Abstract
The initial steps of Venezuelan equine encephalitis virus (VEE) spread from inoculation in the skin to the draining lymph node have been characterized. By using green fluorescent protein and immunocytochemistry, dendritic cells in the draining lymph node were determined to be the primary target of VEE infection in the first 48 h following inoculation. VEE viral replicon particles, which can undergo only one round of infection, identified Langerhans cells to be the initial set of cells infected by VEE directly following inoculation. These cells are resident dendritic cells in the skin, which migrate to the draining lymph node following activation. A point mutation in the E2 glycoprotein gene of VEE that renders the virus avirulent and compromises its ability to spread beyond the draining lymph blocked the appearance of virally infected dendritic cells in the lymph node in vivo. A second-site suppressor mutation that restores viral spread to lymphoid tissues and partially restore virulence likewise restored the ability of VEE to infect dendritic cells in vivo.
Venezuelan equine encephalitis virus (VEE) is a positive-sense, single-stranded RNA Alphavirus belonging to the family Togaviridae. Epizootic strains of VEE cause significant disease in horses and humans, as evidenced by the 1995 to 1996 epizootic in Venezuela and Columbia, during which an estimated 60,000 humans were infected following outbreaks in surrounding horse populations (35). VEE is transmitted by mosquitoes, typically of the subgenus Culex, during the course of a blood meal. Infection by VEE causes a biphasic illness in equines. The first phase is characterized by fever and viral replication in lymphoid tissues, with a high serum viremia (14, 19). In the second phase, in spite of immune system-mediated clearance from peripheral tissues, virus invades the central nervous system and leads to an often lethal encephalitis. Infection in humans results in much milder disease than that seen in equines and ranges from asymptomatic to flu-like symptoms and fever, with approximately 0.1 to 0.7% of cases resulting in encephalitis, most commonly in children and the elderly (18).
Infection in rodent models closely parallels the encephalitic disease seen in horses (14, 23). Following a subcutaneous (s.c.) inoculation in the rear footpad of a mouse, replicating virus is first observed in the draining lymph node within 4 h postinoculation (p.i.). This precedes replication at the site of inoculation in the footpad by 6 to 8 h, suggesting that the primary amplification occurs at the first lymphoid tissue encountered and not at the site of inoculation (J. F. Aronson, N. L. Davis, F. B. Grieder, P. C. Charles, T. A. Knott, K. W. Brown, and R. E. Johnston, unpublished data). Virus titers of 106 to 107 PFU/g of tissue are observed in the draining node as early as 6 h p.i. (2). By 12 to 24 h p.i., high titers are observed in other lymphoid tissues and serum, with lower titers being found in several nonlymphoid tissues. By 48 to 72 h p.i., the titers begin to decline, with necrosis in infected tissues. Complete clearance from the periphery occurs by 72 to 96 h p.i. However, at this point virus has already spread from the blood to the central nervous system via the olfactory and trigeminal nerves, terminating in a fatal encephalitis 7 to 10 days p.i. (6).
Although lymphoid tissues represent a significant site of viral replication in the early stages of VEE pathogenesis (14, 23, 34; Aronson et al., unpublished), specific cell targets for viral replication had not been identified. VEE expression vectors (10, 27) encoding either the green fluorescent protein (GFP) or influenza virus hemagglutinin (HA) were used in conjunction with immunocytochemistry to demonstrate that VEE primarily targets dendritic cells (DC) in the lymph nodes. The use of VEE viral replicon particles (VRP), which can undergo only one round of infection, showed that VEE initially infects Langerhans cells, the resident DC in the skin which migrate to the draining lymph node following activation. A point mutation in the E2 glycoprotein gene of VEE that renders the virus avirulent and compromises its ability to spread beyond the draining lymph node (11, 18) blocked the appearance of virally infected DC in the lymph node in vivo. A second-site suppressor mutation that restores viral spread to lymphoid tissues and partially restores virulence (Aronson et al., unpublished; K. A. Bernard, personal communication) likewise restored the ability of VEE to infect DC in vivo.
MATERIALS AND METHODS
Virus and mice.
This study used viral particles derived directly from transfection of full-length or partial RNA transcripts of VEE cDNA clones into baby hamster kidney (BHK) cells. These clones include pV3000, derived from the Trinidad donkey strain of VEE (11, 12), and two mutated clones, pV3010 (11, 18) and pV3533 (Aronson et al., unpublished). The infectious virus derived from these full-length clones are designated V3000, V3010, and V3533, respectively. V3010 is isogenic with V3000 except at E2 76 (Glu → Lys). V3533 is a second-site revertant of V3010 that was generated by the insertion of a suppressor mutation at E2 116 (Lys → Glu) into the V3010 background. The E2 116 suppressor mutation was identified as a constituent mutation in a V3010 revertant isolated from the serum of a V3010-infected mouse. The replication of V3533 in mice appears identical to that of the in vivo-isolated revertant.
