Peak expiratory flow varies throughout the day in normal subjects, and this diurnal variation is increased in people with asthma.1 Current asthma guidelines recommend that diurnal variability of the peak expiratory flow rate should be calculated when diagnosing asthma and assessing its severity, 2–7 including during exacerbations.3,4 Diurnal variability of peak flow has been used as a marker of airway responsiveness,8,9 particularly in epidemiological studies,10,11 and as an outcome measure in clinical asthma trials.12 However, there are problems associated with its use.
Summary points
Variation in peak flow over days or weeks provides helpful information about asthma control
Asthma guidelines recommend that diurnal peak flow variability is calculated to provide an index of airway lability
These calculations are too time consuming for normal clinical practice
Factors such as the time of recording or recent use of β2 agonist drugs result in minor changes in peak flow, but can cause large errors in diurnal variability
Diurnal variability may fail to detect important changes in lung function
An alternative, simpler index of peak flow variation such as the lowest morning peak flow expressed as percentage of the patient’s personal best peak flow value should be evaluated for inclusion in asthma guidelines
Cumbersome calculations
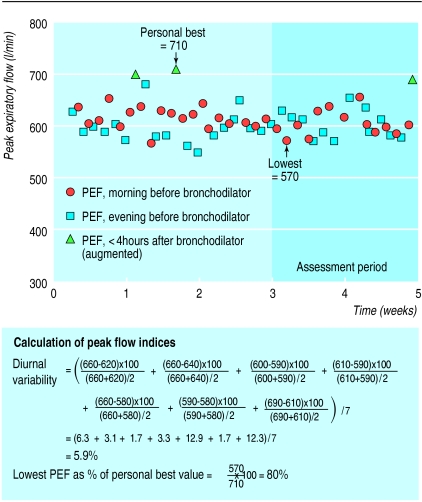
Although diurnal variability in peak flow has been included in asthma guidelines for many years, doctors in primary and secondary care settings rarely use it, because of the cumbersome calculations involved. Several alternative equations may be used. The most common are the amplitude percentage mean ((maximum−minimum)/mean) or the amplitude percentage maximum ((maximum−minimum)/maximum), calculated for each day, and then averaged over a period of 1 to 2 weeks.13 Determining the amplitude percentage mean from as few as 7 days of twice daily peak flow readings for one patient (see fig 1) is complicated and tedious, even if calculator shortcuts (which may increase the possibility of error) are used. Furthermore, the calculations take too long for a standard medical consultation. Electronic recording and computerised processing of peak flow data are still prohibitively expensive for general practice, and also have pitfalls. For example, if a program is written to calculate daily amplitude as (evening−morning) instead of (maximum−minimum), some daily values may be negative, resulting in an underestimation of average diurnal variability. It should be noted that the various equations currently used to estimate diurnal variability give results that are not directly comparable.
Figure 1.
Peak expiratory flow (PEF) chart of a 36 year old man. Diurnal variability been calculated for the last 7 days only
Number of daily observations
Most asthma guidelines state that diurnal variability should be calculated from two sets of peak flow readings each day—taken in the morning and afternoon/evening.2–7 However, several studies have now shown that diurnal variability is grossly underestimated unless peak flow is recorded four or more times a day.14,15 For example, one study showed that only 20%-45% of “true” diurnal variability (based on 13 daily peak flow readings) was detected from two daily peak flow readings.15 Two hourly peak flow readings are currently used in diagnosing occupational asthma, but patients in normal clinical practice often have difficulty recording peak flow even twice daily,16 resulting in bias through non-compliance.17
Impact of timing
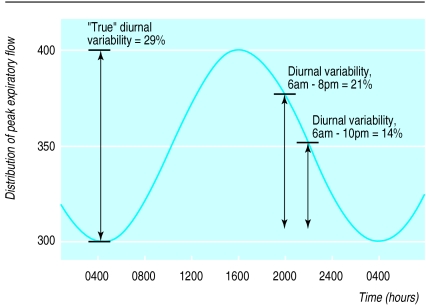
A corollary of the above is that if patients record only two peak flow sessions per day, the time at which peak flow is recorded may have a major impact on the estimated diurnal variability. In most patients, the acrophase (peak) of peak flow occurs at approximately 1400 to 1600,1 but asking patients to record peak flow at this time on workdays is generally impractical. A delay in recording the second daily set of peak flow readings which leads to a difference of only 50 l/min in absolute peak flow may cause an underestimation of diurnal variability for that day of as much as 50% (fig 2). Clinical trials often try to standardise by specifying time “windows” within which peak flow measurements should be performed. Doing this may, however, introduce more error, because the timing of the peak flow acrophase seems to be determined by the time at which the patient wakes on that day, and, unlike other circadian rhythms, changes almost immediately with a change in waking time.18 In one study, peak flow determinations performed “immediately upon waking” were recorded, on average, 81 minutes later on weekends than on weekdays.19 Gannon et al found that the peak flow acrophase occurred more than 2 hours later on rest days than on workdays,14 presumably because of differences in waking time. Thus, normal lifestyle variations can have a substantial impact on estimated diurnal variability.
