions for rats in the 3.0 mg/kg ketanserin group was steady throughout the 6 hr of SA, indicating ketanserin induced a consistent increase in heroin SA (Supplemental Figure 2). After 17D abstinence, rats received IP injection of 0.1, 1, or 3.0 mg/kg ketanserin 15 min prior to relapse tests on three consecutive days (Figure 2I–K). One-way ANOVA on active lever responses revealed a significant effect of ketanserin treatment on relapse days 1 and 2 (one-way ANOVA, R1: F (3, 28) = 5.116, p=.006; R2: F (3, 28) = 4.548, p=0.010; Figure 2I). Rats treated with 3.0 mg/kg ketanserin made more active lever presses compared to vehicle controls at the first two relapse tests (Tukey post hoc test, active lever vehicle vs 3.0 mg/kg ketanserin, R1: p=0.004; R2: p=0.017; Figure 2I). RM-ANOVA of lever pressing over the course of all three relapse tests revealed a significant main effect of 3 mg/kg ketanserin, as well as a significant time X 3 mg/kg ketanserin treatment interaction for active lever responses, and a significant main effect of 3 mg/kg ketanserin treatment for inactive lever responses (RM-ANOVA, active lever responses R1–3, 3 mg/kg ketanserin treatment effect: F(1, 18) = 8.333, p=0.010; time X treatment effect, active lever: F(2,36) = 10.67, p=0.0002; main effect of ketanserin treatment for inactive lever: F(1, 18) = 7.134, p=0.016; Figure 2J, K). Thus, ketanserin may exacerbate heroin seeking at the 3.0 mg/kg dose, which is in alignment with the increase in lever pressing and infusions observed during heroin SA. Importantly, we verified that administration of ketanserin or psilocybin at the time points tested did not induce overall gross changes in locomotor behavior (Supplemental Figure 3).
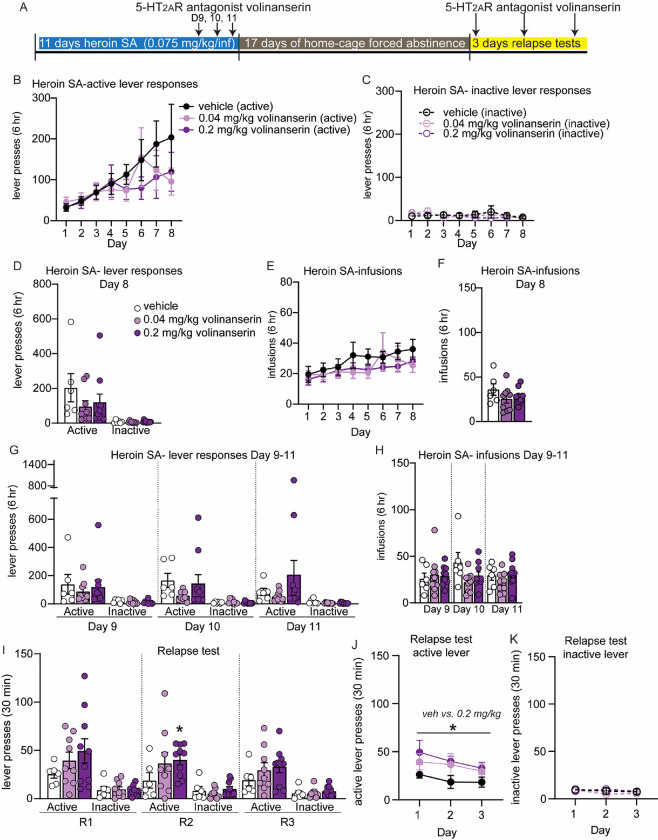
Figure 3: The selective 5-HT2AR antagonist volinanserin increases heroin relapse.
(A) Overview of experiment. Animals underwent heroin SA for 8 days. On days 9–11, animals were pretreated with 5-HT2AR antagonist volinanserin IP 15 min prior to SA. After 17D abstinence, animals underwent 3 relapse tests on consecutive days and were pretreated with volinanserin 15 min prior to the test. (B-H) Shown are drug-paired active or inactive lever presses (B-D, G) and heroin infusions during SA (E, F, H). (B-F) SA behavior for each treatment group, prior to volinanserin treatment for days 1–8. Animals were split into treatment groups on day 8, with no significant differences observed in lever responses (D) or heroin infusions (F). (G-H) Heroin SA lever responses (G) and infusions (H) on days 9–11, following pretreatment with volinanserin. (I-K) Active and inactive lever presses during relapse tests on subsequent days (R1, R2, R3) for animals that were pretreated with volinanserin prior to the relapse test. (J, K) Shown are active lever presses from panel I combined. Error bars indicate mean +/− S.E.M. * above solid line denotes p<0.05 for RM-ANOVA between .2 mg/kg volinanserin and vehicle; * directly above histogram indicates unpaired t-test between 0.2 mg/kg volinanserin and vehicle with p<0.05. n=6–10/group.
Ketanserin has been widely used in behavioral pharmacology as a 5-HT2AR antagonist, despite the fact that it also has some activity at 5-HT2CR. Given this fact, coupled with the published studies demonstrating that 5-HT2CR antagonism may be beneficial for reducing drug seeking, we chose to repeat our studies using the more selective 5-HT2AR antagonist volinanserin. Rats underwent identical procedures, as described above for ketanserin, except were treated with either 0.04 or 0.2 mg/kg volinanserin or vehicles, 15 min prior to each behavior examined. Similar to our reported findings with psilocybin and ketanserin, volinanserin-treated rats did not differ in their heroin SA compared to vehicle-treated control rats (Figure 3B–H). Rats underwent 17D forced abstinence and were then treated with volinanserin or vehicle 15 min prior to the cue-induced relapse test. One-way ANOVA of active and inactive lever responses during each relapse day did not yield statistical significance. However, a trend for an increase in active lever responses was observed for rats that received 0.2 mg/kg volinanserin compared to vehicle-treated rats, with a significant increase in active lever responses observed on relapse day 2 (unpaired t-test, 0.2 mg/kg volinanserin vs vehicle, t(14) = 2.352, p=0.034; Fig. 3I). RM-ANOVA of lever responses over all three days of relapse tests revealed a significant main effect of 0.2 mg/kg volinanserin treatment compared to vehicle treated rats, with this dose of volinanserin significantly elevating active, but not inactive, lever responses (RM-ANOVA, F(1, 15) = 5.575, p=0.0322; Figure 3J, K). These data lead us to conclude that antagonism of 5-HT2AR prior to cue-induced relapse increases heroin seeking behavior.
A single exposure to psilocybin alters the PFC transcriptome.
