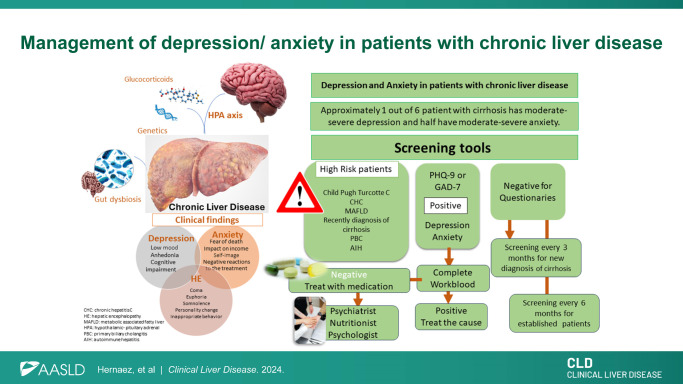
Cirrhosis, depression, and anxiety often co-exist in our patient population. Approximately 1 in 6 patients with cirrhosis have moderately severe to severe depression, and half have moderate severe anxiety.1 Cross-sectional studies have shown a positive, independent association between chronic liver diseases and depression. For example, data from the National Health and Nutrition Examination Surveys (2005–2010) demonstrated a positive association of depression with chronic hepatitis C (OR 2.87, 95% CI: 1.78–4.62).2 In the 11,877 National Health and Nutrition Examination Survey participants (2007–2016) with metabolic dysfunction–associated steatotic liver disease, the weighted prevalence of depression was 11.3% (95% CI: 10.5–12.3).3 Gungabissoon et al found in patients with primary biliary cholangitis a prevalence of 21.3% of depression and a 28.1% of anxiety in the US IBM MarketScan Commercial Claims and Medicare Supplemental Database during 2016.4 As expected, diagnosis of cirrhosis was associated with increased incidence of depression in cohort studies: Seo and Yoo, using a claims database in Korea, that the first 3 months nearly doubled the incidence of depression (incidence rate ratio of 1.24 before the diagnosis and 2.12 after diagnosis, p<0.001).5 What are the risk factors for depression/anxiety among those patients with cirrhosis? We recently provided a glimpse of an answer. In a baseline survey of 1021 patients with compensated cirrhosis across 3 US institutions, we showed that self-reported poor health, being widowed, fear of HCC, higher household income, and Hispanic ethnicity were associated with moderately severe to severe depression. Further, male sex, self-reported poor health, and fear of HCC were associated with high anxiety.1 Understanding how to assess and manage such conditions in patients with cirrhosis can improve our patient’s quality of life and adherence to medical treatment. A preliminary assessment and screening for depression/anxiety should be incorporated into hepatologist approach to care.
PATHOPHYSIOLOGY OF DEPRESSION AND ANXIETY IN PATIENTS WITH CHRONIC LIVER DISEASE
Depression and anxiety are more common among individuals with liver disease and cirrhosis compared to the general population.1,6 Depression and anxiety are major contributors to increased morbidity and mortality, reduced health-related quality of life, and decreased functional status.7
Inflammatory links in depression and cirrhosis
Neuroinflammation is a highly correlated pathophysiological pathway to this relationship. Elevated glucocorticoid levels can lead to the release of inflammatory markers and changes in gut permeability and can activate the hypothalamic-pituitary-adrenal axis, which further increases the release of glucocorticoid, elevating the likelihood of developing or worsening depressive symptoms.8 Further, gut dysbiosis may also be a pathogenic link, with a similar reduction in beneficial bacteria, increase in pathogens, and decreased bacterial diversity observed in both HE and depression.9
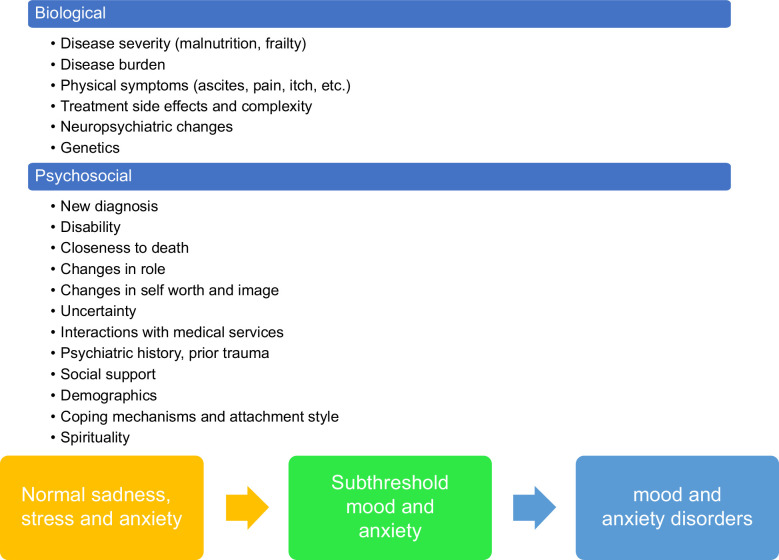
Anxiety and depression are on a continuum of the mood spectrum
Individuals with chronic medical conditions, including liver disease, are more likely to have anxiety disorders, and many patients with anxiety symptoms do not present for mental health services and report poor quality of life and medication adherence. Unfortunately, most patients with anxiety disorders remain undiagnosed. Distinguishing between normal and pathological anxiety is crucial for an accurate diagnosis.10 Some of the common psychological stress reactions to cirrhosis may include uncertainty regarding the diagnosis, course of illness, and prognosis, anxiety about one’s body, fear of death, anxiety about the impact the disease can have on the income, self-image and worth, fears about strangers and being alone in the hospital, and anxiety about negative reactions from the treatment teams Depression is also a stronger predictor of poor adherence to medical treatment and may even lead to patients refusing diagnostic or surgical procedures, and/or sign out of the hospital against medical advice.11,12 Normal sadness and grief represent one end of the spectrum of depression in these patients, in addition to an adjustment disorder, which appears when a patient has symptoms of depression in reaction to a stressor, such as the diagnosis of cirrhosis (Figure 1).