Figure 2.
A sample stylised cosinor distribution of peak flow with a bathyphase (trough) of 300 l/min at 0400 and an acrophase (peak) of 400 l/min at 1600. “True” diurnal variability (amplitude percentage mean) is (400-300)×100/350=29%. Diurnal variability for peak flow recorded at 0600 and 2000 is (375-305)×100/340=21%, while that for peak flow recorded at 0600 and 2200 is (350-305)×100/328=14%
Effect of drug regimens
The validity of diurnal variability as a measure of asthma severity was originally established when the recommended treatment regimen for β2 agonist drugs was routine inhalation two to four times daily, but its continuing validity cannot be assumed now that β2 agonists are usually taken only on demand. If a β2 agonist drug is inhaled to relieve symptoms rather than at a routine time, the elapsed time before the next scheduled peak flow recording is likely to vary from day to day, and errors caused by the augmentation of peak flow by a residual bronchodilator effect may occur. Once again (as in fig 2), a small change in the absolute peak flow can cause a large change in the calculated diurnal variability.
Several asthma guidelines specify that in order to standardise recording conditions peak flow should be recorded before the inhalation of β2 agonist drugs. This is also standard practice in most clinical trials. However, in clinical practice, patients with asthma that is poorly controlled cannot be asked to delay taking a needed β2 agonist for several hours so that a “pretreatment” peak flow can be recorded. In a recent study we found that people who used a bronchodilator more than 2.2 times a day were unable to delay its use for 4 hours before 31% of scheduled peak flow measurements.20 Including these potentially augmented peak flow values resulted in positive bias in the average morning peak flow and average evening peak flow, but diurnal variability was affected in an unpredictable way. It was increased or decreased on any day depending on whether the morning peak flow or evening peak flow, or both, were augmented by bronchodilator use before the recording.21
This problem is less likely to occur after patients begin long acting β2 agonist treatment (provided peak flow is recorded consistently before this medication is taken), as the frequency of use of short acting β2 agonist drugs usually falls. Long acting β2 agonists themselves increase evening and, to a greater extent, morning peak flow,22 with a resulting reduction in peak flow amplitude, and hence a reduction in diurnal variability.
Diurnal variability and exacerbations
Calculating diurnal variability in peak flow during exacerbations of asthma is included in two current guidelines. The guidelines of the British Thoracic Society recommend that diurnal variability should be used to assess whether a patient admitted to hospital for an exacerbation of asthma can be discharged home safely. However, not all asthma exacerbations are associated with increased variability in peak flow. During presumed viral asthma exacerbations in patients with previously well controlled asthma, diurnal variability did not increase despite an average fall in morning peak flow of 27%.23 In these exacerbations, both daily peak flow amplitude and mean peak flow fell, so that the amplitude percentage mean was unchanged. Diurnal variability may thus fail to detect important and sustained changes in lung function, and cannot be recommended for assessing the severity of asthma exacerbations.
