Abstract
In the polyphagous insect Monolepta signata (M. signata) (Coleoptera: Chrysomelidae), antennae are important for olfactory reception used during feeding, mating, and finding a suitable oviposition site. Based on NextSeq 6000 Illumina sequencing, we assembled the antennal transcriptome of mated M. signata and described the first chemosensory gene repertoire expressed in this species. The relative expression levels of some significant chemosensory genes were conducted by quantitative real-time PCR. We identified 114 olfactory-related genes based on the antennal transcriptome database of M. signata, including 21 odorant binding proteins (OBPs), six chemosensory proteins (CSPs), 46 odorant receptors (ORs), 15 ionotropic receptors (IRs), 23 gustatory receptors (GRs) and three sensory neuron membrane proteins (SNMPs). Blastp best hit and phylogenetic analyses showed that most of the chemosensory genes had a close relationship with orthologs from other Coleoptera species. Overall, this study provides a foundation for elucidating the molecular mechanism of olfactory recognition in M. signata as well as a reference for the study of chemosensory genes in other species of Coleoptera.
1. Introduction
Monolepta signata (M. signata) belongs to the subfamily Galerucinae of the family Chrysomelidae of Coleoptera and is mainly distributed in East Asia and Southeast Asia [1]. The species is distributed in most areas of China, and it occurs at high densities in northern China [2]. M. signata is an herbivorous pest, with adults feeding on the leaves and flowers of host plants and causing damage to a variety of cash crops, such as corn, cotton, sunflowers, soybeans, rice, grains, sorghum, peanuts, potatoes, and cruciferous vegetables [3]. The beetle is characterized by an extensive damage period, high population density, and rapid spread within crop areas [4]. Similar to most insects of Chrysomelidae, M. signata identifies and locates host plants based on the volatile substances released by the plants, but research on this beetle has primarily focused on the biological characteristics and control effects [5–7]. The molecular mechanisms of interactions with host plants and their targets have not been reported. Identifying the olfactory-related genes of M. signata is a prerequisite for studying the olfactory recognition process of this beetle at the molecular level.
The co-evolution of herbivorous insects and plants has defined the range of host plants for herbivorous insects and the defenses of plants, and the insects can distinguish between host and non-host plants. This ensures the growth and reproduction of insects, and it prevents their poisoning and malnutrition from feeding on non-host plants [8]. The chemoreception process involves the reception, binding, transport, and inactivation of odorants, ultimately activating receptor neurons and converting chemical signals into electrical signals to the insect brain [9]. Interactions between different types of proteins determine the high sensitivity and specificity of the insect olfactory system [10]. Odorant binding proteins (OBPs) and chemosensory proteins (CSPs) are involved with binding, and transport of odorant substances. Odorant receptors (ORs), gustatory receptors (GRs), and ionotropic receptors (IRs) can recognize specific odor molecules in complex environments, then covert the recognized chemical signal into electrical signals through receptor-mediated signal transduction. Peripheral neuron depolarization is then transmitted through axonal processes to the central nervous system of the brain, and sensory neuronal membrane proteins (SNMPs) identify and transport lipophilic odor molecules [11]. With the application of high-throughput sequencing technology in insect functional gene mining, a variety of insect chemosensory genes has been identified. In total 26 OBP genes, 15 CSP genes, 37 OR genes, 10 IRs, and three SNMP genes were identified in the antennal transcriptome of Leptinotarsa decemlineata [12]. Twenty-six OBPs, 12 CSPs, 4 SNMPs, 43 ORs, 9 IRs, and 10 GRs were identified in the antennal transcriptome of Colaphellus bowringi [13]. Transcriptome sequencing and olfactory-related gene mining of the antenna and leg of Ambrostoma quadriimpressum Motschulsky yielded 16 OBPs, 10 CSPs, 34 ORs, 20 IRs, and two SNMPs [14].
Insect recognition of volatile substances emitted by plants is an important factor affecting insect feeding [15]. By measuring the electrophysiological responses of the antennae, it was found that certain concentrations of geraniol, 3-carene, β-pinene, α-erythro-myrcene, and leaf alcohol could produce strong stimulating effects in M. signata [16, 17]. Behavioral bioassays showed that 10 μg/mL β-pinacolone had a significant attraction effect on the females and a significant avoidance effect on the males, while γ-terpinene and D-limonene had significant attraction effects on the males and a significant avoidance effect on the females [18, 19]. Additionally, various types of antennal chemoreceptor sensilla (notably sensilla trichodea, sensilla basiconica, sensilla coeloclnica, and sensilla campaniformia) have been recently characterized in M. signata [20]. Thus, understanding the molecular basis of M. signata chemoreception, in particular the types and number of olfactory-related genes, is likely to provide new information that can be used to increase the biological control efficacy for this pest and further clarify the relationship between the pest and the host plant.
In this study, using the antenna transcriptome data of M. signata adults assembled in our laboratory (BioProject Accession Number: PRJNA960895), the chemosensory genes of M. signata were identified and examined through bioinformatic analyses. Moreover, the relative expression levels of some chemosensorygenes were analyzed by quantitative real-time PCR (RT-qPCR). The results provide theoretical support for future studies on the mechanism of chemoreception of M. signata and ultimately allow us to identify potential targets for disrupting odorant perception in M. signata that could lead to new pest management techniques.
2. Materials and methods
2.1. Insect rearing and antenna collection
Adults of M. signata were collected from a corn field in Shawan city, Xinjiang province, China (85°48′E, 44°03′N), before being cultured at the laboratory and fed with fresh cotton leaves every day. For transcriptome sequencing, three male and three female biological replicates were collected, each consisting of 100 pairs of antennae. For the antennal qRT-PCR study, three male and three female biological replicates were collected, each consisting of 100 pairs of antennae. These samples were immediately frozen in liquid nitrogen and stored at −80°C until they were used.
2.2. RNA extraction and cDNA library construction
Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA), the concentration of RNA was determined with a ND-2000 Spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA), and UV absorption values were recorded at 260 / 280 nm to test the purity of RNA products. RNA integrity was monitored on 1% agarose gel electrophoresis. The Illumina sequencing of the samples was performed by Berry Genomics (Beijing, China). The cDNA library was synthetized with NEBNext® Ultra mRNA Library Prep Kit for Illumina (NEB, Ipswich, MA, USA) following manufacturer’s instructions. The mRNAs were enriched from total RNA using Oligo(dT)-attached magnetic beads and mRNAs were fragmented into short sequences within an RNA fragmentation buffer. Next, the first-strand cDNA was generated using random hexamer-primed reverse transcription, followed by synthesis of the second-strand cDNA using the buffer, dNTPs, RNaseH and DNA polymerase I. Then, end repair was performed on these double strands of cDNA with a dA-tail was added. After the end repair and ligation of adaptors, the PCR was performed to enrich the cDNA. Finally, cDNA library construction and Illumina sequencing of the samples were performed at Novogene Bioinformatics Technology Co., Ltd (Beijing, China).
