Abstract
The assembly of human cytomegalovirus (HCMV) is thought to be similar to that which has been proposed for alphaherpesviruses and involve envelopment of tegumented subviral particles at the nuclear membrane followed by export from the cell by a poorly defined pathway. However, several studies have shown that at least two tegument virion proteins remain in the cytoplasm during the HCMV replicative cycle, thereby suggesting that HCMV cannot acquire its final envelope at the nuclear envelope. We investigated the assembly of HCMV by determining the intracellular trafficking of the abundant tegument protein pp150 (UL32) in productively infected human fibroblasts. Our results indicated that pp150 remained within the cytoplasm throughout the replicative cycle of HCMV and accumulated in a stable, juxtanuclear structure late in infection. Image analysis using a variety of cell protein-specific antibodies indicated that the pp150-containing structure was not a component of the endoplasmic reticulum, (ER), ER-Golgi intermediate compartment, cis or medial Golgi, or lysosomes. Partial colocalization of the structure was noted with the trans-Golgi network, and it appeared to lie in close proximity to the microtubule organizing center. Two additional tegument proteins (pp28 and pp65) and three envelope glycoproteins (gB, gH, and gp65) localized in this same structure late infection. This compartment appeared to be relatively stable since pp150, pp65, and the processed form of gB could be coisolated following cell fractionation. Our findings indicated that pp150 was expressed exclusively within the cytoplasm throughout the infectious cycle of HCMV and that the accumulation of the pp150 in this cytoplasmic structure was accompanied by at least five other virion proteins. These results suggested the possibility that this virus-induced structure represented a cytoplasmic site of virus assembly.
As for other human herpesviruses, the assembly of the infectious human cytomegalovirus (HCMV) particle is a complex and poorly understood process. Extracellular virions produced by HCMV-infected cells appear structurally similar to those of other herpesviruses in that HCMV virions consist of three major structural regions, the capsid, the tegument, and a lipid-containing envelope (29). While it is generally accepted that capsid assembly occurs in the nuclei of infected cells, the cellular compartments in which tegument assembly takes place remain incompletely defined (29). Several seemingly mutually exclusive pathways of envelopment have been proposed for herpesviruses (6, 9, 11, 13, 16, 18, 20, 25, 27, 28, 33, 34, 39–42). The earliest model for envelopment of herpes simplex virus (HSV) proposed that the viral particle was enveloped during passage through the inner nuclear membrane (18, 29, 32). Glycoproteins within the viral envelope would then be modified as the particle traversed the Golgi (9, 18). More recent models have suggested that envelopment and deenvelopment occur at the nuclear envelope and that final envelopment of the tegumented nucleocapsid occurs in the trans-Golgi (TGN) following fusion with membranes containing processed envelope glycoproteins (6, 11, 13, 14, 16, 20, 25, 27, 39–43).
Addition of the tegument has been assumed to take place in the nucleus in both models, although formal proof of such a site of tegument acquisition is lacking. Previous studies of HCMV assembly utilizing electron microscopy described coated particles budding into cytoplasmic vacuoles; however, the site of tegument addition was not defined (32, 36). A more recent study of the assembly of human herpesvirus 6 suggested that tegumentation of the particle took place by budding of nuclear capsids into cytoplasmic invaginations in the nucleus (28). Some evidence consistent with the possibility that tegument proteins are incorporated into the viral particle outside of the nucleus has been presented in a study of the UL11 tegument protein of HSV. Electron micrographs of HSV-infected cells suggested that this myristoylated protein was located in the nuclear membrane, but the polarity of this association was not readily apparent (2). Furthermore, the phenotype of a UL11 deletion virus was characterized by significantly decreased infectious virion production and accumulation of tegument-containing capsids on the inner nuclear membrane (2). Recent studies of the morphogenesis of a related alphaherpesvirus, varicella-zoster virus, have suggested that the tegument of this virus is also partially assembled in the cytoplasm of the infected cell, perhaps concurrently with final envelopment of the maturing particle at the TGN (11, 42, 43). Studies of the envelopment of HSV have suggested that if a cytoplasmic envelopment follows envelopment/deenvelopment at the nuclear membrane, then this step most likely occurs in the Golgi (9). A more definitive analysis of the envelopment of pseudorabies virus suggested that two pools of a major envelope glycoprotein, gE, were present within the infected cell (35). One pool, derived by retrieval of gE from the plasma membrane by the endocytic pathway, was not necessary for assembly of infectious virions, suggesting that a second intracellular pool of gE was incorporated into progeny virions in an as yet undefined cytoplasmic compartment (35).
In HCMV-infected cells, the cellular distribution of several of the better-characterized tegument proteins has suggested that tegument assembly might occur in two different compartments, the nucleus and the cytoplasm. Tegument proteins encoded by the UL25, UL99 (pp28), and UL32 (pp150) open reading frames (ORFs) have been shown to be present in the cytoplasm of infected cells late in the viral replicative cycle (3, 17, 21). Ultrastructural analysis by electron microscopy has demonstrated nonenveloped particles apparently acquiring an additional structural layer by budding into cytoplasmic structures (12, 32, 36). This layer is assumed to be the envelope; however, direct evidence for this is lacking, and it remains to be determined whether these observations are relevant to the assembly of infectious particles or represent a default pathway for noninfectious particles destined for intracellular or extracellular degradation.
In this study, we examined the subcellular site of tegument acquisition, using well-characterized HCMV-specific monoclonal and polyclonal antibodies to describe both the expression and cellular distribution of several tegument proteins. Our findings indicated that tegument proteins were added to the subviral particle in both nuclear and cytoplasmic compartments, suggesting that the final envelopment of HCMV occurred in the cytoplasm of infected cells. Moreover, we have identified a juxtanuclear structure which contained at least three virion glycoproteins and three tegument proteins. This structure colocalized with the microtubule organizing center (MTOC) and could be disrupted with the microtubule-depolymerizing agent nocadazole but not by brefeldin A. Although this structure failed to colocalize with several markers of intracellular compartments, the viral protein-containing structure was surrounded by elements of the Golgi, suggesting a close proximity of soluble viral proteins and Golgi-localized, transmembrane-containing viral glycoproteins. The association of the viral proteins within this structure appeared to be relatively stable since fractionation of infected cells by discontinuous sucrose gradient centrifugation resulted in the enrichment of both tegument and envelope proteins in a single fraction. Interestingly, this fraction contained at least one viral glycoprotein but was devoid of markers for the endoplasmic reticulum (ER)-Golgi intermediate compartment (ERGIC) or the Golgi, suggesting that viral proteins might be sequestered in a membrane subdomain from which cellular proteins were excluded. The presence of virion tegument and envelope proteins within a single cytoplasmic compartment suggested that tegumentation and envelopment likely occurred in the cytoplasm and that the juxtanuclear compartment might represent a site of virion assembly.
MATERIALS AND METHODS
Cells, viruses, plasmids, and antibodies.
Primary human foreskin fibroblasts (HF cells) were prepared, propagated, and infected as previously described (30). HCMV strain AD169 was used for all experiments. Infectious stock were prepared from supernatants of infected HF cells which exhibited 100% cytopathic effect and were titered as described elsewhere (1); fractions collected from cell fractionation gradients were titered by the same method. The ORFs encoding pp150 (UL32) and pp28 (UL99) were cloned into the expression vector pcDNA 3 (Invitrogen, San Diego, Calif.). Orientation and sequence were verified by nucleotide sequencing. Human 293T cells or monkey Cos 7 cells were used for transient expression of pp150 (UL32) following calcium phosphate-mediated transfection (8).