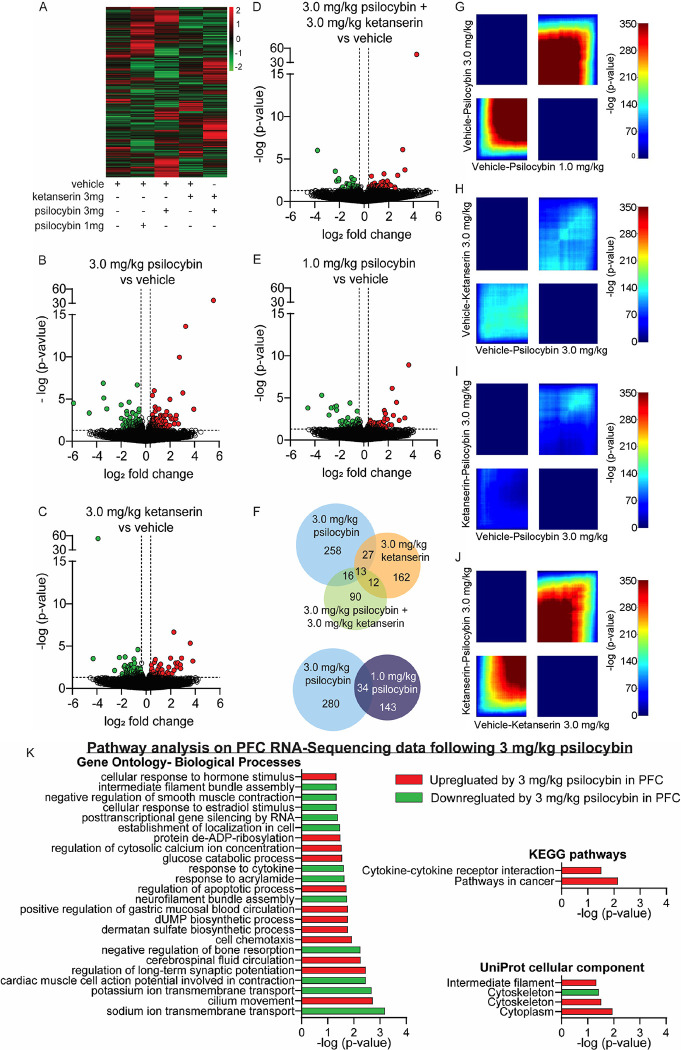
To discern a molecular mechanism for psilocybin-mediated inhibition of heroin seeking, we measured mRNA expression of genes in the PFC (prelimbic and infralimbic medial prefrontal cortex) following acute exposure to psilocybin. The PFC mediates drug seeking behavior 32, 47, 48, and psilocybin impacts PFC circuit activation 45, 49, 50. Given the observed reduction in heroin-seeking behavior after acute 3.0 mg/kg psilocybin treatment but not 1 mg/kg, we decided to examine psilocybin-induced transcriptional changes that occur in the PFC following the 3 mg/kg dose. RNA sequencing was performed on PFC tissue from drug-naïve rats that received IP injection of 1.0 mg/kg psilocybin + vehicle, 3.0 mg/kg psilocybin + vehicle, 3.0 mg/kg ketanserin + vehicle, 3.0 mg/kg psilocybin + 3.0 mg/kg ketanserin, or both vehicles alone (Figure 4 and Supplemental Excel Tables SE1–8). Each animal received two injections to account for exposure to psilocybin (saline) or ketanserin (5% tween-80 in saline) vehicles. The choice to use drug-naïve rats was made to eliminate confounding factors related to chronic heroin exposure and withdrawal periods, ensuring a clear setting for pharmacological comparison. This setting allowed for an unambiguous evaluation of the effects of the 3 mg psilocybin treatment against the non-effective 1 mg dose and the 3 mg ketanserin treatment, which produced opposite results. 3.0 mg/kg psilocybin regulated expression of 314 genes in the PFC, while ketanserin regulated 214 (Figure 4B–C and Supplemental excel tables SE1–4). Coadministration of 3.0 mg/kg ketanserin/psilocybin blocked regulation of >90% of psilocybin-regulated genes in the PFC, while 1.0 mg/kg psilocybin regulated expression of only 177 genes (Figure 4D–E). Venn diagrams of genes regulated in more than one condition indicated that coadministration of 3.0 mg/kg ketanserin/psilocybin blocked ketanserin-specific or psilocybin-specific genes, but 90 genes were uniquely regulated by the combination of the two drugs (Figure 4F). An alluvial plot demonstrated that most genes regulated by 1.0 or 3.0 mg/kg psilocybin were muted by dual application of 3.0 mg/kg psilocybin/ketanserin or 3.0 mg/kg ketanserin alone (Supplemental Figure 4 and Supplemental Excel Tables SE6–8). While a quarter of genes were regulated by both 1.0 and 3.0 mg/kg psilocybin, the two doses resulted in unique in molecular consequences. We performed rank-rank hypergeometric overlap (RRHO) analysis 51 and observed coordinated gene expression between the two doses of psilocybin (Figure 4G and Supplemental Excel Table SE5). Conversely, an RRHO analysis of 3.0 mg/kg psilocybin vs 3.0 mg/kg ketanserin or 3.0 mg/kg psilocybin/ketanserin vs 3.0 mg/kg psilocybin alone revealed that treatment with ketanserin blocks PFC gene expression induced by psilocybin (Figure 4H–I). Interestingly RRHO analysis of 3.0 mg/kg psilocybin/ketanserin vs 3.0 mg/kg ketanserin alone suggested that psilocybin may not combat many of the effects of ketanserin (Figure 4J).
Figure 4: Psilocybin induces unique gene expression profiles in the PFC at varying doses that are blocked by ketanserin.
(A) Heatmap of PFC gene expression profiles obtained from tissue collected 24 hours after a single injection of psilocybin and/or ketanserin. (B-E) Volcano plots depicting differential gene expression in the PFC between vehicle treated animals and treatment with 3.0 mg/kg psilocybin (B), 3.0 mg/kg ketanserin (C), 3.0 mg/kg psilocybin and ketanserin (D), or 1.0 mg/kg psilocybin (E). (F) Venn diagram of total genes regulated by psilocybin and/or ketanserin in the PFC and the number of genes regulated by more than one treatment group. (G-H) RRHO plots were generated to compare all gene expression data between animals treated with 3.0 mg/kg psilocybin and 1.0 mg/kg psilocybin (G), 3.0 mg/kg ketanserin and 3.0 mg/kg psilocybin (H), 3.0 mg/kg ketanserin + 3.0 mg/kg psilocybin and psilocybin 3mg/kg alone (I), or 3.0 mg/kg ketanserin + 3.0 mg/kg psilocybin and ketanserin 3mg/kg alone (J). Lower left quadrant represents genes up-regulated in both groups while upper right quadrant represents genes down-regulated in both groups. (K) Gene ontology, KEGG pathway analysis and Uniprot cellular compartments of genes regulated in the PFC following a single exposure of 3.0 mg/kg psilocybin in drug-naïve animals. n=4/group.
Pathway analysis and gene ontology assessment of genes significantly altered in the PFC following acute psilocybin revealed psilocybin-regulated genes belonged to gene ontology terms that included ‘sodium ion transmembrane transport,’ ‘regulation of long-term synaptic potentiation,’ ‘neurofilament bundle assembly,’ and ‘response to cytokine’ (Figure 4K). However, only two KEGG pathways were significantly regulated by psilocybin, ‘Cytokine-cytokine receptor interaction’ and ‘Pathways in cancer.’ Assessment of the Uniprot cellular component of the psilocybin-regulated genes revealed significant enrichment for terms related to ‘Cytoplasm,’ ‘Cytoskeleton’ and ‘Intermediate filament.’
Pre-treatment with a single dose of psilocybin reduced cue-induced heroin seeking.
Psilocybin-mediated reduction of heroin seeking at the 3.0 mg/kg dosage (Figure 1I–K) represents an intriguing finding, as psilocybin has been proposed as a potentially effective, low-risk, long-lasting therapeutic tool for treating patients with substance use disorders (SUD) 52–55 and just one exposure to psilocybin has long-lasting behavioral and molecular consequences 56. To further explore the impact of psilocybin on heroin seeking in another translationally-relevant administration protocol, we evaluated the temporal aspect of a single psilocybin exposure on heroin relapse by treating additional groups of rats with 3.0 mg/kg psilocybin once, either 4 or 24 hr prior to a relapse test (Figure 5A). All rats acquired heroin SA over 10 days (Figure 5B–C) and were divided into experimental groups after SA, with lever response and heroin intake during SA balanced (Figure 5D–E). After 21D abstinence, rats were injected IP with 3.0 mg/kg psilocybin or saline vehicle either 4 hr or 24 hr prior to a single 30-minute relapse test. Vehicle-treated animals at each timepoint performed almost identically in both SA as well as the relapse test, and were therefore collapsed into a single vehicle group (Supplemental Figure 5; relapse active lever presses for vehicle 4 hr = 77.14 +/− 11.21; vehicle 24 hr= 76.25 +/− 15.73). One-way ANOVA analysis of active lever response during the relapse test revealed that a single exposure to 3 mg/kg psilocybin, either 4 or 24 hours prior, was sufficient to significantly reduce cue-induced heroin seeking behavior following forced abstinence (Kruskal-Wallis test: KW statistic = 7.657, p=0.025; Dunn’s tests: vehicle vs 3 mg/kg psilocybin 4 hr, p=0.0379; vehicle vs 3 mg/kg psilocybin 24 hr, p=0.0148; Figure 5G). No significant differences between treatment groups were observed for inactive lever responses during the relapse test.
