Abstract
The role of viral immediate-early (IE) gene expression in herpes simplex virus type 1 (HSV-1) latency was investigated. The HSV-1 multiple mutant in1312, defective for the expression of the virion transactivator VP16 and the IE proteins ICP0 and ICP4, was used as the parent for these studies. The coding sequences of the Escherichia coli lacZ gene, preceded by the encephalomyocarditis virus internal ribosome entry site, were inserted into the region of in1312 that encodes the latency-associated transcripts (LATs) such that transcription of the transgene was controlled by the LAT promoter. This insert has previously been shown to direct long-term latent-phase expression of β-galactosidase in a wild-type HSV-1 genome (R. H. Lachmann and S. Efstathiou, J. Virol. 71, 3197–3207, 1997). The resulting recombinant, in1388, was apathogenic after inoculation into mice via the footpad and did not detectably replicate in dorsal root ganglia (DRG) or footpads. Mutant in1388 established latency in DRG, and β-galactosidase was expressed in increasing numbers of neurons over the first 25 days of infection. During latency, more than 1% of neurons in ganglia that innervate the footpad expressed β-galactosidase, with the number of positive cells remaining constant for at least 5 months. Rescue of the VP16, ICP0, or ICP4 mutations of in1388 did not affect the number of β-galactosidase-expressing neurons detected during latency. The results demonstrate that HSV-1 mutants severely impaired for IE gene expression are capable of establishing latency and efficiently expressing a foreign gene product under control of the LAT promoter.
Infection with herpes simplex virus type 1 (HSV-1) normally results in productive replication of virus and death of the host cell. Neurons, however, are able to survive infection and retain the HSV-1 genome in a latent state for the lifetime of the host. Reactivation of latent virus and, in some instances, reappearance of disease occur in response to stimuli that cause stress to the neuron or to the host organism (reviewed in references 43, 54, 60).
Transcription of the HSV-1 genome is largely controlled by the immediate-early (IE) proteins ICP4 (Vmw175) and ICP0 (Vmw110) and by the virion protein VP16 (Vmw65 or α-TIF). ICP4 is a transcription activator that is absolutely required for productive infection. Early and late gene transcription does not occur after infection with virus mutants lacking functional ICP4 (10, 38, 62). ICP0 alters the intranuclear environment such that entry of HSV-1 into the lytic cycle is facilitated (17, 18). Infection at low multiplicity of infection (MOI) with viruses possessing mutations that inactivate ICP0 results in only a small proportion of infected cells supporting replication and most viral genomes being retained in a quiescent state (16, 40, 44, 45, 56, 57). The absence of ICP0 can, however, be overcome by carrying out infection at a high MOI (16, 44, 57). Transcription of the IE genes is stimulated by VP16, a component of the incoming virus particle which interacts with the cell factors Oct-1 and HCF to form a multiprotein complex at the TAATGARAT (R is a purine nucleotide) sequences found in all IE promoters (reviewed in reference 36). The virus mutant in1814, which expresses nonfunctional VP16, exhibits a phenotype similar to that of ICP0 mutants, with most cells infected at low MOI failing to initiate early or late gene expression and retaining the HSV-1 genome in a quiescent state (1, 23). The absence of functional ICP4, ICP0, or VP16 therefore arrests the HSV-1 lytic cycle at early stages.
During latency in humans or animals, gene expression characteristic of productive replication cannot be detected; instead only one portion of the genome, located within the long repeat (RL) region, is transcribed to yield the latency-associated transcripts (LATs) (20, 55). The factors controlling the repression of viral lytic gene expression and the selective transcription of the LATs are unclear. It is thought that the lytic and latent outcomes of infection are mutually exclusive and that a major commitment to one or the other pathway is made early after infection (30, 33, 52). The exact point at which the pathways diverge is not known at present, although studies using virus mutants in animal models of latency point to a major early decision, at the level of IE gene expression or IE protein function. ICP4 is not required for latency or production of LATs, since viral DNA and neurons containing LATs can be detected after infection with viral ICP4 deletion mutants (11, 26, 49). It was found that mutant in1814, defective for VP16 function, established latency in mice apparently as efficiently as wild-type HSV-1 when comparisons were made on the basis of PFU in the inoculum, demonstrating that functional VP16 is not absolutely required for latency (12, 53). Similarly, viral mutants lacking functional ICP0 were able to establish latency and to reactivate, although they were attenuated for replication in mice (4, 7, 31). For VP16- and ICP0-deficient mutants, however, the quantitative assessment of latency establishment is imprecise because the mutants are impaired for replication at the site of inoculation and in ganglia. In addition, only 0.1 to 1.0% of virus particles in a preparation can form plaques, compared with the number obtained when VP16 or ICP0 is provided exogenously to complement the effects of the mutations (1, 16, 24, 56). For example, in experiments in which animals were inoculated with equivalent doses (as PFU) of in1814 or a revertant, two factors would have affected the amount of virus reaching the sensory ganglia. First, the mutant inoculum contained 100- to 1,000-fold more viral genomes than the revertant inoculum, and second, the revertant replicated more efficiently than the mutant at the site of inoculation, compensating to some extent for the lower number of genomes added. In addition, replication in the neuron and spread in the nervous system may have affected the ultimate number of cells harboring latent viral genomes (8, 13, 27, 58). Therefore, when a VP16 or ICP0 mutant is compared with a wild-type or revertant virus, differences in replication at the periphery or in the ganglion may obscure true effects of the genetic defect on the establishment of latency. In view of these factors, although it is clear that latency can be established in the absence of VP16, ICP0, or ICP4, it has not been possible to make a quantitative evaluation of the contribution of these proteins to the establishment of latency in terms of events within the neuron.
One way of overcoming the complexities introduced by differential replication of mutant and wild-type viruses in experimental animals is the use of in vitro model systems to study latency. In one approach, primary cultures of sympathetic or sensory neurons are infected with HSV-1 in the presence of acyclovir to prevent virus replication. Latency is established, provided nerve growth factor (NGF) is present in the cell culture medium, and many cells express LATs (50, 63, 64). Upon withdrawal of NGF or upon activation of signal transduction pathways in the presence of NGF, reactivation occurs and virus replication rapidly resumes (51, 63, 64, 66). A second approach has been to infect human fibroblasts with mutants lacking VP16 and/or ICP0 function (23, 24, 40, 45, 46, 56). In these cases, the majority of viral genomes do not initiate productive replication and are retained in a quiescent state that resembles latency in many respects. The viral genome is sequestered in a nonlinear configuration, as in vivo, and no gene expression is detected; indeed promoters in the viral genome become repressed within the first 24 h after infection (40, 45). Reversal of repression in fibroblasts requires the presence of ICP0 (18, 40, 45, 56). There is a major difference between the interaction of HSV-1 with the host cell in the two model systems in terms of the effect of ICP0. In cultured neurons, viruses with mutations that inactivate ICP0 established latency 1,000-fold less efficiently than wild-type virus, on a virus particle basis (65), whereas in fibroblasts the absence of ICP0 aids the retention of quiescent viral genomes due to reduction of cytotoxicity and increased propensity to enter the quiescent state (24, 39, 40, 45, 46). The difference in the requirement for ICP0 forms a point of focus which distinguishes the two model latency systems.