HCMV-encoded proteins were detected with monoclonal antibodies (MAbs) as previously described (30). MAbs used in this study included those specific for IE-1 (UL123, MAb P63-27), MCP (UL86, MAb 28-4), ppUL69 (UL69, MAb 69), pp65 (UL83, MAbs 28-19 and 65-8), pp150 (UL32, MAb 36-14), pp28 (UL99, MAb 41-18), gB (UL55, MAb 58-15), gH (UL75, MAb 14-4b), and gp65 (MAb 14-16) (4, 5, 7, 26, 30, 31). The generation of the monospecific rabbit anti-serum reactive with pp150 has been previously described (15). MAb 36-14 was generated from spleen cells obtained from mice immunized with prokaryotic expressed fragments of pp150.
The antibodies reactive with cellular markers included a MAb specific for ERGIC53 (generously provided by Peter Hauri, University of Basel, Basel, Switzerland), a rabbit antiserum specific for mannosidase II (provided by Marilyn Farquhar, University of California, San Diego), a rabbit antiserum against TGN46 (from George Banting, University of Manchester, Manchester, United Kingdom), and a rabbit antiserum reactive with the lysosomal membrane protein LAMP-1 (from Minoru Fukuda, La Jolla Cancer Research Center, La Jolla, Calif.). The rabbit antiserum against the Golgi protein GM130 and the murine MAb reactive with the ER-resident protein RAP have been described previously (24). A murine MAb specific for vimentin, clone V9, was purchased from Sigma Chemical Co., St. Louis, Mo. The murine MAb reactive with tubulin was obtained from Tom Howard, University of Alabama, Birmingham. Actin was stained with fluorochrome-conjugated phalloidin (Molecular Probes, Eugene, Oreg.). Fluorochrome-conjugated secondary antibodies, including murine immunoglobulin G (IgG) subclass antibodies, were purchased from Southern Biotechnology Associates, Birmingham, Ala.
SDS-PAGE and immunoblotting.
Electrophoresis under reducing conditions and immunoblotting were carried out as described elsewhere (30). Virus-infected cell proteins were obtained from HCMV-infected HF cells grown in 35-mm-diameter tissue culture dishes. Following washing in phosphate-buffered isotonic saline (PBS; pH 7.4), the cells were lysed in sample buffer containing 5% 2-mercaptoethanol and 2% sodium dodecyl sulfate (SDS) and heated to 100°C. The solubilized proteins were then subjected to SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to either nitrocellulose membranes or polyvinylidene fluoride filters. Murine MAbs or in some cases a 1:500 dilution of the IgG fraction of the rabbit anti-pp150 serum were used to detect specific proteins. Antibody binding was detected by 125I-protein A followed by autoradiography or alternatively by reaction with horseradish peroxidase-conjugated goat anti-mouse or rabbit IgG followed by enhanced chemiluminescence (ECL) fluorography (ECL kit; Amersham, Arlington Heights, Ill.).
Immunofluorescence microscopy.
HF cells were grown in 24-well tissue culture plates containing a 13-mm-diameter coverslip. After the cells had reached at least 90% confluency, the cells were infected with HCMV strain AD169 for 1 to 2 h and then washed once and incubated for the indicated time. The coverslips were harvested by first washing the cells with PBS and then fixing them for 30 min at room temperature in 2% paraformaldehyde (PFA) freshly prepared in PBS. In some cases the cells were incubated for 5 min prior to PFA fixation in a 100 mM HEPES-buffered solution containing 5 mM EGTA and 5% polyethylene glycol (pH 6.9) to stabilize microtubules. The coverslips were then washed in PBS and permeabilized with 0.05% NP-40 in PBS at 4°C for 10 min. The coverslips were then blocked with PBS containing 20% normal goat serum for 45 min at 37°C. After a wash in PBS, primary antibody was added and the coverslips were incubated at 37°C for 60 min. The coverslips were then washed in PBS and then incubated for 45 min in fluorochrome-conjugated secondary antibody diluted in PBS containing 10% normal goat serum. Following repeated washings in PBS the coverslip was fixed in 2% PFA as described above and then washed once in PBS immediately prior to viewing.
The fixed coverslips were incubated for 5 min in Slowfade (Molecular Probes) and then inverted and sealed on a glass microscope slide with fingernail polish. The sealed coverslips were viewed under a Leitz Dialux epifluorescence microscope at a magnification of ×50, and the images were captured with a digital camera (Photometrics, Tucson, Ariz.). The images were processed with Image Pro software (Media Cybernetics, Silver Spring, Md.). Deconvolution was accomplished with Hazebuster (Vaytek, Fairfield, Iowa).
Cell fractionation.
Three 150-cm2 flasks of HF cells were infected at a multiplicity of infection (MOI) of 3 and harvested on day 6 postinfection by scraping the cells from the flask. The cell pellet was washed twice with cold PBS plus 5 mM EDTA and then resuspended in 1 ml of Tris-buffered isotonic saline (pH 7.4) containing 5 mM EDTA and 0.25 M sucrose. The cell suspension was repeatedly passed through a 27-gauge needle until there were no intact cells in the suspension as determined by light microscopy. This material was then centrifuged at 1,000 × g for 10 min, and the supernatant was transferred to a new tube. This solution, termed the postnuclear supernatant, was then applied to a preformed sucrose step gradient consisting of the following amounts of sucrose in 10 mM Tris-buffered isotonic saline, pH 7.4: (i) 0.5 ml, 2 M; (ii) 1.5 ml, 1.6 M; (iii) 2.5 ml, 1.4 M; (iv) 3.5 ml, 1.2 M; and (v) 1.5 ml, 0.8 M. The postnuclear supernatant was layered on the top of the gradient and centrifuged for 3 h at 100,000 × g in an SW41 rotor at 4°C. The gradient was fractionated from the bottom, and individual fractions were assayed for virus infectivity as described above and for viral protein by immunoblotting.
RESULTS
pp150 is expressed late during HCMV infection.
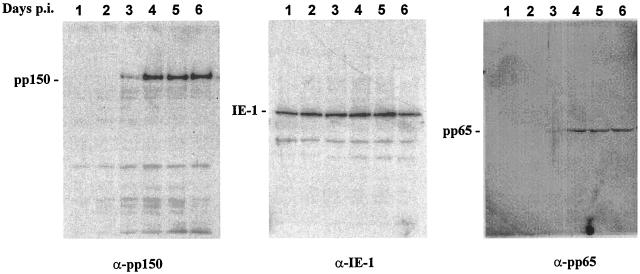
The kinetics of pp150 expression were initially studied by immunoblotting of HF cell lysates harvested on days 1 to 6 postinfection. HCMV-specific MAbs reactive with the major immediate-early protein, IE-1 (UL123, pp72), and the tegument protein, pp65 (UL83), were used to detect proteins of the immediate and early-late kinetic classes, respectively. As previously shown, the IE-1 protein was expressed by day 1 postinfection and could be detected at approximately similar levels throughout the duration of the infection (Fig. 1). In contrast, pp65 was first detected on day 3 and appeared to increase in amount during subsequent days (Fig. 1). Although incoming virion pp65 has been reported to be detectable immediately after infection, at the low MOI used in this experiment, we failed to detect pp65 until day 3 postinfection. Similarly, pp150 was first detected on day 3 and appeared to increase in amount during subsequent days (Fig. 1). These results suggested that pp150 was expressed with kinetics consistent with either an early-late or a late protein. The lack of detectable pp150 in infected HF cells treated with phosphonoformic acid further supported the classification of pp150 as an early-late protein (data not shown).
FIG. 1.
Time course of pp150 (UL32) expression in virus-infected HF cells. HF cells were infected with HCMV strain AD169 at an MOI of approximately 0.5 and harvested on the indicated day postinfection (p.i.). Following solubilization, the cell lysates were subjected to SDS-PAGE and transferred to polyvinylidene difluoride membranes. The membranes were then developed with antibodies reactive with the pp72 (UL123) major immediate-early protein, and antibody binding was detected by ECL as described in Materials and Methods. The membrane was then stripped of bound antibody, and a 1:500 dilution of rabbit antibodies reactive with the carboxyl terminus of pp150 was used to detect pp150. Similarly, the membrane was stripped and reacted a third time with antibodies reactive with pp65 (UL83).
pp150 accumulates in a juxtanuclear structure in HCMV-infected cells.