2.3. De novo assembly and gene annotation
After sequencing, clean reads were obtained after the quality control of raw data and then spliced using Trinity software (version 2.6.6) to generate a set of transcripts. BUSCO was used to verify the completeness of the assemblage [21]. These transcripts were annotated according to the following databases: NCBI nonredundant protein sequences (NCBI-nr), NCBI nucleotide sequences (NCBI-nt), Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG-Ontology), Protein family database (Pfam), EuKaryotic Ortholog Groups / Clusters of Orthologous Groups (KOG / COG) and Swiss-Prot databases by Blast alignment with a cut-off E-value of 10−5.
2.4. Identification of chemosensory genes and bioinformatic analysis
Based on functional annotation information of the antennae transcriptome sequencing data of M. signata, preliminary candidate chemosensory gene nucleic acid sequences were obtained. The candidate chemosensory protein gene sequences were further compared and verified by Blastp at NCBI (www.ncbi.nlm.nih.gov), and the expected e-value was < 10−5. Open reading frames (ORFs) of candidate olfactory-related protein genes were predicted and verified by ORF finder in NCBI (https://www.ncbi.nlm.nih.gov/orffifinder). The default parameters of SignalP 5.0 Server online software (https://services.healthtech.dtu.dk) were utilized to predict signal peptides for candidate chemosensory protein genes. Transmembrane domains of both MsigORs, MsigIRs and MsigGRs were predicted with the TMHMM Server Version 2.0 (https://services.healthtech.dtu.dk/services/TMHMM-2.0/). The physical and chemical properties of proteins were predicted by Expasy online software (https://web.expasy.org/protparam). DNAMAN software (version 6.03) was used to analyze the protein sequence characteristics and sequence consistency. The nucleotide sequences of chemosensory gene transcripts in M. signata are listed in S1 File. The amino acid sequences of M. signata are listed in S2 File.
2.5. Phylogenetic analysis of olfactory-related genes from M. signata and other insects
The amino acid sequence alignment of the candidate OBPs, CSPs, ORs, GRs, and SNMPs of M. signata and other Coleopteran insects and Drosophila melanogaster was performed using the ClustalW [22] program implemented in the Mega V7.0 software package [23]. The phylogenetic tree was constructed using the neighbor-joining (NJ) method [24] with P-distance modeling [25] and pairwise deletion of gaps being performed, and a bootstrap procedure of 1000 replicates was used to evaluate the node support. Phylogenetic trees were visualized using Evolview (www.evolgenius.info/evolview-v2). The data sets of olfactory-related genes chosen from other species of Coleoptera are listed in S3 File.
2.6. RT-qPCR validation of MsigOBPs, MsigCSPs, and MsigORs
Based on FPKM values (S1 Table) and sequence analysis, expression profiles of nine OBPs, three ORs, and three CSPs were identified using RT-qPCR. The total RNA of each tissue sample of M. signata was extracted following the manufacturer’s protocol by using a Trizol reagent kit (Servicebio). First-stand cDNA was synthesized from 2 μg of total RNA using the Servicebio® RT First Strand cDNA Synthesis Kit (Servicebio). RT-qPCR was performed using 2 × SYBR Green qPCR Master Mix (Servicebio). RT-qPCR reactions were conducted in 15 μL reaction volumes containing 7.5 μl of 2× qPCR Mix, 1.5 μl of forward and reverse primers (2.5 μM), 2 μL of cDNA, and 4 μL Water Nuclease-Free. Gene-specific primers (S2 Table) were designed using the Primer designing tool (https://www. ncbi. nlm. nih. gov/ tools/ primer-blast/ index. cgi) and sequenced after PCR. RT-qPCR conditions were as follows: one cycle of 95°C for 30 s followed by 40 cycles of 95°C for 15 s and 60°C for 30 s. The RT-qPCR was performed on a CFX Connect Real-time PCR Detection System (Bio-Rad, Hercules, CA, USA). The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was selected as a reference gene to normalize the relative expression levels of OBP, CSP, and OR genes. The Ct values of divergent antennae from RT-qPCR were normalized to that of the endogenous control in the same tissue and presented as fold change relative to the expression level in the female antennae. Three biological replicates of each sample were analyzed, and relative expression levels of selected genes across the sample were measured using the 2−ΔΔCT method [26]. The difference between antennae of males and females was established by independent sample t-tests using SPSS (SPSS Institute 20.0, SPSS Inc, Chicago, IL, USA).
3. Results
3.1. Transcriptome overview
We carried out next-generation sequencing on a cDNA library constructed from the adult antennae of M. signata using the Illumina NovaSeq 6000 platform. In total, each cDNA library was deep sequenced to yield 6.0 Gb of clean data. After clustering and redundancy filtering, we identified 73, 050 unigenes with the average length of 1140 bp. The length of N50 and N90 were 1896 bp and 440 bp, respectively. The Q20 and Q30 accounted for more than 97.75% and 93.23%, respectively. Through annotation by Blast using the NR, NT, GO, KEGG, Pfam, KOG/COG and Swiss-Prot databases, 34, 233 unigenes were matched to known proteins (S4 File). The raw data were deposited in the NCBI Short Read Archive (SRA) database with BioProject accession number: PRJNA960895.
3.2. Identification of candidate odorant binding proteins
We identified 21 OBP candidate genes based on the antennal transcriptome data for M. signata; these were named MsigOBP1-MsigOBP21. Among the candidate genes, 20 unigenes were likely to represent full-length genes as they contained a complete ORF, and all of the unigenes had predicted signal peptide sequences. All of the candidate OBP sequence Blastp best hits were similar to known Coleoptera OBPs. The length of all of the putative full-length MsigOBPs ranged from 120 to 180 amino acids (S3 Table). Nine MsigOBPs (MsigOBP2, 3, 5, 11, 12, 13, 15, 16, and 17) contained six conserved cysteine residues, and the sequence features were C1-X24-29-C2-X3-C3-X37-42-C4-X8-10-X8-C6 (X represents any amino acid except cysteine), a pattern that belonged to the “Classic OBP” subfamily (Fig 1). Eight MsigOBPs (MsigOBP1, 4, 7, 8, 14, 18, 19, and 20) belonged to the “Minus-C OBP” subfamily (Fig 2). Compared with ORs, insect OBPs were highly conserved. MsigOBP15 shared 86% identity with the OBP of Diabrotica virgifera virgifera (NCBI ID: XP_028129817.1), and MsigOBP10 shared 73% identity with PmacOBP31 in Pyrrhalta maculicollis. A phylogenetic tree was generated to infer the relationships between 21 OBPs of M. signata and 98 OBPs with known functions from seven Coleopteran insects. The results showed that MsigOBP1 and CbowOBP22, MsigOBP3 and MaltOBP4, MsigOBP6 and CbowOB, and MsigOBP17 and CbowOBP14 were clustered together with a high degree of homology (Fig 3).
Fig 1. Multiple amino acid sequence alignment of Classic OBPs in M. signata.
The six conserved cysteine residues are highlighted in black ground and red border. Amino acids that are more than 50% identical in all sequences are marked with cyan color highlights, and more than 75% identical are marked with pink highlights.
Fig 2. Multiple amino acid sequence alignment of Minus-C OBPs in M. signata.