There is considerable interest in the potential use of HSV-1 as a vector for gene therapy, particularly for the treatment of neurological diseases (reviewed in references 14, 22, and 29). The ability of latent virus to be retained for the lifetime of an individual, coupled with the use of the LAT promoter to achieve long-term expression of foreign gene products, is central to this approach. Problems arise in the development of HSV-1-derived vectors, however, due to the toxicity of HSV-1 for mammalian cells and the complexity of the LAT transcription unit. Cytotoxicity is due mainly to the expression of viral proteins in the infected cell, and it has been shown that IE proteins are toxic when introduced by transfection of plasmids or infection with viral mutants which express only the IE genes (25, 39, 67). Toxicity can be overcome by severely reducing IE gene expression, and viruses with mutations in VP16 and IE genes are promising vehicles for the development of gene therapy vectors (39, 45). The problem of long-term expression of foreign gene products has recently been addressed by a novel modification of the LAT region (28). Insertion of a reporter gene cassette (lacZ or a fusion of lacZ and neomycin phosphotransferase named β-geo) at a position 1.5 kbp downstream of the 5′ end of the primary LAT transcript resulted in the maintenance of all cis-acting sequence elements for authentic latent expression. By linking the transgene to the internal ribosome entry site (IRES) of encephalomyocarditis virus, it was possible to achieve efficient translation of the resulting transcript, which contains a long 5′ leader sequence. Upon inoculation of mice with such a recombinant virus, expression of β-galactosidase activity was detected only at low levels in dorsal root ganglion (DRG) neurons during the early stages of infection but increased during the establishment of latency, as would be expected for a transgene under authentic latent control.
In the experiments reported here, we have introduced the IRES–β-geo construct into the LAT region of a multiply defective HSV-1 mutant impaired for the production of functional VP16, ICP0, and ICP4 and have analyzed β-galactosidase expression after inoculation into mice. The experiments were designed to answer three questions. First, can latency be established efficiently after infection of mice with a multiply defective virus that is unable to replicate at the site of inoculation or in the sensory ganglia that innervate that site? Second, do the viral transactivator proteins affect the establishment of latency and/or the expression of LATs? Inherent in this question is a resolution of whether ICP0 is required for efficient establishment of latency in vivo, as it is in cultured neurons, or if ICP0 is not required, as found in studies with fibroblasts. Third, can long-term gene expression in neurons be achieved by use of the IRES–β-geo construct inserted into the LAT region of an HSV-1 mutant defective for IE gene expression? The final question is relevant to the potential for using multiply defective mutants as starting points for the construction of HSV-1 vectors for long-term gene expression in neurons.
MATERIALS AND METHODS
Plasmids.
Plasmid pSLAT1βgeo contains IRES–β-geo (with the Moloney murine leukemia virus long terminal repeat terminator) cloned between the HpaI sites in the major LATs (nucleotides 120,300 and 120,466 in the inverted long repeat [37]), as described by Lachmann and Efstathiou (28). Plasmid pGX158 is the HSV-1 BamHI f fragment, containing VP16 coding sequences, cloned into pAT153, and pGX58 is the HSV-1 XhoI c fragment, which contains the ICP4 coding sequences, cloned into the XhoI site of pMK16. Plasmid pAR28 was prepared by cloning a 4,596-bp HpaI/SstI fragment (nucleotides 120,466 to 125,062) from pCP2461 (41) between the SstI and HincII sites of pUC18.
Cells.
Baby hamster kidney (BHK) cells were grown in Eagle medium supplemented with 10% newborn calf serum, 10% tryptose phosphate, and 100 U of penicillin and 100 μg of streptomycin per ml (ETC10). Human osteosarcoma U2OS cells were propagated in Dulbecco medium supplemented with 5% fetal calf serum, 5% newborn calf serum, and 100 U of penicillin and 100 μg of streptomycin per ml.
Construction of recombinant viruses.
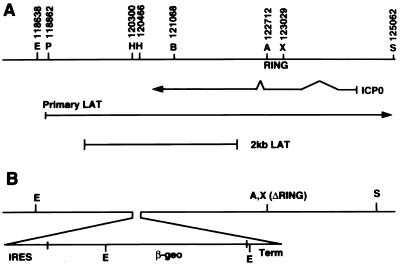
The locations of restriction endonuclease cleavage sites and fragments used for virus construction are shown in Fig. 1. HSV-1 mutants tsK and in1312 have been described previously (9, 38, 41). To construct in1388, pSLAT1βgeo was cleaved with XhoI and ScaI and transfected into BHK cells together with DNA isolated from in1312. To identify recombinants containing the IRES–β-geo insert, DNA was prepared from pooled or single plaque isolates and cleaved with EcoRV and XhoI. DNA samples were analyzed by Southern hybridization with the 4,166-bp PstI/XhoI fragment (nucleotides 118,862 to 123,029) from pJR3 (15) as the probe. After four rounds of plaque purification and screening by Southern hybridization, an isolate containing 3,425- and 2,250-bp fragments from pSLAT1βgeo, with no detectable 4,074-bp fragment from the in1312 parent, was obtained and named in1388. To rescue the ICP0 mutation, in1388 DNA was cotransfected with HindIII-cleaved pAR28 and DNA from plaque isolates was prepared. Samples were cleaved with BstEII and XhoI and probed with a 1,961-bp fragment (nucleotides 121,068 to 123,029) from pAR28, which hybridized to a 1,674-bp fragment from in1388 and the 1,961-bp fragment itself from the rescuant (the difference represents the 317-bp deletion of the RING domain). An isolate with no detectable in1388-derived fragment was named in1365. Restoration of ICP0 function was confirmed by the demonstration that superinfection with in1365 reversed the quiescent state of another in1312-based virus (40; C. M. Preston, unpublished observations). The VP16 mutation was rescued by cotransfection of in1388 DNA with HindIII-cleaved pGX158 and subsequent screening for loss of the BamHI site in the VP16 coding sequences (1). A pure isolate was named in1366. Infection with in1366 activated an HSV-1 IE promoter present in the in1312 genome, demonstrating that VP16 function was restored (C. M. Preston, unpublished observations). The ICP4 mutation was rescued by cotransfection of in1388 DNA with XhoI-cleaved pGX58 and plaque purification of viruses capable of replication at 38.5°C. An isolate that formed plaques equally efficiently at 38.5 and 31°C was named in1368. The properties of the mutants used are summarized in Table 1.
FIG. 1.