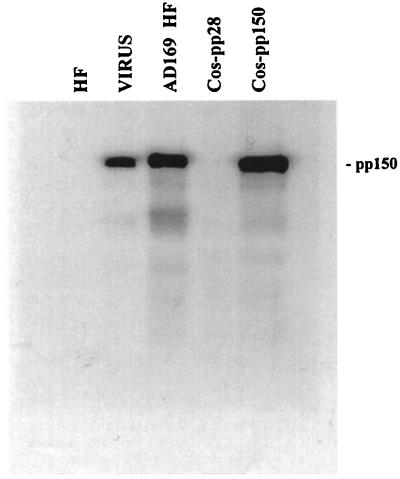
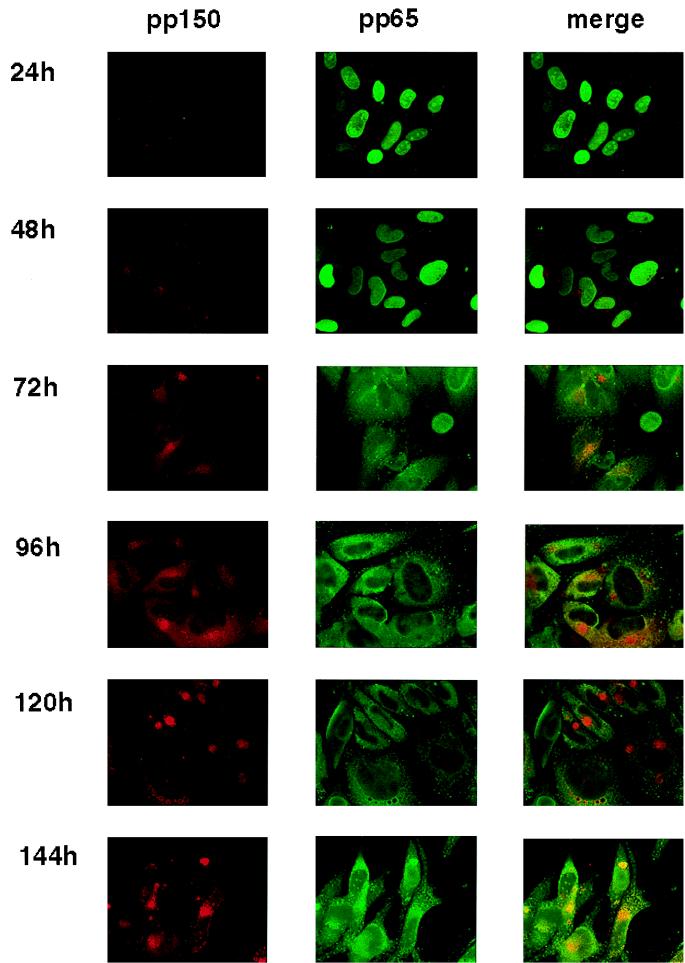
To confirm the immunoblotting results and to examine the cellular distribution of pp150 within HF cells at various times after infection, a murine MAb reactive with pp150 was generated. This antibody was specific for pp150; it detected viral pp150 in virions and infected cells as well as recombinant pp150 produced in transfected Cos 7 cells as determined by immunoblot analysis (Fig. 2). The MAb did not cross-react with cellular proteins in Cos 7 and HF cells or cells expressing pp28 (Fig. 2). The anti-pp150 MAb and a MAb specific for pp65 were used to examine the localization of the corresponding antigens during productive infection of HF cells. As noted previously by other investigators, pp65 was rapidly translocated to the nucleus and could be readily detected in the nucleus as early as 120 min postinfection when cells were infected at a high MOI (data not shown). Within 24 h postinfection, pp65 was detectable in the nucleus of almost every cell in the culture (Fig. 3). In contrast, pp150 was present in small punctate structures scattered throughout the cytoplasm. Although some punctate structures could be seen overlying the nucleus, these were not localized to the nucleus when multiple focal planes of the same image were collected (data not shown). Within 48 h postinfection, pp65 expression continued to be restricted to the nucleus (Fig. 3). At that time, pp150 was still detected in clearly separate punctate structures but with an increase in the intensity of the signal in an area adjacent to the nucleus. At 72 and 96 h postinfection, pp65 was present in both the nucleus and the cytoplasm of infected cells. At these times postinfection, pp150 localization was still limited to the cytoplasm, but it appeared that the punctate structures coalesced into a large juxtanuclear structure. In the early periods postinfection, we could not distinguish between incoming virion pp150 and newly synthesized pp150; however, the cytoplasmic distribution and the increased intensity of the signal between 48 and 72 h postinfection suggested that newly synthesized pp150 was accumulating in the juxtanuclear structure (Fig. 3). In the interval between 120 and 144 h postinfection, pp65 was present in both the cytoplasm and the nucleus, with partial overlap with pp150 at 144 h postinfection. In this final time interval, pp150 remained exclusively cytoplasmic, the intensity of the signal associated with the juxtanuclear structure increased, and the structures appeared more compact (Fig. 3). Additional analysis by immunoelectron microscopy was consistent with these findings in that anti-pp150 antibodies labeled particles only in the cytoplasm and not in the nucleus (data not shown). These results suggested that the abundant tegument protein pp150 was expressed as a cytoplasmic protein throughout the productive infection of HF cells with HCMV. Significantly, the intracellular distribution of pp150 varied with the time postinfection, ranging from a dispersed punctate distribution early in infection, to a concentration of the protein in a large juxtanuclear structure late in infection.
FIG. 2.
Characterization of a murine MAb reactive with pp150 (UL32). Cell lysates from uninfected HF cells (HF), HCMV-infected HF cells (AD169 HF), Cos 7 cells transfected with an expression plasmid encoding pp28 (Cos-pp28), or Cos 7 cells transfected with an expression plasmid encoding pp150 (Cos-pp150) were solubilized, subjected to SDS-PAGE, and transferred to a nitrocellulose membrane. Gradient-purified HCMV strain AD169 virions (VIRUS) were solubilized in a similar fashion and loaded in the same gel. Following transfer, the membrane was incubated with MAb 36-14, and antibody binding was detected as described in Materials and Methods.
FIG. 3.
Time course of pp150 (UL32) expression in virus-infected HF cells. HF cells grown on glass coverslips were infected at an MOI of between 3 and 5 and harvested at 24-h intervals. Following fixation, the expression of pp65 (UL83) and pp150 (UL32) was determined by using murine MAbs followed by IgG subclass-specific fluorochrome-conjugated second antibodies. The signals from the red and green channels were merged to determine colocalization.
The juxtanuclear structure containing pp150 is not a secretory or a degradative cellular compartment.