Even in a less acute clinical situation, calculation of diurnal variability may not reliably identify short term reductions in peak expiratory flow because of the effect of averaging over one or two weeks.24 The Global Initiative for Asthma guidelines include diurnal variability in a self management plan based on peak flow measurement, but given the arithmetic complexity of calculating diurnal variability, this hardly seems feasible for the average patient.4
Other indices of variation in peak flow
Despite the problems with diurnal variability discussed above, visual inspection of peak flow charts suggests that peak flow variation over a period of days or weeks can provide helpful information about the severity of asthma and the response to treatment.25–27 This process of visual inspection has been validated for occupational asthma,28 but requires considerable experience, and is thus not appropriate for inclusion in asthma guidelines for general clinical practice. Other measures of peak flow variation such as the standard deviation or coefficient of variation have been examined,24 but these indices require computerised processing and are therefore not currently suitable for normal clinical practice.
Expressing the lowest peak expiratory flow (usually on waking) over 1 or 2 weeks as a percentage of the patient’s “personal best” measurement recorded on the same peak flow meter (lowest % personal best), is an alternative numerical estimate of variation in peak flow that can be quickly calculated. The resulting peak flow index is illustrated in figure 1. In a study of 46 adults with a wide range of asthma control, the lowest % personal best was 77.6% (range 46.9-97.8, 95% confidence limits 73.9 to 81.3).29 Preliminary evidence suggests that the correlations between lowest % personal best and airway hyperresponsiveness, symptoms, and bronchodilator use are similar to those for other indices of peak flow variation,27,29 and that lowest % personal best index increases appropriately as asthma improves with inhaled corticosteroid treatment.20 This index is not noticeably affected by bronchodilator use20 or (provided peak flow is recorded upon waking) by the time of recording.18 In keeping with the recent recommendation for once daily peak flow monitoring,2 the lowest % personal best index can be based on a single daily peak flow session performed upon waking, once the personal best value has been established.30 It will be necessary to determine the optimal criteria for establishing personal best values, and we are currently undertaking such a study. The lowest % personal best index corresponds to the peak flow index used in many current asthma action plans, in which the morning peak flow is compared with peak flow “zones” calculated as a percentage of the patient’s personal best peak flow measurement.31,32
Conclusions
In the absence of a “gold standard,” clinical practice guidelines for assessing asthma severity and monitoring asthma control usually include several measures such as symptoms, lung function, and airway lability. Airway lability is estimated by diurnal variability when bronchial provocation testing is unavailable. These clinical practice guidelines must be scientifically valid, but the extent of their implementation, and hence their effectiveness, also depends greatly on how simple and straightforward they are in practice.33 Diurnal variability seems to be deficient on both counts—its calculation is subject to errors and is impractical clinically in that it takes too long to calculate. We propose that asthma guidelines on peak expiratory flow monitoring be reviewed, and that a simpler measure such as the lowest % personal best should be evaluated as an index of peak flow lability in assessing asthma severity and monitoring of asthma control.
Acknowledgments
HR is a research scholar with the National Health and Medical Research Council of Australia. CJ is chairman of the National Asthma Campaign of Australia. AW is a member of the executive of the Global Initiative for Asthma and was a participant and author for the conference report, “The assessment and treatment of asthma.”