Structure of in1388 in RL. (A) Organization of the inverted long repeat from nucleotides 118,000 to 125,000, including the locations of the ICP0 mRNA, the primary LAT (terminating outside the region represented), and the stable 2-kb species. (B) Insertion of IRES–β-geo and the Moloney murine leukemia virus long terminal repeat terminator (Term), plus the deletion of the ICP0 RING domain. Restriction sites used for cloning and for preparing probes are labelled as follows: E, EcoRV; P, PstI; H, HpaI; B, BstEII; A, Asp 718; X, XhoI; S, SstI. The PstI site at nucleotide 118,659 and the BstEII sites at nucleotides 119,194 and 120,091 are not shown.
TABLE 1.
Characteristics of mutants used
| Mutant | Mutations |
|---|---|
| in1312 | VP16−, ICP0−, tsICP4 |
| in1388 | VP16−, ICP0−, tsICP4, IRES–β-geo insertiona |
| in1365 | VP16−, tsICP4, IRES–β-geo insertion |
| in1366 | ICP0−, tsICP4, IRES–β-geo insertion |
| in1368 | VP16−, ICP0−, IRES–β-geo insertion |
The IRES–β-geo insertion was 1.5 kbp downstream from the start site of the primary LAT, resulting in failure to produce the major 2-kb transcript.
Virus assay.
The titer of in1388 was measured by plaque formation on BHK cells at 31°C in the presence of 3 mM hexamethylene bisacetamide (HMBA) (34). Direct comparison of the titers of rescuants with that of in1388 by this method is not informative, because the presence of VP16 or ICP0 results in more-efficient plaque formation per virus particle of the inoculum. A method described by Cai and Schaffer, based on estimation of viral DNA in virus preparations, was therefore used for comparison between mutants (3). A sample of each virus preparation was mixed with a fixed amount of in1332, a mutant containing an insertion of Escherichia coli lacZ in the thymidine kinase (TK) coding sequences (39). DNA was prepared from the mixtures, cleaved with EcoRI, and analyzed by Southern hybridization, with the HSV-1 EcoRI n fragment as a probe. DNA from in1388 and rescuants gave a single 2.4-kbp band (EcoRI n itself), whereas in1382 DNA gave 1.85- and 1.0-kbp bands due to the lacZ insertion. Quantification was achieved by phosphorimage analysis of autoradiographs and comparison of the signals from the in1388-derived mutants with the signal from the in1382 internal control. In a further test, mutants were titrated on U2OS cells, on which ICP0-deficient and VP16-deficient mutants form plaques with normal efficiency (C. M. Preston, unpublished observations; 68), at 31°C in the presence of 3 mM HMBA. The relative titers from this approach agreed well with the values obtained by hybridization. In the experiments presented here, the titer of in1388 is presented as the value on BHK cells with 3 mM HMBA present, and equivalent amounts of the other mutants were injected, with the DNA contents of inocula as the basis for normalization.
Animal experiments.
Five-week-old female BALB/c mice were infected unilaterally via the right rear footpad with 25 μl of cell-released virus suspension, diluted in ETC10. At various times postinfection the ipsilateral DRG from lumbar levels L1 to L6 were partially dissected within a hemiblock of vertebral column from which the spinal cord and spinal nerve trunks had been removed, uncovering the underlying DRG within the intervertebral foramina. Subsequent fixing (4% paraformaldehyde in phosphate-buffered saline [PBS] for 1 h on ice), washing (twice for 15 min in ice-cold PBS), and a whole-mount β-galactosidase assay (overnight incubation at 37°C in a staining solution consisting of 1 mg of 5-bromo-4-chloro-3-indolyl-β-d-galactoside/ml in PBS containing 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 2 mM MgCl2, 0.1% [vol/vol] NP-40, 0.1% [wt/vol] sodium deoxycholate) was performed with the DRG in situ. DRG were removed from the intervertebral foramina for counting blue (β-galactosidase-expressing) neurons, after clarification by immersion overnight in PBS containing 20% (vol/vol) glycerol. Individual DRG were placed between two microscope slides for photography at ×45 magnification without counterstaining. Feet were dissected from mice proximal to the ankle joint and frozen at −70°C prior to processing. Individual feet were minced with fine scissors, disrupted in glass homogenizers, and homogenized again in 1 ml of ETC10. The homogenate was transferred to screw-cap vials and subjected to two cycles of freezing and thawing at −70 and 37°C. Samples were sonicated and titrated on U2OS cells at 31°C with 3 mM HMBA present. DRG from each mouse from levels L3 to L5 were dissected, pooled into 400 μl of ETC10 in glass screw-cap vials, and stored at −70°C. Samples were thawed and homogenized on ice with an Omni μH hand-held homogenizer (Camlab Ltd.). Homogenates were sonicated briefly and transferred to 1.5-ml microcentrifuge tubes, and cell debris was pelleted. Supernatants were titrated on U2OS cells at 31°C, with 3 mM HMBA present.
RESULTS
Construction of HSV-1 mutant in1388.
The starting point for the studies presented here was mutant in1312, which contains three mutations: an inactivating insertion in the coding sequences for VP16, a deletion of the RING domain of ICP0, and the ICP4 temperature-sensitive (ts) mutation derived from tsK (41). The last is a tight mutation which reduces virus replication by approximately 105-fold at 38°C, the core temperature of the mouse (35, 61; C. M. Preston, unpublished observations). In preliminary experiments, mice were inoculated via the footpad with 106 PFU of tsK and DRG were screened for the presence of virus, by sonication and titration at 31°C, 1 and 4 days later. No virus was detected at either time, demonstrating that the ts mutation effectively inactivated the function of ICP4 in ganglia. Plasmid pSLAT1βgeo, containing the IRES–β-geo cassette in the LAT region, was recombined with in1312 DNA, and progeny plaques were screened by Southern hybridization through four rounds of purification until no parental sequences were detectable on long autoradiographic exposures, indicating that the insertion was present in both copies of RL. The resulting virus was named in1388.
Latent expression of β-galactosidase by in1388.