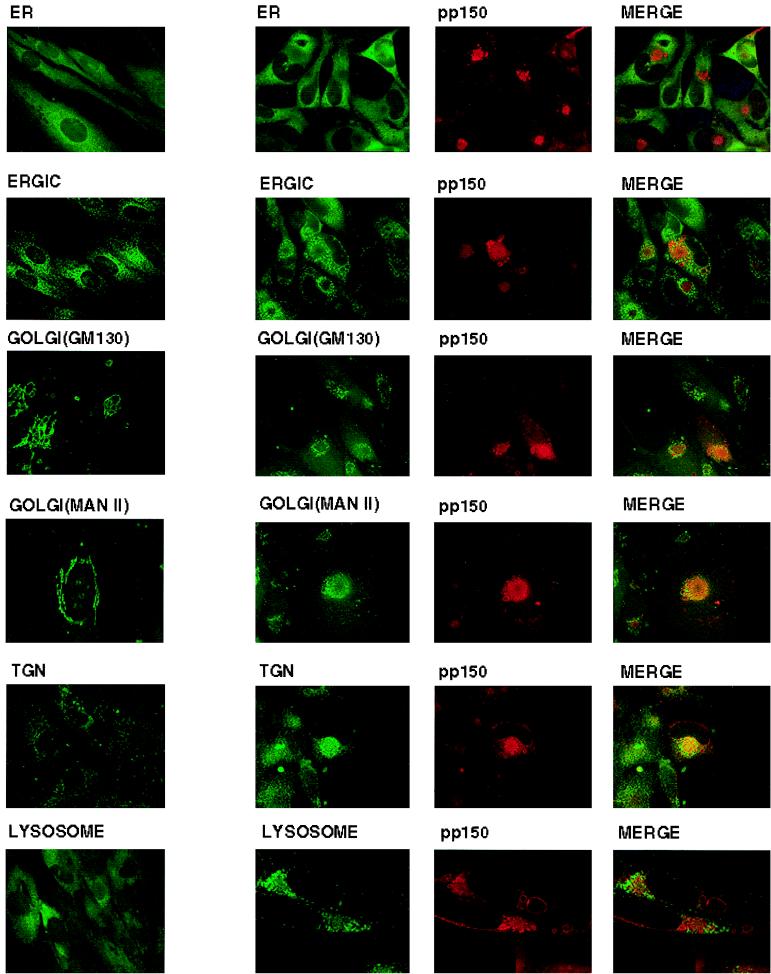
Late in infection, during the time interval of maximal production of infectious progeny virions, pp150 was localized predominantly to a large juxtanuclear compartment. We attempted to characterize this structure by using a panel of antibodies reactive with specific subcellular marker proteins. Although the juxtanuclear structure did not have the morphologic appearance of the ER, we used an antibody reactive with a resident lumenal ER protein, RAP, to demonstrate that the juxtanuclear structure did not represent a structurally aberrant ER (Fig. 4). The pp150-containing structure did not colocalize with ERGIC53, an integral membrane protein of the ERGIC positioned between the ER and the Golgi (Fig. 4). Similarly, the pp150-containing compartment was not labeled with antibodies reactive with two Golgi marker proteins, GM130 and mannosidase II (Fig. 4). Antibodies to the TGN marker TGN 46 revealed overlap in signal with the pp150-containing structure, although there was not complete colocalization (Fig. 4). Interestingly, the distribution of the ERGIC, Golgi, and TGN markers appeared displaced by the juxtanuclear pp150-containing structure and resulted in the accumulation of the markers on the periphery of the virus induced structure (Fig. 4). The intracellular distribution of the ERGIC53 appeared most similar to that seen in uninfected cells, yet even this protein appeared to be more concentrated in the periphery of the pp150-containing juxtanuclear structure instead of its more diffuse distribution in uninfected cells (Fig. 4). The changes in the morphology of the Golgi and the TGN in infected cells were very striking, with change of the normal lacy, tubular appearance in uninfected cells to more compact fragments surrounding the juxtanuclear structure in infected cells. The appearance of the Golgi and the TGN suggested that they were displaced radially by the pp150-containing structure. Together, these results indicated that the morphology of the intracellular compartments of the secretory system was altered during the late phases of HCMV infection.
FIG. 4.
Accumulation of pp150 (UL32) in a juxtanuclear compartment. HF cells grown on glass coverslips were infected with HCMV at an MOI of 3 to 5 and fixed on day 6 postinfection. Uninfected HF cells were processed in a similar fashion. The various cellular compartments were stained with the following antibodies: ER, anti-RAP; ERGIC, anti-ERGIC53; Golgi, anti-GM130; Golgi, anti-mannosidase (MAN) II; TGN, anti-TGN46; and lysosome, anti-LAMP-1. Infected cells were also reacted with the anti-pp150 MAb, 36-14. The cellular markers were detected with a fluorescein isothiocyanate-conjugated secondary antibody, and pp150 was detected with a Texas red-conjugated secondary antibody. Colocalization is indicated by a yellow signal in the merge column. (A) Uninfected cells; (B) infected cells.
An obvious possibility that could account for the accumulation of pp150 in the perinuclear location was that this compartment represented a degradative pathway for viral proteins overexpressed during productive viral infection. We examined this by attempting to colocalize the LAMP-1 protein, an integral membrane protein of lysosomes, with the pp150-containing structure. No colocalization was observed (Fig. 4). Furthermore, comparison of the distributions of LAMP-1-containing structures in infected and uninfected cells indicated no significant alteration of distribution (Fig. 4).
The juxtanuclear structure containing pp150 surrounds the MTOC but is not an aggresome.
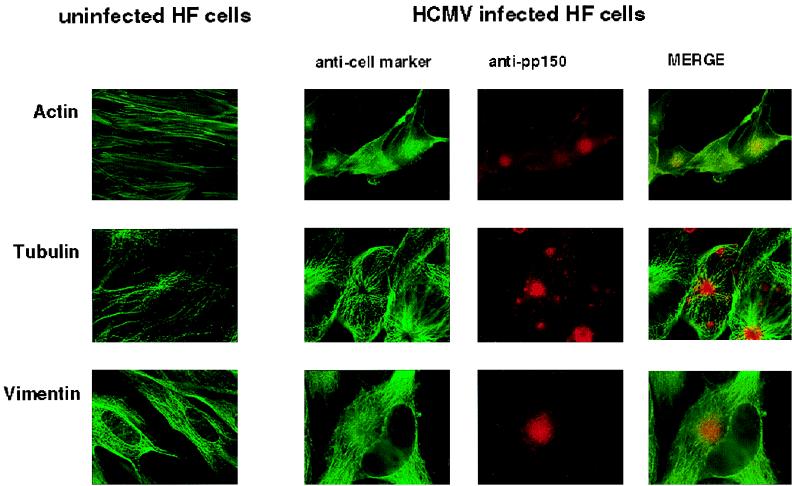
The displacement of Golgi elements that usually surround the MTOC to around the pp150-containing structure suggested that the viral proteins might be concentrated in the region of the MTOC. In agreement, the pp150-containing structure did not colocalize with the cytoskeletal proteins, actin, or vimentin but appeared to be in close proximity to a structure whose morphologic appearance was suggestive of that of the MTOC (Fig. 5). A signal overlap between the base of microtubules arising from the periphery of this structure and the pp150-containing juxtanuclear structure was also noted (Fig. 5).
FIG. 5.
The pp150 (UL32)-containing juxtanuclear compartment is associated with the MTOC. HF cells were infected with HCMV as described for Fig. 4. Immediately prior to fixation, the cells were incubated in a microtubule-stabilizing solution and then fixed as described for Fig. 4. In all cases pp150 (UL32) was detected with MAb 36-14 followed by a Texas red-conjugated secondary antibody. Actin was stained with phalloidin, tubulin was stained with an anti-α-tubulin MAb, and vimentin was stained with an antivimentin MAb as described in Materials and Methods.