The four conserved cysteine residues are highlighted in black ground and red border. Amino acids that are more than 50% identical in all sequences are marked with cyan color highlights, and more than 75% identical are marked with pink highlights.
Fig 3. Phylogenetic tree of candidate MsigOBPs with known Coleopteran OBP sequences.
Amal, Agrilus mali (N = 2); Cbow, Colaphellus bowringi (N = 25); Hobl, Holotrichia oblita (N = 9); Hpar, Holotrichia parallela (N = 3); Malt, Monochamus alternatus (N = 7); Pyas, Pachyrhinus yasumatsui (N = 2); Szea, Sitophilus zeamais (N = 2); Tcas, Tribolium castaneum (N = 48). Bootstrap values >50 are shown.
3.3. Identification of candidate chemosensory proteins
Bioinformatic analysis led to the identification of six different sequences of candidate CSPs in the M. signata antennal transcriptome; these were named MsigCSP1-MsigCSP6. Four sequences were predicted with a putative full-length ORF, and all of the unigenes had predicted signal peptide sequences. The lengths of all of the putative full-length MsigCSPs ranged from 113 to 130 amino acids (S3 Table). In addition, all of the MsigCSPs followed the highly conserved pattern with four cysteine residues arranged with an exact spacing of C1-X6-C2-X18-C3-X2-C4 (X represents any amino acid except cysteine) (Fig 4). Insect CSPs are more conserved than ORs or OBPs, and all of the MsigCSPs amino acid sequences have more than 48% identity with CSPs of P. maculicollis, C. bowringi, Galeruca daurica, and Ophraella communa. Notably, the identity between MsigCSP6 and PmacCSP6 was as high as 94%. A phylogenetic tree was generated to infer the relationships between six CSPs of M. signata and 66 CSPs from nine species of Coleoptera. Homology analysis showed that the MsigCSPs were dispersed in different branches of the phylogenetic tree. All of the MsigOBPs were orthologs of known OcomCSPs (OcomCSP1, 7, 8, 10, 11, and 12) (Fig 5).
Fig 4. Multiple amino acid sequence alignment of CSPs in M. signata.
The four conserved cysteine residues are highlighted in black ground and red border. Amino acids that are more than 50% identical in all sequences are marked with cyan color highlights, and more than 75% identical are marked with pink highlights.
Fig 5. Phylogenetic tree of candidate MsigCSPs with known Coleopteran CSP sequences.
Amal, Agrilus mali (N = 4); Bhor, Batocera horsfieldi (N = 1); Cfor, Cylas formicarius (N = 3); Darm, Dendroctonus armandi (N = 9); Hpar, Holotrichia parallela (N = 1); Lory, Lissorhoptrus oryzophilus (N = 5); Malt, Monochamus alternatus (N = 12); Ocom, Ophraella communa (N = 12); Pyas, Pachyrhinus yasumatsui (N = 1); Tcas, Tribolium castaneum (N = 18). Bootstrap values >50 are shown.
3.4. Identification of candidate odorant receptors
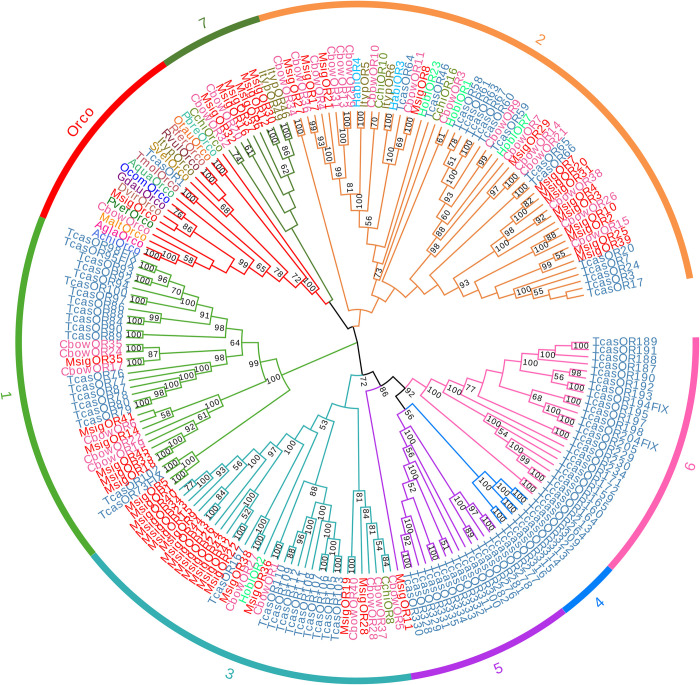
We found 46 candidate OR transcripts in the M. signata antennal transcriptome, including a highly conserved co-receptor (Orco) and 45 typical odorant receptors. Forty-two MsigORs contained a putative full-length ORF, with five to seven TMDs were predicted. The lengths of all of the putative full-length MsigORs ranged from 302 to 459 amino acids (S4 Table). The MsigOrco gene was easily identified because it had an intact ORF and seven transmembrane domains, features characteristic of typical insect ORs. The amino acid sequences of MsigOrco shared 91% identity with the odorant co-receptor of O. communa. The phylogenetic tree was generated to infer the relationships between the 46 MsigORs and 151 ORs from 20 species of Coleoptera. The results showed that the OR sequence were clustered into several subgroups according to previous studies. MsigORs were only present within the previously defined coleopteran OR subgroup 1, 2, 3, 5, and 7 as well as the Orco subgroup. Notably, MsigOrco was clustered with DvirOrco, GdauOrco, OcomOrco, CbowOrco, MaltOrco, AchiOrco, AglaOrco, AquaOrco, TmolOrco, SvelOrco, ItypOrco, RvulOrco, RferOrco, OtauOrco, CchiOrco, PverOrco, PbreOrco, and TcasOR1 which are Orco homologs. In addition, MsigOR4 and CbowOR38, MsigOR8 and CbowOR11, MsigOR10 and CbowOR26, MsigOR17 and CbowOR36, MsigOR19 and CbowOR40, MsigOR27 and CbowOR34, MsigOR28 and CchiOR8, MsigOR29 and HoblOR7, MsigOR32 and CbowOR32, MsigOR35 and CbowOR17, MsigOR36 and CbowOR1, MsigOR38 and CbowOR37, were grouped with a high degree of homology (Fig 6).
Fig 6. Phylogenetic tree of candidate MsigORs with known Coleopteran OR sequences.
Achi, Anoplophora chinensis (N = 1); Agla, Anoplophora glabripennis (N = 1); Aqua, Ambrostoma quadriimpressum (N = 1); Cbow, Colaphellus bowringi (N = 31); Cchi, Callosobruchus chinensis (N = 4); Dvir, Diabrotica virgifera virgifera (N = 1); Gdau, Galeruca daurica (N = 1); Habi, Hylobius abietis (N = 2); Hobl, Holotrichia oblita (N = 4); Ityp, Ips typographus (N = 5); Malt, Monochamus alternatus (N = 1); Ocom, Ophraella communa (N = 1); Otau, Onthophagus taurus (N = 1);Pbre, Protaetia brevitarsis (N = 1); Pver, Plagiodera versicolora (N = 1); Rfer, Rhynchophorus ferrugineus (N = 1); Rvul, Rhynchophorus vulneratus (N = 1); Svel, Sympiezomias velatus (N = 1); Tcas, Tribolium castaneum (N = 91); Tmol, Tenebrio molitor (N = 1). Bootstrap values >50 are shown.