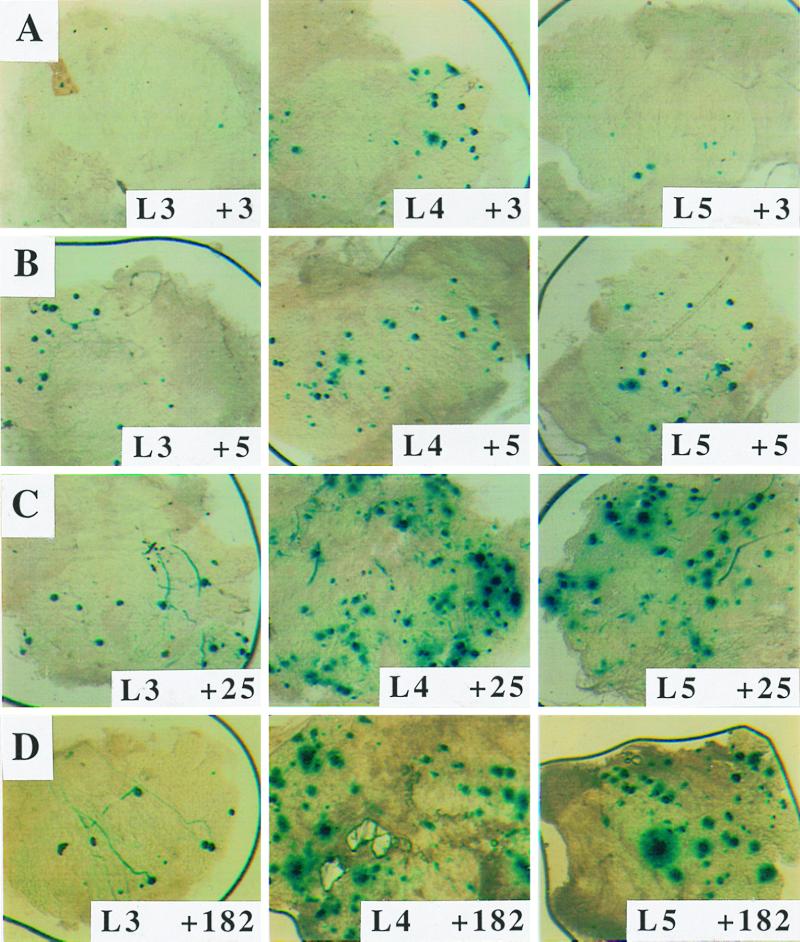
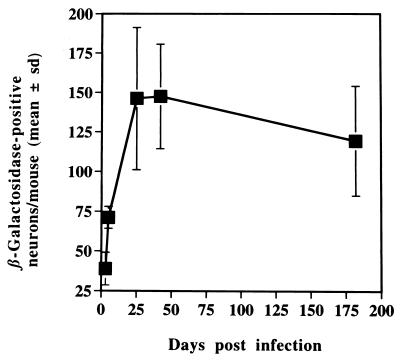
Mice were inoculated with 8 × 105 PFU of in1388, and ganglia were examined by histochemical staining for β-galactosidase at various times (Fig. 2 and 3). This assay quantifies the establishment of latency by monitoring the activity of the LAT promoter to direct the synthesis of transcripts containing the β-geo sequences. Expression of β-galactosidase was readily detected in L3, L4, and L5 DRG, and the number of positive cells increased through 3, 5, and 25 days, remaining at undiminished levels until at least 6 months postinoculation. Only ganglia containing neurons which innervate the footpad were positive, and staining was observed exclusively in cells which morphologically resembled neurons. No β-galactosidase was detected in ganglia innervating the uninoculated foot. The relationship between input virus dose and number of positive neurons was investigated (Table 2). A 10-fold reduction of in1388 dose, to 8 × 104 PFU per mouse, gave an approximately 2-fold decrease in the number of expressing neurons, and a further 10-fold reduction to 8 × 103 PFU per mouse gave numbers additionally 6-fold lower, close to the limit of reliable quantification.
FIG. 2.
Expression of β-galactosidase in DRG neurons. Mice were infected with 8 × 105 PFU of in1388, and L3, L4, and L5 DRG were histochemically stained for the presence of β-galactosidase at 3 (A), 5 (B), 25 (C), and 182 (D) days after inoculation.
FIG. 3.
Time course of β-galactosidase expression. Mice were infected with 8 × 105 PFU of in1388, and the positive neurons in DRG were counted at various times. The values for L3, L4, and L5 DRG combined per mouse are presented, with standard deviations (sd) shown.
TABLE 2.
Effect of input dose on expression of β-galactosidase
| Dosea (PFU) | β-Galactosidase-expressing neurons
|
|
|---|---|---|
| No. (SD)b | Range (no. of mice) | |
| 8 × 105 | 160 (39) | 99–217 (8) |
| 8 × 104 | 84 (27) | 45–126 (7) |
| 8 × 103 | 14 (7) | 9–23 (4) |
Mice were inoculated with various amounts of in1388 in a total volume of 25 μl.
The mean number of β-galactosidase-expressing neurons per mouse in L3, L4, and L5 DRG was determined 42 days after infection.
Effects of rescuing mutations of in1388.
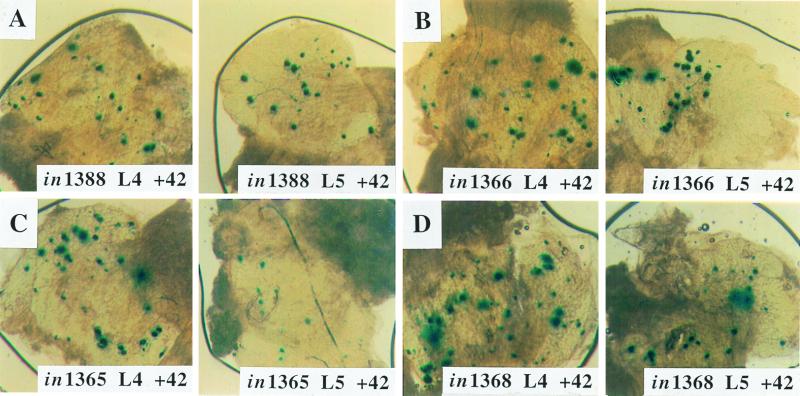
To assess whether VP16, ICP0, or ICP4 functions affected the establishment of latency, the mutations in the genes encoding these proteins were rescued, giving viruses in1365 (ICP0 rescued), in1366 (VP16 rescued), and in1368 (ICP4 rescued). Mice were inoculated with 8 × 104 or 8 × 103 PFU of in1388 or equivalent amounts of the rescuants, and β-galactosidase-positive neurons were counted at 42 days postinfection (Fig. 4 and Table 3). The doses were chosen because, as shown in Table 2, they represent points at which the dose-response curve was almost linear. In all cases, the numbers of positive neurons in animals inoculated with the rescuants were indistinguishable from those of animals inoculated with in1388, and no differences in intensity of staining were observed.
FIG. 4.
Expression of β-galactosidase by rescuants of in1388. Mice were infected with 8 × 104 PFU of in1388 (A) and an equivalent amount of in1365 (B), in1366 (C), or in1368 (D). DRG were stained for the presence of β-galactosidase at 42 days after inoculation. The L4 and L5 DRG are shown.
TABLE 3.
Expression of β-galactosidase after inoculation with rescuants of in1388
| Mutant | Dosea | β-Galactosidase-expressing neurons
|
|
|---|---|---|---|
| No. (SD)b | Range (no. of mice) | ||
| in1388 | A | 70 (33) | 39–166 (16) |
| in1365 | A | 94 (42) | 29–144 (8) |
| in1366 | A | 66 (28) | 24–144 (8) |
| in1368 | A | 66 (15) | 47–98 (8) |
| in1388 | B | 16 (11) | 5–44 (16) |
| in1365 | B | 21 (11) | 0–38 (10) |
| in1366 | B | 14 (11) | 0–39 (10) |
| in1368 | B | 17 (10) | 5–30 (8) |
Mice were inoculated with 8 × 104 PFU (A) or 8 × 103 PFU (B) of in1388 or with an equivalent amount of rescuants, in a total volume of 25 μl.