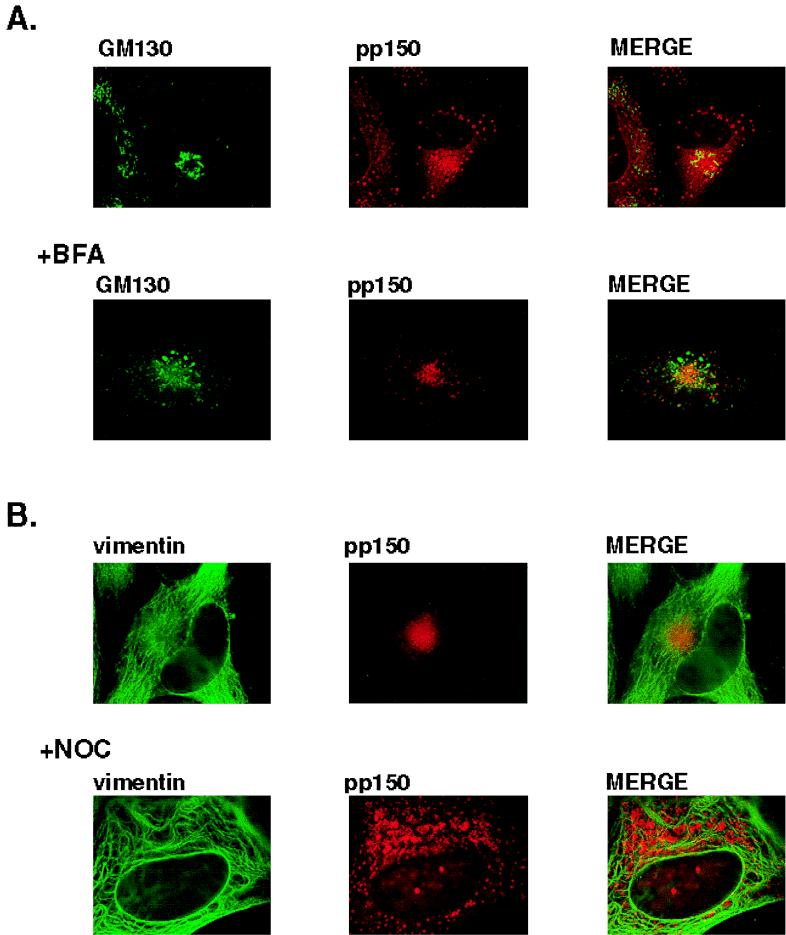
Although we could not colocalize the juxtanuclear structure with cellular markers of the Golgi, the localization of pp150 in the proximity of the MTOC appeared similar to that of the Golgi. To further explore the possibility that the juxtanuclear structure colocalized with the Golgi, we incubated infected cells with brefeldin A and compared the distribution of the pp150 to that of the Golgi marker, GM130. Brefeldin A treatment resulted in the vesiculation of the Golgi as revealed by staining of the cells with anti-GM130 but failed to cause a similar change in the appearance of the pp150-containing juxtanuclear structure compared to control, untreated infected cells (Fig. 6A).
FIG. 6.
The pp150-containing juxtanuclear structure is resistant to treatment with brefeldin A but is dispersed by treatment with nocadazole. (A) Infected cells were incubated in the presence of 2 μg of brefeldin A (BFA) per ml for 2 h or left untreated and then processed as described for Fig. 4. (B) Infected cells were incubated in 2 μM nocadazole for 1.5 h prior to fixation or left untreated and processed as described for Fig. 5. Vimentin and pp150 were detected as described for Fig. 5.
In recent studies of COS cells transfected with expression vectors encoding mutant proteins, a novel subcellular compartment has been identified as the site of accumulation of misfolded and overexpressed proteins (19). This compartment, termed an aggresome, appeared to represent a cellular site for storage of abundantly expressed proteins. Characteristics of the aggresome included its stability and a capacity to remain as a morphologically defined structure following treatment with the microtubule-destabilizing drug nocadazole (19). In addition, the aggresome has been shown to be encased in a vimentin cage. We analyzed the pp150-containing juxtanuclear structure formed in the late stages of HCMV infection in HF cells for these properties of aggresomes. Treatment of infected cells with nocadazole dispersed the juxtanuclear structure containing pp150 (Fig. 6B). When we examined the effect on multiple fields from nocadazole-treated cells, we could not find an intact juxtanuclear pp150-containing structure (data not shown). Furthermore, the pp150-containing structures were not encased in vimentin cages, and the vimentin pattern in infected cells was not significantly different from that in uninfected cells (Fig. 6B). Together, these data support the conclusion that the juxtanuclear structures that contained viral proteins were not collections of lysosomes or aggresomes. Moreover, these results indicated that integrity of the microtubule network is essential for maintenance of the pp150-containing structure, a finding consistent with the association of this structure with the MTOC.
Tegument and envelope HCMV proteins colocalize in the juxtanuclear structure containing pp150.
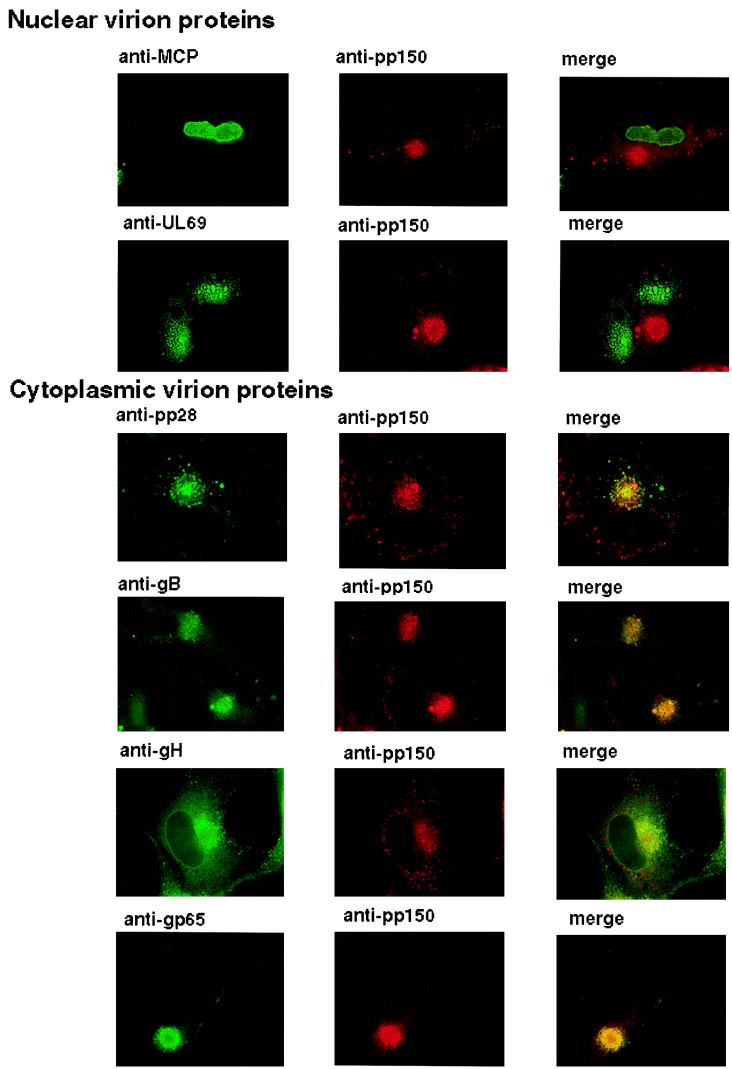
The finding of an abundant virion protein within a discrete cytoplasmic compartment raised the possibility that other virion proteins could also be present within this structure. We used several different antibodies reactive with both structural and nonstructural HCMV-encoded proteins along with antibodies against pp150 to compare the intracellular distribution of these proteins. The nonstructural proteins IE-1 and the polymerase accessory protein UL44 were nuclear in their distribution and did not colocalize with pp150 in the perinuclear structure (data not shown). Similarly, we failed to detect a signal from antibodies reactive with the nuclear tegument protein UL69 or the major capsid protein (UL86) in the perinuclear structure (Fig. 7). In contrast, we have already shown that pp65 colocalized with pp150 (Fig. 3) and could readily colocalize another tegument pp28 (UL99) within the pp150-containing juxtanuclear structures (Fig. 7). Likewise, we could also colocalize signals from three virion envelope glycoproteins, gB, gH, and gp65, within the perinuclear pp150-containing structure (Fig. 7). Together, these data indicated that virion proteins of the tegument and the envelope of HCMV accumulated in juxtanuclear structures late in infection and raised the possibility that this structure has a role in the cytoplasmic assembly of subviral particles or infectious virions.
