Abstract
Background:
In some hidradenitis suppurativa (HS) clinical trial study arms, there is an unexpected decline in efficacy between the penultimate visit and the prespecified primary endpoint week, which we have termed a “wobble.”
Objective:
We aimed to establish how often study arms in HS programs wobble.
Methods:
In a retrospective review, we identified HS clinical trials listed on ClinicalTrials.gov testing systemic, nonantibiotic medications that utilized Hidradenitis Suppurativa Clinical Response (HiSCR) as an outcome measure. We identified study arms demonstrating greater improvement in a visit prior to the primary endpoint week. Baseline subject characteristics were compared between studies with HiSCR wobble and no HiSCR wobble.
Results:
A total of 21 studies (randomized control trial [RCT], n = 14; open-label, n = 7) with 35 study drug arms (RCT, n = 27; open-label, n = 8) and 14 placebo arms were identified. HiSCR wobble occurred significantly more often in RCT compared to open-label study drug arms (11/27 [40.7%] vs 0/8 [0%]). In RCT study arms with HiSCR wobble, baseline draining fistula counts were significantly lower (2.3 vs 3.2), and numerically fewer Hurley stage 3 patients (33.2% vs 42.5%), lower weighted total abscess and nodule counts (12.1 vs 12.6), lower weighted dermatology life quality index scores (12.5 vs 14.5), and a higher proportion of female patients (63.9% vs 58.3%) were observed.
Limitations:
Include low number of HS clinical trials and insufficient data reported in many studies to assess for wobble, degree of wobble, and to compare all baseline characteristics.
Conclusion:
Nonlinear improvement in study arm response occurs in some HS RCTs. Potential contributing factors include a higher proportion of less severe patients at baseline and more female patients.
Keywords: acne inversa, biological products, clinical trial protocol, hidradenitis suppurativa, randomized controlled trial
What is known about this subject in regard to women and their families?
In the United States, hidradenitis suppurativa is diagnosed approximately 3 times more often in women than men and can often affect multiple family members.
Hidradenitis suppurativa is an incredibly difficult condition to treat with currently only one Food and Drug Administration-approved treatment. Several clinical trials are ongoing to increase effective treatment options.
What is new from this article as messages for women and their families?
This article uncovers nonlinear improvements found in hidradenitis suppurativa clinical trials, explores factors that may play a role, and suggests some strategies to help reduce this unexpected data outcome in future trials.
Introduction
Hidradenitis suppurativa (HS) is a chronic debilitating skin disorder characterized by recurrent inflammatory nodules, abscesses, fistulas, and scars.1 Adalimumab (a tumor necrosis factor-alpha inhibitor) was approved by the Food and Drug Administration in 2015.2,3 Over the past several years, clinical trials for HS increased dramatically, leading to the approval of secukinumab by the European Commission in 2023.4 HS clinical trials typically utilize a placebo-controlled design using the Hidradenitis Suppurativa Clinical Response (HiSCR or HiSCR 50), at least a 50% reduction in total abscess and nodule (AN) count with no increase in abscess or draining fistula (DF) count, as the primary efficacy endpoint at either 12 or 16 weeks.2
We have observed that in some HS studies, there is an unexpected declining efficacy between the penultimate visit and the prespecified primary endpoint week. For example, adalimumab’s phase 2 study used a primary endpoint of 16 weeks, but both study drug arms demonstrated a higher HiSCR response at week 12.2 Subsequently, adalimumab’s phase 3 study used a primary endpoint week of 12 with maximum HiSCR response at week 12.3 In this study, we established how often nonlinear improvement occurs in HS programs and explored possible trial design and data analysis implications.
Methods
A retrospective review of randomized control trials (RCTs) and open-label studies of systemic, nonantibiotic medications for HS on ClinicalTrials.gov as of March 27, 2023, was performed. Study results were obtained from ClinicalTrials.gov and augmented with press releases and dermatology conference presentations. Inclusion criteria included the employment of HiSCR as an outcome measure and the availability of HiSCR data at the primary endpoint week and at least one additional time point prior to the primary endpoint week. Studies were excluded if they included conditions other than HS, had a primary endpoint week before 12 weeks, did not investigate a systemic medication, or were not an RCT or open-label study.