FIGURE 1.
Factors associated with mood and anxiety disorder presence and development.
DIFFERENTIAL DIAGNOSIS AND SCREENING TOOLS AVAILABLE FOR DEPRESSION/ANXIETY IN PATIENTS WITH CHRONIC LIVER DISEASE
There are many differential diagnoses when we face a patient with suspected depression/anxiety, for example, endocrinopathies (hypothyroidism or hypercortisolism), substance use disorder (dependency or withdrawal), and neurodegenerative disorders. Grade I (covert) HE may include signs and symptoms with neuropsychiatric manifestations that can easily be confused with primary disorders: lack of awareness, shortened attention span, mood disturbances/anxiety, and altered sleep cycle. QuickStroop, a test that takes <1 minute to apply, can help predict the development of overt HE, hospitalization, and death.13 The Montreal Cognitive Assessment, can also help to determine the prevalence of cognitive impairment in patients listed for liver transplantation.14 Berry et al14 found that each point increase in the pretransplant Montreal Cognitive Assessment was associated with 26% lower odds of post-liver transplantation cognitive impairment (adjusted OR: 0.74, 95% CI: 0.63–0.87, p<0.001), after adjusting for recipient age, history of HE, and time since liver transplantation. In Table 1, we provide some tests to narrow the differential on anxiety/depression.
TABLE 1.
Suggested laboratory workup in patients with suspected depression and anxiety
| Test | Rationale |
|---|---|
| Complete blood count with differential | To rule out symptomatic anemia, infection and less likely hematological malignancy. Elevated mean corpuscular volume is common in decompensated cirrhosis but also may be important to rule out hypothyroidism, and other causes of megaloblastic anemia (eg, alcohol use disorder associated with severe nutritional deficiencies) |
| Comprehensive metabolic panel with INR | To assess Child-Pugh-Turcotte, MELD 3.0, and rule out electrolyte abnormalities (sodium, calcium), or worsening kidney function, hypo/hyperglycemia. Particularly important rapid serum sodium shifts to avoid the development of central pontine myelinolysis |
| 25-hydroxy vitamin D, B12 | To rule out nutritional deficiency in the setting of severe alcohol use disorder |
| Anti-nuclear antibody, ESR, CRP, auto-antibodies | While not specific of chronic liver disease, some of these test could point toward autoimmune hepatitis and will be important to consider when treating depression associated with autoimmune hepatitis |
| AUDIT-C (Alcohol Use Disorders Identification Test), urine drug screen, plasma phosphatidylethanol (PEth) | The patient encounter will provide clues to ascertain potential substance use disorder |
| Urinalysis | To exclude urinary tract infection and/or renal pathology, in particular, in the setting of acute decompensation |
| Neuroimaging (non contrast CT, first) | HE is a diagnosis of exclusion and is critical to have a baseline imaging to exclude other causes of encephalopathy (eg, hemorrhage and masses) |
| STOP BANG | To identify people who might have obstructive sleep apnea and includes snoring, tiredness, observed apnea, presence of high blood pressure, body mass index >35 kg/m2, age 50+, neck circumference >16 inches and sex (male), being 3+ indication to refer for more studies |
| Medication review history | In particular, medications associated with depressions (eg, beta-blockade) or anxiety (eg, corticosteroids) |
Bold shows first-line testing.