FIG. 7.
Distribution of virion proteins in infected HF cells. HF cells were infected and processed as described for Fig. 4. Following fixation, the glass coverslips were incubated in MAbs specific for pp150 and the antibodies against the indicated virus-encoded proteins. pp150 reactivity was detected with a Texas red-conjugated secondary antibody, and the second viral protein was detected with fluorescein isothiocyanate conjugated secondary antibody. Colocalization was indicated by a yellow signal in the merge channel.
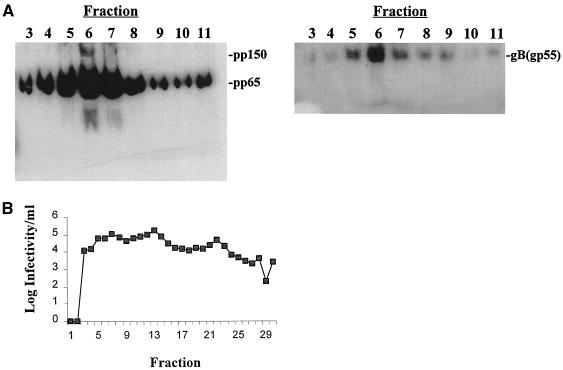
To determine whether the juxtanuclear structure containing viral proteins represented a distinct and stable subcompartment, we sedimented a postnuclear supernatant of HCMV infected cells harvested late in infection over a discontinuous sucrose gradient and then analyzed individual fractions for the presence of three viral proteins, the tegument proteins pp150 and pp65 and the envelope glycoprotein gB. We could detect all three proteins, including the proteolytically cleaved form of gB, in few fractions near the bottom of the gradient (Fig. 8). Analysis of the remaining fractions failed to show the presence of either pp150 or gB; however, lesser amounts of pp65 were detected throughout the entire gradient, a finding consistent with the widespread cellular distribution of this protein late in infection as observed in our imaging studies. Consistent with our immunofluorescence imaging findings, we could not detect the Golgi marker protein GM130 within the fraction (fraction 6) that contained the three viral proteins, although GM130 could be detected in other fractions (fractions 12 to 14) recovered from the gradient (data not shown).
FIG. 8.
Cell fractionation of HCMV-infected HF cells. (A) HF cells were infected with HCMV at an MOI of 3 to 5 and harvested on day 6 postinfection as described in Materials and Methods. The postnuclear supernatant was applied to a discontinuous sucrose gradient; following centrifugation at 100,000 × g for 3 h, individual 0.3-ml fractions were collected from the bottom of the gradient. An aliquot of each fraction was either analyzed by immunoblotting or titered for virus infectivity. Murine MAbs specific for pp150 (UL32) and pp65 (UL83) were used to probe the membranes, and antibody was detected as described in Materials and Methods. The filter probed with anti-gB was made by cutting the original filter into two pieces such that proteins migrating faster than 100 kDa were present in the membrane probed with anti-gB and those above 100 kDa probed were present in the membrane probed with anti-TAP antibody. The positions of migration of pp150, pp65, and gB are indicated at the right. (B) Infectivity of the gradient fractions.
Although the high sucrose concentrations and relative centrifugal field used in this cell fractionation were not sufficient to sediment HCMV virions, we explored the possibility that this profile of virus-encoded proteins reflected the concentration of assembled virions instead of cosedimentation of several virion proteins within an intracellular compartment. To test this possibility, we determined the titer of infectious virus in each fraction. Although a large amount of infectious virus was found associated with the fraction containing pp150, pp65, and gB, this fraction did not represent the peak fraction of viral infectivity (Fig. 8). Infectious virus of similar titers could be found throughout most of the gradient. Thus, it appeared that following fractionation of infected HF cells, we could enrich for a cellular compartment which contained virion structural proteins.
DISCUSSION
The assembly of HCMV, specifically the envelopment of the infectious particle, has been proposed to follow an assembly pathway similar to that for HSV, for which acquisition of the tegument proteins occurs within the nucleus and envelopment proceeds at the nuclear membrane (29). Yet little if any recent experimental data have been presented to support this model of assembly. As noted previously, recent studies of varicella-zoster virus and pseudorabies virus have suggested an alternate assembly pathway, in which envelopment is followed by deenvelopment at the nuclear membrane and final envelopment occurs in a cytoplasmic compartment (11, 14, 20, 39, 42). Similarly, studies of HCMV showed that at least two virion tegument proteins could be localized exclusively within the cytoplasm of infected cells, a finding contradictory with assembly at the nuclear membrane (3, 21). Furthermore, the cleavage of the major HCMV envelope glycoprotein, gB, into its mature forms has been shown to be accomplished by the proteolytic activity of host furin, an enzyme found within the late secretory/endocytic pathway, suggesting that envelopment was unlikely to occur at the nuclear membrane (23, 37, 38). Together, these findings strongly support a cytoplasmic phase in the assembly of the HCMV virion.
The results presented in this report suggested that the tegumentation and envelopment steps of virus assembly might occur within a cytoplasmic compartment. Specifically, we demonstrated that one of the most abundant and well-studied virion tegument protein, pp150, was detected only in the cytoplasm of infected cells throughout the course of infection. Rarely, pp150 could be detected within the nucleus of an infected cell, but this was evident only very late in infection, when excessive cytopathology was apparent in the cell. The findings in our study were not due to an antibody reactivity artifact since two different antibody preparations, a heterologous monospecific antiserum and a murine MAb, both detected pp150 only in the cytoplasm. In addition, after viewing countless number of infected cells with these reagents and only very infrequently noting a single cells with what could be considered as having a signal within the nucleus, we could not explain the discrepancy between our findings and those of an earlier report which suggested that pp150 was a nuclear protein (17). Furthermore, when the pp150 ORF was transiently expressed in Cos 7 cells, the protein remained entirely within the cytoplasm (data not shown). Taken together, the results indicated that at least two abundant virion tegument proteins, pp28 and pp150, remained exclusively cytoplasmic during the replicative cycle of HCMV. Since these proteins and the product of the UL25 ORF appeared to be restricted to the cytoplasm throughout the replicative cycle, our results suggested that tegumentation and envelopment (assuming that envelopment of the mature virion occurs after tegument acquisition) stages of virus assembly were likely completed within the cytoplasm.
Our results were consistent with these processes occurring within a single compartment, since pp150 and a number of other tegument and envelope viral proteins accumulated in a cytoplasmic juxtanuclear structure. We attempted to define the cellular compartment(s) that contributed to the formation of this juxtanuclear structure by using markers of known components of various cellular compartments. We observed that the ERGIC and the Golgi were altered in the infected cells late in infection, such that protein markers for these cellular compartments appeared to be localized on the periphery of the viral protein-containing juxtanuclear structure. Interestingly, pp150 within the virus-induced structure appeared to overlap with microtubules emanating from the MTOC, a finding suggesting that it was microtubule associated. In agreement, we found that the structural integrity of the virus-induced structure was dependent on microtubules, as demonstrated by its disruption following treatment with nocadazole. Thus, this intracellular collection of viral proteins appeared to displace normal cellular constituents from a compartment which would normally localize to an area adjacent to the MTOC. In uninfected cells, such a compartment would be consistent with either the Golgi, TGN, and/or the Golgi-ERGIC interface.