Data collected included baseline patient characteristics, HiSCR response rates, dermatology life quality index (DLQI) scores, HS pain, and the International HS severity score system (IHS4). In RCT studies, study drug arms were considered “successful” if the HiSCR response at the primary endpoint week was significant compared with placebo or “failed” if found nonsignificant. The maximum value for each outcome measure was assessed through week 16 by comparing values reported in tables or text, if available, or peak graphical points. A “wobble” was defined as outcome measure efficacy that peaked prior to the primary endpoint week.
The proportions of study drug arms that displayed a wobble were compared using Fisher exact test in RStudio (2022.07.01, Boston, Massachusetts). Significance was set to a P < .05. Means, weighted based on sample size, were calculated for baseline characteristics, Hurley stage, AN count, total DF count, pain scores, DLQI scores, and proportion of females, in study drug arms and compared between groups with HiSCR wobble and no HiSCR wobble using a Mann–Whitney U test in Microsoft Excel 2016 (16.0.5378.1000). Study arms that reported HiSCR response data beyond the primary endpoint were also assessed for the timing of maximum response. The proportion of study drug arms that displayed a wobble in quality of life (QoL) measures (DLQI and pain scores) and IHS4 was also assessed.
Results
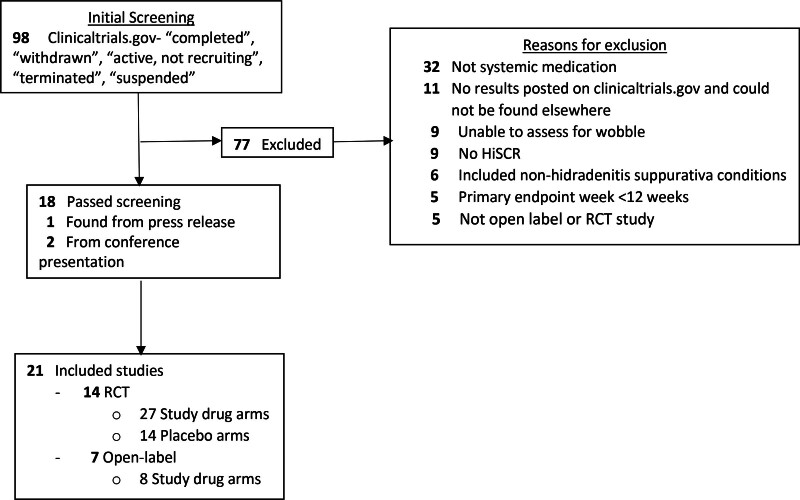
A total of 21 studies (RCT, n = 14; open-label, n = 7), with 35 study drug arms (RCT, n = 27; open-label, n = 8) and 14 placebo arms, were included (Table 1; Fig. 1).2–19 Among RCTs, 9 studies (21 study drug arms, 9 placebo arms) designated the primary endpoint at week 16, and 5 studies (6 study drug arms, 5 placebo arms) designated at week 12 (Table 1). Significance tests/designation as success or failure was not performed for the adalimumab arm in Glatt et al., since it was used as a comparator arm and not designed for statistical testing. In total, 14 study drug arms were considered successful and 12 failed.
Table 1.