Figure 5: Psilocybin pretreatment blunts heroin relapse and regulates gene expression of inflammatory cytokine and chemokines in the PFC during inhibition of heroin seeking.
(A) Overview of experiment. Animals underwent heroin SA for 10 days, 21D forced abstinence and then a 30-minute relapse test. Animals were pretreated with a single dose of 3.0 mg/kg psilocybin or saline vehicle either 4 or 24 hr prior to the test. Animals were euthanized within 1 hour of completion of the relapse test. (A-F) Shown are drug-paired active or inactive lever presses (B, E) and heroin infusions during SA (C, D, F) for each group, prior to any psilocybin treatment. Animals were balanced for lever responses and heroin infusions (D-F) on day 10 of SA. (G) Active and inactive lever presses during the relapse test for rats that received 3 mg/kg psilocybin 4 or 24 hr prior to testing. Asterisk above solid line denotes p<0.05 for Kruskal-Wallis test; * directly above histogram denotes p<0.05 vs vehicle for Post hoc test. Error bars indicate mean +/− S.E.M. n=8–15/group. (H-I) Volcano plot depicting inflammatory cytokine and chemokine genes, obtained by qPCR array, that were differentially expressed in the PFC of rats that were treated with 3.0 mg/kg psilocybin 4 hr (H) or 24 hr (I) prior to a relapse test, vs vehicle treated animals. Horizontal dotted line denotes p value of 0.05. Vertical dotted line denotes fold change of +/− 30%. Grey circles represent genes that were not significantly altered. Red dots represent genes that meet criteria for significance (J-K) Results of correlation analysis of relapse behavior of rats (from G) with inflammatory cytokine or chemokine gene expression data for the PFC that had psilocybin 4 hr (J) or 24 hr (K) prior to a relapse test. Italicized, bolded values were statistically significant.
Psilocybin-mediated inhibition of heroin seeking is accompanied by modulation of inflammatory chemokines and cytokines in the PFC and NAc
The gene ontology and KEGG pathway analysis overlapped to indicate that psilocybin alters PFC expression of genes related to cytokines signaling pathways. We focused further on psilocybin-induced regulation of inflammatory molecules because activation of 5-HT2AR by agonists produces anti-inflammatory effects; cytokine and chemokine signaling is involved in the rewarding effects of drugs; and human transcriptomics data demonstrated heroin use results in dysregulation of PFC and NAc inflammatory pathways 29, 30, 57, 58. A focused qPCR array on 84 inflammatory cytokines and chemokines was performed using PFC tissue from rats that underwent the relapse test in Figure 6G after either 4 or 24 hr pretreatment with 3.0 mg/kg psilocybin (Figure 5H–I). In the PFC of rats pretreated 4 hr before the relapse test with psilocybin, psilocybin-mediated inhibition of heroin relapse was accompanied by significant downregulation of Interleukin 17A (Il17a), and upregulation of C-X-C Motif Chemokine Receptor 1 (Cxcr1) and TNF Superfamily Member 10 (Tnfsf10) (unpaired t-test, vehicle vs 3.0 mg/kg psilocybin: Il17a, t(12) = 2.39, p=0.034; Cxcr1, t(12) = 3.02, p=0.011; Tnfsf10, t(12) = 2.40, p=0.036; Figure 5H). Interestingly, the Il17a receptor, Il17ra, was among the most significantly downregulated genes in the PFC in our transcriptome analysis following 3 mg/kg psilocybin (Supplemental excel file). In animals pretreated 24 hr before the relapse test with psilocybin, psilocybin-mediated inhibition of heroin seeking was associated with significant downregulation of C-X3-C Motif Chemokine Ligand 1 (Cx3cl1) in the PFC (unpaired t-test, vehicle vs. 3.0 mg/kg psilocybin: t(14)= 2.37, p=0.032; Figure 5I).
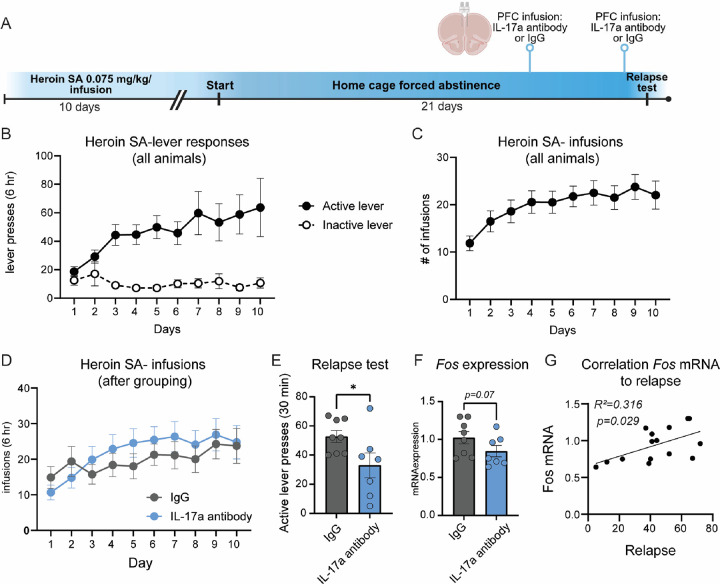
Figure 6: Selective inhibition of IL-17a signaling in the PFC is sufficient to reduce heroin seeking following forced abstinence.
(A) Experimental overview: rats underwent jugular vein catheterization and implantation of PFC cannula. Following recovery, rats underwent heroin SA, forced abstinence and a relapse test. 72 and 24 hours prior to the relapse test, rats received infusion of 50 ng/hemisphere of IL-17a antibody or an IgG control directly into the PFC. (B) Active and inactive lever responses during heroin SA, prior to PFC infusion of IL-17a antibody. (C, D) Heroin infusions made during heroin SA for all rats (C) and depiction of infusions for each treatment group after regrouping into balanced treatment groups (D). (E) Active lever presses during a 30 min relapse test for rats that received IL-17a antibody into the PFC and IgG controls. (F) Fos mRNA in the PFC of rats that received IL-17a antibody prior to a relapse test. (G) Pearson correlation of Fos mRNA expression with active lever responses during the relapse test for rats that received IL-17a antibody or IgG into the PFC prior to the relapse test. Error +/− S.E.M. *p<0.05. n=7–8/group.
Correlation analysis of relapse performance with gene expression revealed 7 genes were positively correlated with active lever responses in the PFC of heroin SA animals pretreated with saline, but not psilocybin 4 hr prior: C-C Motif Chemokine Receptor 2 (Ccr2), Colony Stimulating Factor 1 (Csf1), C-X-C Motif Chemokine Ligand 6 (Cxcl6), Interleukin 10 Receptor Subunit Alpha (Il10ra), Interleukin 1 Receptor Type 1 (Il1r1), Lymphotoxin Alpha (Lta) and Tnfrsf11b (Figure 5J). Conversely, expression of C-C motif chemokine ligand 12 (Ccl12) was negatively correlated with relapse in rats that were pretreated 4 hr prior with psilocybin, but not saline, while expression of TNF Superfamily Member 13b (Tnfsf13b) was positively correlated with relapse only in psilocybin-treated rats (Figure 5J). Following the 24 hr pretreatment with psilocybin, significant negative correlation was observed between relapse behavior and Ccl22, as well as Aminoacyl TRNA Synthetase Complex Interacting Multifunctional Protein 1 (Aimp1) (Figure 5K).