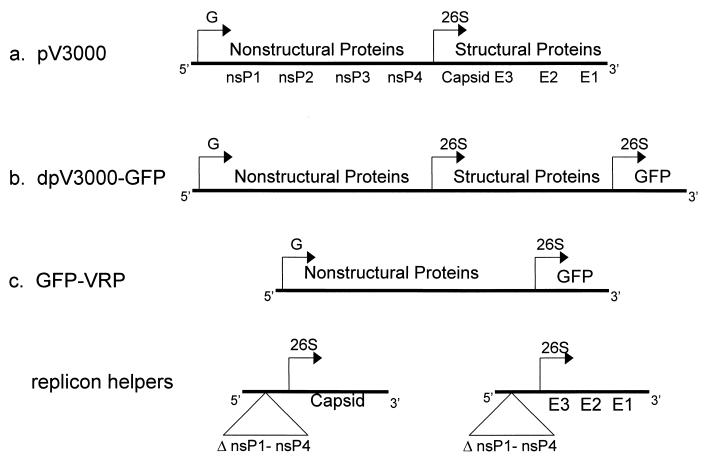
Two types of VEE expression vectors derived from V3000 were used in these studies (Fig. 1): a double-promoter vector (dpV3000) (10) and a replicon vector (27). dpV3000 contains a second copy of the 26S subgenomic promoter inserted near the 3′ end of the V3000 genome. dpV3000-GFP was generated by inserting a copy of a mutated form of GFP (mut2GFP) (7) downstream of the second 26S promoter. This vector produces propagation-competent virus particles which express GFP following infection and which can spread from cell to cell. In contrast, the replicon expression vectors have had the structural genes directly replaced by a heterologous gene (10, 27) and were used to produce propagation-defective viral particles, which are limited to one round of infection.
FIG. 1.
Genetic maps of V3000 clones and vectors. Diagrams of the parental virus, V3000, depicting positions of the genomic promoter (G), the sub-genomic 26S promoter (26S) and the nonstructural (nsP1-nsP4), capsid, and glycoprotein genes (E1 to E3) (a), the double promoter expression vector, dpV3000-GFP (b), and the replicon expression vector system with pGFP-VRP, capsid, and glycoprotein helpers (c). In the expression replicon, the structural protein genes were deleted and replaced with the mut2GFP gene immediately downstream of the 26S promoter. The VEE capsid gene and the glycoprotein genes were supplied on separate helper RNAs in which the 5′ and 3′ ends of the VEE genome were retained but from which most of the nonstructural gene region, including the encapsidation signal, was deleted (7). When coelectroporated into the same cell, these three RNAs supply all the required VEE functions resulting in the assembly and release of infectious VRP encoding GFP into the culture media.
Two replicon vectors, one expressing influenza virus HA (pHA-VRP) (27) and one expressing mut2GFP (pGFP-VRP), were used in these studies. pGFP-VRP was generated by cloning mut2GFP directly behind the 26S promoter in the place of the structural genes. Infectious VRP were produced from these vectors by coelectroporation into BHK cells of RNA transcripts from the pGFP-VRP or pHA-VRP, respectively, with two VEE helper vectors expressing VEE capsid and glycoprotein genes (Fig. 1c). Both helper vectors lack the viral packaging signal, which results in only the pGFP-VRP or pHA-VRP genome being packaged in VRP. GFP-VRP-3000 and HA-VRP-3000 were produced by electroporation with helpers expressing the wild-type (V3000) glycoproteins, whereas the mutated VRPs, GFP-VRP-3010 and GFP-VRP-3533, were packaged by using glycoprotein helpers containing the V3010 and V3533 mutations, respectively. While VRP are fully infectious, they can undergo only one round of infection and were used to study the initial events of infection in vivo without the production and spread of viral progeny. The titer was determined on BHK cells as infectious units (IU) by either immunofluorescence or immunocytochemistry.
Female CD-1 or C57BL/6 mice, 7 to 8 weeks old (Charles River Laboratories, Wilmington, Mass., and Jackson Laboratory, Bar Harbor, Maine, respectively) were inoculated s.c. in the footpad with 103 to 104 PFU of virus or IU of VRP.
GFP detection and immunocytochemistry.
At 7 to 48 h p.i., mice were perfused with 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS), and the draining popliteal lymph nodes were removed. For leg sections, whole legs were decalcified in 8% EDTA–4% PFA for 6 weeks before the sectioning was performed. GFP-positive cells were visualized by fluorescence microscopy of fixed frozen sections by using a fluorescein isothiocyanate filter set. The following antibodies (Abs) were used for immunostaining: rabbit anti-VEE (kind gift of J. Smith, Ft. Detrick, Fredrick, Md.), anti-DEC 205 (NLDC145; American Type Culture Collection) and anti-CD11C (N418; American Type Culture Collection) for DC, anti-CR-1 (PharMingen) and anti-FcγIII/IIR (PharMingen) for follicular DC; anti-CD11B (MAC-1) for macrophages, anti-MHC II IAd+b (clone 25.9.175; both kindly provided by S. Virgin, Washington University, St. Louis, Mo.), anti-CD45R (B220; PharMingen) for B cells, and anti-CD5 specific for T cells and Ly1 B cells (PharMingen). Rabbit anti-flu (H1N1; kindly provided by R. Webster, St. Jude's Children Hospital, Memphis) was used to detect influenza virus HA. Species-matched normal sera or isotype-matched monoclonal Ab (MAb) (PharMingen) was used in all immunostaining steps as the negative control primary Ab. To optimize DEC 205 reactivity, fixed frozen sections were steamed for 25 min (or, in the case of tissues with GFP, warmed to 55°C for 1 h) in 10 mM citrate (pH 6.0). Immunostaining patterns obtained by epitope retrieval methods were confirmed by using fresh tissue sections fixed in acetone (10 min). Sections were blocked in 10% normal serum (with 0.1% Tween 20 in PBS for DEC 205) for 1 h, washed in PBS, and incubated with the primary Ab overnight at 4°C. Sections were incubated with a secondary Ab with the appropriate fluorochrome (CY2, CY3, or Texas Red) either directly conjugated to the Ab or through an avidin-biotin bridge (Jackson ImmunoResearch Laboratories).