Randomized control trial study arm baseline characteristics, wobble data, and HiSCR at weeks 12 and 16
| Author/sponsor | Drug name | Dosing | n | Age, mean (SD) | F, % | Duration years, mean (SD) | Hurley 1, 2, 3; (%) | BL AN count, mean (SD) | BL DF count, mean (SD) | BL pain, mean (SD) | BL DLQI, mean (SD) | Wobble-peak week | %HiSCR week 12 | %HiSCR week 16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glatt et al., 20217 | Bimekizumab | 320 mg Q2Wa | 46 | 37.4 (11.9) | 65.2 | 9 (8.8) | 0, 50, 50 | 14.5 (11.9) | - | 3.7 (2.4) | 11.7 (8) | Yes-10 | 62.5b | - |
| Glatt et al. 20217 | Adalimumab | 40 mg Q2Wa | 21 | 31.1 (9.4) | 81 | 8.6 (5.7) | 0, 48, 52 | 20 (11.5) | - | 5.8 (2.7) | 14.5 (7.9) | No | 66.7 | - |
| Glatt et al. 20217 | Placebo | Placebo | 21 | 40.7 (12.8) | 67 | 9.5 (8.4) | 0, 48, 52 | 12.7 (5.7) | - | 5.6 (2.7) | 12.7 (5.7) | Yes-4 | 27.8 | - |
| InflaRx GmbH 20198 | IFX-1 | 400 mg Q4W | 34 | 39 (-) | 47.1 | - | - | 9 (-) | - | - | - | Yes-12 | 45.2 | 40 |
| InflaRx GmbH 20198 | IFX-1 | 800 mg Q4W | 35 | 35 (-) | 51.4 | - | - | 8 (-) | - | - | - | No | 36.4 | 51.5 |
| InflaRx GmbH 20198 | IFX-1 | 800 mg Q2W | 36 | 37 (-) | 55.6 | - | - | 10 (-) | - | - | - | Yes-12 | 40.6 | 38.7 |
| InflaRx GmbH 20198 | IFX-1 | 1200 mg Q2W | 36 | 33.5 (-) | 63.9 | - | - | 12.5 (-) | - | - | - | No | 40.6 | 45.5 |
| InflaRx GmbH 20198 | Placebo | Placebo | 36 | 34.5 (-) | 58.3 | - | - | 9.5 (-) | - | - | - | No | 41.2 | 47.1 |
| Bechara et al. 20219 | Adalimumab | 40 mg Q1Wa | 103 | 38.5 (11.71) | 49.5 | - | 0, 51, 49 | 10.3 (7.5) | 3.6 (4) | - | 13.6 (7.3) | No | 48b | - |
| Bechara et al. 20219 | Placebo | Placebo | 103 | 36.8 (10.81) | 53.4 | - | 0, 52, 49 | 11.3 (12.6) | 4 (5.4) | - | 12.9 (7.1) | No | 34 | - |
| Kimball et al. 20162 | Adalimumab | 40 mg Q2Wa | 45 | 36.1 (12.77) | 73.3 | - | 15.6, 55.6, 28.9 | - | - | - | - | Yes-8 | 35.6 | 33.3b |
| Kimball et al. 20162 | Adalimumab | 40 mg Q1Wa | 44 | 36.6 (10.68) | 70.5 | 12.1 (9.34) | 18.2, 56.8, 25 | - | - | - | - | Yes-12 | 59.1 | 54.5b |
| Kimball et al. 20162 | Placebo | Placebo | 43 | 37.7 (12.01) | 67.4 | 13.3 (9.53) | 14, 55.8, 30.2 | - | - | - | - | Yes-8 | 16.3 | 25.6 |
| Kimball et al. 20163 | Adalimumab | 40 mg Q1Wa | 153 | 36.2 (10.8) | 59.5 | 8.8 (-) | 0, 52.3, 47.7 | 14.3 (11.9) | 4.6 (5.2) | 6 (1.8) | 16.3 (6.6) | No | 41.8b | - |
| Kimball et al. 20163 | Placebo | Placebo | 154 | 37.8 (11.3) | 68.2 | 9.4 (-) | 0, 52.6, 47.4 | 14.4 (14.8) | 3.8 (4.4) | 6 (2) | 16 (7.1) | No | 26 | - |
| Kimball et al. 20163 | Adalimumab | 40 mg Q1Wa | 163 | 34.9 (10) | 66.3 | 9 (-) | 0, 52.8, 47.2 | 10.7 (8.1) | 3 (4.1) | 5.7 (1.9) | 14.1 (7.7) | No | 58.9b | - |
| Kimball et al. 20163 | Placebo | Placebo | 163 | 36.1 (12.2) | 69.3 | 9.9 (-) | 0, 54.6, 45.4 | 11.9 (11) | 3.7 (5.2) | 6.2 (1.9) | 14.9 (7.3) | No | 27.6 | - |