Abbreviations: AUDIT-C, Alcohol Use Disorders Identification Test; CRP, c-reactive protein; ESR, erythrocyte sedimentation rate; INR: International Normalized Ratio; MELD, Model for End-Stage Liver Disease; PEth, phosphatidylethanol.
It is unknown how frequently the hepatologist applies screening tools for depression and anxiety despite many studies showing chronic liver disease frequently co-occurring with depression/anxiety.15 Whitsett and colleagues summarized some screening tools to assess depression and anxiety in patients with chronic liver disease. Of these, we recommend the Patient Depression Questionnaire-9 and the General Anxiety Disorder-7 to screen for depression and anxiety, respectively. These are free tools, patient-administered with adequate sensitivity and specificity to detect depression and anxiety (sensitivity >85% and specificity >76%)15
SPECIFIC CONSIDERATIONS FOR MANAGEMENT OF DEPRESSION/ANXIETY IN PATIENTS WITH LIVER DISEASE
The standard of care for patients with cirrhosis not only includes targeting the etiological factor to reverse underlying fibrosis (eg, hepatitis C treatment) and treating complications (eg, lactulose for HE) but also monitoring side effects from these treatments. Nonselective beta-blockade use has been associated with fatigue and depressed mood, hence, we recommend dose-adjustment or discontinuation to assess whether this medication is a contributor to patient’s depressed mood.
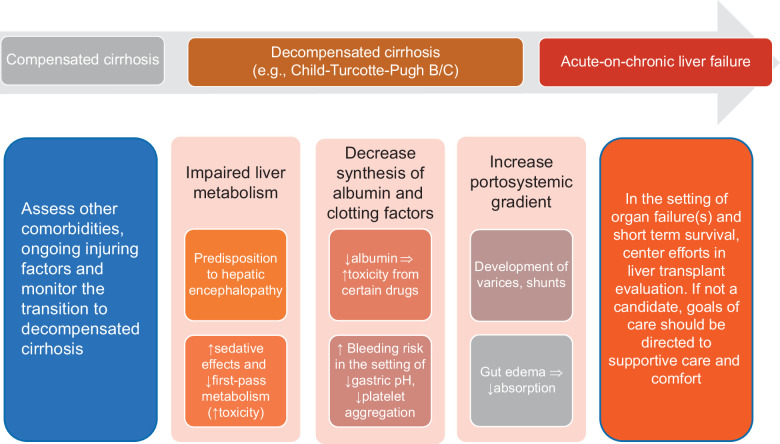
Drug choice is dependent on the classic Child-Turcotte-Pugh status
Clinicians should consider the following when prescribing psychotropic medications for patients with liver disease: the severity of the disease, the medication itself, the range of therapeutic to toxic plasma levels, and whether the patient has or is at high risk of HE (Figure 2). Generally, we try to avoid or use strong caution when using drugs with a narrow therapeutic window, like lithium. Drug dosing recommendations are guided by the Child-Turcotte-Pugh (CTP) (Table 2).16,17 Most patients rated as having CTP-A can tolerate 75%–100% of a standard initial dose. For patients with CTP-B, a reduction of 50%–75% in the normal starting dose may be appropriate. Patients with CTP-C cirrhosis will likely present with some degree of HE, and medications must be closely monitored to avoid toxicity or deteriorating mental status. Benzodiazepines should be avoided in patients at risk of HE, except in the setting of seizures or for delirium tremens treatment/prevention in the setting of alcohol-associated hepatitis. In such circumstances, lorazepam, temazepam, or oxazepam are preferred. Further, drug-drug interactions are always essential to check in these patients with frequent polypharmacy (Table 2).