Isolation of VEE-infected cells.
Mice were inoculated in all four footpads with 103 PFU of dpV3000-GFP, and 2 h later the popliteal, lateral iliac, accessory axial, and proper axial lymph nodes were removed and minced into 1-mm2 pieces on ice in complete medium (RPMI-C: RPMI 1640, 10% fetal bovine serum FBS, 25 mM HEPES [pH 7.2], 100 U of penicillin per ml, 100 μg of streptomycin per ml, 0.25 mg of amphotericin B per ml, 2 mM l-Gln, 50 μM 2-mercaptoethanol, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids). Tissue fragments were digested with collagenase (Sigma; type IA, 100 μg/ml) and DNase (Sigma; 30 U/ml) for 90 min at 37°C. At 30-min intervals during the digestion, the fragments were gently pipetted and the suspension cells were removed to ice, with fresh enzymes being added to the remaining fragments. The final cell suspension was passed through a 70-μm nylon cell sieve (Falcon) and washed twice. DC and macrophages were separated from lymphocytes by density centrifugation of a suspension of 0.5 × 106 to 1 × 106 cells/ml over a 14.5% Nycodenz (GIBCO BRL) cushion at 600 × g for 20 min at 27°C. Low-density cells at the interphase (macrophages and DC) were separated from pelletted lymphocytes, and both fractions were cultured in RPMI-C overnight (14 to 16 h) at 37°C. DC were removed from the low-density cell culture by rigorous pipetting, leaving the strongly adherent macrophages. The dendritic, macrophage, and lymphocyte fractions were fixed in 4% PFA, and the number of GFP-positive cells in each fraction was counted by fluorescence microscopy. The purity of the DC and lymphocyte fractions was checked by immunofluorescence with DEC 205-, CD11B-, CD11C-, B220-, and CD5-specific Ab. Macrophages, T cells, and B cells represented 0.3, 1.0, and 5.8% of the DC fraction, respectively. Likewise, DC and macrophages represented 0.6 and 0.9%, respectively, of the lymphocyte fraction.
Kinetics of migration of VEE-infected cells.
To determine the movement of VEE-infected cells from the site of inoculation to the draining lymph node, mice were inoculated s.c. with 103 IU of GFP-VRP-3000 in the rear footpad. At the times specified, two mice were sacrificed by cervical dislocation, the femoral arteries were severed, and the footpad and draining lymph nodes were surgically removed. Tissue explants were cultured at 37°C in RPMI-C for a total of 12 h (in vivo plus ex vivo) prior to fixation in 4% PFA. Tissues were serially sectioned, and the total number of GFP-positive cells in each tissue was determined by fluorescence microscopy.
RESULTS
VEE infects DC in the draining lymph node.
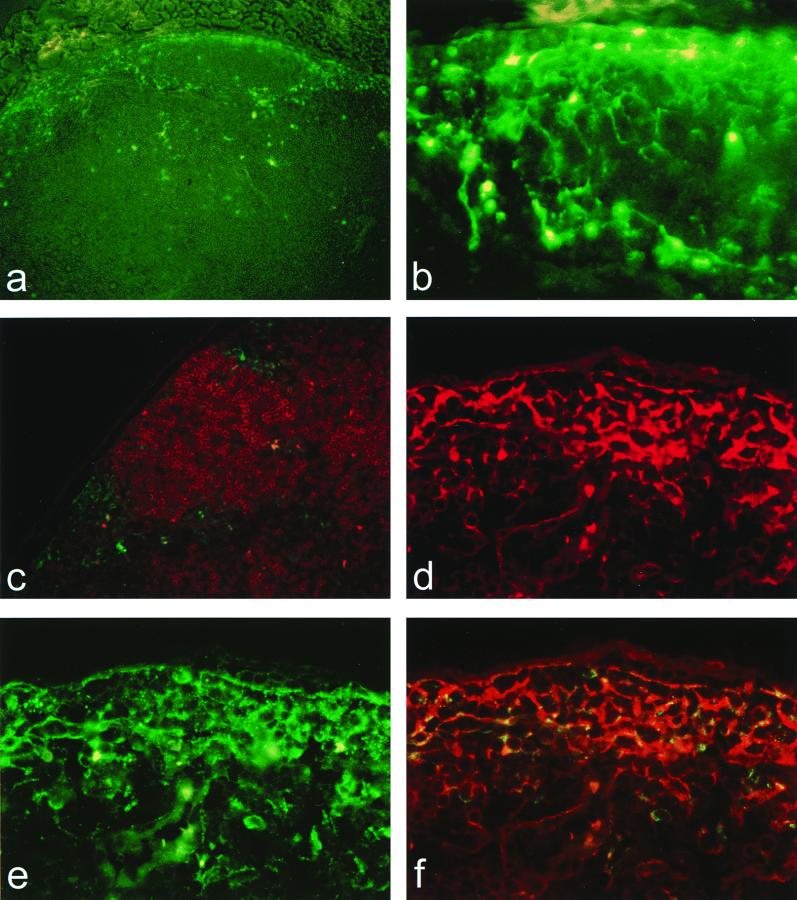
The dpV3000-GFP vector encodes all virus genes in addition to GFP and allows viral spread from cell to cell over time to be studied by identification of cells expressing GFP. Draining lymph nodes were harvested from mice at 12, 24, and 48 h after inoculation with dpV3000-GFP. At 12 h p.i., GFP-positive cells with morphology similar to DC were observed in the paracortex of the lymph node (Fig. 2a and b). No GFP signal was detected in tissues from mice inoculated with dpV3000-GFP having the GFP gene cloned in the antisense orientation. Immunostaining with the B-cell-specific Ab, B220, revealed that the GFP-positive cells were restricted to the interfollicular regions of the paracortex and in close proximity to B-cell follicles (Fig. 2c). By 24 and 48 h p.i., GFP-positive cells were seen deeper in the cortex. As at 12 h p.i., only a few positive cells were seen in the medulla. No apparent change in the cell type infected was observed at these later time points. At all time points, occasional positive cells were observed in the lumen of the medullary cords and possibly represented emigration of infected cells into the lymphatics.