We conducted further analysis in the NAc, another region sensitive to the modulatory action of inflammatory factors in drug-seeking behaviors59, 60, of animals that had completed the relapse test 4 hours following the acute administration of 3.0 mg/kg psilocybin to determine the specificity of cytokine and chemokine regulation following psilocybin-mediated inhibition of heroin seeking. In the NAc, psilocybin pre-treatment prior to relapse tests upregulated 4 genes: Chemokine (C-C motif) receptor 3 (Ccr3), Chemokine (C-X-C motif) receptor 3 (Cxcr3), Interleukin 1 beta (Il1b), and Tumor necrosis factor receptor superfamily, member 11b (Tnfrsf11b) (unpaired t-test, vehicle vs 3.0 mg/kg psilocybin: Ccr3, t(12) = 2.56, p=0.025; Cxcr3, t(12) = 2.45, p=0.031; Il1b, t(12) = 3.12, p=0.009; Tnfrsf11b, t(12) = 3.92, p=0.002; Supplemental Figure 6A). In the NAc, 7 genes were negatively correlated with relapse behavior in rats pretreated with psilocybin, but not saline: C-C Motif Chemokine Ligand 7 (Ccl7), C-C Motif Chemokine Receptor 10 (Ccr10), Ccr2, Fas Ligand (Faslg), Interleukin 33 (Il33), Platelet Factor 4 (Pf4), and Tnfrsf11b (Supplemental Figure 6B). These results demonstrate that psilocybin-mediated inhibition of heroin seeking is accompanied by regulation of inflammatory cytokines and chemokines in the NAc and PFC.
Selective inhibition of Il17a in the PFC is sufficient to reduce heroin relapse
In multiple molecular analyses, we identified regulation of the IL-17 signaling pathway following psilocybin: 3 mg/kg psilocybin reduced Il17ra, the receptor for IL-17a, 24 hr later in the PFC; and pretreatment with 3 mg/kg psilocybin prior a relapse test reduced Il17a in the PFC during psilocybin-mediated inhibition of heroin relapse. Based on these data, we hypothesized that IL-17a signaling may function downstream of psilocybin to reduce heroin seeking behavior. To begin to explore this hypothesis, we selectively reduced IL-17a signaling in the PFC during heroin relapse. Rats were implanted with jugular vein catheters, then underwent heroin SA for 10D and forced abstinence for 21D (Figure 6A). Rats were grouped into IL-17a antibody or IgG control groups based on their SA behavior and did not differ in drug infusions or lever presses (Figure 6B–D). During abstinence, PFC cannula were implanted. 48 and 4 hr prior to a 30 min cue-induced relapse test, rats received infusion of 50 ng/hemisphere of IL-17a antibody or IgG control directly into the PFC (Fig. 6A). Selective infusion of IL-17a antibody into the PFC significantly reduced heroin seeking behavior during the relapse test (unpaired t-test, t(13) = 2.187, p=0.048; Fig. 6E). Rat brains were collected within 90 min of the relapse test and Fos mRNA expression was measured in the PFC, as a measure of activity. Rats infused with the IL-17a antibody into the PFC displayed a trend for reduced Fos activity in the PFC that did not reach statistical significance (Mann-Whitney test U= 12.50, p=0.07; Fig. 6F). However, a correlation analysis revealed a significant negative correlation between Fos mRNA expression in the PFC following IL-17a antibody infusion and active lever responses during the relapse test (Pearson correlation: R2=0.316, p=0.0291; Fig. 6G). These data indicate that inhibiting PFC IL-17a with monoclonal antibodies reduces heroin relapse. This reduction in relapse is correlated with decreased Fos mRNA in the PFC, suggesting lower PFC reactivity during heroin seeking after forced abstinence.
Discussion:
This study is the first to demonstrate that psilocybin reduces heroin seeking in a model of forced abstinence. Although we also pretreated rats with psilocybin during SA and did not observe a regulation of heroin intake, we excluded contribution of prior psilocybin exposure during SA to the relapse test results by replicating our findings of psilocybin-mediated inhibition of heroin seeking after a single injection of 3.0 mg/kg psilocybin only prior to the relapse test. Additionally, our study design allowed us to evaluate the effect of repeated psilocybin administration on heroin relapse, in comparison to acute psilocybin administration. Both administration paradigms are translationally relevant, as psilocybin can induce long-lasting changes in behavior and plasticity following a single exposure56, yet reports of ‘microdosing’, which has not been examined in models of drug seeking, may have therapeutic value for drug seeking behavior by protecting against stress-induced phenotypes61, such as stress-induced relapse.
We examined three doses of psilocybin and observed a pattern of decreased heroin seeking only in rats treated with 3.0 mg/kg. Lack of efficacy from the 1.0 mg/kg psilocybin dose on heroin relapse may be due to the fact that occupancy of 5-HT2AR is estimated to only be ~30% with 1.0 mg/kg IP dosages 62. The 1 mg/kg dose is among the most extensively studied doses in rodents for both behavioral and molecular effects 44–46, while 3 mg/kg falls within the range of therapeutic doses for humans 18. While psilocybin does induce head-twitch responses in rodents, the 3 mg/kg dose has been reported to induce less head-twitch responses compared to the 1 mg/kg dose over time but 3 mg/kg induces a rapid, short-lived head-twitch response in rodents that may be further indicative of unique molecular and behavioral consequences that arise from the two doses 39, 40. Accordingly, our analyses revealed that the transcriptional consequences were distinct between the 1.0 and 3.0 mg/kg doses of psilocybin. In our study, rats underwent three relapse tests following 21D forced abstinence from heroin SA and we observed a rapid reduction of heroin seeking behavior in rats that received a pretreatment with 3 mg/kg psilocybin prior to the relapse test. The 30-minute relapse test functions similarly to extinction training, due to the fact that rats receive all drug-associated cues in the same context that they previously self-administered heroin. Therefore, the reduction of active lever responses in rats treated with 3 mg/kg psilocybin prior to the relapse test suggests that psilocybin may also facilitate extinction of opioid-associated drug memories. Conversely, ketanserin and volinanserin may delay extinction. The repeated relapse testing in Figures 1 and 2 highlight the potential for psilocybin to be used in combination with behavioral therapy to rapidly reduce drug seeking behavior and facilitate extinction of drug seeking.
Our observation of psilocybin-mediated inhibition of heroin seeking is in alignment with published studies that demonstrated that the 5-HT2AR psychedelic agonist 2,5-dimethoxy-4-iodoamphetamine (DOI) decreased demand for fentanyl and a non-hallucinogenic 5-HT2AR agonist tabernanthalog (TBG) reduced both heroin SA and seeking behavior 23, 25, 26. In contrast, a similar dose of 2.5 mg/kg psilocybin had no enduring impact on re-initiation of alcohol taking 18. This divergence likely arises from distinct methodological approaches, as we utilized a cue-induced relapse protocol without reintroduction of heroin, and administered a single psilocybin dose, as opposed to their two-dose regimen spaced a week apart. Subsequent investigation into the impact of psilocybin on alcohol SA demonstrated that a single psilocybin injection 4 hr before the relapse test effectively reduced alcohol SA 19. This suggests that the efficacy of psilocybin in reducing relapse may be contingent upon experimental conditions, dosing strategies, and drug re-exposure. Conversely, nicotine and cocaine studies demonstrate that inhibition of 5-HT2AR generally reduces seeking behavior, which suggests divergence of 5-HT2AR signaling with different drug classes 10–12, 15–17. However, human studies indicate that psilocybin may reduce nicotine craving 3, and therefore, it is possible that psilocybin may have therapeutic efficacy through non-5-HT2AR-mechanisms 63, or that humans have unique psychedelic experiences from psilocybin that may not be recapitulated in all rodent models. Recent studies suggest that the therapeutic effects of serotonergic psychedelics may be dissociable from their hallucinogenic effects45, 64. Specifically, it has been observed that while pretreatment with ketanserin can block the acute effects of psilocybin, the plasticity-promoting and antianhedonic effects of psilocybin were not reversed with ketanserin64. However, a recent study by Vargas et al.65 has revealed that the effects of psychedelics on synaptic plasticity can be inhibited by ketanserin at a dose ten times higher than the dose of psychedelic used. This finding suggests that additional research using 5-HT2AR knockout animals and/or different doses/timing of ketanserin administration in models of opioid SA may be necessary to further explore this significant, yet open question. However, it is important to note that the dose of psilocybin (3 mg/kg) that reduced heroin seeking in our model is higher than the 1 mg/kg dose of psilocybin used in the aforementioned studies that was sufficient for antidepressant and plasticity-promoting effects. Therefore, it is challenging to draw conclusions from these discrepancies, and it is possible that different mechanisms may underlie the antidepressant and anti-drug seeking effects. In our study, we offer a different perspective, demonstrating that the transcriptional effects of psilocybin are largely, though not completely, inhibited by ketanserin administration. Hence, the current study, together with cumulative findings from the addiction field, leads us to conclude that compounds that target 5-HT2AR signaling are excellent candidates for the modification of drug seeking behavior. Because psilocybin is a 5-HT2AR agonist, these results suggest that modulation of 5-HT2AR signaling may be critical for maintaining abstinence from opioid seeking or for reducing opioid-related craving behavior.