FIGURE 2.
Pathophysiological considerations in patients with different stages of cirrhosis when prescribing medications for depression and anxiety.
TABLE 2.
Selected medications used in the treatment of mood disorders and anxiety and cirrhosis
| Recommendations for dose adjustments | LiverTox likelihood score of toxicitya | |
|---|---|---|
| Antidepressants | ||
| Fluoxetine (SSRI-selective serotonin reuptake inhibitors) | Reduce dose by 50% and use with caution | C |
| Paroxetine (SSRI) | Immediate release: Initial: 10 mg/d; increase if needed by 10 mg/d increments at intervals of at least 1 wk; maximum dose: 40 mg/d. Extended release: initial: 12.5 mg/d; increase if needed by 12.5 mg/d increments at intervals of at least 1 wk to a maximum of 50 mg/d (major depressive disorder, panic disorder). |
B |
| Sertraline (SSRI) | CTP-A/B: oral: initial: administer 50% of the usual recommended indication-specific dose; may increase based on response and tolerability in ≤25 mg increments at ≥2-week intervals. Maximum dose: 100 mg/d. CTP-C: not recommended |
B |
| Escitalopram (SSRI) | CTP-A/B: Initial: 5 mg/d for 2 wk; may increase to a maximum of 10 mg/d based on response and tolerability. CTP-C: not recommended |
C |
| Duloxetine [selective noradrenergic reuptake inhibitor (SNRI)] | Avoid in any degree of hepatic impairment | B |
| Venlafaxine | Initiate at 37.5–75 mg daily with gradual titration (maximum 150 mg daily). Avoid in decompensated cirrhosis (CTP-B/C). | B |
| Desvenlafaxine | CTP-A: No dosage adjustment necessary. CTP-B/C: Initial: 50 mg once daily; maximum dose: 100 mg once daily |
E |
| Amitriptyline (tricyclic antidepressant-TCA) | Reduce initial and maintenance dose to half. Monitor carefully. Considering second-generation TCA such as nortriptyline or desipramine because increase of sedation. | B |
| Bupropion | CTP-A/B do not exceed 75 mg daily (immediate release), 100 mg (sustaine release), or 150 mg every other day (extended release). Avoid in CTP-C. | C |
| Trazodone (serotonin antagonist and reuptake inhibitor) | No dosing guidelines. Avoid in patients with HE | B |
| Mirtazapine (noradrenergic and specific serotonergic antidepressant) | Initiate at half dose or regular starting dose. Titrate with caution. Avoid with other serotonergic agents as it can cause serotonin syndrome | C |
| Anxiolytics | ||
| Alprazolam | Reduce by 50% and avoid in CTP-C | D |
| Diazepam | There are no dosage adjustments provided in the manufacturer’s labeling; use with caution because distribution and half-life may increase, and clearance may decrease significantly. CTP-C: contraindicated |
D |
| Lorazepam, oxazepam | The preferred agents in cirrhosis, without specific dose adjustments but recommend to avoid in patients with HE | D, (oxazepam -E) |
| Chlordiazepoxide | There are no dosage adjustments on initial dosing but proceed with caution in the setting of HE | C |
| Buspirone | Reduce dose and frequency by 50% in CTP-A/B. CTP-C: Use not recommended. |
E |
Note: For all these medications, we recommend starting with a drug-drug interaction check and looking at the monography to check for specific circumstances (eg, EKG for QTc measure in the case of fluoxetine and QTc prolongation).
Recommendations adapted from Menon et al.16
Available at LiverTox https://www.ncbi.nlm.nih.gov/books/NBK547852/ A 5-point scale [A–E] that estimates whether a medication is a of cause liver injury: A=well-known cause; B=highly likely cause; C=probable cause; D=possible cause; E=unlikely cause; E*=suspected but unproven cause; X=unknown.
Abbreviations: CTP, Child-Turcotte-Pugh; SNRI, Serotonin and norepinephrine reuptake inhibitors; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant.