FIG. 2.
VEE infects DC in the draining lymph node. Sections of the draining popliteal lymph node 12 h following s.c. inoculation in the rear footpad either with 103 PFU of dpV3000-GFP (a to c), showing the distribution and morphology of GFP-positive cells in the cortex of the draining popliteal lymph node (a and b) (magnification, ×100 and ×400, respectively) and around B-cell follicles immunostained with the B-cell-specific Mab, B220 (c) (magnification, ×200), or with 103 PFU of V3000 (d to f), showing double immunostaining with VEE-specific Ab (CY2; green) and the DC-specific Ab, DEC 205, (Texas Red; red) visualized by using either Texas Red filters (d), fluorescein isothiocyanate filters (e), or triple-pass filters allowing simultaneous visualization of CY2 and Texas Red (f; magnification, ×400).
The cytoplasmic expression of GFP made it difficult to confirm the identity of infected cells by using surface markers. Therefore, mice were inoculated with the parental virus, V3000, and lymph node sections were doubly immunostained with VEE-specific Ab in combination with lymphocyte or DC-specific Ab. A similar pattern of VEE positive cells in the paracortex was observed with V3000 as was initially observed with the dpV3000-GFP vector. Double immunostaining revealed that cells positive for VEE also were positive for the DC-specific marker, DEC 205 (Figure 2d to f). VEE-positive cells, however, were negative for the B-cell marker, B220 (data not shown), consistent with the localization of GFP-positive cells outside of B-cell follicles. VEE-positive cells also were negative for CD5, a pan-T-cell and Ly1 B-cell marker (data not shown). Immunostaining with serum and isotype-matched control Ab confirmed the specificity of this staining (data not shown). Similar results were found when tissue sections from mice inoculated with dpV3000-GFP were immunostained with these Ab. The status of CD11C reactivity of infected cells could not be determined under the fixation conditions required for inactivating propagative virus. These results indicate that DEC 205-positive DC in the paracortex represent a major cell target in the draining lymph node for infection by VEE.
GFP-positive cells cofractionate with the DC population from lymph nodes of mice infected with VEE.
To confirm the targeting of VEE to DC, lymph nodes were removed from mice soon after inoculation with dpV3000-GFP. Tissue homogenates were fractionated into lymphocytes, macrophages, and DC by density centrifugation, cultured overnight, and scored for the number of GFP-positive cells (Table 1). The DC fraction consistently contained 2 orders of magnitude more GFP-positive cells than did the lymphocyte fraction. No positive cells were observed in the macrophage population in one experiment, and the four positive cells observed in the second experiment were loosely adherent, most probably representing DC that had not been removed from the dish. These results are consistent with the immunocytochemical observations indicating that DC represent a major target for infection by VEE.
TABLE 1.
VEE-infected cells cofractionate with DC from the lymph node
| Expt | Cell fraction | No. of GFP-positive cells per 107 cells | Total no. of GFP-positive cells countedc |
|---|---|---|---|
| 1 | DC | 660b | 178 |
| Lymphocytes | 0.4a | 1 | |
| Macrophages | 0b | 0 | |
| 2 | DC | 556b | 139 |
| Lymphocytes | 4a | 16 | |
| Macrophages | 4b | 4 |
Number of GFP-positive cells counted per 107 lymphocytes plated.
Number of GFP-positive cells counted per 107 low-density cells plated.
Total number of cells counted per fraction.
Langerhans cells are the first cells infected by VEE following s.c. inoculation.
VRP are propagation-defective viral particles and can undergo only one round of infection. Therefore, VRP-infected cells necessarily represent the population of cells first infected by the virus. VRP expressing either GFP or HA (Fig. 1c) were used to identify the initial set of cells which are infected by the virus following an s.c. injection in the footpad. Mice were inoculated with a low dose (103 IU) of GFP-VRP-3000 s.c. in the rear footpad, and serial sections of either the whole leg or the draining popliteal lymph node were examined. Examination of whole-leg serial sections at 12 and 24 h p.i. revealed that GFP-positive cells were found exclusively in the draining popliteal lymph node in the subcapsular region of the cortex (Fig. 3a and b). No signal was observed in any other tissues, including the footpad. Identical results were found when mice, inoculated with dpV3000-GFP, were sacrificed at 7.5 h p.i., the earliest time at which GFP could be detected (data not shown). The large size of the GFP-positive cells (cell bodies 20 to 30 μm in diameter) and their morphology with extensive processes that could be tracked through three to four serial 30-μm-thick sections were similar to those of Langerhans cells found in the skin. These GFP-positive cells only rarely were observed deeper in the node, even at 24 to 48 h p.i. and persisted up to 5 days following inoculation without showing significant changes in morphology. The phenotype of these cells was confirmed by double immunostaining in lymph nodes from mice inoculated with VRP expressing HA as a cell surface marker (HA-VRP-3000). Cells that were positive for HA (Fig. 3c and e) were weakly positive for DEC 205 and for major histocompatibility complex (MHC) class II (Fig. 3d and f) but were negative for lymphocyte and macrophage markers (CD5 and CD11B) as well as the DC marker, CD11C (data not shown). A 10-fold-higher dose of VRP (104 IU) resulted in a small proportion of HA-positive cells in the paracortex that were negative for CD5 and B220 but were positive for CD11C and CD11B, indicating that at higher doses other subsets of antigen-presenting cells, including macrophages, could be infected.