| Kimball et al. 20234 | Secukinumab | 300 mg Q4Wa | 180 | 35.5 (11.4) | 57 | 8.2 (8.4) | 3, 59, 38 | 13.3 (8.8) | 2.5 (3.5) | 4.6 (2.5) | - | Yes-12 | 51 | 46.1b |
| Kimball et al. 20234 | Secukinumab | 300 mg Q2Wa | 180 | 37.3 (11.5) | 54 | 7.1 (7) | 3, 51, 46 | 13.9 (9.9) | 3 (3.6) | 4.8 (2.4) | - | No | 39.3 | 42.3b |
| Kimball et al. 20234 | Placebo | Placebo | 183 | 36.2 (11.3) | 57 | 7 (6.7) | 2, 60, 38 | 12.8 (8.5) | 2.6 (3.2) | 4.7 (2.4) | - | No | 24.7 | 31.2 |
| Kimball et al. 20234 | Secukinumab | 300 mg Q4Wa | 180 | 35.7 (11.7) | 56 | 6.6 (6.7) | 6, 59, 35 | 12.6 (8.4) | 2.5 (3.5) | 4.2 (2.5) | - | No | 39.5 | 41.8 |
| Kimball et al. 20234 | Secukinumab | 300 mg Q2Wa | 181 | 37.1 (12.5) | 56 | 7.4 (8) | 4, 58, 39 | 12.9 (9.6) | 2.9 (3.4) | 4.5 (2.5) | - | No | 43.3 | 45b |
| Kimball et al. 20234 | Placebo | Placebo | 180 | 35.5 (10.8) | 57 | 7.5 (7) | 4, 67, 28 | 12.8 (8.2) | 2.4 (3.2) | 4.3 (2.5) | - | No | 28.6 | 33.7 |
| Kimball et al. 20245 | Brepocitinib | 45 mg QD | 52 | - | - | - | - | - | - | - | - | No | - | 51.9b |
| Kimball et al. 20245 | Zimlovisertib | 400 mg QD | 47 | - | - | - | - | - | - | - | - | Yes-4 | - | 34 |
| Kimball et al. 20245 | Ropsacitinib | 400 mg QD | 47 | - | - | - | - | - | - | - | - | Yes-6 | - | 37 |
| Kimball et al. 20245 | Placebo | Placebo | 48 | - | - | - | - | - | - | - | - | Yes-8 | - | 33.3 |
| Kirby et al. 20246 | Povorcitinib | 15 mg QD | 52 | 36.5 (-) | 71.2 | 9.9 (8.1) | 5.8, 71.2, 23.1 | 11.8 (7.1) | 2.4 (4.4) | 4.6 (2.4) | 11.2 (7.1) | No | 42.3 | 48.1 |
| Kirby et al. 20246 | Povorcitinib | 45 mg QD | 52 | 35 (-) | 75 | 11.2 (11.5) | 7.7, 69.2, 23.1 | 12.9 (12.3) | 2.2 (4) | 5.1 (2.3) | 13 (7.6) | Yes-6,12 | 48.1 | 44.2 |
| Kirby et al. 20246 | Povorcitinib | 75 mg QD | 53 | 38 (-) | 73.6 | 12.1 (9.7) | 7.5, 69.8, 22.6 | 10.6 (7.2) | 1.6 (2.9) | 5.2 (2.7) | 12.1 (7.3) | Yes-12 | 58.5 | 45.3 |
| Kirby et al. 20246 | Placebo | Placebo | 52 | 33.5 (-) | 82.7 | 8.1 (6.5) | 7.7, 69.2, 23.1 | 11.2 (5.9) | 2.4 (4) | 5.4 (2.8) | 12.7 (7.3) | Yes-12 | 30.8 | 28.8 |
| Tzanetakou et al. 201610 | Anakinra | 100 mg QD | 9 | 42.8 (13.8) | 44.4 | 12.3 (6.7) | 0, 66.6, 33.3 | - | 3 (-) | 54.4 (22.9)c | 20.7 (5.9) | No | 77.8b | - |
| Tzanetakou et al. 201610 | Placebo | Placebo | 10 | 36 (11.3) | 50 | 11.1 (6.8) | 0, 40, 60 | - | 2 (-) | 60.5 (21.7)c | 14.3 (8.4) | No | 30 | - |
| Vossen et al. 201911 | Apremilast | 30 mg BID | 15 | 35.7 (13) | 80 | 21.6 (13) | - | 6.1 (1.7) | - | 6.4 (2.4) | 14.6 (7.6) | Yes-2 | 53.3 | 53.3 |
| Vossen et al. 201911 | Placebo | Placebo | 5 | 33.4 (8.2) | 100 | 16 (7.1) | - | 5.8 (2.4) | - | 5.8 (2.2) | 11.8 (5.9) | Yes-2 | 0 | 0 |
| UCB 202312 | Bimekizumab | 320 mg Q2W | 289 | - | - | - | - | - | - | - | - | Nod | - | 47.8b |
| UCB 202312 | Bimekizumab | 320 mg Q4W | 144 | - | - | - | - | - | - | - | - | Nod | - | 45.3 |
| UCB 202312 | Placebo | Placebo | 72 | - | - | - | - | - | - | - | - | Nod | - | 28.7 |