The mean number of β-galactosidase expressing neurons per mouse in L3, L4, and L5 DRG was determined 42 days after inoculation.
Absence of detectable in1388 replication in DRG or footpads.
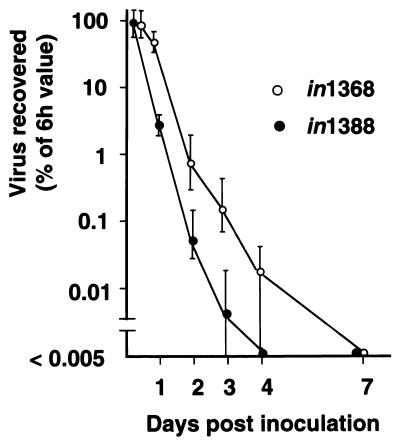
The retention and possible replication of in1388 in the footpad and ganglion were examined and compared with those of in1368, in which ICP4 is fully functional (Fig. 5). The titer of in1388, measured by titration on U2OS cells in the presence of 3 mM HMBA, declined rapidly from 6 h postinfection (a time before virus replication would be expected), with no virus detectable by 4 days postinoculation. The titer of in1368 also declined, but less rapidly than that of in1388 such that low levels of virus were present at 4 days, but not at 7 days, postinoculation. Infectious virus could not be detected in DRG at any time after infection with in1388. For in1368-inoculated mice, all ganglion samples were negative with the exception of pooled DRG from one animal of four at 3 days postinfection (1 PFU in L3, L4, and L5 combined) and two animals of four at 4 days postinfection (1 PFU in each animal). Therefore, no in1388 and only very low levels of in1368 were detected in DRG, indicating that extensive replication of mutants, even when ICP4 was functional, did not occur. In the footpad, the decline in the titer of in1388 was consistent with clearance of the initial inoculum without significant increase in virus load due to replication, suggesting that the inoculum provided the source of virus to nerve termini. The lower rate of decline of in1368 suggested that, as expected, limited replication of this mutant occurred but, again, that the amount of virus in the footpad was primarily determined by the input inoculum.
FIG. 5.
Virus titers in footpads. Mice (four per point) were inoculated with 8 × 104 PFU of in1388 or an equivalent amount of in1368. At various times after inoculation (the first point, defined as 100%, was at 6 h), feet were homogenized and virus titers on U2OS cells in the presence of 3 mM HMBA were determined. The ranges of values are shown.
DISCUSSION
We describe HSV-1 mutant in1388, which is severely impaired for the production of IE gene products and additionally contains an insertion of IRES–β-geo in the LAT region, thereby allowing latently infected neurons to be detected by the histochemical staining of ganglia. After inoculation of in1388 into the mouse footpad, the numbers of neurons expressing β-galactosidase increased up to 25 days postinfection and remained constant over a further 5 months. Therefore, as found with a wild-type virus containing the IRES–β-geo construct (LβB), transgene expression was controlled by a latency-active promoter (28). The numbers of β-galactosidase-expressing neurons after inoculation with the two higher doses of in1388 used were comparable to those observed in corresponding experiments using LβB and ear inoculation (28), although in1388 gave much greater consistency between mice, probably due to the absence of virus replication at the periphery or in the ganglion.
To a first approximation, in1388 established latency in the DRG with an efficiency comparable to that of a VP16 mutant (in1851), which is unable to replicate in neurons due to an insertion that disrupts the TK coding sequences (12), since the number of β-galactosidase-positive neurons after inoculation with in1388 is similar to the number of LAT-containing cells in in1851-infected mice when the same routes of inoculation and approximately equal titers of virus are used. Between 1 and 2% of DRG neurons (based on a value of 10,000 neurons in L3, L4, and L5 DRG [12]) expressed β-galactosidase by 25 days postinoculation, although this value underestimates the proportion of positive neurons in the infected population. Many DRG neurons project to parts of the limb other than the foot, and even within the foot not all nerve endings would be exposed to the inoculum. The large amount of virus that can be injected with apathogenic mutants can compensate to a considerable extent for the absence of input virus amplification by replication, although in other studies the numbers of LAT-positive neurons were greater than 1 to 2% after inoculation of replication-competent HSV-1 (12, 33). The absence of detectable virus in the DRG shows that the combination of VP16, ICP0, and ICP4 mutations prevents progression to the lytic route of infection; indeed, the VP16 and ICP0 mutations together, as in in1368, are sufficient to block replication in the footpad and ganglion almost completely. It appears, therefore, that in1388 genomes reaching the sensory neurons are exclusively directed to latency and that the lytic route of infection is not operative. It would not be expected that the disruption of the LAT region is an important factor affecting the establishment of latency by in1388, since recent studies suggest that any early functions of LATs in establishment of latency are concerned with prevention of lytic replication, possibly by interference with IE gene expression and function, thereby preventing death of neurons due to virus replication (5, 21, 32, 47, 59). In the absence of ICP0 and ICP4, infection would not proceed as far as the expression of IE gene products and thus this property of LATs would not be relevant.
The results formally demonstrate that, as in cultured neurons (65), a functional ICP0 is not essential for the activity of the LAT promoter in mice, a point that has not been made previously since all ICP0 mutants used to date for animal latency studies also have all or part of LATs deleted.
It is important to note that our studies use the activity of the LAT transcription unit as a measure of latency establishment. Reactivation has not been investigated, although this parameter of latency would be difficult to address quantitatively because the debilitating nature of the mutations present in the in1388 genome would severely reduce virus replication once reactivation had occurred.
The data presented here strongly suggest that no peripheral replication is required for efficient latency establishment. Although we cannot exclude the possibility that very limited replication of in1388 occurred in the foot, the effect of progeny from such replication would be negligible compared with the large amount of virus delivered in the inoculum. Even with ICP4 function fully restored, as in in1368, viral titers in the foot declined over 4 days after inoculation. If the differences in titers between the two curves of Fig. 5 are considered to be a measure of the amount of new virus produced by replication of in1368, it can be calculated that replication increased the virus load provided by the input inoculum by no more than 50%.
The establishment of latency was not detectably affected by restoration of VP16, ICP0, or ICP4 coding sequences. This observation is compatible with the view that the natural block to lytic gene expression is at the IE level, since if later functions were required, enhancement of IE gene expression would be expected to increase the establishment of latency. The lack of effect of VP16 was not surprising, since previous studies have suggested that this protein does not function in neurons (48). Functional ICP4 represses LAT expression during lytic infection of neurons (19), but no differences in the expression of β-geo by in1388 and in1368 were observed, again emphasizing that initiation of the lytic pathway of infection is not operational with the mutants described here. Repair of the ICP0 deletion did not affect the establishment of latency in our assay, showing that ICP0 is not required for the establishment of stable, LAT-positive latency. It is important to note, however, that our results show only the absence of major effects on the establishment of latency and that the variation between animals precludes detection of small differences between the behavior of mutants.