3.5. Identification of candidate ionotropic receptors
We found 15 candidate IR transcripts in the M. signata antennal transcriptome. 13 MsigIRs contained a putative full-length ORF, with three to four TMDs were predicted. Lengths of all of the candidate MsigIRs ranged from 283 to 947 amino acids (S4 Table). Based on the Blastp results, MsigIRs had 44–92% sequence homology with previously identified IRs from other Coleopteran insects. A phylogenetic tree was generated to infer the relationships between the 15 MsigIRs and 100 IRs from nine species of Coleoptera. The results showed that MsigIRs were grouped into different clades with high-level bootstrap values. Four iGluR genes were identified, these were named MsigGluR, MsigGluR1, MsigGluR2, and MsigGluR3. MsigGluR2 and BlonGluR2, MsigIR64a, MsigIR64a.1 and BmelIR64a, and MsigIR75c and BmelIR75c were clustered together with a high degree of homology (Fig 7).
Fig 7. Phylogenetic tree of candidate MsigIRs with known Coleopteran IR sequences.
Blon, Brontispa longissima (N = 13); Bmel, Basilepta melanopus (N = 15); Cbow, Colaphellus bowringi (N = 7); Cfor, Cylas formicarius (N = 13); Dpon, Dendroctonus ponderosae (N = 5); Otau, Onthophagus taurus (N = 3); Pver, Plagiodera versicolora (N = 4); Tcas, Tribolium castaneum (N = 33); Tmol, Tenebrio molitor (N = 7). Bootstrap values >50 are shown.
3.6. Identification of candidate gustatory receptors
We found 23 candidate GR transcripts in the M. signata antennal transcriptome; these were named MsigGR1-MsigGR23. Eighteen MsigGRs contained a putative full-length ORF with five to eight TMDs. Lengths of all of the candidate MsigGRs ranged from 278 to 482 amino acids were predicted (S4 Table). Based on the Blastp results, six MsigGRs had more than 50% identity with GRs of D. v. virgifera, Pyrrhalta aenescens, Leptinotarsa decemlineata, and P. maculicollis. A phylogenetic tree was generated to infer the relationships between the 23 MsigGRs and 87 GRs of Phyllotreta striolata, Tribolium castaneum and D. melanogaster. Notably, three MsigGRs (MsigGR7, 11, and 21) were homologous to known sugar receptors, while no MsigGRs were homologous to other known carbon dioxide receptors (Fig 8).
Fig 8. Phylogenetic tree of candidate MsigGRs with known Coleopteran GR sequences.
Dmel, Drosophila melanogaster(N = 67); Pstr, Phyllotreta striolata (N = 12); Tcas, Tribolium castaneum (N = 11). Bootstrap values >50 are shown.
3.7. Identification of candidate sensory neuron membrane proteins
We identified three SNMP genes in the antennal transcriptome. The lengths of all of the candidate MsigSNMPs were over 522 amino acids, and all were predicted to have a putative full-length ORF (S3 Table). Furthermore, all of the MsigSNMPs had more than 61% identity with SNMPs of D. v. virgifera. A phylogenetic tree was generated to infer the relationships between the three MsigSNMPs and 38 SNMPs from 14 species of Coleoptera. The results showed that MsigSNMP1a, MsigSNMP1b, and MsigSNMP2 were classified into SNMP1 and SNMP2, respectively (Fig 9).
Fig 9. Phylogenetic tree of candidate MsigSNMPs with known Coleopteran SNMP sequences.
Achi, Anoplophora chinensis (N = 3); Bmel, Basilepta melanopus (N = 4); Cbow, Colaphellus bowringi (N = 4); Cfor, Cylas formicarius (N = 3); Dpon, Dendroctonus ponderosae (N = 3); Dvir, Diabrotica virgifera virgifera (N = 1); Gdau, Galeruca daurica (N = 2); Ityp, Ips typographus (N = 2); Ocom, Ophraella communa (N = 4); Paen, Pyrrhalta aenescens (N = 2); Pmac, Pyrrhalta maculicollis (N = 2); Pstr, Phyllotreta striolata (N = 2); Pver, Plagiodera versicolora (N = 4); Tcas, Tribolium castaneum (N = 2).Bootstrap values > 50 are shown.
3.8. Expression levels of MsigOBP, MsigCSP, and MsigOR genes by RT-qPCR
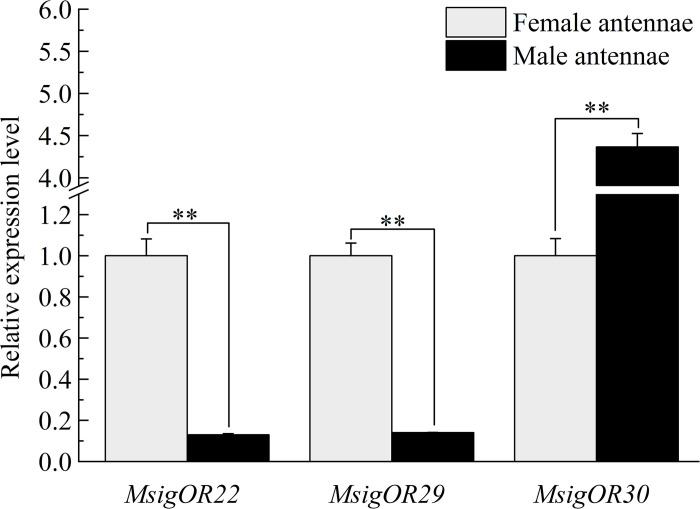
Some chemosensory genes were selected based on FPKM values and functional annotation for additional RT-qPCR analyses, including nine MsigOBPs (MsigOBP2, 3, 5, 6, 8, 15, 18, 20, and 21), three MsigCSPs (MsigCSP1, 2, and 6) and three MsigORs (MsigOR22, 29, and 30). The RT-qPCR results indicated that six MsigOBPs (MsigOBP2, 6, 15, 18, 20, and 21) were expressed at higher levels in the female antennae than male antennae, while only MsigOBP3 and MsigOBP8 were more highly expressed in the male antennae than in female antennae. Notably, MsigOBP5 was not differentially expressed in male and female antennae (Fig 10). The expression levels of MsigCSP1, MsigCSP2 and MsigCSP6 in female antennae were higher than those in male antennae (Fig 11). MsigOR22 and MsigOR29 were highly expressed in the female, while MsigOR30 was highly expressed in the male antennae (Fig 12).
Fig 10. The relative expression levels of OBPs in male and female antennae of M. signata by RT-qPCR.
FA: female antennae; MA: male antennae. The GAPDH gene was used to normalize expression levels in each sample, and the female antennae were selected as the calibrator to normalize the gene expression levels in various tissues. ** Indicates that the difference is extremely significant (P<0.01), * indicates that the difference is significant (P<0.05), ns means no significant difference (P>0.05). Standard errors are represented by the error bars.