Opioids trigger a proinflammatory response 66, 67 and published studies have demonstrated the utility of reducing inflammatory signaling to inhibit drug seeking behaviors 68, 69. Inhibition of toll-like receptor 4 (TLR4), which is associated with opioid addiction and can activate TNF-α/NF-kB pathways, inhibits opioid seeking behaviors 70–72. Intriguingly, activation of 5-HT2AR with the agonist (R)-DOI abolished the inflammatory effects mediated by TNF-α systemic administration in mice 27. Moreover, opioid-induced TNF-α dysregulation was reversed in peripheral blood samples from OUD patients treated with the medications buprenorphine or methadone 68. Hence, targeting this receptor may be a viable option for modulation of inflammatory signaling pathways, such as those that are impacted by chronic opioid exposure, as indicated by our observation of a negative relationship between heroin relapse behavior and expression of 8 inflammatory cytokines or chemokines in the brain when animals were pretreated with psilocybin.
Surprisingly, we did not observe significant changes in gene expression across the qPCR panel, which included numerous cytokines, chemokines, and their respective receptors, that mirrored the RNA sequencing data when psilocybin was administered 24 hr prior to a relapse test. This lack of significant variation may be attributed to the dynamic nature and rapid turnover of chemokines and cytokines, coupled with the short half-life of these inflammatory factors 73, 74. Moreover, it is important to consider that our analysis was confined to mRNA levels rather than protein levels. Given the dynamic and pulsatile behavior of these inflammatory factors, it is plausible that their mRNA levels had returned to baseline after 24 hours, which might not directly reflect changes at the protein level. Future studies could benefit from examining the protein pool to provide deeper insights into these discrepancies. The consistency in behavioral outcomes between the 4-hour and 24-hour pretreatment groups might be explained by the possibility that protein levels, which can persist longer than their mRNA transcripts, differ from the mRNA profiles. Therefore, while mRNA levels provide valuable information, they may not fully capture the changes at the protein level that are crucial for mediating the behavioral effects observed. Alternatively, the transcriptomic changes observed at the inflammatory factor level could affect pathways not directly assessed in our current study, but which play critical roles in modulating behavior. These pathways may include, but are not limited to, those involved in synaptic plasticity, and neurotransmitter signaling. While our study focused on specific markers of inflammation, the broader impact of these transcriptomic changes may extend to these critical pathways, influencing behavioral outcomes in ways not captured by our initial analysis.
However, we did observe regulation in the IL-17a pathway in both PFC qPCR and sequencing datasets of psilocybin-treated rats: In animals that displayed psilocybin-mediated inhibition of heroin seeking following a 4 hr acute psilocybin pretreatment, 3 mg/kg psilocybin decreased PFC Il17a; while Il17ra, the receptor for IL-17a, was downregulated following a single exposure to psilocybin 24 hr prior in our RNA sequencing study. Alcohol-dependent mice treated with anti-IL-17a antibodies reduced their voluntary alcohol intake 75, suggesting that modulation of IL-17a may impact drug seeking behavior. Additionally, IL-17a is proinflammatory and was reported upregulated in the blood of mice following long-access exposure to fentanyl vapor 76, as well as in the PFC after methamphetamine exposure 77. Treatment with a purinergic P2X7 receptor antagonist, which blocks pro-inflammatory signaling 78, inhibits methamphetamine-induced upregulation of IL-17a in the PFC, as well as methamphetamine-induced conditioned place preference 77. We conducted an additional experiment showing that selective inhibition of IL-17a signaling in the PFC before a relapse test significantly reduced heroin-seeking behavior in animals after 21 days of forced abstinence from heroin SA. This finding corroborates previous research suggesting that targeting IL-17a signaling could be an effective strategy for reducing drug-seeking behaviors. Additionally, this experiment supports our hypothesis that modulating inflammatory pathways in the PFC may be one of the mechanisms through which psilocybin achieves its therapeutic effects, particularly in diminishing drug-seeking behaviors. Therefore, studying other inflammatory pathways could also be helpful. For example, psilocybin also increased NAc Tnfrsf11b, which encodes the Opg protein. This protein is elevated by anti-inflammatory drugs 79–81, and has been studied for neuroprotective and inflammatory effects 82, although not for drug seeking behavior. Future studies exploring downstream signaling consequences of psilocybin will provide additional pathways that may be therapeutic targets for reduction of opioid seeking.
In this study, we have directed our molecular investigations downstream of psilocybin exposure towards inflammatory cytokines and chemokines, driven by emerging evidence that emphasizes reports of opioid-induced regulation of inflammatory modulators as a major neuroadaptation observed in the molecular foundations of OUD 30, 83. Additionally, multiple lines of evidence demonstrate that inflammation significantly impacts the regulation of synaptic plasticity84–86 and it is posited that psychedelics such as psilocybin may induce long-lasting therapeutic effects by altering the nature of neural circuits through neuronal plasticity. Psychedelic compounds may facilitate structural changes within the nervous system, rendering it more receptive to environmental stimuli. This heightened receptivity may enable extensive modifications at the synaptic, circuit, and behavioral levels, through induction of a specific form of synaptic plasticity termed metaplasticity, which supports the restoration and stabilization of healthy neural function87, 88. Psilocybin can modulate neuroimmune factors and this interaction could represent one of the mechanisms through which psychedelics facilitate changes in synaptic plasticity, potentially leading to durable therapeutic effects. Intriguingly, this positions psychedelics as potential therapeutic agents in OUD by potentially resetting maladaptive neural circuits and reducing neuroinflammation. This dual action highlights the innovative potential of psychedelics in redefining treatments for addiction and other psychiatric disorders where neuroinflammation and synaptic dysfunction play critical roles. As such, further research into the interplay between psychedelic-induced neuroplasticity and neuroimmune modulation is crucial for developing targeted therapies that harness these complex biological interactions.