FIG. 3.
Langerhans cells are the first cells infected following inoculation. (a and b) A leg section showing the draining popliteal lymph node 12 h following s.c. inoculation in the rear footpad with 103 IU of GFP-VRP-V3000 (magnification, ×100 and ×400, respectively). (c to f) Draining lymph node sections from mice 8 h after being infected with 103 IU of HA-VRP-3000 were doubly immunostained with Ab specific for either influenza virus (c and e), DC (d; DEC 205), or MHC class II (f), and 0.5-μm-thick sections were analyzed by confocal microscopy (magnification, ×600).
To determine the kinetics of migration of infected cells and/or free virus from the site of inoculation to the draining lymph node, footpads and draining popliteal lymph nodes from mice inoculated with 103 IU of GFP-VRP-3000 were surgically removed at time points between 7 min and 3 h p.i. and placed in organ culture to allow the expression of GFP. Tissues were cultured for a total time of 12 h (in vivo plus in vitro) and fixed, and the total number of GFP-positive cells in serial sections was determined (Fig. 4). No GFP-positive cells were recovered in the media or adherent to the plastic in any of the cultures. The number of GFP-positive cells in the footpad declined to 0 by 30 min after inoculation, suggesting a rapid emigration of infected cells from the site of inoculation. Likewise, a significant number of GFP-positive cells were observed in the lymph node at 30 min and reached a plateau between 1 and 2 h. This time course is consistent with VEE infection of Langerhans cells at the site of inoculation and subsequent movement of these cells to the draining lymph node. It appears that this is an efficient process, since approximately 45% of the inoculated VRP could be recovered as GFP-positive cells in the lymph node.
FIG. 4.
Kinetics of the movement of VEE-infected cells. Numbers of GFP-positive cells in the footpad (hatched) and in the draining popliteal lymph node (solid) were determined immediately following s.c. inoculation in the rear footpad with 103 IU of GFP-VRP-3000. At the times specified, tissues were surgically removed and cultured as tissue explants at 37°C for a total of 12 h (in vivo plus ex vivo). Tissues were serially sectioned, and the total number of GFP-positive cells in each tissue was determined by fluorescent microscopy. Each time point represents the averages of two paired footpads and draining lymph nodes.
A single mutation in the VEE E2 glycoprotein gene prevents infection of DC in the lymph node.
A single mutation introduced into the E2 glycoprotein gene of V3000 to generate the V3010 mutant severely affects both the virulence of the virus and the ability of the virus to spread beyond the draining lymph node (11, 18). VRP were packaged in V3010 structural proteins to determine the effect of this mutation on the targeting of Langerhans cells. Examination of serial sections through entire draining lymph nodes demonstrated that this mutation effectively prevents the appearance of GFP-positive Langerhans cells in the draining lymph node. Occasionally, small, irregular GFP-positive cells were observed deep in the medulla (Fig. 5a). These cells also were found at low frequencies in lymph nodes from mice inoculated with V3000. No GFP-positive cells were observed in other tissues of the lower leg, including the site of inoculation in the footpad.
FIG. 5.
A single mutation in the VEE E2 glycoprotein gene prevents infection in vivo of DC, while a second-site suppressor mutation restores the ability of VEE to infect DC. Sections of the draining lymph node 12 h following s.c. inoculation in the rear footpad with 103 IU of either GFP-VRP-V3010 (magnification, ×600) (a), GFP-VRP-V3533 (magnification, ×400) (b), or HA-VRP-V3533 (c to f). HA-GFP-V3533 sections were doubly immunostained with Ab specific for influenza virus and FcγIII/IIR and analyzed by confocal microscopy (magnification, ×600) (c and d) or for influenza virus and CR-1 (e and f) and analyzed by fluorescence microscopy (magnification, ×600) (e and f).
A second-site suppressor mutation restores the ability of VEE to infect DC in vivo.
The introduction of a second-site suppressor mutation into the V3010 E2 glycoprotein gene (clone V3533), originally isolated as an in vivo reversion, restores the ability of the virus to spread and replicate in lymphoid tissues beyond the draining lymph node and partially restores viral virulence (Aronson et al., unpublished; Bernard, personal communication). We tested the effect of this second mutation on cell targeting in vivo by inoculating mice with VRP packaged in V3533 glycoproteins. At a dose of 103 IU, GFP-VRP-V3533 infected cells with a DC-like morphology located within B-cell follicles (Fig. 5b). These cells were positive for FcγIII/IIR (Fig. 5d) and CR-1 (Fig. 5f), consistent with markers of follicular DC (22), but were negative for DEC 205, CD11B, CD11C, and lymphocyte markers. At a higher dose of 104 IU, GFP-VRP-3533 infected cells that were positive for DEC 205, CD11B, and CD11C (data not shown), similar to VRP-3000 at 104 IU. These results demonstrate that at lower doses, the V3533 revertant has acquired the ability to infect a new subset of DC, but at higher doses, it infects a cell population comparable to that infected by the wild-type virus.