Psychotherapy
There are multiple psychosocial interventions designed and/or proven to be effective in treating depression and anxiety in the medical setting. Filipovic et al,18 found cognitive behavioral therapy as an effective method in the treatment of NAFLD, obesity, and comorbid sleep apnea. Further, most of the treatments for metabolic-associated fatty liver disease not only include nutritional and lifestyle modifications but also psychotherapeutic interventions such as motivational interviewing, behavioral activation, and problem-solving therapy.19 The treatment of alcohol-associated liver disease should be mainly focused on addressing the underlying etiology, the alcohol use disorder, hence psychotherapy is the backbone of such conditions20
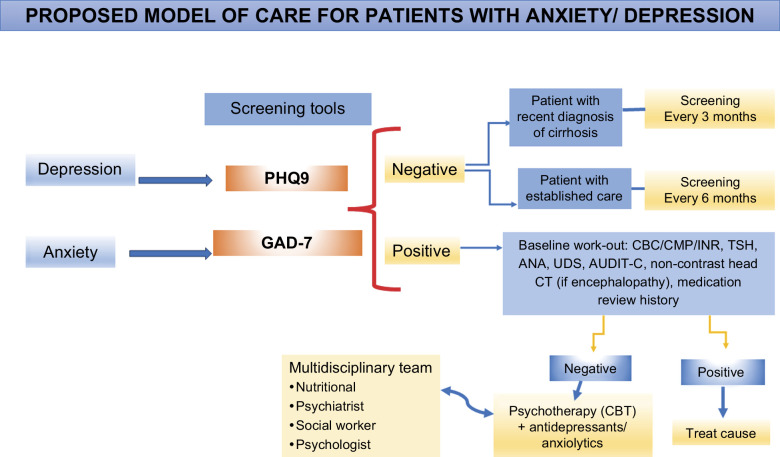
PROPOSED MODEL OF CARE FOR PATIENTS WITH ANXIETY/DEPRESSION
Our patients with cirrhosis have concurrent mental and substance abuse disorders, which worsen the patient’s health outcomes if they are untreated. While we center cirrhosis care on clinical outcomes such as the management of decompensation and improved transplantation rate, there is an unmet need for patient-centered care, including mental health services. We adapted the framework proposed by Verma et al,21 including outpatient, inpatient, rehabilitation, and supportive care, such as nutrition, social work, and behavioral health (Figure 3).
FIGURE 3.
Models of care in patients with cirrhosis, integrating of mental health services. Abbreviations: ANA, antinuclear antibodies; AUDIT-C, Alcohol Use Disorders Identification Test; CBC, complete blood count; CBT, cognitive behavioral therapy; CMP, comprehensive metabolic panel; GAD-7, General Anxiety Disorder; INR, International Normalized Ratio; PHQ9, Patient Depression Questionnaire; TSH, thyroid stimulating hormone; UDS, urinary drug screen.
In summary, depression and anxiety are common in patients with cirrhosis. While screening and ruling out other comorbidities is part of the routine care in hepatology, we need to consider early referral and/or initiation of medication to co-manage these patients with mental health providers.
Acknowledgments
FUNDING INFORMATION
The work is also supported in part by the Center for Innovations in Quality, Effectiveness and Safety (CIN 13-413), Michael E. DeBakey VA Medical Center, Houston, TX.
CONFLICTS OF INTEREST
The authors have no conflicts to report.
Footnotes
Abbreviations: ACT, acceptance and commitment therapy; CTP, Child-Turcotte-Pugh; MELD, Model for End-Stage Liver Disease.
Mauro Garcia-Altieri and Keila Carrera-Mejias are joint authors.
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government.
Contributor Information
Mauro Garcia-Altieri, Email: mauro.garcia-altieri@bcm.edu.
Keila Carrera-Mejias, Email: keila.carreramejias@utsouthwestern.edu.
Ruben Hernaez, Email: ruben.hernaez@bcm.edu.