Fig 11. The relative expression levels of CSPs in male and female antennae of M. signata by RT-qPCR.
FA: female antennae; MA: male antennae. The GAPDH gene was used to normalize expression levels in each sample, and the female antennae were selected as the calibrator to normalize the gene expression levels in various tissues. ** Indicates that the difference is extremely significant (P<0.01), * indicates that the difference is significant (P<0.05), ns means no significant difference (P>0.05). Standard errors are represented by the error bars.
Fig 12. The relative expression levels of ORs in male and female antennae of M. signata by RT-qPCR.
FA: female antennae; MA: male antennae. The GAPDH gene was used to normalize expression levels in each sample, and the female antennae were selected as the calibrator to normalize the gene expression levels in various tissues. ** Indicates that the difference is extremely significant (P<0.01), * indicates that the difference is significant (P<0.05), ns means no significant difference (P>0.05). Standard errors are represented by the error bars.
4. Discussion
With the application of a new generation of transcriptome sequencing technology to functional gene mining in non-model insects [27], chemosensory protein genes of more than 30 species of Coleoptera have been identified. As an important agricultural pest, the identification, characterization, and expression analysis of chemosensory genes of M. signata facilitate a better understanding of the mechanism of the olfactory system in Chrysomelidae of Coleoptera. Based on the antennal transcriptome data, we screened 111 candidate olfactory-related genes and analyzed their sequence characteristics and phylogenetic relationships in M. signata. These included 21 OBPs, 6 CSPs, 46 ORs, 23 GRs, 15 IRs, and 3 SNMPs. The total number (114) of chemosensory protein genes in M. signata was greater than what has been reported in Pyrrhalta aenescens (92), P. maculicollis (87) [28], and Ophraella communa (105) [29]. This phenomenon may be explained by the evolution of divergent physiological behaviors of different insects during the process of adaptation to various environments [30]. Genetic and phylogenetic analyses were performed on these genes to examine similarities and differences between related genes. Tissue distribution patterns can provide key clues to the functions of olfactory proteins, and it is commonly thought that a high and specific antennal expression pattern suggests a chemosensory role of the genes. The results of this study provide a molecular foundation for further research on the olfactory system of M. signata as well as a reference for similar studies.
OBPs are a critical first step in the olfactory process of insects. Binding with fat-soluble odor molecules in the environment is the major biochemical mechanism of insect-specific odor recognition [31, 32]. To a certain extent, the differences in OBP reflect the evolutionary process of diverse insect chemosensory systems [33]. We identified 21 transcripts encoding OBP genes in the M. signata antennal transcriptome. The number of OBPs was clearly lower than in Rhynchophorus palmarum (37 OBPs) [34], P. maculicollis (36 OBPs), and P. aenescens (31 OBPs) [28] but higher than those of A. quadriimpressum (16 OBPs) [14] and Callosobruchus maculatus (12 OBPs) [27]. Analysis of the properties of the OBP amino acid sequences revealed eight Minus-C OBPs in M. signata, suggesting that these genes might play an important role in chemosensory function in Coleoptera. The phylogenetic analysis showed that MsigOBP15 and MaltOBP3 were clustered in same branch, suggesting that they may be involved in host plant selection [35]. MsigOBP2, MsigOBP5, MsigOBP11, and MsigOBP13 were grouped in the same branch; MsigOBP8, MsigOBP14, and MsigOBP19 were grouped in the same branch, indicating that they may be duplicated or have evolved from the same ancestral gene. According to the RT-qPCR results, the expression levels of the six MsigOBPs were higher in the female antennae than in the male antennae, indicating that they may play an important role in the identification of male pheromones or the oviposition process [36]. OBP genes have been developed as an important target for pest control due to their importance for insects to recognize odors in the external environment. Further functional studies of MsigOBPs would be helpful in providing target genes for green prevention and control of M. signata.
CSPs are involved in the first step of olfaction in insects as OBPs, and hence CSP gene families have received considerable attention [37, 38]. Our survey for these gene families revealed a total of six CSPs in M. signata. The high-level similarities found in Blastp best-hit results demonstrated that CSPs were highly conserved proteins among insects. Comparing MsigCSPs (six CSPs) gene numbers with those in other Coleopteran species, there were fewer than in Leptinotarsa decemlineata (15 CSPs) [12] and Glenea cantor Fabricius (14 CSPs) [39], and a similar number of CSP genes in C. maculatus (seven CSPs) [27]. In the phylogenetic tree, six MsigCSPs were distributed in different branches, suggesting that these genes may have undergone rapid evolution in adaptation to ecological changes. Notably, MsigCSP2 and OcomCSP12 were clustered in the same branch, indicating that they may be involved in mediating reproduction [40]. MsigCSP1 was identified with significantly higher expression levels in female antennae than male antennae, suggesting that it may be involved in the detection of odors that regulate female-specific behaviors. In conclusion, the study of sequence characteristics and expression profiles of MsigCSP genes may contribute in further investigating their specific function.
ORs play an important role in the olfactory system of insects, as they determine the sensitivity and specificity of odorant reception, being the centerpiece of the peripheral olfactory reception in insects [11]. The number of MsigORs (46 ORs) was greater than in G. daurica (10 ORs) [41] but was similar to Brontispa longissima (48 ORs) [42], Holotrichia parallela (47 ORs) [43], Holotrichia oblita Faldermann (44 ORs) [44] and C. bowringi (43 ORs) [13]. MsigOrco was on the same branch and was highly homologous with Orco of other species. This was consistent with the fact that Orco genes are highly conserved in insects. MsigOrco shared 91% identity with OcomOrco, indicating that MsigOrco could help other MsigORs localize to the dendritic membrane or better associate with odor molecules [29]. Further functional work in MsigOrco would allow a better understanding of ion channel formation. The highly conserved sequence of Orco and its unique position in olfactory recognition indicates that it could potentially be used as a target gene for the future development of novel and effective control strategies [45–47]. RT-qPCR results showed that MsigOR22 and MsigOR30 were preferentially expressed in female antennae, which may be involved in the regulation of female oviposition.
Compared with ORs, IRs are another type of olfactory-related protein [48]. ORs bind and respond to pheromones and plant volatiles, regulating insect behaviors such as mating and host-plant selection, while IRs respond to acids and amines [49, 50]. A total of 15 IRs were identified in the antennal transcriptomes of M. signata. This is considerably lower than the numbers found in R. palmarum (28 IRs) [34], Harmonia axyridis (Pallas) (27 IRs) [51], and Eucryptorrhynchus brandti (Harild) (25 IRs) [52], but greater than those in Plagiodera versicolora (seven IRs) [53] and Dendroctonus valens (three IRs) [54]. All of the MsigIRs identified here have orthologs in other Coleoptera. IRs of insects have a variety of functions, including taste, olfactory, temperature, and humidity perception. Like Orco, both IR8a and IR25a were thought to act as co-receptors since they are co-expressed along with other IRs. The phylogenetic tree revealed that MsigIR8a and MsigIR25a belong to the co-expression IR group. The use of molecular biology, behavior and other methods, proved that the host-seeking behavior of Anastatus japonicus is related to peripheral olfactory receptors, and mainly related to the olfactory co-receptor Orco, and not related to ionotropic co-receptors IR8a and IR25a [55]. The molecular mechanisms and functions of most insect IR genes have not been reported, and thus MsigIR genes have a huge research space and potential for functional studies.