| UCB 202312 | Bimekizumab | 320 mg Q2W | 291 | - | - | - | - | - | - | - | - | Nod | - | 52b |
| UCB 202312 | Bimekizumab | 320 mg Q4W | 144 | - | - | - | - | - | - | - | - | Nod | - | 53.8b |
| UCB 202312 | Placebo | Placebo | 74 | - | - | - | - | - | - | - | - | Nod | - | 32.2 |
AN, total abscess and nodule count; BID, two times a day; BL, baseline; DF, draining fistula; DLQI, dermatology quality life index; F, female; HiSCR, Hidradenitis Suppurativa Clinical Response; Q2W, every 2 weeks; Q4W, every 4 weeks; QD, every day; SD, standard deviation.
Used a loading dose.
Found to be significant compared to placebo arm.
Baseline pain measured on a scale of 100 rather than 10.
Wobble data based on a graph that displayed HiSCR for all observed patients. HiSCR recorded in the table for this study arms based on patients treated with new antibiotics during the trial were considered nonresponders.
Fig. 1.
Inclusion/exclusion algorithm. HiSCR, Hidradenitis Suppurativa Clinical Response; RCT, randomized control trial.
HiSCR wobble occurred significantly more often in RCTs compared with open-label study drug arms (11/27 [40.7%] vs 0/8 [0%], respectively, P = .0292) (Table 2). Within RCTs, HiSCR wobble occurred significantly more often in study drug arms with sample sizes of less than 50 patients than in those with more than 50 patients (8/12 [66.7%] vs 3/15 [20%], respectively, P = .022). Among RCTs, HiSCR wobble occurred numerically more often in failed versus successful study drug arms (7/12 [58.3%] vs 4/14 [28.6%], respectively, P = .2329) and in those with a primary endpoint week of 16 versus 12 (10/21 [47.6%] vs 1/6 [16.7%], respectively, P = .3497). HiSCR wobble also occurred in 5 (35.7%) of the placebo arms (Table 2).
Table 2.
Wobble breakdown based on HiSCR
| Wobble | No wobble | P value/u value | |
|---|---|---|---|
| Open-label (n = 8), n (%) | 0 (0) | 8 (100) | .0292a |
| RCT | |||
| Placebo arms (n = 14), n (%) | 5 (35.7) | 9 (64.3) | |
| Study drug arms (n = 27)b, n (%) | 11 (40.7) | 13 (48.1) | |
| Successful (n = 14), n (%) | 4 (28.6) | 10 (71.4) | .2329 |
| Failed (n = 12), n (%) | 7 (58.3) | 5 (41.7) | |
| Primary endpoint week 12 (n = 6), n (%) | 1 (16.7) | 5 (83.3) | .3497 |
| Primary endpoint week 16 (n = 21), n (%) | 10 (47.6) | 11 (52.4) | |
| Sample size >50 (n = 15), n (%) | 3 (20) | 12 (80) | .022 |
| Sample size <50 (n = 12), n (%) | 8 (66.7) | 4 (33.3) | |
| Baseline characteristicsc | |||
| Hurley 1/2, mean % | 66.8 | 57.7 | 14.5 |
| Hurley 3, mean % | 33.2 | 42.5 | 13.5 |
| AN count, mean | 12.1 | 12.6 | 26.5 |
| DF count, mean | 2.3 | 3.2 | 1d |
| Pain score, mean | 4.7 | 5 | 18.5 |
| DLQI score, mean | 12.5 | 14.5 | 7 |
| Female, mean % | 63.9 | 58.3 | 31 |
AN, total abscess and nodule count; DF, draining fistula; DLQI, dermatology life quality index; HiSCR, Hidradenitis Suppurativa Clinical Response; RCT, randomized control trial.
