Oliguria and renal dysfunction are common in critically ill patients. In most cases the kidney is an innocent bystander affected secondarily by the primary disease process. As patients with acute renal failure usually have multiple organ dysfunction and often require respiratory or circulatory support, they are increasingly referred to intensive care units rather than to specialist renal units. Nevertheless, close liaison with nephrologists is advisable, particularly when primary renal disease is suspected. It is rare for patients to develop acute renal failure after admission to intensive care unless a new problem has occurred or the primary process has not been controlled.
Renal failure is not an acceptable cause of death unless a conscious decision has been made not to treat it in the face of another non-recoverable disease
Physiology
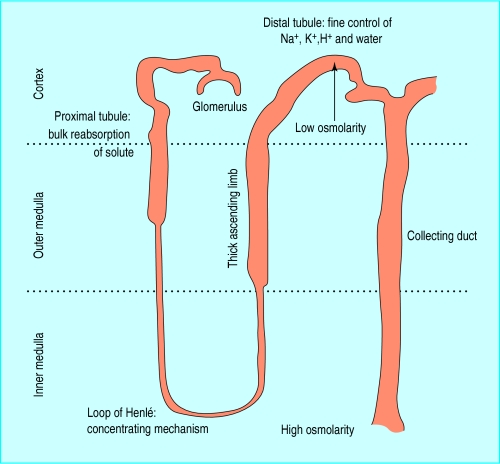
Urine is produced by glomerular filtration, which depends on the maintenance of a relatively high perfusion pressure within the glomerular capillary and an adequate renal blood flow.
Role of kidneys in maintaining the internal environment
Elimination of water soluble waste products of metabolism other than carbon dioxide
Control of fluid and electrolyte homeostasis
Elimination of water soluble drugs
Endocrine function (erythropoietin, vitamin D, renin)
Glomerular blood flow is autoregulated by the pre-glomerular arteriole until the mean arterial pressure falls to 80 mm Hg. Below this pressure the flow decreases. The autoregulation is achieved by arteriolar dilatation (partly mediated by prostaglandins and partly myogenic) as pressure falls and by vasoconstriction as pressure rises. If perfusion pressure continues to fall glomerular filtration pressure is further maintained by constriction of post-glomerular arterioles, which is mediated by angiotensin II.
The proximal tubules reabsorb the bulk of the filtered solute required to maintain fluid and electrolyte balance, but elimination of potassium, water, and non-volatile hydrogen ions is regulated in the distal tubules. As renal perfusion and glomerular filtration diminish, reabsorption of water and sodium by the proximal tubules rises from approximately 60% of that filtered to over 90% so that minimal fluid reaches the distal tubule. This explains why hypotensive or hypovolaemic patients cannot excrete potassium, hydrogen ions, and water. Similar defects in excretion of potassium and hydrogen ions occur in patients with distal tubular damage caused by drugs or obstructive uropathy.
The energy required for tubular function comes from aerobic metabolism within the mitochondria of the tubular cells. Tubular cells deep within the medulla operate at the limit of oxidative metabolism and are particularly sensitive to the effects of ischaemia and hypoxia. Blood flow to the medulla is threatened as renal perfusion falls and is maintained by the action of prostaglandins produced by the medullary interstitial cells. The cells of the thick ascending limb of the loop of Henlé are the most metabolically active in the deep medulla and thus the most vulnerable.
Acute renal failure
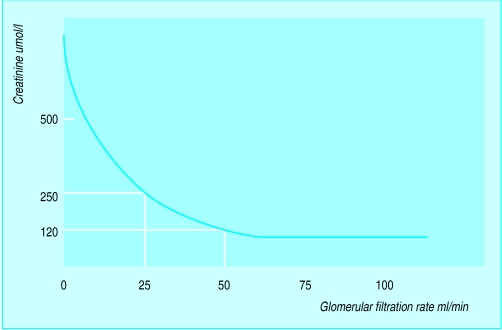
Acute renal failure is defined as a sudden, normally reversible impairment of the kidneys’ ability to excrete the body’s nitrogenous waste products of metabolism. Acute renal failure is usually accompanied by oliguria. However, a daily urine volume above 500 ml does not necessarily imply normal renal function in critically ill patients. The plasma urea concentration rises with the breakdown of soft tissue or blood (which may be within the gut) or a high protein intake. Uraemia is a less reliable indicator of underlying renal function than creatinine concentration. The rate of production of creatinine is related to lean body mass, except in rhabdomyolysis. The concentration of creatinine in the blood reaches the upper limit of normal after 50% of function is lost and then doubles for each further 50% reduction in renal function.