DISCUSSION
The initial events in the course of viral infection often determine the outcome and severity of viral disease. We have characterized the initial steps of VEE spread and pathogenesis from the s.c. site of inoculation to the draining lymph node. Through the use of propagation-defective VEE vectors, we have demonstrated that Langerhans cells, DC of the skin, were the first cells infected via this route of inoculation. Identical results were found when mice were inoculated with propagation-competent virus expressing GFP and were sacrificed at the earliest time when GFP could be detected. GFP expression in these cells unambiguously confirms a productive viral infection as opposed to antigen trapped on cell surfaces. While it is possible that DC have taken up GFP via phagocytosis of other infected cell types, both the uniform distribution of GFP in the cytoplasm and the limited phagocytic characteristics of lymph node DC argue against this.
At later time points, virus spreads to DC that are strongly positive for DEC 205 in the paracortex of the draining lymph node. DEC 205 is typically associated with interdigitating DC, a subset of DC which is associated with the T-cell-rich regions of the deep cortex of lymph nodes (29). The presence of virally infected cells that are DEC 205 positive in the subcortical region between B-cell follicles may be a direct consequence of VEE infection or may represent a distinct subset of DC which share this marker with interdigitating DC. The cofractionation of GFP-positive cells in the DC fraction of lymph nodes further indicates that DC, as opposed to lymphocytes and macrophages, represent the major target of infection in these tissues.
The targeting of VEE to both Langerhans cells and DC is consistent with data indicating that Langerhans cells differentiate into DC when they migrate from the skin to the lymph node. Langerhans cells are the resident DC of the skin, where they act as scavengers or sentinels for foreign antigens (1, 31, 33). These cells are nonmobile and express low levels of MHC class II and DEC 205. Upon activation by antigen uptake or by cytokines such as tumor necrosis factor alpha, they differentiate into mobile “veiled cells” and migrate through the lymph to the draining lymph node. In the lymph node, they further differentiate into potent antigen-presenting cells which express high levels of MHC class II and DEC 205 (1, 8, 9, 29, 31, 33). Langerhans cells infected with VRP appeared to have retained the characteristics of skin Langerhans cells in the lymph node without further maturation, even over the 5-day period during which they can be detected in the node. Alphaviruses inhibit host protein synthesis in cultured cells 4 to 6 h after infection (30). It is possible that VRP infection of Langerhans cells perturbed cellular protein synthesis to the extent that the maturation of these cells in the lymph node was halted at an intermediate differentiation state. If this is the case, VEE may provide a valuable tool in studying Langerhans cell differentiation in vivo and in vitro.
Although a number of viruses are able to infect DC in vitro, a limited number of organisms have actually been demonstrated to infect DC in vivo. These include human immunodeficiency virus (13, 28), lymphocytic choriomeningitis virus (LCMV) (3), African swine fever virus (16), adenovirus (15), murine retrovirus SL3-3 (32), and the protozoan parasite Leishmania major (24). In the case of adenovirus, human immunodeficiency virus, and L. major, cell types other than DC represent additional significant targets of infection. In LCMV, however, a mutation introduced into the Armstrong strain (clone 13) shifts cell tropism from macrophages primarily to DC in the spleen.
Pathogen infection of DC, as described in several systems, can promote potent antipathogen immunity due to efficient antigen presentation and/or immunosuppression due to impairment or depletion of infected DC (2). Persistent L. major infection of DC is correlated with healing of lesions, lifelong immunity, and resistance to reinfection (24), whereas the shift in cell tropism in the LCMV clone 13 results in a broad immunosuppression due to the depletion of infected DC (3). Measles virus infection of DC in vitro results in a loss of antigen-presenting capacity and has been proposed as a major cause of the transient immunosuppression seen following measles virus infections in vivo (2, 20). As with L. major, VEE infection in mice or humans is not associated with immunosuppression. Virulent and attenuated clones of VEE, as well as VEE vaccine vectors expressing specific antigens, are strongly immunogenic (4, 5, 10, 21, 27).
Time course studies of the disappearance of infected cells in the footpad and the appearance of infected cells in the lymph node suggest that VEE-infected Langerhans cells in the skin rapidly migrate to the draining lymph node, where they seed the infection of other DC. A similar series of steps has been described for L. major, where infected Langerhans cells from the skin traffic parasites to the draining lymph node (25). The studies reported here, however, cannot distinguish between Langerhans cells becoming infected in the skin and migrating to the lymph node and the possibility that Langerhans cells constantly circulate to the node and become infected by free virus carried by the lymph. Recent studies indicate a size restriction of particle movement into the paracortex of the lymph node, with large molecules and particles the size of VEE being shunted directly to areas proximal to high endothelial venules (17). VEE infection of Langerhans cells may provide a critical mechanism providing access of the virus to the region of the lymph node where subsequent rounds of infection occur. In addition, it is possible that migration of infected DC plays an important role in trafficking the virus to other lymphoid tissues.
Results of studies with the mutated V3010 and its cloned revertant, V3533, further illustrate the importance of Langerhans cells in the spread of VEE. The mutation in V3010 abrogated the appearance of infected Langerhans cells in the draining lymph node, whereas the suppressor mutation in V3533 restored the ability of virus to infect DC in vivo. These results are strongly correlated with the loss (V3010) and subsequent recovery (V3533) of viral spread and partial virulence. In addition, these mutations identify the E2 glycoprotein as a primary determinant of VEE cell tropism in vivo. Because the replicon genomes of VRP lack the glycoprotein genes, it is highly likely that the mutations exact their targeting effects at the level of viral spread, attachment to a target cell, penetration, and/or uncoating.