We found fewer GRs than ORs in antennae in M. signata because insect antennae are not primary gustatory organs. The maxillary palps may be responsible for expressing more GRs [56]. GRs are involved in the detection of sugars, bitter compounds, carbon dioxide, and contact pheromones [57–59]. The number of predicted MsigGRs (23 GRs) was higher than that in the antennal transcriptomes of O. communa (17 GRs) [29] and P. versicolora (13 GRs) [53]. In the phylogenetic tree, MsigGR7, MsigGR11, and MsigGR21 were clustered with the sugar receptor families, indicating that they may play an important role in host plant selection.
SNMPs are transmembrane domain proteins, and their main function is the recognition and transport of lipophilic odor molecules [60]. SNMP genes are expressed in the dendrite membrane of olfactory receptor neurons, and they play an important role in pheromone recognition [61, 62]. Most studies have shown two SNMP genes in insects, namely, SNMP1 and SNMP2. However, multiple SNMPs have been identified in a variety of Coleopteran species. Three SNMPs were identified in the antennal transcriptomes of M. signata, the same as in Dendroctonus ponderosae and Ips typographus (three SNMPs) [63]. According to the phylogenetic tree of the SNMPs from M. signata and various Coleopterans, we observed that MsigSNMP1a, MsigSNMP1b, and MsigSNMP2 were clustered separately in a branch, indicating that they may play a different role in M. signata.
5. Conclusions
In this study, to better understand the molecular mechanisms of the olfactory recognition process in M. signata, a total of 114 candidate olfactory-related genes were annotated and identified in the antennal transcriptome, including 21 OBPs, 6 CSPs, 46 ORs, 15 IRs, 23 GRs, and three SNMPs. As the first step towards understanding gene functions, we conducted a comprehensive and comparative phylogenetic analysis and examined the relative expression levels of some olfactory-related genes by RT-qPCR. Our study provided a foundation for the functional study of olfactory-related genes in M. signata and a basis for elucidating the molecular mechanism of olfactory recognition in insects as well as potential target genes for the development of novel behavior regulation techniques based on olfactory recognition.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(DOCX)
Acknowledgments
Thank you to Professor Jianping Zhang, Dr. Zhiping Cai, Dr. Jie Su and Dr. Jie Zhao for their guidance in experimental design and methods. Thank you to academic Jing Chen from Shihezi University for their help in the experiment. We are grateful to the reviewer and the editor for comments and suggestions.
Data Availability
All relevant data are within the paper and its Supporting Information files. https://doi.org/10.1371/journal.pone.0301177. All raw data are available from the NCBI Short Read Archive (SRA) database (accession number, PRJNA960895). https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA960895.
Funding Statement
This study was funded by the Xinjiang Uygur Autonomous Region Key Research and Development Task Special (2022B02043-2), the National Natural Science Funds of China (No. 31960541 and NO. 31460473), Xinjiang Uygur Autonomous Region Key Science and Technology Task Special (2023A02009), and Xinjiang Production and Construction Corps Guiding Science and Technology Plan Project (2022ZD010). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Yu PY, Wang SY, Yang XK. Economic entomology of China: Volume 54, Coleptera: Chrysomeloidea (二) [M]. Beijing: Science Press, 1996. 82–169 p. [Google Scholar]
- 2.Chen GH, Yin W, Li Q, Hu HY. Research progress on Monolepta hieroglyphica (Motschulsky). China Plant Prot. 2016; 36: 19–26. [Google Scholar]
- 3.Tian YH, Zhang JP, Chen J, Ouyang DH, Li GW. Occurring characteristic and preventions and control strategy of Monolepta hieroglyphica (Motschulsky)—a new pest of the cotton field in Xinjiang. Anhui Agri Sci Bull. 2017; 10: 120–1+230. [Google Scholar]
- 4.Du JJ, Yun L. Characteristics and control measures for the occurrence and damage of Monolepta hieroglyphica. Shaanxi J Agric Sci. 2009; 55: 202–203. [Google Scholar]
- 5.Li GW, Chen XL. Studies on biological characteristics and population dynamics of Monolepta hieroglyphica in cotton in Xinjiang. China Plant Prot. 2010; 30: 8–10. [Google Scholar]
- 6.Zhang X, Zhang R, Li L, Yang Y, Ding Y, Guan H, et al. Negligible transcriptome and metabolome alterations in RNAi insecticidal maize against Monolepta hieroglyphica. Plant Cell Rep. 2020; 39(11):1539–1547. doi: 10.1007/s00299-020-02582-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng F, Jiang H, Jia J, Wang R, Zhang Z, Xu H. Effect of dimethoate in controlling Monolepta hieroglyphica (Motschulsky) and its distribution in maize by drip irrigation. Pest Manag Sci. 2020; 76(4):1523–1530. doi: 10.1002/ps.5670 . [DOI] [PubMed] [Google Scholar]
- 8.Du YJ, Yan FS. Mechanisms of the role of plant volatile secondary substances in the relationship between phytophagous insects, host plants and natural enemies of insects. Acta Entomol Sin. 1994; 2: 233–250. [Google Scholar]
- 9.Zhao Y, Ding J, Zhang Z, Liu F, Zhou C, Mu W. Sex- and tissue-specific expression profiles of odorant binding protein and chemosensory protein genes in Bradysia odoriphaga (Diptera: Sciaridae). Frontiers in physiology. 2018; 9: 107. doi: 10.3389/fphys.2018.00107 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weng XQ, Zheng RP, Huang CY. A model of insect olfactory chemosensory proteins and their associated roles. Agric Sci& Technol Equip. 2017; 5: 15–16. [Google Scholar]
- 11.Leal WS. Odorant reception in insects: roles of receptors, binding proteins, and degrading enzymes. Annu Rev Entomol. 2013; 58: 373–391. doi: 10.1146/annurev-ento-120811-153635 . [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Sun L, Cao D, Walker W, Zhang Y, Wang G. Identification of candidate olfactory genes in Leptinotarsa decemlineata by antennal transcriptome analysis. Front Ecol Evol. 2015; 3: 60. 10.3389/fevo.2015.00060. [DOI] [Google Scholar]
- 13.Li XM, Zhu XY, Wang ZQ, Wang Y, He P, Chen G, et al. Candidate chemosensory genes identified in Colaphellus bowringi by antennal transcriptome analysis. BMC Genomics. 2015; 16: 1028. doi: 10.1186/s12864-015-2236-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Chen Q, Zhao H, Ren B. Identification and comparison of candidate olfactory genes in the olfactory and non-olfactory organs of elm pest Ambrostoma quadriimpressum (Coleoptera: Chrysomelidae) based on transcriptome analysis. PLoS ONE. 2016; 11(1): e0147144. doi: 10.1371/journal.pone.0147144 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin JD. The physiological basis of phytophagous insect feeding. Acta Entomol Sin. 1980; 1: 106–122. [Google Scholar]