We considered the possibility that the juxtanuclear structure represented a cellular storage area or site for protein degradation. A recently described cellular response to overexpression of misfolded (and perhaps normal) proteins is the formation of a vimentin caged storage area, the aggresome (19). The location of the aggresome and its relative size was consistent with the virion protein-containing juxtanuclear structure which appeared late in infection, at a time when viral promoters would drive high expression of a number of viral proteins. However, two of the defining characteristics of aggresomes, the reorganization of cellular vimentin and the resistance of these structures to the microtubule-depolymerizing agent nocadazole, were not properties of the viral protein-containing juxtanuclear structure. It was unlikely that the juxtanuclear viral structure represented a degradative compartment since we failed to colocalize the lysosomal protein LAMP-1 to the pp150-containing structure and, perhaps more importantly, found no gross alteration in the cellular distribution of LAMP-1 in infected compared to uninfected cells. Thus, it appeared that the viral protein-containing juxtanuclear structure was not a known cellular site of protein storage or degradation.
The viral protein-containing structure appeared relatively stable since a number of tegument and envelope glycoproteins cosedimented in sucrose gradients following fractionation of infected cells. A trivial explanation for this finding was that this cofractionation represented an accumulation of cytoplasmic virions or subviral particles and that the results of our biochemical analysis represented a signal obtained from proteins incorporated into progeny virions which cosediment with a specific cellular membrane. However, the distribution of viral infectivity in all fractions of the gradient was not consistent with this interpretation. This was not unexpected because HCMV virions and/or noninfectious particles such as dense bodies would not be expected to sediment into specific fractions in the gradient and relative centrifugal field conditions used for separation of cellular membranes (22).
The finding of the cleaved form of gB within the fraction containing pp150 and pp65 was intriguing, as it suggested that this mature envelope glycoprotein was retrieved from a cellular compartment distal to the Golgi (10, 37). Although an explanation for this result was not obvious, the apparent proximity of the TGN and the pp150-containing juxtanuclear structure raised the possibility that regions of the TGN and the virion protein-containing structure can interface with one another. Finally, it should be noted that the juxtanuclear structure developed late in infection, at a time when the production of progeny virus was increasing rapidly. Interestingly, an abundant tegument protein and a true late protein encoded by HCMV, pp28 (UL99), was also transported to this juxtanuclear structure late in infection. In fact, pp28 was not readily detectable in infected cells until this structure developed, suggesting that accumulation of virion structural proteins within the juxtanuclear structure and the assembly of progeny virus might be temporally associated. Admittedly, several of these interpretations were based on correlative data, but they were consistent with the concept that assembly of HCMV included cytoplasmic tegumentation and envelopment.
Based on the obtained results, we proposed that the juxtanuclear accumulation of tegument and envelope proteins might represent a cytoplasmic site of virion assembly. It appears inherently reasonable that the host cell would sequester tegument and envelope viral proteins destined for cytoplasmic virion assembly into a compartment adjacent to a cellular site where ERGIC or Golgi membranes and microtubular transport converged. In support of our hypothesis, we found that three tegument proteins (UL99, UL83, and UL32) whose primary sequence did not contain motifs which have been described as signals for entry into the cellular secretory pathway and that viral envelope glycoproteins which do transit the secretory pathway could be colocalized to this juxtanuclear compartment. The proximity of this structure to ERGIC or Golgi elements suggested that tegument proteins could interface with envelope glycoproteins within such structures.
It remains unclear how infectious particles would leave this juxtanuclear compartment unless membrane-bound vacuoles containing mature virions could enter the exocytic pathway. Although we and other have observed such cytoplasmic collections of virions, often in the site of a morphologically altered Golgi, we cannot argue with any certainty that this represented a major route of virion production (data not shown). However, the finding of relatively high titers of infectious virus in the membrane fraction containing pp150, pp65, and gB was consistent with assembly of virions within this juxtanuclear compartment followed by export from the cell as membrane-bound virus-containing vacuoles. Taken together, our results strongly supported a cytoplasmic model of HCMV tegumentation and envelopment. Future studies will be aimed at further characterizing the nature of the viral protein-containing compartment in which virion assembly is likely to occur.
ACKNOWLEDGMENTS
V. Sanchez and K. D. Greis contributed equally to this study.
This work was supported by grant R01 AI35602 to W.J.B. from NIAID, National Institutes of Health.
REFERENCES
- 1.Andreoni M, Faircloth M, Vugler L, Britt W J. A rapid microneutralization assay for the measurement of neutralizing antibody reactive with human cytomegalovirus. J Virol Methods. 1989;23:157–168. doi: 10.1016/0166-0934(89)90129-8. [DOI] [PubMed] [Google Scholar]
- 2.Baines J D, Roizman B. The UL11 gene of herpes simplex virus 1 encodes a function that facilitates nucleocapsid envelopment and egress from cells. J Virol. 1992;66:5168–5174. doi: 10.1128/jvi.66.8.5168-5174.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Battista M C, Bergamini G, Boccuni M C, Campanini F, Ripalti A, Landini M P. Expression and characterization of a novel structural protein of human cytomegalovirus, pUL25. J Virol. 1999;73:3800–3809. doi: 10.1128/jvi.73.5.3800-3809.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Billstrom M A, Britt W J. Postoligomerization folding of human cytomegalovirus glycoprotein B: identification of folding intermediates and importance of disulfide bonding. J Virol. 1995;69:7015–7022. doi: 10.1128/jvi.69.11.7015-7022.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Britt W J, Auger D. Identification of a 65,000 dalton virion envelope of human cytomegalovirus. Virus Res. 1985;4:31–36. doi: 10.1016/0168-1702(85)90018-8. [DOI] [PubMed] [Google Scholar]
- 6.Browne H, Bell S, Minson T, Wilson D W. An endoplasmic reticulum-retained herpes simplex virus glycoprotein H is absent from secreted virions: evidence for reenvelopment during egress. J Virol. 1996;70:4311–4316. doi: 10.1128/jvi.70.7.4311-4316.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chee M, Rudolf S, Plachter B, Barrell B, Jahn G. Identification of the major capsid protein gene of human cytomegalovirus. J Virol. 1989;63:1345–1353. doi: 10.1128/jvi.63.3.1345-1353.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Lazarro C, Campadelli-Fiume G, Torrisi M R. Intermediate forms of glycoconjugates are present in the envelope of herpes simplex virions during their transport along the exocytic pathway. Virology. 1995;214:619–623. doi: 10.1006/viro.1995.0073. [DOI] [PubMed] [Google Scholar]
- 10.Fish K N, Soderberg-Naucler C, Nelson J A. Steady-state plasma membrane expression of human cytomegalovirus gB is determined by the phosphorylation state of Ser900. J Virol. 1998;72:6657–6664. doi: 10.1128/jvi.72.8.6657-6664.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gershon A A, Sherman D L, Zhu Z, Gabel C A, Ambron R T, Gershon M. Intracellular transport of newly synthesized varicella-zoster virus: final envelopment in the trans-Golgi network. J Virol. 1994;68:6372–6390. doi: 10.1128/jvi.68.10.6372-6390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibson W. Structure and assembly of the virion. Intervirology. 1996;39:389–400. doi: 10.1159/000150509. [DOI] [PubMed] [Google Scholar]