References
- 1.Hetzel MR, Clark TJH. Comparison of normal and asthmatic circadian rhythms in peak expiratory flow rate. Thorax. 1980;35:732–738. doi: 10.1136/thx.35.10.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Asthma Education and Prevention Program. Guidelines for the diagnosis and management of asthma expert panel. Report 2. Bethesda, MD: National Institutes of Health; 1997. (NIH publication No 97-4051, NHLBI.) [Google Scholar]
- 3.British Thoracic Society; National Asthma Campaign and Royal College of Physicians of London in association with the General Practitioner in Asthma Group; British Association of Accident and Emergency Medicine; British Paediatric Respiratory Society; Royal College of Paediatric and Child Health. The British guidelines on asthma management. 1995 review and position statement. Thorax. 1996;52(suppl 1):S1–21. [Google Scholar]
- 4.Global strategy for asthma management and prevention. Bethesda, MD: National Institutes of Health; 1995. Global Initiative for Asthma. (NIH publication No 96-3659A, NHLBI.) [Google Scholar]
- 5.National Heart Lung and Blood Institute. International consensus report on the diagnosis and management of asthma. Clin Exp Allergy. 1992;22(suppl 1):1–72. [Google Scholar]
- 6.Newhouse MT, editor. The assessment and treatment of asthma: a conference report. J Allergy Clin Immunol. 1990;85:1098–1111. doi: 10.1016/0091-6749(90)90056-a. [DOI] [PubMed] [Google Scholar]
- 7.National Asthma Campaign [Australia] Asthma management handbook. Melbourne: National Asthma Campaign; 1998. [Google Scholar]
- 8.Ryan G, Latimer KM, Dolovich J, Hargreave FE. Bronchial responsiveness to histamine: relationship to diurnal variation of peak flow rate, improvement after bronchodilator, and airway calibre. Thorax. 1982;37:423–429. doi: 10.1136/thx.37.6.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neukirch F, Liard R, Segala C, Korobaeff M, Henry C, Cooreman J. Peak expiratory flow variability and bronchial responsiveness to methacholine. Am Rev Respir Dis. 1992;146:71–75. doi: 10.1164/ajrccm/146.1.71. [DOI] [PubMed] [Google Scholar]
- 10.Lebowitz MD, Krzyzanowski M, Quackenboss JJ, O’Rourke MK. Diurnal variation of PEF and its use in epidemiological studies. Eur Respir J. 1997;10 (suppl 24):49–56s. [PubMed] [Google Scholar]
- 11.Higgins BG, Britton JR, Chinn S, Cooper S, Burney PGJ, Tattersfield AE. Comparison of bronchial reactivity and peak expiratory flow variability measurements for epidemiologic studies. Am Rev Respir Dis. 1992;145:588–593. doi: 10.1164/ajrccm/145.3.588. [DOI] [PubMed] [Google Scholar]
- 12.Toogood JH, Andreaou P, Baskerville J. A methodological assessment of diurnal variability of peak flow as a basis for comparing different inhaled steroid formulations. J Allergy Clin Immunol. 1996;98:555–562. doi: 10.1016/s0091-6749(96)70089-1. [DOI] [PubMed] [Google Scholar]
- 13.Connolly CK. The effect of bronchodilators on diurnal rhythms in airway obstruction. Br J Dis Chest. 1981;75:197–203. doi: 10.1016/0007-0971(81)90053-x. [DOI] [PubMed] [Google Scholar]
- 14.Gannon PFG, Newton DT, Pantin CFA, Burge PS. Effect of the number of peak expiratory flow readings per day on the estimation of diurnal variation. Thorax. 1998;53:790–792. doi: 10.1136/thx.53.9.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Alonzo GE, Volker WS, Keller A. Measurements of morning and evening airflow grossly underestimate the circadian variability of FEV1 and peak expiratory flow rate in asthma. Am J Resp Crit Care Med. 1995;152:1097–1099. doi: 10.1164/ajrccm.152.3.7663789. [DOI] [PubMed] [Google Scholar]
- 16.Côté J, Cartier A, Malo J-L, Rouleau M, Boulet L-P. Compliance with peak expiratory flow monitoring in home management of asthma. Chest. 1998;113:968–972. doi: 10.1378/chest.113.4.968. [DOI] [PubMed] [Google Scholar]