- 16.Liu H, Chi DF, Chen HY, Yu J, Li XC. EAG and behavioral responses of Monolepta hieroglyphica (Motschulsky) to several volatile compounds. For Res. 2013; 26(4): 488–493. [Google Scholar]
- 17.Zhang ZH, Guo DD, Chen J, Han XQ. Tentacular electrophysiological responses of Monolepta hieroglyphica (Motschulsky) to 10 volatiles from cotton and maize. Xinjiang Agric Sci. 2018; 55: 285–292. [Google Scholar]
- 18.Guo DD, Zhang ZH, Chen J, Wang SS. Electrophysiological and behavioral responses of Monolepta hieroglyphica (Motschulsky) to 7 cotton and corn volatiles. Chin J Appl Entomol. 2018; 55: 79–86. [Google Scholar]
- 19.Yang C, Li L, Arman., Duan X, Zhang JP, Chen J. Electrophysiological responses of Monolepta hieroglyphica (Motschulsky) to 13 volatiles electrophysiological response of antennae. Xinjiang Agric Sci. 2021; 58: 1282–1290. [Google Scholar]
- 20.Zhang C, Wang ZY, He KL, Bai SX. Scanning electron microscopy studies of antennal sensilla of Monolepta hieroglyphica. Chin J Appl Entomol. 2012; 49: 756–761. [Google Scholar]
- 21.Simão FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015, 31: 3210–3212. doi: 10.1093/bioinformatics/btv351 [DOI] [PubMed] [Google Scholar]
- 22.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007; 23(21): 2947–2948. doi: 10.1093/bioinformatics/btm404 . [DOI] [PubMed] [Google Scholar]
- 23.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for Bigger Datasets. Mol Biol Evol. 2016; 33(7): 1870–1874. doi: 10.1093/molbev/msw054 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987; 4(4): 406–425. doi: 10.1093/oxfordjournals.molbev.a040454 . [DOI] [PubMed] [Google Scholar]
- 25.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013; 30(12): 2725–2729. doi: 10.1093/molbev/mst197 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25(4): 402–408. doi: 10.1006/meth.2001.1262 . [DOI] [PubMed] [Google Scholar]
- 27.Tanaka K, Shimomura K, Hosoi A, Sato Y, Oikawa Y, Seino Y, et al. Antennal transcriptome analysis of chemosensory genes in the cowpea beetle, Callosobruchus maculatus (F.). PLoS ONE. 2022; 17(1): e0262817. doi: 10.1371/journal.pone.0262817 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang B, Zhang W, Nie RE, Li WZ, Segraves KA, Yang XK, et al. Comparative transcriptome analysis of chemosensory genes in two sister leaf beetles provides insights into chemosensory speciation. Insect Biochem Mol Biol. 2016; 79: 108–118. doi: 10.1016/j.ibmb.2016.11.001 . [DOI] [PubMed] [Google Scholar]
- 29.Ma C, Zhao C, Cui S, Zhang Y, Chen G, Chen H, et al. Identification of candidate chemosensory genes of Ophraella communa LeSage (Coleoptera: Chrysomelidae) based on antennal transcriptome analysis. Sci Rep. 2019; 9(1): 15551. doi: 10.1038/s41598-019-52149-x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldman-Huertas B, Mitchell RF, Lapoint RT, Faucher CP, Hildebrand JG, Whiteman NK. Evolution of herbivory in Drosophilidae linked to loss of behaviors, antennal responses, odorant receptors, and ancestral diet. Proc Natl Acad Sci U S A. 2015; 112(10): 3026–3031. doi: 10.1073/pnas.1424656112 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou X, Slone JD, Rokas A, Berger SL, Liebig J, Ray A, et al. Phylogenetic and transcriptomic analysis of chemosensory receptors in a pair of divergent ant species reveals sex-specific signatures of odor coding. PLoS Genet. 2012; 8(8): e1002930. doi: 10.1371/journal.pgen.1002930 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu XQ, Jiang HB, Fan JY, Liu TY, Meng LW, Liu Y, et al. An odorant-binding protein of Asian citrus psyllid, Diaphorina citri, participates in the response of host plant volatiles. Pest Manag Sci. 2021; 77(7): 3068–3079. doi: 10.1002/ps.6352 . [DOI] [PubMed] [Google Scholar]
- 33.Hu YY, Xu SF, Abebe JW, Li W, Guo ZB, Zhou T. Research advance of olfactory proteins and olfactory mechanism in insects. Genomics Appl Biol. 2013; 32: 667–676. [Google Scholar]
- 34.Gonzalez F, Johny J, Walker WB, Guan Q, Mfarrej S, Jakše J, et al. Antennal transcriptome sequencing and identification of candidate chemoreceptor proteins from an invasive pest, the American palm weevil, Rhynchophorus palmarum. Sci Rep. 2021; 11(1): 8334. doi: 10.1038/s41598-021-87348-y . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao X, Wang M-Q. A cDNA library from the antenna of Monochamus alternatus Hope and binding properties of odorant-binding proteins. J Appl Entomol. 2015; 139(3): 229–236. http://doi.org/ 10.1111/jen.12136. [DOI] [Google Scholar]
- 36.Zhao HX, Xiao WY, Ji CH, Ren Q, Xia XS, Zhang XF, et al. Candidate chemosensory genes identified from the greater wax moth, Galleria mellonella, through a transcriptomic analysis. Sci Rep. 2019; 9(1):10032. doi: 10.1038/s41598-019-46532-x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gong DP, Zhang HJ, Zhao P, Lin Y, Xia QY, Xiang ZH. Identification and expression pattern of the chemosensory protein gene family in the silkworm, Bombyx mori. Insect Biochem Mol Biol. 2007; 37(3): 266–277. doi: 10.1016/j.ibmb.2006.11.012 . [DOI] [PubMed] [Google Scholar]
- 38.Zhang YN, Ye ZF, Yang K, Dong SL. Antenna-predominant and male-biased CSP19 of Sesamia inferens is able to bind the female sex pheromones and host plant volatiles. Gene. 2014; 536(2): 279–286. doi: 10.1016/j.gene.2013.12.011 . [DOI] [PubMed] [Google Scholar]
- 39.Wu G, Su R, Ouyang H, Zheng X, Lu W, Wang X. Antennal transcriptome analysis and identification of olfactory genes in Glenea cantor Fabricius (Cerambycidae: Lamiinae). Insects. 2022;13(6): 553. doi: 10.3390/insects13060553 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma C, Cui S, Tian Z, Zhang Y, Chen G, Gao X, et al. OcomCSP12, a Chemosensory protein expressed specifically by ovary, mediates reproduction in Ophraella communa (Coleoptera: Chrysomelidae). Frontiers in physiology. 2019; 10: 1290. doi: 10.3389/fphys.2019.01290 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li L, Zhou YT, Tan Y, Zhou XR, Pang BP. Identification of odorant-binding protein genes in Galeruca daurica (Coleoptera: Chrysomelidae) and analysis of their expression profiles. Bull Entomol Res. 2017; 107(4): 550–561. doi: 10.1017/S0007485317000402 . [DOI] [PubMed] [Google Scholar]