In summary, the results of these experiments reveal several new aspects of VEE pathogenesis. First, the initial site of virus replication is not at the site of inoculation but, rather, in the lymph node draining the inoculation site. Second, the initial cells infected by VEE are Langerhans cells, either in the lymph node or, more likely, at the site of the inoculation, from where they migrate rapidly to the draining lymph node. Finally, the specificity of this process is controlled by the VEE E2 glycoprotein, since it functions in early interactions with target cells in vivo. These observations were made possible by the genetic manipulation of VEE to yield wild-type and mutant virions capable of only a single cycle of replication, thus unequivocally identifying the earliest pathogenic events following inoculation.
ACKNOWLEDGMENTS
This research was supported by grants NS26681 and A122186 from the Public Health Service and by the U.S. Army Research and Development Command under contract DAMD17-94-J-4430. G.H.M. gratefully acknowledges support from National Research Service Award NIH F32-AI09778.
We acknowledge the excellent technical support of Cherice Connor, Jacque Bailey, and Dwayne Muhammed.
REFERENCES
- 1.Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Bhardwaj N. Interactions of viruses with dendritic cells: a double-edged sword. J Exp Med. 1997;186:795–799. doi: 10.1084/jem.186.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borrow P, Evans C F, Oldstone M B. Virus-induced immunosuppression: immune system-mediated destruction of virus-infected dendritic cells results in generalized immune suppression. J Virol. 1995;69:1059–1070. doi: 10.1128/jvi.69.2.1059-1070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caley I J, Betts M R, Irlbeck D M, Davis N L, Swanstrom R, Frelinger J A, Johnston R E. Humoral, mucosal, and cellular immunity in response to a human immunodeficiency virus type 1 immunogen expressed by a Venezuelan equine encephalitis virus vaccine vector. J Virol. 1997;71:3031–3038. doi: 10.1128/jvi.71.4.3031-3038.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charles P C, Brown K W, Davis N L, Hart M K, Johnston R E. Mucosal immunity induced by parenteral immunization with a live attenuated Venezuelan equine encephalitis virus vaccine candidate. Virology. 1997;228:153–160. doi: 10.1006/viro.1996.8381. [DOI] [PubMed] [Google Scholar]
- 6.Charles P C, Walters E, Margolis F, Johnston R E. Mechanism of neuroinvasion of Venezuelan equine encephalitis virus in the mouse. Virology. 1995;208:662–671. doi: 10.1006/viro.1995.1197. [DOI] [PubMed] [Google Scholar]
- 7.Cormack B P, Valdivia R H, Falkow S. FACS-optimized mutants of the green fluorescent protein (GFP) Gene. 1996;173:33–38. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- 8.Cumberbatch M, Kimber I. Dermal tumour necrosis factor-alpha induces dendritic cell migration to draining lymph nodes, and possibly provides one stimulus for Langerhans' cell migration. Immunology. 1992;75:257–263. [PMC free article] [PubMed] [Google Scholar]
- 9.Cumberbatch M, Kimber I. Tumour necrosis factor-alpha is required for accumulation of dendritic cells in draining lymph nodes and for optimal contact sensitization. Immunology. 1995;84:31–35. [PMC free article] [PubMed] [Google Scholar]
- 10.Davis N L, Brown K W, Johnston R E. A viral vaccine vector that expresses foreign genes in lymph nodes and protects against mucosal challenge. J Virol. 1996;70:3781–3787. doi: 10.1128/jvi.70.6.3781-3787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis N L, Powell N, Greenwald G F, Willis L V, Johnson B J, Smith J F, Johnston R E. Attenuating mutations in the E2 glycoprotein gene of Venezuelan equine encephalitis virus: construction of single and multiple mutants in a full-length cDNA clone. Virology. 1991;183:20–31. doi: 10.1016/0042-6822(91)90114-q. [DOI] [PubMed] [Google Scholar]
- 12.Davis N L, Willis L V, Smith J F, Johnston R E. In vitro synthesis of infectious Venezuelan equine encephalitis virus RNA from a cDNA clone: analysis of a viable deletion mutant. Virology. 1989;171:189–204. doi: 10.1016/0042-6822(89)90526-6. [DOI] [PubMed] [Google Scholar]
- 13.Frankel S S, Wenig B M, Burke A P, Mannan P, Thompson L D, Abbondanzo S L, Nelson A M, Pope M, Steinman R M. Replication of HIV-1 in dendritic cell-derived syncytia at the mucosal surface of the adenoid. Science. 1996;272:115–117. doi: 10.1126/science.272.5258.115. [DOI] [PubMed] [Google Scholar]
- 14.Gleiser C A, Gochenour W S, Jr, Berge T O, Tigertt W D. The comparative pathology of experimental Venezuelan equine encephalomyelitis virus infection in different animal hosts. J Infect Dis. 1961;110:80–97. doi: 10.1093/infdis/110.1.80. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Villamandos J C, Bautista M J, Carrasco L, Hervas J, Sierra M A. Electron microscopic evidence for infection of splenic dendritic cells by adenovirus in psittacine birds. Res Virol. 1995;146:389–395. doi: 10.1016/0923-2516(96)80898-3. [DOI] [PubMed] [Google Scholar]