- 17.Higgins BG, Britton JR, Chinn S, Jones TD, Jenkinson D, Burney PGJ, et al. The distribution of peak flow variability in a population sample. Am Rev Respir Dis. 1989;140:1368–1372. doi: 10.1164/ajrccm/140.5.1368. [DOI] [PubMed] [Google Scholar]
- 18.Clark THJ, Hetzel MR. Diurnal variation of asthma. Br J Dis Chest. 1977;71:87–92. doi: 10.1016/0007-0971(77)90087-0. [DOI] [PubMed] [Google Scholar]
- 19.Reddel HK, Ware SI, Salome CM, Jenkins CR, Woolcock AJ. Pitfalls in processing electronic spirometric data in asthma. Eur Respir J. 1998;12:853–858. doi: 10.1183/09031936.98.12040853. [DOI] [PubMed] [Google Scholar]
- 20.Reddel HK, Ware SI, Salome CM, Marks GB, Jenkins CR, Woolcock AJ. Standardization of ambulatory peak flow monitoring: the importance of recent beta 2-agonist inhalation. Eur Respir J. 1998;12:309–314. doi: 10.1183/09031936.98.12020309. [DOI] [PubMed] [Google Scholar]
- 21.Salome C, Reddel H, Ware S, Jenkins C, Woolcock A. Diurnal variability from twice daily PEF is unreliable for assessment of asthma severity. Am J Resp Crit Care Med. 1998;157:A631. [Google Scholar]
- 22.Dahl R, Earnshaw JS, Palmer JB. Salmeterol: a four week study of a long-acting beta-adrenoceptor agonist for the treatment of reversible airways disease. Eur Respir J. 1991;4:1178–1184. [PubMed] [Google Scholar]
- 23.Reddel HK, Ware SI, Marks GB, Salome CM, Jenkins CR, Woolcock AJ. Differences between asthma exacerbations and poor asthma control. Lancet. 1999;353:364–369. doi: 10.1016/S0140-6736(98)06128-5. . (Erratum. Lancet 1999;353:758.) [DOI] [PubMed] [Google Scholar]
- 24.Siersted HC, Hansen HS, Hansen N-CG, Hyldebrandt N, Mostgaard G, Oxhoj H. Evaluation of peak expiratory flow variability in an adolescent population sample. The Odense schoolchild study. Am J Resp Crit Care Med. 1994;149:598–603. doi: 10.1164/ajrccm.149.3.8118624. [DOI] [PubMed] [Google Scholar]
- 25.Turner-Warwick M. On observing patterns of airflow obstruction in chronic asthma. Br J Dis Chest. 1977;71:73–86. doi: 10.1016/0007-0971(77)90086-9. [DOI] [PubMed] [Google Scholar]
- 26.Cross D, Nelson HS. The role of the peak flow meter in the diagnosis and management of asthma. J Allergy Clin Immunol. 1991;87:120–128. doi: 10.1016/0091-6749(91)90223-b. [DOI] [PubMed] [Google Scholar]
- 27.Brand PL, Duiverman EJ, Postma DS, Waalkens HJ, Kerrebijn KF, Van Essen-Zandvliet EE. Peak flow variation in childhood asthma: relationship to symptoms, atopy, airways obstruction and hyperresponsiveness. Eur Respir J. 1997;10:1242–1247. doi: 10.1183/09031936.97.10061242. [DOI] [PubMed] [Google Scholar]
- 28.Côté J, Kennedy S, Chan-Yeung M. Quantitative versus qualitative analysis of peak expiratory flow in occupational asthma. Thorax. 1993;48:48–51. doi: 10.1136/thx.48.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reddel HK, Salome CM, Peat JK, Woolcock AJ. Which index of peak expiratory flow is most useful in the management of stable asthma? Am J Resp Crit Care Med. 1995;151:1320–1325. doi: 10.1164/ajrccm.151.5.7735580. [DOI] [PubMed] [Google Scholar]
- 30.Connolly C, Prescott R, Alcock S, Gatnash A. Actual over best function as an outcome measure in asthma. Respir Med. 1994;88:453–459. doi: 10.1016/s0954-6111(05)80049-6. [DOI] [PubMed] [Google Scholar]
- 31.Meijer RJ, Kerstjens HAM, Postma DS. Comparison of guidelines and self-management plans in asthma. Eur Respir J. 1997;10:1163–1172. doi: 10.1183/09031936.97.10051163. [DOI] [PubMed] [Google Scholar]
- 32.Fishwick D, Beasley R. Use of peak-flow based self-management plans by adult asthmatic patients. Eur Respir J. 1996;9:861–865. doi: 10.1183/09031936.96.09050861. [DOI] [PubMed] [Google Scholar]
- 33.Grol R, Dalhuijsen J, Thomas S, Veld C, Rutten G, Mokkink H. Attributes of clinical guidelines that influence use of guidelines in general practice: observational study. BMJ. 1998;317:858–861. doi: 10.1136/bmj.317.7162.858. [DOI] [PMC free article] [PubMed] [Google Scholar]