Open-label compared to RCT study drug arms.
Total number of study drug arms is one greater than successful and failed trials because Glatt et al. contained an extra study drug arm that served as a comparator and no statistics were run for significance compared to the placebo arm.
Not all study drug arms reported baseline characteristics. For those that displayed a wobble: 6/11 supplied Hurley stage breakdown, 7/11 AN count, 3/11 DF, 5/11 pain score, 9/11 female percentage. For those that displayed no wobble: 9/16 supplied Hurley stage breakdown, 10/16 AN count, 8/16 DF, 8/16 pain score, 11/16 female percentage.
DF counts were found to be significantly different between the 2 groups.
Baseline DF counts were significantly lower in study drug arms with HiSCR wobble (2.3 vs 3.2, u = 1, P < .05). Study drug arms where HiSCR wobble occurred had less severe populations, that is, fewer Hurley stage 3 patients (33.2% vs 42.5%), lower weighted AN counts (12.1 vs 12.6), lower weighted pain scores (4.7 vs 5), lower weighted DLQI scores (12.5 vs 14.5), and a higher proportion of female patients (63.9% vs 58.3%) (Table 2).
Twelve of the RCT study drug arms across 7 trials reported HiSCR response data beyond their original primary endpoint week as part of an extension study. The majority of these extensions were not placebo-controlled (10/12 [83.3%]). Most study drug arms had greater HiSCR responses beyond their primary endpoint week (10/11 [90.9%]). Two of the study drug arms from a single study had their extension periods pooled together.
Fifteen study drug arms reported sufficient data to assess QoL measures for wobble including DLQI (n = 7) and pain scores (n = 9). DLQI wobble occurred in 2/7 (28.6%) of study arms, and pain score wobble occurred in 8/9 (88.9%) of study arms. QoL outcome measures reached maximum improvement earlier than maximum HiSCR response in 6 (40%) instances, at the same time in 5 (33.3%) instances, and after in 4 (26.7%) instances.
Six RCT study drug arms in 2 studies used the IHS4 with a primary endpoint week of 16, and half displayed an IHS4 wobble.5,6
Discussion
In these studies, nonlinear improvement or wobble was observed in drug and placebo arms in HS studies across multiple outcomes including HiSCR, IHS4, and patient-reported outcome measures. Factors associated with HiSCR wobble included smaller sample sizes, RCT study design, a primary endpoint week of 16, study drugs failing to demonstrate significance compared to their placebo arm, and studies including patients with lower baseline disease severity (lower DF counts and more Hurley 1/2 vs Hurley 3 patients) or female gender, although only the first 2 were significant.
The finding that study drug arms with less severe patients displayed greater HiSCR wobble could be due to the prior observation that less severe patients at baseline may be more susceptible to HS disease fluctuation and variability compared with patients with more severe disease at baseline. Indeed, Frew et al.20 demonstrated that increasing inclusion criteria to 7 nodules may decrease the placebo rate in HS studies by reducing variability; however, they did not recommend this approach due to the potential reduction in external validity of future trials. In our experience, folliculonodular disease, which may affect women more frequently, is characterized by relapsing/remitting primarily inflammatory nodules and papules sensitive to hormonal triggers. The higher proportion of female patients noted in study drug arms that displayed HiSCR wobble is consistent with the concept that hormonal fluctuations may contribute to disease flares and disease variability in trials with HiSCR wobble.1 Currently, disease triggers and clinically relevant HS phenotypes are not captured in HS clinical trials. Validation and subsequent incorporation of phenotypes in clinical trials, along with tracking of disease triggers such as dates of menses, could add further insight into the interpretation of placebo responses, HiSCR wobble, and differences in treatment response based on disease phenotype in HS trials. These data also reinforce the clinical concept that patients with HS can experience significant variability in their disease, even as their overall trajectory is improving. Long-term studies, especially, against active controls may help us better understand and predict their improvement course.