- 42.Bin SY, Qu MQ, Li KM, Peng ZQ, Wu ZZ, Lin JT. Antennal and abdominal transcriptomes reveal chemosensory gene families in the coconut hispine beetle, Brontispa longissima. Sci Rep. 2017; 7(1): 2809. doi: 10.1038/s41598-017-03263-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ju Q, Li X, Guo XQ, Du L, Shi CR, Qu MJ. Two odorant-binding proteins of the dark black chafer (Holotrichia parallela) display preferential binding to biologically active host plant volatiles. Frontiers in physiology. 2018; 9: 769. doi: 10.3389/fphys.2018.00769 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li K, Wei H, Shu C, Zhang S, Cao Y, Luo C, et al. Identification and comparison of candidate odorant receptor genes in the olfactory and non-olfactory organs of Holotrichia oblita Faldermann by transcriptome analysis. Comp Biochem Physiol, Part D: Genomics Proteomics. 2017; 24: 1–11. doi: 10.1016/j.cbd.2017.07.001 . [DOI] [PubMed] [Google Scholar]
- 45.He P, Ma YF, Wang MM, Wang H, Youssef D, Nesreen AEM, et al. Silencing the odorant coreceptor (Orco) disrupts sex pheromonal communication and feeding responses in Blattella germanica: toward an alternative target for controlling insect-transmitted human diseases. Pest Manage Sci. 2020; 77: 1674–1682. doi: 10.1002/ps.6187 [DOI] [PubMed] [Google Scholar]
- 46.Paulo DF, Junqueira ACM, Arp AP, Vieira AS, Ceballos J, Skoda SR, et al. Disruption of the odorant coreceptor Orco impairs foraging and host finding behaviors in the New World screwworm fly. Sci Rep. 2021; 11(1): 11379. doi: 10.1038/s41598-021-90649-x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang X, Liu P, Qin Q, Li M, Meng R, Zhang T. Characterizing the role of Orco gene in detecting aggregation pheromone and food resources in Protaetia brevitarsis Leiws (Coleoptera: Scarabaeidae). Front Physiol. 2021; 12: 649590. doi: 10.3389/fphys.2021.649590 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wicher D, Miazzi F. Functional properties of insect olfactory receptors: ionotropic receptors and odorant receptors. Cell Tissue Res. 2021; 383(1): 7–19. doi: 10.1007/s00441-020-03363-x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu Z, Bin Y, Jie YU, Bao-ping P, Gui-rong W. Expression profiles and functional prediction of ionotropic receptors in Asian corn borer, Ostrinia furnacalis (Lepidoptera: Crambidae). Journal of Integrative Agriculture. 2022; 21(2): 474–485. 10.1016/S2095-3119(20)63427-X. [DOI] [Google Scholar]
- 50.Ni L. The structure and function of ionotropic receptors in Drosophila. Front Mol Neurosci. 2020; 13: 638839. doi: 10.3389/fnmol.2020.638839 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rondoni G, Roman A, Meslin C, Montagné N, Jacquin-Joly E. Antennal transcriptome analysis and identification of candidate chemosensory genes of the harlequin ladybird beetle, Harmonia axyridis (pallas) (coleoptera: Coccinellidae). Insects. 2021; 12, 209. doi: 10.3390/insects12030209 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wen X, Wang Q, Gao P, Wen J. Identification and comparison of chemosensory genes in the antennal transcriptomes of Eucryptorrhynchus scrobiculatus and E. brandti fed on Ailanthus altissima. Front physiol. 2018; 9: 1652. doi: 10.3389/fphys.2018.01652 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu X, Tong N, Wu Z, Li Y, Ma M, Liu P, et al. Identification of chemosensory genes based on the antennal transcriptomic analysis of Plagiodera versicolora. Insects. 2021; 13(1): 306. doi: 10.3390/insects13010036 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gu XC, Zhang YN, Kang K, Dong SL, Zhang LW. Antennal transcriptome analysis of odorant reception genes in the red turpentine beetle (RTB), Dendroctonus valens. PLoS ONE. 2015; 10(5): e0125159. doi: 10.1371/journal.pone.0125159 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li J. Research on the olfactory mechanism of parasitic behaviour in Anastatus japonicus. Master dissertation. Jilin: Northeast Normal University. 2018. [Google Scholar]
- 56.Engsontia P, Satasook C. Genome-wide identification of the gustatory receptor gene family of the invasive pest, red palm weevil, Rhynchophorus ferrugineus (Olivier, 1790). Insects. 2021; 12(7): 611. doi: 10.3390/insects12070611 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rimal S, Sang J, Dhakal S, Lee Y. Cucurbitacin B activates bitter-sensing gustatory receptor neurons via gustatory receptor 33a in Drosophila melanogaster. Mol Cells. 2020; 43(6): 530–538. doi: 10.14348/molcells.2020.0019 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sato K, Tanaka K, Touhara K. Sugar-regulated cation channel formed by an insect gustatory receptor. Proc Natl Acad Sci U S A. 2011; 108(28): 11680–11685. doi: 10.1073/pnas.1019622108 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kwon JY, Dahanukar A, Weiss LA, Carlson JR. The molecular basis of CO2 reception in Drosophila. Proc Natl Acad Sci U S A. 2007; 104(9): 3574–3578. doi: 10.1073/pnas.0700079104 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vogt RG, Miller NE, Litvack R, Fandino RA, Sparks J, Staples J, et al. The insect SNMP gene family. Insect Biochem Mol Biol. 2009; 39(7): 448–456. doi: 10.1016/j.ibmb.2009.03.007 . [DOI] [PubMed] [Google Scholar]
- 61.Forstner M, Gohl T, Gondesen I, Raming K, Breer H, Krieger J. Differential expression of SNMP-1 and SNMP-2 proteins in pheromone-sensitive hairs of moths. Chem Senses. 2008; 33(3): 291–299. doi: 10.1093/chemse/bjm087 . [DOI] [PubMed] [Google Scholar]
- 62.Cassau S, Sander D, Karcher T, Laue M, Hause G, Breer H, et al. The sensilla-specific expression and subcellular localization of SNMP1 and SNMP2 reveal novel insights into their roles in the antenna of the desert locust Schistocerca gregaria. Insects. 2022; 13(7): 579. doi: 10.3390/insects13070579 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Andersson MN, Grosse-Wilde E, Keeling CI, Bengtsson JM, Yuen MM, Li M, et al. Antennal transcriptome analysis of the chemosensory gene families in the tree killing bark beetles, Ips typographus and Dendroctonus ponderosae (Coleoptera: Curculionidae: Scolytinae). BMC Genomics. 2013; 14: 198. doi: 10.1186/1471-2164-14-198 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files. https://doi.org/10.1371/journal.pone.0301177. All raw data are available from the NCBI Short Read Archive (SRA) database (accession number, PRJNA960895). https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA960895.