Criteria for diagnosis of acute renal failure
Fall in urine volume to less than 500 ml per day
Rising plasma urea and creatinine concentrations
Rising plasma potassium and phosphate plus falling calcium and venous bicarbonate
Urine dipstick testing can detect haematuria and proteinuria, which may signify primary renal disease or other systemic disease. If primary glomerular disease is suspected a urine sample should be sent for microscopy. Although there are now direct tests for myoglobinuria, microscopy can help diagnose rhabdomyolysis and haemolysis. The stick test is strongly positive for haem pigment but no red cells are visible on microscopy.
Investigations that may help to differentiate renal hypoperfusion from acute renal failure in oliguric patients
| Measurement | Renal hypoperfusion | Acute renal failure |
|---|---|---|
| Fractional excretion of sodium (%) | <1 | >4 |
| Urinary sodium (mmol/l) | <20 | >40 |
| Urine:plasma urea ratio | >20 | <10 |
| Urine:plasma creatinine ratio | >40 | <10 |
| Urine:plasma osmolality ratio | >2 | <1.2 |
Simultaneous measurement of urinary and plasma urea, creatinine, and sodium concentrations and osmolality may help differentiate physiological oliguria of renal hypoperfusion from acute renal failure. Concurrent drug treatment—for example, diuretics or dopamine—will make values difficult to interpret. However, the findings will not generally alter management greatly. Patients with absolute anuria must be assumed to have lower urinary tract obstruction until proved otherwise. Always remember to check for a blocked catheter.
Established acute renal failure is confirmed by the lack of response to correction of any cardiorespiratory deficit, urinary tract obstruction, or septic process and rising concentrations of urea and creatinine. In critically ill patients it commonly results from a number of combined insults: hypovolaemia (absolute or relative), impaired renal perfusion (low perfusion pressure, low cardiac output), sepsis, drugs (including radiocontrast agents), hepatic dysfunction, obstruction of the collecting system (partial or complete), vascular occlusion (large or small vessel), or primary renal disease.
Standard guidelines exist for intensive care of patients with established or impending renal dysfunction. A window of opportunity exists between the onset of the insult(s) and the onset of established acute renal failure. Rapid identification and correction of these insults is essential and further potential insults must be avoided.
Guidelines for immediate management of patients with oliguria or anuria
Assess and correct any respiratory or circulatory impairment
Manage any life threatening consequences of renal dysfunction (hyperkalaemia, salt and water overload, severe uraemia, extreme acidosis)
Exclude obstruction of the urinary tract
Establish underlying cause(s) and institute prompt remedial action
Get a drug history and alter prescriptions appropriately
Get help from senior appropriately trained specialists
Correct circulation
Once hypoxaemia has been corrected (by using controlled ventilation if necessary) meticulous attention must be paid to cardiovascular function. Adequate intravascular volume, cardiac output, and perfusion pressure must be ensured before patients are given any diuretic or other drug purported to generate production of urine.
Correct metabolic acidosis
Severe metabolic acidosis secondary to renal tubular dysfunction can be corrected over 24-36 hours with isotonic sodium bicarbonate provided that the patient does not have a salt overload. Acidosis related to tissue hypoxia should be treated by addressing the underlying cause.
The cause of acidosis will determine the treatment
Tissue hypoxia/lactic acidosis—optimise circulation and oxygenation
Salt and water depletion—normal saline
Established renal failure (acute or chronic)—sodium bicarbonate, ?dialysis
Poisoning (methanol, ethylene glycol, salicylate)—sodium bicarbonate, ?dialysis
Liver failure—sodium bicarbonate, ?haemofiltration
Diabetes mellitus—insulin, saline
Exclude and relieve any urinary tract obstruction
Any obstruction at the bladder neck or urethra is relatively easily corrected by urethral or suprapubic catheterisation. Obstructions of the upper collecting system can be relieved at the bedside by percutaneous nephrostomy under ultrasonography.