In contrast to clinical care, where providers often overlap therapies to avoid flaring disease, HS clinical trials require washout periods of systemic and topical treatments that often last from 4 to 12 weeks prior to enrollment. For biologics, this time frame is often 5 half-lives or 12 weeks, whichever is longer.2,3 Thus, patients enrolled into placebo arms of placebo-controlled trials may go without treatment for months, resulting in rebound, recurrence, and unknown impact on their long-term prognosis or disease progression. Because long washout periods can unpredictably disrupt stable moderate to severe disease in both placebo subjects and those on active therapy, we recommend reducing washout periods to no longer than 5 half-lives or 12 weeks, whichever is shorter. Since we found that a large proportion of study drug arms with primary endpoint at week 16 demonstrated HiSCR wobble, with many peaking at week 12, it may also be appropriate to limit placebo-controlled periods to 12 weeks or consider active-controlled arms, which would also allow for much longer controlled periods.
Limitations
Limitations include low number of HS clinical trials and insufficient/incomplete data reported in many studies to assess for wobble, degree of wobble, and to compare all baseline characteristics. Further, we were unable to assess if trials with primary endpoint week 12 would have met maximum response at a later week and the dataset was limited to trials listed on ClinicalTrials.gov.
Conclusion
Nonlinear improvement in study arm response occurs in HS RCTs across several outcome measures. Potential contributing factors include a higher proportion of less severe patients at baseline and more female patients, which could represent a particular HS phenotype that has more underlying variability. Study designers may wish to incorporate 12-week endpoints to help mitigate this problem, bearing in mind that some drugs may not have reached peak efficacy at this time.
Conflicts of interest
The authors made the following disclosures: A.B.K.’s institution received grants from Abbvie, Admirx, Anapyts Bio, Aristea, Bristol Myers Squibb, Chemocentryx, Eli Lilly, Incyte, Janssen, Moonlake, Novartis, Pfizer, Prometheus, UCB; Sonoma Bio. she received consulting fees from Abbvie, Alumis, Bayer, Bristol Myers Squibb, Boehringer Ingelheim, Eli Lilly, Janssen, Moonlake Novartis, Pfizer, Priovant, Sonoma Bio, Sanofi, UCB; Target RWE, Ventyx; and serves on the board of directors of Almirall. M.L.P. is a consultant and/or investigator for Abbvie, Alumis, Anaptys Bio, Aristea, Bayer, Bristol Meyers Squibb, Janssen, Eli Lilly, Moonlake, Novartis, Pfizer, Prometheus, Trifecta Clinical, UCB, Regeneron, Innovoderm, Bayer, Prometheus, and Incyte. S.X.C. is an investigator for Novartis, Moonlake, Prometheus, and UCB. R.S.G.’s fellowship was funded through the National Psoriasis Foundation. R.S.G. is an investigator for Abbvie, Janssen, Regeneron, Eli Lilly, Novartis, UCB, Aristea, Incyte, Innovoderm, Bayer, and Moonlake. C.L.S. declares no conflicts of interest.
Funding
None.
Study approval
N/A
Author contributions
CLS: Participated in research design, data collection, data analysis, writing of the manuscript, and manuscript review. RSG and SXC: Participated in writing of the manuscript and manuscript review. MLP and ABK: Participated in research design, writing of the manuscript, and manuscript review.