The finding that multiply impaired mutants establish latency efficiently and that ICP0 did not affect this process supports the view that latency arises when IE gene expression (or function) is insufficient to trigger the lytic cycle, as found after infection of fibroblasts with mutants impaired for VP16, ICP0, and ICP4 function (40, 45). These observations are not apparently compatible with the strong reduction in HSV-1 genome retention in cultured neurons when ICP0 is absent (65). It should be noted, however, that neurons in culture may produce more ICP0 than the cells in vivo, because VP16 is added with the inoculum to cultures whereas transactivation is thought not to occur in the animal (48). On the other hand, the quiescent state reached by VP16, ICP0, and ICP4 triple mutants in fibroblasts appears to be analogous to latency by in1388 in vivo, differing only in expression from the LAT promoter, which is controlled by neuron-specific elements (2, 6, 69). The relevance of cell culture models to latency in vivo is discussed in a recent review (42).
The experiments described here show that the advantage of long-term expression, achieved by use of the IRES–β-geo insertion in the LAT locus, can be harnessed in a multiple mutant in which cytotoxicity and other adverse effects of IE proteins are largely eliminated. This result provides “proof of principle” that HSV-1 mutants severely impaired for the three major transactivators can direct long-term expression of a foreign protein under latent control, provided that the appropriate construction is made in the LAT region. Previous studies showed that the LAT promoter, when present in a wild-type HSV-1 genome, is active in central nervous system neurons which connect to the relevant ganglionic sites after peripheral inoculation (28). The highly attenuated nature of in1388 suggests that injection of this virus into specific areas of the brain may result in long-term expression of β-galactosidase, which would represent an important advance in the development of therapeutically useful HSV-1 vectors.
ACKNOWLEDGMENTS
We acknowledge support from the Medical Research Council to R.H.L. and S.E.
K. R. Marshall, A. Rinaldi, and C. M. Preston are members of the Medical Research Council Virology Unit.
REFERENCES
- 1.Ace C I, McKee T A, Ryan J M, Cameron J M, Preston C M. Construction and characterization of a herpes simplex virus type 1 mutant unable to transinduce immediate-early gene expression. J Virol. 1989;63:2260–2269. doi: 10.1128/jvi.63.5.2260-2269.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Batchelor A H, O'Hare P. Localization of cis-acting sequence requirements in the promoter of the latency-associated transcript of herpes simplex virus type 1 required for cell type-specific activity. J Virol. 1992;66:3573–3582. doi: 10.1128/jvi.66.6.3573-3582.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai W, Schaffer P A. A cellular function can enhance gene expression and plating efficiency of a mutant defective in the gene for ICP0, a transactivating protein of herpes simplex virus type 1. J Virol. 1991;65:4078–4090. doi: 10.1128/jvi.65.8.4078-4090.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai W, Astor T L, Liptak L M, Cho C, Coen D M, Schaffer P A. The herpes simplex virus type 1 regulatory protein ICP0 enhances viral replication during acute infection and reactivation from latency. J Virol. 1993;67:7501–7512. doi: 10.1128/jvi.67.12.7501-7512.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen S-H, Kramer M F, Schaffer P A, Coen D M. A viral function represses accumulation of transcripts from productive-cycle genes in mouse ganglia latently infected with herpes simplex virus. J Virol. 1997;71:5878–5884. doi: 10.1128/jvi.71.8.5878-5884.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X, Schmidt M C, Goins W F, Glorioso J C. Two herpes simplex virus type 1 latency-active promoters differ in their contributions to latency-associated transcript expression during lytic and latent infections. J Virol. 1995;69:7899–7908. doi: 10.1128/jvi.69.12.7899-7908.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clements G B, Stow N D. A herpes simplex virus type 1 mutant containing a deletion within immediate early gene 1 is latency-competent in mice. J Gen Virol. 1989;70:2501–2506. doi: 10.1099/0022-1317-70-9-2501. [DOI] [PubMed] [Google Scholar]
- 8.Coen D M, Kosz-Vnenchak M, Jacobson J G, Leib D A, Bogard C L, Schaffer P A, Tyler K L, Knipe D M. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc Natl Acad Sci USA. 1989;86:4736–4740. doi: 10.1073/pnas.86.12.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davison M-J, Preston V G, McGeoch D J. Determination of the sequence alteration in the DNA of the herpes simplex virus type 1 temperature-sensitive mutant ts K. J Gen Virol. 1984;65:859–863. doi: 10.1099/0022-1317-65-5-859. [DOI] [PubMed] [Google Scholar]
- 10.DeLuca N A, Schaffer P A. Activation of immediate-early, early, and late promoters by temperature-sensitive and wild-type forms of herpes simplex virus type 1 protein ICP4. Mol Cell Biol. 1985;5:558–570. doi: 10.1128/mcb.5.8.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dobson A T, Margolis T P, Sederati F, Stevens J G, Feldman L T. A latent, nonpathogenic HSV-1-derived vector stably expresses β-galactosidase in mouse neurons. Neuron. 1990;5:353–360. doi: 10.1016/0896-6273(90)90171-b. [DOI] [PubMed] [Google Scholar]
- 12.Ecob-Prince M S, Preston C M, Rixon F J, Hassan K, Kennedy P G E. 1814. J. Gen. Virol. 74:985–994. 1993. Neurons containing latency-associated transcripts are numerous and widespread in dorsal root ganglia following footpad inoculation of mice with herpes simplex virus type 1 mutant. [DOI] [PubMed] [Google Scholar]
- 13.Efstathiou S, Kemp S, Darby G K, Minson A C. The role of herpes simplex virus type 1 thymidine kinase in pathogenesis. J Gen Virol. 1989;70:869–879. doi: 10.1099/0022-1317-70-4-869. [DOI] [PubMed] [Google Scholar]
- 14.Efstathiou S, Minson A C. Herpes virus-based vectors. Br Med Bull. 1995;51:45–55. doi: 10.1093/oxfordjournals.bmb.a072952. [DOI] [PubMed] [Google Scholar]
- 15.Everett R D. Transactivation of transcription by herpes virus products: requirement for two HSV-1 immediate-early polypeptides for maximum activity. EMBO J. 1984;3:3135–3141. doi: 10.1002/j.1460-2075.1984.tb02270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Everett R D. Construction and characterisation of herpes simplex virus type 1 mutants with defined lesions in immediate early gene 1. J Gen Virol. 1989;70:1185–1202. doi: 10.1099/0022-1317-70-5-1185. [DOI] [PubMed] [Google Scholar]