While these exciting findings demonstrate psilocybin reduces heroin relapse, limitations of the study include use of only male animals, given that sex-specific effects of psilocybin on mouse ethanol drinking 46 and responses to 5-HT2AR psychedelics have been reported 89–91. In our initial investigation, we employed a subchronic treatment with high doses of psilocybin, an approach that may lack translational validity, to enable direct comparisons with both the microdosing protocol and the ketanserin antagonist treatment. Nevertheless, we subsequently replicated the experiments using a single high-dose psilocybin administration at two distinct time points. The consistent results from these tests, even at the later time point (24 hr), suggest that psilocybin’s positive effects on drug-seeking behavior may be long lasting. Secondly, for the initial molecular studies, we used naive animals, avoiding any variables associated with voluntary heroin use or withdrawal. This approach provided a clear baseline for pharmacological comparisons, yet it also attenuated the translational potential of our transcriptomic observations in the context of relapse-tested animals with a history of heroin self-administration. To address this limitation, we expanded our analysis focusing on key pathways revealed by RNA-seq in samples from animals that underwent relapse tests at different time points. Although we have initially focused our study on the transcriptional alterations in only the PFC and NAc following psilocybin exposure, psilocybin has been shown to impact many other brain regions 92. We acknowledge that the sample size used in Figure 1 to investigate the impact of 0.1 mg/kg and 3 mg/kg psilocybin on heroin SA may be underpowered to detect small differences and future studies replicating our data in larger cohorts are undoubtedly necessary. While considering these potential limitations, the findings indicated that inflammatory mediators may play a role in the psilocybin-induced reduction of heroin-seeking behaviors. Future studies into putative sex-specific responses to psilocybin during heroin relapse are warranted. Finally, psilocybin can also activate other receptor systems, such as 5-HT2CR, 5-HT1AR and TrkB 61, 63, 93, and whether the effects of psilocybin on heroin relapse are mediated entirely through 5-HT2AR, or if non-5-HT2AR mechanisms also contribute to psilocybin-mediated reduction of heroin relapse is currently unknown. Ketanserin may also function as a 5-HT2CR antagonist and future studies with additional 5-HT2AR/5-HT2CR antagonists in combination with psilocybin are needed. Irrespective of these potential limitations, our data suggests that psilocybin has a promising role in modulating heroin-seeking behavior, potentially offering a novel therapeutic avenue for the treatment of opioid addiction.
Supplementary Material
Acknowledgements:
Heroin was provided by the National Institute on Drug Abuse drug supply program. We thank all members of the Daws lab for helpful discussion on the interpretation of data.
Funding:
This work was supported by NIDA/NIH grants DP1DA051550 (SD), T32DA007237 (KD), and P30DA013429 (Temple).
Footnotes
Competing Interests: We have no competing interests to disclose.
References
- 1.Hedegaard H, Miniño AM, Warner M. Co-involvement of opioids in drug overdose deaths involving cocaine and psychostimulants. NCHS Data Brief 2021; 406: 1–8. [PubMed] [Google Scholar]
- 2.Bogenschutz MP, Forcehimes AA, Pommy JA, Wilcox CE, Barbosa PC, Strassman RJ. Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J Psychopharmacol 2015; 29(3): 289–299. [DOI] [PubMed] [Google Scholar]
- 3.Johnson MW, Garcia-Romeu A, Griffiths RR. Long-term follow-up of psilocybin-facilitated smoking cessation. Am J Drug Alcohol Abuse 2017; 43(1): 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vargas MV, Meyer R, Avanes AA, Rus M, Olson DE. Psychedelics and Other Psychoplastogens for Treating Mental Illness. Front Psychiatry 2021; 12: 727117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Donnell KC, Mennenga SE, Owens LT, Podrebarac SK, Baron T, Rotrosen J et al. Psilocybin for alcohol use disorder: Rationale and design considerations for a randomized controlled trial. Contemp Clin Trials 2022; 123: 106976. [DOI] [PubMed] [Google Scholar]
- 6.López-Giménez JF, González-Maeso J. Hallucinogens and Serotonin 5-HT(2A) Receptor-Mediated Signaling Pathways. Curr Top Behav Neurosci 2018; 36: 45–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim K, Che T, Panova O, DiBerto JF, Lyu J, Krumm BE et al. Structure of a Hallucinogen-Activated Gq-Coupled 5-HT(2A) Serotonin Receptor. Cell 2020; 182(6): 1574–1588.e1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrett FS, Zhou Y, Carbonaro TM, Roberts JM, Smith GS, Griffiths RR et al. Human Cortical Serotonin 2A Receptor Occupancy by Psilocybin Measured Using [Front Neuroergon 2021; 2: 784576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao D, Yu J, Wang H, Luo Z, Liu X, He L et al. Structure-based discovery of nonhallucinogenic psychedelic analogs. Science 2022; 375(6579): 403–411. [DOI] [PubMed] [Google Scholar]
- 10.Nic Dhonnchadha BA, Fox RG, Stutz SJ, Rice KC, Cunningham KA. Blockade of the serotonin 5-HT2A receptor suppresses cue-evoked reinstatement of cocaine-seeking behavior in a rat self-administration model. Behav Neurosci 2009; 123(2): 382–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pockros LA, Pentkowski NS, Swinford SE, Neisewander JL. Blockade of 5-HT2A receptors in the medial prefrontal cortex attenuates reinstatement of cue-elicited cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2011; 213(2–3): 307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sholler DJ, Stutz SJ, Fox RG, Boone EL, Wang Q, Rice KC et al. The 5-HT(2A) Receptor (5-HT(2A)R) Regulates Impulsive Action and Cocaine Cue Reactivity in Male Sprague-Dawley Rats. J Pharmacol Exp Ther 2019; 368(1): 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fletcher PJ, Grottick AJ, Higgins GA. Differential effects of the 5-HT(2A) receptor antagonist M100907 and the 5-HT(2C) receptor antagonist SB242084 on cocaine-induced locomotor activity, cocaine self-administration and cocaine-induced reinstatement of responding. Neuropsychopharmacology 2002; 27(4): 576–586. [DOI] [PubMed] [Google Scholar]
- 14.Anastasio NC, Sholler DJ, Fox RG, Stutz SJ, Merritt CR, Bjork JM et al. Suppression of cocaine relapse-like behaviors upon pimavanserin and lorcaserin co-administration. Neuropharmacology 2020; 168: 108009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schenk S, Foote J, Aronsen D, Bukholt N, Highgate Q, Van de Wetering R et al. Serotonin antagonists fail to alter MDMA self-administration in rats. Pharmacol Biochem Behav 2016; 148: 38–45. [DOI] [PubMed] [Google Scholar]
- 16.Levin ED, Slade S, Johnson M, Petro A, Horton K, Williams P et al. Ketanserin, a 5-HT2 receptor antagonist, decreases nicotine self-administration in rats. Eur J Pharmacol 2008; 600(1–3): 93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burmeister JJ, Lungren EM, Kirschner KF, Neisewander JL. Differential roles of 5-HT receptor subtypes in cue and cocaine reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology 2004; 29(4): 660–668. [DOI] [PubMed] [Google Scholar]
- 18.Meinhardt MW, Güngör C, Skorodumov I, Mertens LJ, Spanagel R. Psilocybin and LSD have no long-lasting effects in an animal model of alcohol relapse. Neuropsychopharmacology 2020; 45(8): 1316–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meinhardt MW, Pfarr S, Fouquet G, Rohleder C, Meinhardt ML, Barroso-Flores J et al. Psilocybin targets a common molecular mechanism for cognitive impairment and increased craving in alcoholism. Sci Adv 2021; 7(47): eabh2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berquist MD, Fantegrossi WE. Effects of 5-HT2A receptor agonist 2,5-dimethoxy-4-iodoamphetamine on alcohol consumption in Long-Evans rats. Behav Pharmacol 2021; 32(5): 382–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sáiz PA, García-Portilla MP, Arango C, Morales B, Martínez-Barrondo S, Alvarez C et al. Association between heroin dependence and 5-HT2A receptor gene polymorphisms. Eur Addict Res 2008; 14(1): 47–52. [DOI] [PubMed] [Google Scholar]