REFERENCES
- 1. Hernaez R, Kramer JR, Khan A, Phillips J, McCallister K, Chaffin K, et al. Depression and anxiety are common among patients with cirrhosis. Clin Gastroenterol Hepatol. 2022;20:194–203.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee K, Otgonsuren M, Younoszai Z, Mir HM, Younossi ZM. Association of chronic liver disease with depression: A population-based study. Psychosomatics. 2013;54:52–9. [DOI] [PubMed] [Google Scholar]
- 3. Kim D, Manikat R, Shaikh A, Cholankeril G, Ahmed A. Depression in nonalcoholic fatty liver disease and all-cause/cause-specific mortality. Eur J Clin Invest. 2024;54:e14087. [DOI] [PubMed] [Google Scholar]
- 4. Gungabissoon U, Gibbons DC, Requena G, Ribeiro de Souza A, Smith H. Disease burden of primary biliary cholangitis and associated pruritus based on a cross-sectional US claims analysis. BMJ Open Gastroenterol. 2022;9:e000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Seo GH, Yoo J. Incidence of major depressive disorder over time in patients with liver cirrhosis: A nationwide population-based study in korea. PLoS One. 2022;17:e0278924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Doyle T, Schmidt B, Scaglione S. Prevalence of depression and anxiety symptoms by liver disease etiology. Clin Liver Dis (Hoboken). 2023;22:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Russ TC, Kivimaki M, Morling JR, Starr JM, Stamatakis E, Batty GD. Association between psychological distress and liver disease mortality: A meta-analysis of individual study participants. Gastroenterology. 2015;148:958–66.e4. [DOI] [PubMed] [Google Scholar]
- 8. Shao Q, Wu Y, Ji J, Xu T, Yu Q, Ma C, et al. Interaction mechanisms between major depressive disorder and non-alcoholic fatty liver disease. Front Psychiatry. 2021;12:711835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kronsten VT, Shawcross DL. Hepatic encephalopathy and depression in chronic liver disease: Is the common link systemic inflammation? Anal Biochem. 2022;636:114437. [DOI] [PubMed] [Google Scholar]
- 10. Sareen J, Jacobi F, Cox BJ, Belik S, Clara I, Stein MB. Disability and poor quality of life associated with comorbid anxiety disorders and physical conditions. Arch Intern Med. 2006;166:2109–2116. [DOI] [PubMed] [Google Scholar]
- 11. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: Meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–7. [DOI] [PubMed] [Google Scholar]
- 12. Garcia-Llana H, Remor E, del Peso G, Celadilla O, Selgas R. Motivational interviewing promotes adherence and improves wellbeing in pre-dialysis patients with advanced chronic kidney disease. J Clin Psychol Med Settings. 2014;21:103–15. [DOI] [PubMed] [Google Scholar]
- 13. Kanagalingam G, Park D, Badal BD, Fagan A, Thacker LR, Bajaj JS. QuickStroop predicts time to development of overt hepatic encephalopathy and related hospitalizations in patients with cirrhosis. Clin Gastroenterol Hepatol. 2024;22:899-901.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berry K, Ruck JM, Barry F, Shui AM, Cortella A, Kent D, et al. Prevalence of cognitive impairment in liver transplant recipients. Clin Transplant. 2024;38:e15229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Whitsett MP, Goswami Banerjee A, Serper M. Assessment of mental health in patients with chronic liver disease. Clin Liver Dis (Hoboken). 2022;20:52–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Menon V, Ransing R, Praharaj SK. Management of psychiatric disorders in patients with hepatic and gastrointestinal diseases. Indian J Psychiatry. 2022;64(suppl 2):S379–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cotter TG, Beresford T. Treatment of mental health in patients with chronic liver disease. Clin Liver Dis (Hoboken). 2022;20:57–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Filipovic BF, Latas M, Kiurski S, Al Kiswani D, Filipovic N, Marjanovic-Haljilji M, et al. The role of psychotherapy in the treatment of patients with non- alcoholic fatty liver disease and obstructive sleep apnea. J Gastrointestin Liver Dis. 2021;30:477–84. [DOI] [PubMed] [Google Scholar]
- 19. Jeeyavudeen MS, Khan SKA, Fouda S, Pappachan JM. Management of metabolic-associated fatty liver disease: The diabetology perspective. World J Gastroenterol. 2023;29:126–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vannier AGL, Przybyszewski EM, Shay J, Patel SJ, Schaefer E, Goodman RP, et al. Psychotherapy for alcohol use disorder is associated with reduced risk of incident alcohol-associated liver disease. Clin Gastroenterol Hepatol. 2023;21:1571–80.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Verma M, Brahmania M, Fortune BE, Asrani SK, Fuchs M, Volk ML. Patient-centered care: Key elements applicable to chronic liver disease. Hepatology. 2023;78:307–18. [DOI] [PubMed] [Google Scholar]