Drugs that induce renal damage
| Damage | Class of drug |
|---|---|
| Decrease in renal perfusion | Diuretics, angiotensin converting enzyme inhibitors, β blockers, vasodilators |
| Impaired intrarenal haemodynamics | Non-steroidal anti-inflammatories, radiocontrast agents |
| Tubular toxicity | Aminoglycosides, amphotericin, cisplatin |
| Allergic interstitial nephritis | β lactams, non-steroidal anti-inflammatories |
Nephrotoxic drugs
Directly nephrotoxic drugs such as aminoglycosides should be avoided when possible. If they are given, blood concentrations should be measured regularly. Many drugs indirectly affect renal function by their effects on the circulation, and their concentration may build up as renal function deteriorates. In critically ill patients, especially those with sepsis, α and β adrenergic blocking drugs, angiotensin converting enzyme inhibitors, other vasodilators, and diuretics will potentiate any systemic circulatory disturbance and impair the intrarenal mechanisms that normally maintain glomerular filtration and medullary blood flow.
Drugs that may cause acute interstitial nephritis in intensive care
-
Antibiotics
- β lactams
- Rifampicin
- Sulphonamides
- Vancomycin
- • Diuretics
- Thiazides
- Frusemide (furosemide)
- • Non-steroidal anti-inflammatory drugs
-
Others
- Ranitidine
- Cimetidine
- Phenytoin
Non-steroidal anti-inflammatory drugs can produce an allergic interstitial nephritis, but more commonly in patients with a septic, systemic inflammatory, or hypovolaemic insult they impair the compensatory mechanisms that maintain glomerular perfusion and medullary blood flow to the ascending limb of the loop of Henlé. A single dose may be sufficient to precipitate failure of a stressed kidney. These drugs are thus contraindicated in critically ill patients.
Other drugs
The pharmacokinetics of many other drugs in critically ill patients with renal failure have not been established. Care must be taken with all drug treatment.
Renal protection
No convincing evidence exists that any of the regimens advocated to protect against or reverse renal failure are superior to salt loading (that is, extracellular fluid volume expansion with saline) and providing optimal renal perfusion (pressure as well as flow).
Mannitol has been suggested for situations such as obstructive biliary disease and vascular surgery, but there is little evidence that it is better than salt loading in humans other than for producing diuresis. In rhabdomyolysis, mannitol combined with aggressive salt loading and alkalinisation of the urine has been shown to reduce the incidence of severe renal damage.
Low dose dopamine has not been shown to improve renal function (glomerular filtration rate not diuresis) in randomised trials. If it does not have a diuretic effect within 24 hours it should be stopped. The use of loop diuretics to reduce oxygen requirements in the distal tubule in the stressed kidney is theoretically attractive but unproved.
Indications for renal replacement therapy
Uncontrollable hyperkalaemia
Severe salt and water overload unresponsive to diuretics
Severe uraemia
Acidaemia
Renal replacement therapy
Renal replacement therapy should be started early for patients who present with an absolute indication. The concentration of plasma urea at which renal replacement therapy should be started depends on the patient’s condition. A patient with single organ failure secondary to a nephrotoxin might not require renal replacement therapy until the urea concentration is well above 30 mmol/l, but a patient with severe intra-abdominal sepsis such as faecal peritonitis with established renal failure should be treated early as urea concentrations will rise rapidly.
Most critically ill patients in the United Kingdom are now treated by semicontinuous methods of haemofiltration with or without dialysis rather than by short term haemodialysis as used in chronic renal replacement therapy. Peritoneal dialysis is used increasingly rarely in intensive care. Semicontinuous methods of treatment cause less fluctuation in the patient’s biochemistry, which seems to improve cardiovascular stability. However, it has been difficult to prove that patient outcome has been affected other than in the presence of cerebral oedema—for example, in liver failure. The problems of obtaining access for extracorporeal circuits have been considerably reduced by the use of multilumen percutaneous venous catheters.