- 22.Cao J, Liu X, Han S, Zhang CK, Liu Z, Li D. Association of the HTR2A gene with alcohol and heroin abuse. Hum Genet 2014; 133(3): 357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin DA, Gyawali U, Calu DJ. Effects of 5-HT(2A) receptor stimulation on economic demand for fentanyl after intermittent and continuous access self-administration in male rats. Addict Biol 2021; 26(3): e12926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maguire DR, Li JX, Koek W, France CP. Effects of 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane (DOM) and quipazine on heroin self-administration in rhesus monkeys. Psychopharmacology (Berl) 2013; 225(1): 173–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cameron LP, Tombari RJ, Lu J, Pell AJ, Hurley ZQ, Ehinger Y et al. A non-hallucinogenic psychedelic analogue with therapeutic potential. Nature 2021; 589(7842): 474–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heinsbroek JA, Giannotti G, Bonilla J, Olson DE, Peters J. Tabernanthalog Reduces Motivation for Heroin and Alcohol in a Polydrug Use Model. Psychedelic Med (New Rochelle) 2023; 1(2): 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nau F, Yu B, Martin D, Nichols CD. Serotonin 5-HT2A receptor activation blocks TNF-α mediated inflammation in vivo. PLoS One 2013; 8(10): e75426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nau F, Miller J, Saravia J, Ahlert T, Yu B, Happel KI et al. Serotonin 5-HT2 receptor activation prevents allergic asthma in a mouse model. Am J Physiol Lung Cell Mol Physiol 2015; 308(2): L191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu B, Becnel J, Zerfaoui M, Rohatgi R, Boulares AH, Nichols CD. Serotonin 5-hydroxytryptamine(2A) receptor activation suppresses tumor necrosis factor-alpha-induced inflammation with extraordinary potency. J Pharmacol Exp Ther 2008; 327(2): 316–323. [DOI] [PubMed] [Google Scholar]
- 30.Seney ML, Kim SM, Glausier JR, Hildebrand MA, Xue X, Zong W et al. Transcriptional Alterations in Dorsolateral Prefrontal Cortex and Nucleus Accumbens Implicate Neuroinflammation and Synaptic Remodeling in Opioid Use Disorder. Biol Psychiatry 2021; 90(8): 550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saad MH, Rumschlag M, Guerra MH, Savonen CL, Jaster AM, Olson PD et al. Differentially expressed gene networks, biomarkers, long noncoding RNAs, and shared responses with cocaine identified in the midbrains of human opioid abusers. Sci Rep 2019; 9(1): 1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology 2010; 35(1): 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zanda MT, Floris G, Daws SE. Drug-associated cues and drug dosage contribute to increased opioid seeking after abstinence. Sci Rep 2021; 11(1): 14825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Floris G, Gillespie A, Zanda MT, Dabrowski KR, Daws SE. Heroin Regulates Orbitofrontal Circular RNAs. Int J Mol Sci 2022; 23(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dabrowski KR, Floris G, Gillespie A, Daws SE. Orbitofrontal intronic circular RNA from Nrxn3 mediates reward learning and motivation for reward. Prog Neurobiol 2023: 102546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25(4): 402–408. [DOI] [PubMed] [Google Scholar]
- 37.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 2014; 15(12): 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.KALBERER F KREIS W, RUTSCHMANN J. The fate of psilocin in the rat. Biochem Pharmacol 1962; 11: 261–269. [DOI] [PubMed] [Google Scholar]
- 39.Klein AK, Chatha M, Laskowski LJ, Anderson EI, Brandt SD, Chapman SJ et al. Investigation of the Structure-Activity Relationships of Psilocybin Analogues. ACS Pharmacol Transl Sci 2021; 4(2): 533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shahar O, Botvinnik A, Esh-Zuntz N, Brownstien M, Wolf R, Lotan A et al. Role of 5-HT2A, 5-HT2C, 5-HT1A and TAAR1 Receptors in the Head Twitch Response Induced by 5-Hydroxytryptophan and Psilocybin: Translational Implications. Int J Mol Sci 2022; 23(22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jefsen O, Højgaard K, Christiansen SL, Elfving B, Nutt DJ, Wegener G et al. Psilocybin lacks antidepressant-like effect in the Flinders Sensitive Line rat. Acta Neuropsychiatr 2019; 31(4): 213–219. [DOI] [PubMed] [Google Scholar]
- 42.Saito K, Toyo’oka T, Fukushima T, Kato M, Shirota O, Goda Y. Determination of psilocin in magic mushrooms and rat plasma by liquid chromatography with fluorimetry and electrospray ionization mass spectrometry. Analytica Chimica Acta 2004; 527(2): 149–156. [Google Scholar]
- 43.Halberstadt AL, Koedood L, Powell SB, Geyer MA. Differential contributions of serotonin receptors to the behavioral effects of indoleamine hallucinogens in mice. J Psychopharmacol 2011; 25(11): 1548–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davis M, Walters JK. Psilocybin: biphasic dose-response effects on the acoustic startle reflex in the rat. Pharmacol Biochem Behav 1977; 6(4): 427–431. [DOI] [PubMed] [Google Scholar]
- 45.Shao LX, Liao C, Gregg I, Davoudian PA, Savalia NK, Delagarza K et al. Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron 2021; 109(16): 2535–2544.e2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alper K, Cange J, Sah R, Schreiber-Gregory D, Sershen H, Vinod KY. Psilocybin sex-dependently reduces alcohol consumption in C57BL/6J mice. Front Pharmacol 2022; 13: 1074633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci 2007; 8(11): 844–858. [DOI] [PubMed] [Google Scholar]
- 48.Lüscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron 2011; 69(4): 650–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mason NL, Kuypers KPC, Müller F, Reckweg J, Tse DHY, Toennes SW et al. Me, myself, bye: regional alterations in glutamate and the experience of ego dissolution with psilocybin. Neuropsychopharmacology 2020; 45(12): 2003–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mertens LJ, Wall MB, Roseman L, Demetriou L, Nutt DJ, Carhart-Harris RL. Therapeutic mechanisms of psilocybin: Changes in amygdala and prefrontal functional connectivity during emotional processing after psilocybin for treatment-resistant depression. J Psychopharmacol 2020; 34(2): 167–180. [DOI] [PubMed] [Google Scholar]
- 51.Plaisier SB, Taschereau R, Wong JA, Graeber TG. Rank-rank hypergeometric overlap: identification of statistically significant overlap between gene-expression signatures. Nucleic Acids Res 2010; 38(17): e169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones G, Ricard JA, Lipson J, Nock MK. Associations between classic psychedelics and opioid use disorder in a nationally-representative U.S. adult sample. Sci Rep 2022; 12(1): 4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jones GM, Nock MK. Exploring protective associations between the use of classic psychedelics and cocaine use disorder: a population-based survey study. Sci Rep 2022; 12(1): 2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nichols DE, Johnson MW, Nichols CD. Psychedelics as Medicines: An Emerging New Paradigm. Clin Pharmacol Ther 2017; 101(2): 209–219. [DOI] [PubMed] [Google Scholar]
- 55.DiVito AJ, Leger RF. Psychedelics as an emerging novel intervention in the treatment of substance use disorder: a review. Mol Biol Rep 2020; 47(12): 9791–9799. [DOI] [PubMed] [Google Scholar]
- 56.Calder AE, Hasler G. Towards an understanding of psychedelic-induced neuroplasticity. Neuropsychopharmacology 2023; 48(1): 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nishiyama T. Acute effects of sarpogrelate, a 5-HT2A receptor antagonist on cytokine production in endotoxin shock model of rats. Eur J Pharmacol 2009; 614(1–3): 122–127. [DOI] [PubMed] [Google Scholar]