Footnotes
Published online 7 June 2024
References
- 1.Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol 2020;82:1045–58. [DOI] [PubMed] [Google Scholar]
- 2.Kimball AB, Sobell JM, Zouboulis CC, et al. HiSCR (Hidradenitis Suppurativa Clinical Response): a novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo-controlled portion of a phase 2 adalimumab study. J Eur Acad Dermatol Venereol 2016;30:989–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med 2016;375:422–34. [DOI] [PubMed] [Google Scholar]
- 4.Kimball AB, Jemec GBE, Alavi A, et al. Secukinumab in moderate-to-severe hidradenitis suppurativa (SUNSHINE and SUNRISE): week 16 and week 52 results of two identical, multicentre, randomised, placebo-controlled, double-blind phase 3 trials. Lancet 2023;401:747–61. [DOI] [PubMed] [Google Scholar]
- 5.Kimball AB, Peeva E, Forman S, et al. Brepocitinib, Zimlovisertib, and Ropsacitinib in Hidradenitis Suppurativa. NEJM Evid 2024;3:EVIDoa2300155. [DOI] [PubMed] [Google Scholar]
- 6.Kirby JS, Okun MM, Alavi A, et al. Efficacy and safety of the oral Janus kinase 1 inhibitor povorcitinib (INCB054707) in patients with hidradenitis suppurativa in a phase 2, randomized, double-blind, dose-ranging, placebo-controlled study. J Am Acad Dermatol 2024;90:521–529. [DOI] [PubMed] [Google Scholar]
- 7.Glatt S, Jemec GBE, Forman S, et al. Efficacy and safety of bimekizumab in moderate to severe hidradenitis suppurativa: a phase 2, double-blind, placebo-controlled randomized clinical trial. JAMA Dermatol 2021;157:1279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.InflaRx. InflaRx reports additional analysis of the SHINE phase IIb results for IFX-1 in hidradenitis suppurativa. 2019. Available from: https://www.inflarx.de/Home/Investors/Press-Releases/07-2019-InflaRx-Reports-Additional-Analysis-of-the-SHINE-Phase-IIb-Results-for-IFX-1-in-Hidradenitis-Suppurativa-.html [Google Scholar]
- 9.Bechara FG, Podda M, Prens EP, et al. Efficacy and safety of adalimumab in conjunction with surgery in moderate to severe hidradenitis suppurativa: the SHARPS randomized clinical trial. JAMA Surg 2021;156:1001–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol 2016;152:52–9. [DOI] [PubMed] [Google Scholar]
- 11.Vossen ARJV, van Doorn MBA, van der Zee HH, Prens EP. Apremilast for moderate hidradenitis suppurativa: results of a randomized controlled trial. J Am Acad Dermatol 2019;80:80–8. [DOI] [PubMed] [Google Scholar]
- 12.Kimball AB, Jemec GBE, Sayed CJ, et al. Efficacy and safety of bimekizumab in patients with moderate-to-severe hidradenitis suppurativa (BE HEARD I and BE HEARD II): two 48-week, randomised, double-blind, placebo-controlled, multicentre phase 3 trials. The Lancet. Published online May 22, 2024. doi: 10.1016/S0140-6736(24)00101-6. [DOI] [PubMed] [Google Scholar]
- 13.Frew JW, Navrazhina K, Sullivan-Whalen M, Gilleaudeau P, Garcet S, Krueger JG. Weekly administration of brodalumab in hidradenitis suppurativa: an open-label cohort study. Br J Dermatol 2021;184:350–2. [DOI] [PubMed] [Google Scholar]
- 14.Frew JW, Navrazhina K, Grand D, et al. The effect of subcutaneous brodalumab on clinical disease activity in hidradenitis suppurativa: an open-label cohort study. J Am Acad Dermatol 2020;83:1341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morita A, Takahashi H, Ozawa K, et al. Long-term analysis of adalimumab in Japanese patients with moderate to severe hidradenitis suppurativa: open-label phase 3 results. J Dermatol 2021;48:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blok JL, Li K, Brodmerkel C, Horvátovich P, Jonkman MF, Horváth B. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. Br J Dermatol 2016;174:839–46. [DOI] [PubMed] [Google Scholar]
- 17.Casseres RG, Prussick L, Zancanaro P, et al. Secukinumab in the treatment of moderate to severe hidradenitis suppurativa: results of an open-label trial. J Am Acad Dermatol 2020;82:1524–6. [DOI] [PubMed] [Google Scholar]
- 18.Prussick L, Rothstein B, Joshipura D, et al. Open-label, investigator-initiated, single-site exploratory trial evaluating secukinumab, an anti-interleukin-17A monoclonal antibody, for patients with moderate-to-severe hidradenitis suppurativa. Br J Dermatol 2019;181:609–11. [DOI] [PubMed] [Google Scholar]
- 19.Gottlieb A, Natsis NE, Kerdel F, et al. A phase II open-label study of bermekimab in patients with hidradenitis suppurativa shows resolution of inflammatory lesions and pain. J Invest Dermatol 2020;140:1538–45.e2. [DOI] [PubMed] [Google Scholar]
- 20.Frew JW, Jiang CS, Singh N, Navrazhina K, Vaughan R, Krueger JG. Quantifying the natural variation in lesion counts over time in untreated hidradenitis suppurativa: implications for outcome measures and trial design. JAAD Int 2020;1:208–21. [DOI] [PMC free article] [PubMed] [Google Scholar]