- 17.Everett R D, Freemont P, Saitoh H, Orr A, Kathoria M, Parkinson J. The disruption of ND10 during herpes simplex virus infection correlates with the Vmw110- and proteasome-dependent loss of several PML isoforms. J Virol. 1998;72:6581–6591. doi: 10.1128/jvi.72.8.6581-6591.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Everett R D, Orr A, Preston C M. A viral activator of gene expression functions via the ubiquitin-proteasome pathway. EMBO J. 1998;17:7161–7169. doi: 10.1093/emboj/17.24.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farrell M J, Margolis T P, Gomes W A, Feldman L T. Effect of the transcription start region of the herpes simplex virus type 1 latency-associated transcript promoter on expression of productively infected neurons in vivo. J Virol. 1994;68:5337–5343. doi: 10.1128/jvi.68.9.5337-5343.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fraser N W, Block T M, Spivack J G. The latency-associated transcripts of herpes simplex virus: RNA in search of a function. Virology. 1992;191:1–8. doi: 10.1016/0042-6822(92)90160-q. [DOI] [PubMed] [Google Scholar]
- 21.Garber D A, Schaffer P A, Knipe D M. A LAT-associated function reduces productive-cycle gene expression during acute infection of murine sensory neurons with herpes simplex virus type 1. J Virol. 1997;71:5885–5893. doi: 10.1128/jvi.71.8.5885-5893.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glorioso J C, DeLuca N A, Fink D J. Development and application of herpes simplex virus vectors for human gene therapy. Annu Rev Microbiol. 1995;49:675–710. doi: 10.1146/annurev.mi.49.100195.003331. [DOI] [PubMed] [Google Scholar]
- 23.Harris R A, Preston C M. 1814. J. Gen. Virol. 72:907–913. 1991. Establishment of latency in vitro by the herpes simplex virus type 1 mutant. [DOI] [PubMed] [Google Scholar]
- 24.Jamieson D R S, Robinson L H, Daksis J I, Nicholl M J, Preston C M. Quiescent viral genomes in human fibroblasts after infection with herpes simplex virus Vmw65 mutants. J Gen Virol. 1995;76:1417–1431. doi: 10.1099/0022-1317-76-6-1417. [DOI] [PubMed] [Google Scholar]
- 25.Johnson P A, Wang M J, Friedmann T. Improved cell survival by the reduction of immediate-early gene expression in replication-defective mutants of herpes simplex virus type 1 but not by mutation of the virion host shutoff function. J Virol. 1994;68:6347–6362. doi: 10.1128/jvi.68.10.6347-6362.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katz J P, Bodin E T, Coen D M. Quantitative polymerase chain reaction analysis of herpes simplex virus DNA in ganglia of mice infected with replication-incompetent mutants. J Virol. 1990;64:4288–4295. doi: 10.1128/jvi.64.9.4288-4295.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kramer M F, Coen D M. Quantification of transcripts from the ICP4 and thymidine kinase genes in mouse trigeminal ganglia latently infected with herpes simplex virus. J Virol. 1995;69:1389–1399. doi: 10.1128/jvi.69.3.1389-1399.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lachmann R H, Efstathiou S. Utilization of the herpes simplex virus type 1 latency-associated regulatory region to drive stable reporter gene expression in the nervous system. J Virol. 1997;71:3197–3207. doi: 10.1128/jvi.71.4.3197-3207.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lachmann R H, Efstathiou S. The use of herpes simplex virus-based vectors for gene delivery to the nervous system. Mol Med Today. 1997;3:404–411. doi: 10.1016/S1357-4310(97)01106-4. [DOI] [PubMed] [Google Scholar]
- 30.Lachmann R H, Sadarangani M, Atkinson H R, Efstathiou S. An analysis of herpes simplex virus gene expression during latency establishment and reactivation. J Gen Virol. 1999;80:1271–1282. doi: 10.1099/0022-1317-80-5-1271. [DOI] [PubMed] [Google Scholar]
- 31.Leib D A, Coen D M, Bogard C L, Hicks K A, Yager D R, Knipe D M, Tyler K L, Schaffer P A. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J Virol. 1989;63:759–768. doi: 10.1128/jvi.63.2.759-768.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mador N, Goldenberg D, Cohen O, Panet A, Steiner I. Herpes simplex virus type 1 latency-associated transcripts suppress viral replication and reduce immediate-early gene mRNA levels in a neuronal cell line. J Virol. 1998;72:5067–5075. doi: 10.1128/jvi.72.6.5067-5075.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Margolis T P, Sedarati F, Dobson A T, Feldman L T, Stevens J G. Pathways of viral gene expression during acute neuronal infection with HSV-1. Virology. 1992;189:150–160. doi: 10.1016/0042-6822(92)90690-q. [DOI] [PubMed] [Google Scholar]
- 34.McFarlane M, Daksis J I, Preston C M. Hexamethylene bisacetamide stimulates herpes simplex virus immediate early gene expression in the absence of trans-induction by Vmw65. J Gen Virol. 1992;73:285–292. doi: 10.1099/0022-1317-73-2-285. [DOI] [PubMed] [Google Scholar]
- 35.McLennan J L, Darby G. Herpes simplex virus latency: the cellular location of virus in dorsal root ganglia and the fate of the infected cell following virus activation. J Gen Virol. 1980;51:233–243. doi: 10.1099/0022-1317-51-2-233. [DOI] [PubMed] [Google Scholar]
- 36.O'Hare P. The virion transactivator of herpes simplex virus. Semin Virol. 1993;4:145–155. [Google Scholar]
- 37.Perry L J, McGeoch D J. The DNA sequences of the long repeat region and adjoining parts of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988;69:2831–2846. doi: 10.1099/0022-1317-69-11-2831. [DOI] [PubMed] [Google Scholar]
- 38.Preston C M. Control of herpes simplex virus type 1 mRNA synthesis in cells infected with wild-type virus or the temperature-sensitive mutant tsK. J Virol. 1979;29:275–284. doi: 10.1128/jvi.29.1.275-284.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Preston C M, Mabbs R, Nicholl M J. Construction and characterization of herpes simplex virus type 1 mutants with conditional defects in immediate early gene expression. Virology. 1997;229:228–239. doi: 10.1006/viro.1996.8424. [DOI] [PubMed] [Google Scholar]
- 40.Preston C M, Nicholl M J. Repression of gene expression upon infection of cells with herpes simplex virus type 1 mutants impaired for immediate-early protein synthesis. J Virol. 1997;71:7807–7813. doi: 10.1128/jvi.71.10.7807-7813.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Preston C M, Rinaldi A, Nicholl M J. Herpes simplex virus type 1 immediate early gene expression is stimulated by inhibition of protein synthesis. J Gen Virol. 1998;79:117–124. doi: 10.1099/0022-1317-79-1-117. [DOI] [PubMed] [Google Scholar]
- 42.Preston C M. Repression of viral transcription during herpes simplex virus latency. J Gen Virol. 2000;81:1–19. doi: 10.1099/0022-1317-81-1-1. [DOI] [PubMed] [Google Scholar]
- 43.Roizman B, Sears A E. An inquiry into the mechanisms of herpes simplex virus latency. Annu Rev Microbiol. 1987;41:543–571. doi: 10.1146/annurev.mi.41.100187.002551. [DOI] [PubMed] [Google Scholar]