- 58.Ahearn OC, Watson MN, Rawls SM. Chemokines, cytokines and substance use disorders. Drug Alcohol Depend 2021; 220: 108511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Namba MD, Leyrer-Jackson JM, Nagy EK, Olive MF, Neisewander JL. Neuroimmune Mechanisms as Novel Treatment Targets for Substance Use Disorders and Associated Comorbidities. Front Neurosci 2021; 15: 650785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ezeomah C, Cunningham KA, Stutz SJ, Fox RG, Bukreyeva N, Dineley KT et al. Fentanyl self-administration impacts brain immune responses in male Sprague-Dawley rats. Brain Behav Immun 2020; 87: 725–738. [DOI] [PubMed] [Google Scholar]
- 61.Kiilerich KF, Lorenz J, Scharff MB, Speth N, Brandt TG, Czurylo J et al. Repeated low doses of psilocybin increase resilience to stress, lower compulsive actions, and strengthen cortical connections to the paraventricular thalamic nucleus in rats. Mol Psychiatry 2023. [DOI] [PubMed] [Google Scholar]
- 62.Smith RL, Barrett RJ, Sanders-Bush E. Neurochemical and behavioral evidence that quipazine-ketanserin discrimination is mediated by serotonin2A receptor. J Pharmacol Exp Ther 1995; 275(2): 1050–1057. [PubMed] [Google Scholar]
- 63.Moliner R, Girych M, Brunello CA, Kovaleva V, Biojone C, Enkavi G et al. Psychedelics promote plasticity by directly binding to BDNF receptor TrkB. Nat Neurosci 2023; 26(6): 1032–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hesselgrave N, Troppoli TA, Wulff AB, Cole AB, Thompson SM. Harnessing psilocybin: antidepressant-like behavioral and synaptic actions of psilocybin are independent of 5-HT2R activation in mice. Proc Natl Acad Sci U S A 2021; 118(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vargas MV, Dunlap LE, Dong C, Carter SJ, Tombari RJ, Jami SA et al. Psychedelics promote neuroplasticity through the activation of intracellular 5-HT2A receptors. Science 2023; 379(6633): 700–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eisenstein TK. Opioids and the immune system: what is their mechanism of action? Br J Pharmacol 2011; 164(7): 1826–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Johnston IN, Milligan ED, Wieseler-Frank J, Frank MG, Zapata V, Campisi J et al. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J Neurosci 2004; 24(33): 7353–7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sacerdote P, Franchi S, Gerra G, Leccese V, Panerai AE, Somaini L. Buprenorphine and methadone maintenance treatment of heroin addicts preserves immune function. Brain Behav Immun 2008; 22(4): 606–613. [DOI] [PubMed] [Google Scholar]
- 69.Parekh SV, Paniccia JE, Adams LO, Lysle DT. Hippocampal TNF-α Signaling Mediates Heroin Withdrawal-Enhanced Fear Learning and Withdrawal-Induced Weight Loss. Mol Neurobiol 2021; 58(6): 2963–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu R, Li JX. Toll-Like Receptor 4 Signaling and Drug Addiction. Front Pharmacol 2020; 11: 603445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu R, Liu J, Vu J, Huang Y, Dietz DM, Li JX. Interleukin-1 receptor-associated kinase 4 (IRAK4) in the nucleus accumbens regulates opioid-seeking behavior in male rats. Brain Behav Immun 2022; 101: 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Theberge FR, Li X, Kambhampati S, Pickens CL, St Laurent R, Bossert JM et al. Effect of chronic delivery of the Toll-like receptor 4 antagonist (+)-naltrexone on incubation of heroin craving. Biol Psychiatry 2013; 73(8): 729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Whiteside TL. Cytokines and cytokine measurements in a clinical laboratory. Clin Diagn Lab Immunol 1994; 1(3): 257–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu G, Qi M, Hutchinson MR, Yang G, Goldys EM. Recent advances in cytokine detection by immunosensing. Biosens Bioelectron 2016; 79: 810–821. [DOI] [PubMed] [Google Scholar]
- 75.Xu J, Ma HY, Liu X, Rosenthal S, Baglieri J, McCubbin R et al. Blockade of IL-17 signaling reverses alcohol-induced liver injury and excessive alcohol drinking in mice. JCI Insight 2020; 5(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marchette RCN, Carlson ER, Said N, Koob GF, Vendruscolo LF. Extended access to fentanyl vapor self-administration leads to addiction-like behaviors in mice: Blood chemokine/cytokine levels as potential biomarkers. Addict Neurosci 2023; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Potula R, Gentile TA, Meissler JJ, Shekarabi A, Wiah S, Farkas DJ et al. Purinergic P2X7 receptor antagonist inhibits methamphetamine-induced reward, hyperlocomotion, and cortical IL-7A levels in mice: A role for P2X7/IL-17A crosstalk in methamphetamine behaviors? Brain Behav Immun 2023; 107: 47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fernandes NC, Sriram U, Gofman L, Cenna JM, Ramirez SH, Potula R. Methamphetamine alters microglial immune function through P2X7R signaling. J Neuroinflammation 2016; 13(1): 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alraouji NN, Hendrayani SF, Ghebeh H, Al-Mohanna FH, Aboussekhra A. Osteoprotegerin (OPG) mediates the anti-carcinogenic effects of normal breast fibroblasts and targets cancer stem cells through inhibition of the β-catenin pathway. Cancer Lett 2021; 520: 374–384. [DOI] [PubMed] [Google Scholar]
- 80.Jura-Półtorak A, Szeremeta A, Olczyk K, Zoń-Giebel A, Komosińska-Vassev K. Bone Metabolism and RANKL/OPG Ratio in Rheumatoid Arthritis Women Treated with TNF-α Inhibitors. J Clin Med 2021; 10(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Catrina AI, af Klint E, Ernestam S, Catrina SB, Makrygiannakis D, Botusan IR et al. Anti-tumor necrosis factor therapy increases synovial osteoprotegerin expression in rheumatoid arthritis. Arthritis Rheum 2006; 54(1): 76–81. [DOI] [PubMed] [Google Scholar]
- 82.Glasnović A, O’Mara N, Kovačić N, Grčević D, Gajović S. RANK/RANKL/OPG Signaling in the Brain: A Systematic Review of the Literature. Front Neurol 2020; 11: 590480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Phan BN, Ray MH, Xue X, Fu C, Fenster RJ, Kohut SJ et al. Single nuclei transcriptomics in human and non-human primate striatum in opioid use disorder. Nat Commun 2024; 15(1): 878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guyon A. CXCL12 chemokine and its receptors as major players in the interactions between immune and nervous systems. Front Cell Neurosci 2014; 8: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cattaneo A, Macchi F, Plazzotta G, Veronica B, Bocchio-Chiavetto L, Riva MA et al. Inflammation and neuronal plasticity: a link between childhood trauma and depression pathogenesis. Front Cell Neurosci 2015; 9: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Paolicelli RC, Bisht K, Tremblay M. Fractalkine regulation of microglial physiology and consequences on the brain and behavior. Front Cell Neurosci 2014; 8: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nardou R, Sawyer E, Song YJ, Wilkinson M, Padovan-Hernandez Y, de Deus JL et al. Psychedelics reopen the social reward learning critical period. Nature 2023; 618(7966): 790–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Woodburn SC, Kwan AC. Timing is key for behavioural benefits of psychedelics. Nature 2023; 618(7966): 677–678. [DOI] [PubMed] [Google Scholar]
- 89.Effinger DP, Quadir SG, Ramage MC, Cone MG, Herman MA. Sex-specific effects of psychedelic drug exposure on central amygdala reactivity and behavioral responding. Transl Psychiatry 2023; 13(1): 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jaster AM, Younkin J, Cuddy T, de la Fuente Revenga M, Poklis JL, Dozmorov MG et al. Differences across sexes on head-twitch behavior and 5-HT. Neurosci Lett 2022; 788: 136836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vohra HZ, Saunders JM, Jaster AM, de la Fuente Revenga M, Jimenez J, Fernández-Teruel A et al. Sex-specific effects of psychedelics on prepulse inhibition of startle in 129S6/SvEv mice. Psychopharmacology (Berl) 2022; 239(6): 1649–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Davoudian PA, Shao LX, Kwan AC. Shared and Distinct Brain Regions Targeted for Immediate Early Gene Expression by Ketamine and Psilocybin. ACS Chem Neurosci 2023; 14(3): 468–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Roth B, Kroeze W, Patel S, Lopez E. The Multiplicity of Serotonin Receptors: Uselessly diverse molecules or an embarrassment of riches? The Neuroscientist, vol. 62000, pp 252–262. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.