Nutrition
Critically ill patients should not be starved or have their protein intake restricted in an attempt to avoid renal replacement therapy. It is better to accept the need for renal replacement therapy and allow appropriate nutrition. Specially formulated “renal” feeds have no advantage over standard feeding compounds in critically ill patients.
Recovery
The recovery phase is marked by an increase in urine volume as the nephrons recover and renal replacement therapy can be stopped. However, vigilance is still required as the kidney will have a reduced ability to conserve sodium, potassium, bicarbonate, and water. Modern management has made massive electrolyte and water losses uncommon. They may still occur after the relief of urinary tract obstruction because of the severe chronic distal tubular damage. Nephrotoxins and vasoactive drugs must be used with care, and non-steroidal anti-inflammatory drugs should be avoided. Renal function will usually return to within 90% of normal by 6 months after recovery from critical illness.
Patients with pre-existing chronic renal failure have a limited ability to conserve electrolytes and water which depends on their residual functioning renal mass. They cannot concentrate their urine to the normal level and may have an obligatory urine volume of up to 3 litres a day. As their intrarenal vascular compensatory mechanisms are continuously activated, they are more vulnerable to any insult. Careful attention to circulatory stability, electrolyte and water balance, and drug administration is essential.
Key points
Circulation must be corrected before any other specific intervention is started
The cause of renal dysfunction must be determined and if possible treated
Renal replacement therapy should be started and tailored according to the degree of biochemical derangement and the patient’s underlying condition
Primary renal disease is rare in critically ill patients but requires prompt referral to a nephrologist to avoid irreversible renal failure
Figure.
Diagram of nephron and position within kidney
Figure.
Relation of serum creatinine concentration to glomerular filtration rate
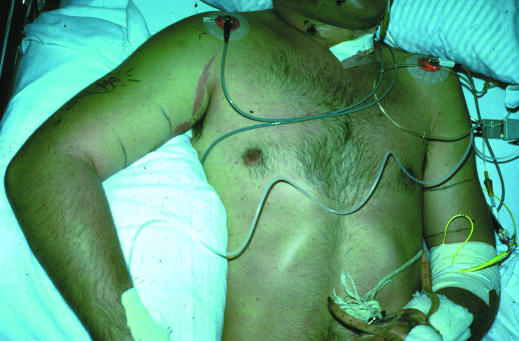
Figure.
Rhabdomyolysis of shoulder and upper arm after prolonged compression secondary to overdose of tricyclic antidepressants. Note the pressure marks close to the axilla
Figure.
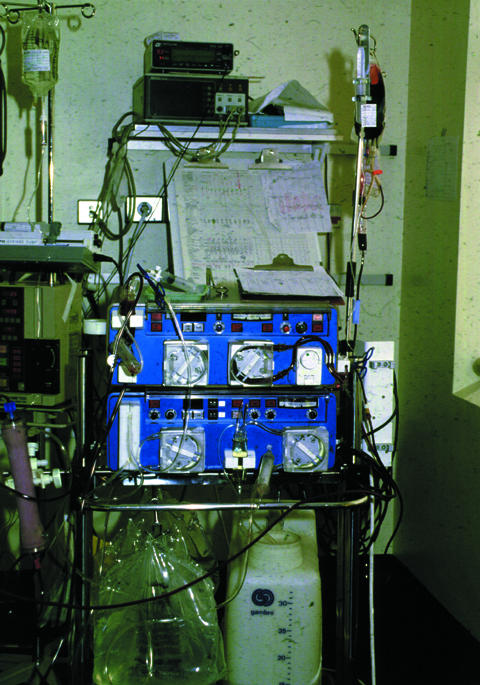
Semicontinuous haemofiltration
Footnotes
Alasdair Short is director of intensive care, Broomfield Hospital, Chelmsford, Essex, and Allan Cumming is consultant nephrologist, Edinburgh Royal Infirmary, Edinburgh.
The ABC of intensive care is edited by Mervyn Singer, reader in intensive care medicine, Bloomsbury Institute of Intensive Care Medicine, University College London, and Ian Grant, director of intensive care, Western General Hospital, Edinburgh. The series was conceived and planned by the Intensive Care Society’s council and research subcommittee.