- 44.Sacks W R, Schaffer P A. Deletion mutants in the gene encoding the herpes simplex virus type 1 immediate-early protein ICP0 exhibit impaired growth in culture. J Virol. 1987;61:829–839. doi: 10.1128/jvi.61.3.829-839.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samaniego L A, Neiderhiser L, DeLuca N A. Persistence and expression of the herpes simplex virus genome in the absence of immediate-early proteins. J Virol. 1998;72:3307–3320. doi: 10.1128/jvi.72.4.3307-3320.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samaniego L A, Wu N, DeLuca N A. The herpes simplex virus immediate-early protein ICP0 affects transcription from the viral genome and infected-cell survival in the absence of ICP4 and ICP27. J Virol. 1997;71:4614–4625. doi: 10.1128/jvi.71.6.4614-4625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sawtell N M, Thompson R L. Herpes simplex virus type 1 latency-associated transcription unit promotes anatomical site-dependent establishment and reactivation from latency. J Virol. 1992;66:2157–2169. doi: 10.1128/jvi.66.4.2157-2169.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sears A E, Hukkanen V, Labow M A, Levine A J, Roizman B. Expression of the herpes simplex virus 1 α transinducing factor (VP16) does not induce reactivation of latent virus or prevent the establishment of latency in mice. J Virol. 1991;65:2929–2935. doi: 10.1128/jvi.65.6.2929-2935.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sedarati F, Margolis T P, Stevens J G. Latent infection can be established with drastically restricted transcription and replication of the HSV-1 genome. Virology. 1993;192:687–691. doi: 10.1006/viro.1993.1089. [DOI] [PubMed] [Google Scholar]
- 50.Smith R L, Escudero J M, Wilcox C L. Regulation of the herpes simplex virus latency-associated transcripts during establishment of latency in sensory neurons in vitro. Virology. 1994;202:49–60. doi: 10.1006/viro.1994.1321. [DOI] [PubMed] [Google Scholar]
- 51.Smith R L, Pizer L I, Johnson E M, Wilcox C L. Activation of second messenger pathways reactivates latent herpes simplex virus in neuronal cultures. Virology. 1992;188:311–318. doi: 10.1016/0042-6822(92)90760-m. [DOI] [PubMed] [Google Scholar]
- 52.Speck P G, Simmons A. Divergent molecular pathways of productive and latent infection with a virulent strain of herpes simplex virus type 1. J Virol. 1991;65:4001–4005. doi: 10.1128/jvi.65.8.4001-4005.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steiner I, Spivack J G, Deshmane S L, Ace C I, Preston C M, Fraser N W. A herpes simplex virus type 1 mutant containing a nontransinducing Vmw65 protein establishes latent infection in vivo in the absence of viral replication and reactivates efficiently from explanted trigeminal ganglia. J Virol. 1990;64:1630–1638. doi: 10.1128/jvi.64.4.1630-1638.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stevens J G. Human herpesviruses: a consideration of the latent state. Microbiol Rev. 1989;53:318–332. doi: 10.1128/mr.53.3.318-332.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stevens J G, Wagner E K, Devi-Rao G B, Cook M L, Feldman L T. RNA complementary to a herpesvirus alpha gene mRNA is predominant in latently infected neurons. Science. 1987;235:1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- 56.Stow E C, Stow N D. Complementation of a herpes simplex virus type 1 Vmw110 mutant by human cytomegalovirus. J Gen Virol. 1989;70:695–704. doi: 10.1099/0022-1317-70-3-695. [DOI] [PubMed] [Google Scholar]
- 57.Stow N D, Stow E C. Isolation and characterisation of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J Gen Virol. 1986;67:2571–2585. doi: 10.1099/0022-1317-67-12-2571. [DOI] [PubMed] [Google Scholar]
- 58.Tenser R B, Hay K A, Edris W A. Latency-associated transcript but not reactivatable virus is present in sensory ganglion neurons after inoculation of thymidine kinase-negative mutants of herpes simplex virus type 1. J Virol. 1989;63:2861–2865. doi: 10.1128/jvi.63.6.2861-2865.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thompson R L, Sawtell N M. The herpes simplex virus type 1 latency-associated transcript gene regulates the establishment of latency. J Virol. 1997;71:5432–5440. doi: 10.1128/jvi.71.7.5432-5440.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wagner E K, Bloom D C. Experimental investigation of herpes simplex virus latency. Clin Microbiol Rev. 1997;10:419–443. doi: 10.1128/cmr.10.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Watson K, Stevens J G, Cook M L, Subak-Sharpe J H. Latency competence of thirteen HSV-1 temperature-sensitive mutants. J Gen Virol. 1980;49:149–159. doi: 10.1099/0022-1317-49-1-149. [DOI] [PubMed] [Google Scholar]
- 62.Watson R J, Clements J B. A herpes simplex virus type 1 function required for early and late virus RNA synthesis. Nature. 1980;285:329–330. doi: 10.1038/285329a0. [DOI] [PubMed] [Google Scholar]
- 63.Wilcox C L, Johnson E M. Nerve growth factor deprivation results in the reactivation of latent herpes simplex virus in vitro. J Virol. 1987;61:2311–2315. doi: 10.1128/jvi.61.7.2311-2315.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilcox C L, Johnson E M. Characterization of nerve growth factor-dependent herpes simplex virus latency in neurons in vitro. J Virol. 1988;62:393–399. doi: 10.1128/jvi.62.2.393-399.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilcox C L, Smith R L, Everett R D, Mysofski D. The herpes simplex virus type 1 immediate-early protein ICP0 is necessary for the efficient establishment of latent infection. J Virol. 1997;71:6777–6785. doi: 10.1128/jvi.71.9.6777-6785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilcox C L, Smith R L, Freed C R, Johnson E M. Nerve growth factor-dependence of herpes simplex virus latency in peripheral sympathetic and sensory neurons in vitro. J Neurosci. 1990;104:1268–1275. doi: 10.1523/JNEUROSCI.10-04-01268.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu N, Watkins S C, Schaffer P A, DeLuca N A. Prolonged gene expression and cell survival after infection by a herpes simplex virus mutant defective in the immediate-early genes encoding ICP4, ICP27, and ICP22. J Virol. 1996;70:6358–6369. doi: 10.1128/jvi.70.9.6358-6369.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yao F, Schaffer P A. An activity specified by the osteosarcoma line U2OS can substitute functionally for ICP0, a major regulatory protein of herpes simplex virus type 1. J Virol. 1995;69:6249–6258. doi: 10.1128/jvi.69.10.6249-6258.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zwaagstra J C, Ghiasi H, Slanina S M, Nesburn A B, Wheatley S C, Lillycrop K, Wood J, Latchman D S, Patel K, Wechsler S L. Activity of herpes simplex virus type 1 latency-associated transcript (LAT) promoter in neuron-derived cells: evidence for neuron specificity and for a large LAT transcript. J Virol. 1990;64:5019–5028. doi: 10.1128/jvi.64.10.5019-5028.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]