- 13.Gong M, Kieff E. Intracellular trafficking of two major Epstein-Barr virus glycoproteins gp350/220 and gp110. J Virol. 1990;64:1507–1516. doi: 10.1128/jvi.64.4.1507-1516.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Granzow H, Weiland F, Jones A, Klupp B G, Karger A, Mettenleitner T C. Ultrastructural analysis of the replication cycle of pseudorabies virus in cell culture: a reassessment. J Virol. 1997;71:2072–2082. doi: 10.1128/jvi.71.3.2072-2082.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greis K D, Gibson W, Hart G W. Site-specific glycosylation of the human cytomegalovirus tegument basic phosphoprotein (UL32) at serine 921 and serine 952. J Virol. 1994;68:8339–8349. doi: 10.1128/jvi.68.12.8339-8349.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grose C. Glycoproteins encoded by varicella-zoster virus: biosynthesis, phosphorylation, and intracellular trafficking. Annu Rev Microbiol. 1990;44:59–80. doi: 10.1146/annurev.mi.44.100190.000423. [DOI] [PubMed] [Google Scholar]
- 17.Hensel G, Meyer H, Gartner S, Brand G, Kern H F. Nuclear localization of the human cytomegalovirus tegument protein pp150 (ppUL32) J Gen Virol. 1995;76:1591–1601. doi: 10.1099/0022-1317-76-7-1591. [DOI] [PubMed] [Google Scholar]
- 18.Johnson D C, Spear P G. O-linked oligosaccharides are acquired by herpes simplex virus glycoproteins in the Golgi apparatus. Cell. 1983;32:987–997. doi: 10.1016/0092-8674(83)90083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnston J A, Ward C L, Kopito R R. Aggresomes: a cellular response to misfolded proteins. J Cell Biol. 1998;143:1883–1898. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones F, Grose C. Role of cytoplasmic vacuoles in varicella-zoster virus glycoprotein trafficking and virion envelopment. J Virol. 1988;62:2701–2711. doi: 10.1128/jvi.62.8.2701-2711.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landini M P, Severi B, Furlini G, Badiali D G L. Human cytomegalovirus structural components: intracellular and intraviral localization of p28 and p65-69 by immunoelectron microscopy. Virus Res. 1987;8:15–23. doi: 10.1016/0168-1702(87)90036-0. [DOI] [PubMed] [Google Scholar]
- 22.Li L, Nelson J A, Britt W J. Glycoprotein H related complexes of human cytomegalovirus: identification of a third protein in the gCIII complex. J Virol. 1997;71:3090–3097. doi: 10.1128/jvi.71.4.3090-3097.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molloy S S, Anderson E D, Jean F, Thomas G. Bi-cycling the furin pathway: from TGN localization to pathogen activation and embryogenesis. Trends Cell Biol. 1999;9:28–35. doi: 10.1016/s0962-8924(98)01382-8. [DOI] [PubMed] [Google Scholar]
- 24.Nelson D S, Alvarez C, Gao Y S, Garcia-Mata R, Fialkowski E, Sztul E. The membrane transport factor TAP/p115 cycles between the Golgi and earlier secretory compartments and contains distinct domains required for its localization and function. J Cell Biol. 1998;143:319–331. doi: 10.1083/jcb.143.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nii S, Yoshida M, Uno F, Kurata T, Ikuta Y, Yamanishi K. Replication of human herpesvirus 6 (HHV-6): morphological aspects. Adv Exp Med Biol. 1990;278:19–28. doi: 10.1007/978-1-4684-5853-4_3. [DOI] [PubMed] [Google Scholar]
- 26.Plachter B, Britt W, Vornhagen R, Stamminger T, Jahn G. Analysis of proteins encoded by IEI regions 1 and 2 of human cytomegalovirus using monoclonal antibodies generated against recombinant antigens. Virology. 1993;193:642–652. doi: 10.1006/viro.1993.1172. [DOI] [PubMed] [Google Scholar]
- 27.Rixon F J, Addison C, McLauchlan J. Assembly of enveloped tegument structures (L particles) can occur independently of virion maturation in herpes simplex type 1-infected cells. J Gen Virol. 1992;73:277–284. doi: 10.1099/0022-1317-73-2-277. [DOI] [PubMed] [Google Scholar]
- 28.Roffman E, Albert J P, Goff J P, Frenkel N. Putative site for the acquisition of human herpesvirus 6 virion tegument. J Virol. 1990;64:6308–6313. doi: 10.1128/jvi.64.12.6308-6313.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roizman B. Herpesviridae. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2221–2230. [Google Scholar]
- 30.Sanchez V, Angeletti P C, Engler J A, Britt W J. Localization of human cytomegalovirus structural proteins to the nuclear matrix of infected human fibroblasts. J Virol. 1998;72:3321–3329. doi: 10.1128/jvi.72.4.3321-3329.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simpson J A, Chow J C, Baker J, Avdalovi N, Yuan S, Au D, Co M S, Vasquez M, Britt W, Coelingh K L. Neutralizing monoclonal antibodies that distinguish three antigenic sites on the human cytomegalovirus glycoprotein H have conformationally-distinct binding sites. J Virol. 1993;67:489–496. doi: 10.1128/jvi.67.1.489-496.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith J D, DeHarven E. Herpes simplex virus and human cytomegalovirus replication in WI-38 cells. I. Sequence of viral replication. J Virol. 1973;12:919–930. doi: 10.1128/jvi.12.4.919-930.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spear P G. Glycoproteins specified by herpes simplex viruses. In: Roizman B, editor. The herpesviruses. Vol. 3. New York, N.Y: Plenum Press; 1985. pp. 315–356. [Google Scholar]
- 34.Stackpole C W. Herpes-type virus of the frog renal adenocarcinoma. I. Virus development in tumor transplants maintained at low temperature. J Virol. 1969;4:75–93. doi: 10.1128/jvi.4.1.75-93.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tirabassi R S, Enquist L W. Mutation of the YXXL endocytosis motif in the cytoplasmic tail of pseudorabies virus gE. J Virol. 1999;73:2717–2728. doi: 10.1128/jvi.73.4.2717-2728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tooze J, Hollinshead M, Reis B, Radsak K, Kern H. Progeny vaccinia and human cytomegalovirus particles utilize early endosomal cisternae for their envelopes. Eur J Cell Biol. 1993;601:163–178. [PubMed] [Google Scholar]
- 37.Vey M, Schafer W, Reis B, Ohuchi R, Britt W, Garten W, Klenk H D, Radsak K. Proteolytic processing of human cytomegalovirus glycoprotein B (gpUL55) is mediated by the human endoprotease furin. Virology. 1995;206:746–749. doi: 10.1016/s0042-6822(95)80002-6. [DOI] [PubMed] [Google Scholar]
- 38.Wan L, Molloy S S, Thomas L, Liu G, Xiang Y, Rybak S L, Thomas G. PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell. 1998;94:205–216. doi: 10.1016/s0092-8674(00)81420-8. [DOI] [PubMed] [Google Scholar]
- 39.Whealy M E, Card J P, Meade R P, Robbins A K, Enquist L W. Effect of brefeldin A on alphaherpesvirus membrane protein glycosylation and virus egress. J Virol. 1991;65:1066–1081. doi: 10.1128/jvi.65.3.1066-1081.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whealy M E, Robbins A K, Enquist L W. The export pathway of the pseudorabies virus gB homolog gII involves oligomer formation in the endoplasmic reticulum and protease processing in the Golgi apparatus. J Virol. 1990;64:1946–1955. doi: 10.1128/jvi.64.5.1946-1955.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whealy M E, Robbins A K, Tufaro F, Enquist L W. A cellular function is required for pseudorabies virus envelope glycoprotein processing and virus egress. J Virol. 1992;66:3803–3810. doi: 10.1128/jvi.66.6.3803-3810.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu Z, Gershon M D, Hao Y, Ambron R T, Gabel C A, Gershon A A. Envelopment of varicella-zoster virus: targeting of viral glycoproteins to the trans-Golgi network. J Virol. 1995;69:7951–7959. doi: 10.1128/jvi.69.12.7951-7959.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu Z, Hao Y, Gershon M D, Ambron R T, Gershon A A. Targeting of glycoprotein I (gE) of varicella-zoster virus to the trans-Golgi network by an AYRV sequence and an acidic amino acid-rich patch in the cytosolic domain of the molecule. J Virol. 1996;70:6563–6575. doi: 10.1128/jvi.70.10.6563-6575.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]