- 16.Gregg D A, Mebus C A, Schlafer D H. Early infection of interdigitating dendritic cells in the pig lymph node with African swine fever viruses of high and low virulence: immunohistochemical and ultrastructural studies. J Vet Diagn Investig. 1995;7:23–30. doi: 10.1177/104063879500700104. [DOI] [PubMed] [Google Scholar]
- 17.Gretz J E, Anderson A O, Shaw S. Cords, channels, corridors and conduits: critical architectural elements facilitating cell interactions in the lymph node cortex. Immunol Rev. 1997;156:11–24. doi: 10.1111/j.1600-065x.1997.tb00955.x. [DOI] [PubMed] [Google Scholar]
- 18.Grieder F B, Davis N L, Aronson J F, Charles P C, Sellon D C, Suzuki K, Johnston R E. Specific restrictions in the progression of Venezuelan equine encephalitis virus-induced disease resulting from single amino acid changes in the glycoproteins. Virology. 1995;206:994–1006. doi: 10.1006/viro.1995.1022. [DOI] [PubMed] [Google Scholar]
- 19.Groot H. The health and economic impact of Venezuelan equine encephalitis (VEE) Pan Am Health Org Sci Publ. 1972;243:7–16. [Google Scholar]
- 20.Grosjean I, Caux C, Bella C, Berger I, Wild F, Banchereau J, Kaiserlian D. Measles virus infects human dendritic cells and blocks their allostimulatory properties for CD4+ T cells. J Exp Med. 1997;186:801–812. doi: 10.1084/jem.186.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hevey M, Negley D, Pushko P, Smith J, Schmaljohn A. Marburg virus vaccines based upon alphavirus replicons protect guinea pigs and nonhuman primates. Virology. 1998;251:28–37. doi: 10.1006/viro.1998.9367. [DOI] [PubMed] [Google Scholar]
- 22.Imai Y, Yamakawa M. Morphology, function and pathology of follicular dendritic cells. Pathol Int. 1996;46:807–833. doi: 10.1111/j.1440-1827.1996.tb03555.x. [DOI] [PubMed] [Google Scholar]
- 23.Jackson A C, SenGupta S K, Smith J F. Pathogenesis of Venezuelan equine encephalitis virus infection in mice and hamsters. Vet Pathol. 1991;28:410–418. doi: 10.1177/030098589102800509. [DOI] [PubMed] [Google Scholar]
- 24.Moll H, Flohe S, Rollinghoff M. Dendritic cells in Leishmania major—immune mice harbor persistent parasites and mediate an antigen-specific T cell immune response. Eur J Immunol. 1995;25:693–699. doi: 10.1002/eji.1830250310. [DOI] [PubMed] [Google Scholar]
- 25.Moll H, Fuchs H, Blank C, Rollinghoff M. Langerhans cells transport Leishmania major from the infected skin to the draining lymph node for presentation to antigen-specific T cells. Eur J Immunol. 1993;23:1595–1601. doi: 10.1002/eji.1830230730. [DOI] [PubMed] [Google Scholar]
- 26.Olmsted R A, Meyer W J, Johnston R E. Characterization of Sindbis virus epitopes important for penetration in cell culture and pathogenesis in animals. Virology. 1986;148:245–254. doi: 10.1016/0042-6822(86)90322-3. [DOI] [PubMed] [Google Scholar]
- 27.Pushko P, Parker M, Ludwig G V, Davis N L, Johnston R E, Smith J F. Replicon-helper systems from attenuated Venezuelan equine encephalitis virus: expression of heterologous genes in vitro and immunization against heterologous pathogens in vivo. Virology. 1997;239:389–401. doi: 10.1006/viro.1997.8878. [DOI] [PubMed] [Google Scholar]
- 28.Rappersberger K, Gartner S, Schenk P, Stingl G, Groh V, Tschachler E, Mann D L, Wolff K, Konrad K, Popovic M. Langerhans' cells are an actual site of HIV-1 replication. Intervirology. 1988;29:185–194. doi: 10.1159/000150045. [DOI] [PubMed] [Google Scholar]
- 29.Steinman R M, Pack M, Inaba K. Dendritic cells in the T-cell areas of lymphoid organs. Immunol Rev. 1997;156:25–37. doi: 10.1111/j.1600-065x.1997.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 30.Strauss J H, Strauss E G. The alphaviruses: gene expression, replication, and evolution. Microbiol Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Udey M C. Cadherins and Langerhans cell immunobiology. Clini Exp Immunol. 1997;107(Suppl. 1):6–8. [PubMed] [Google Scholar]
- 32.Uittenbogaart C H, Law W, Leenen P J, Bristol G, van Ewijk W, Hays E F. Thymic dendritic cells are primary targets for the oncogenic virus SL3-3. J Virol. 1998;72:10118–10125. doi: 10.1128/jvi.72.12.10118-10125.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Wilsem E J, Breve J, Kleijmeer M, Kraal G. Antigen-bearing Langerhans cells in skin draining lymph nodes: phenotype and kinetics of migration. J Investig Dermatol. 1994;103:217–220. doi: 10.1111/1523-1747.ep12393088. [DOI] [PubMed] [Google Scholar]
- 34.Walker D H, Harrison A, Murphy K, Flemister M, Murphy F A. Lymphoreticular and myeloid pathogenesis of Venezuelan equine encephalitis in hamsters. Am J Pathol. 1976;84:351–370. [PMC free article] [PubMed] [Google Scholar]
- 35.Weaver S C, Salas R, Rico-Hesse R, Ludwig G V, Oberste M S, Boshell J, Tesh R B. Re-emergence of epidemic Venezuelan equine encephalomyelitis in South America. VEE Study Group. Lancet. 1996;348:436–440. doi: 10.1016/s0140-6736(96)02275-1. [DOI] [PubMed] [Google Scholar]