Key Points
Question
What is the efficacy of 15 days of nirmatrelvir-ritonavir for improving select symptoms of postacute sequelae of SARS-CoV-2 infection (PASC)?
Findings
This randomized clinical trial including 155 participants with PASC symptoms (≥3 months’ duration) found that a 15-day course of nirmatrelvir-ritonavir in a mostly vaccinated study cohort was generally safe, but did not show significant benefit in improving fatigue, brain fog, body aches, cardiovascular symptoms, shortness of breath, or gastrointestinal symptoms.
Meaning
These findings indicate that further studies are needed to determine the role of antivirals in the treatment of PASC.
Abstract
Importance
There is an urgent need to identify treatments for postacute sequelae of SARS-CoV-2 infection (PASC).
Objective
To assess the efficacy of a 15-day course of nirmatrelvir-ritonavir in reducing the severity of select PASC symptoms.
Design, Setting, and Participants
This was a 15-week blinded, placebo-controlled, randomized clinical trial conducted from November 2022 to September 2023 at Stanford University (California). The participants were adults with moderate to severe PASC symptoms of 3 months or longer duration.
Interventions
Participants were randomized 2:1 to treatment with oral nirmatrelvir-ritonavir (NMV/r, 300 mg and 100 mg) or with placebo-ritonavir (PBO/r) twice daily for 15 days.
Main Outcomes and Measures
Primary outcome was a pooled severity of 6 PASC symptoms (fatigue, brain fog, shortness of breath, body aches, gastrointestinal symptoms, and cardiovascular symptoms) based on a Likert scale score at 10 weeks. Secondary outcomes included symptom severity at different time points, symptom burden and relief, patient global measures, Patient-Reported Outcomes Measurement Information System (PROMIS) measures, orthostatic vital signs, and sit-to-stand test change from baseline.
Results
Of the 155 participants (median [IQR] age, 43 [34-54] years; 92 [59%] females), 102 were randomized to the NMV/r group and 53 to the PBO/r group. Nearly all participants (n = 153) had received the primary series for COVID-19 vaccination. Mean (SD) time between index SARS-CoV-2 infection and randomization was 17.5 (9.1) months. There was no statistically significant difference in the model-derived severity outcome pooled across the 6 core symptoms at 10 weeks between the NMV/r and PBO/r groups. No statistically significant between-group differences were found at 10 weeks in the Patient Global Impression of Severity or Patient Global Impression of Change scores, summative symptom scores, and change from baseline to 10 weeks in PROMIS fatigue, dyspnea, cognitive function, and physical function measures. Adverse event rates were similar in NMV/r and PBO/r groups and mostly of low grade.
Conclusions and Relevance
The results of this randomized clinical trial showed that a 15-day course of NMV/r in a population of patients with PASC was generally safe but did not demonstrate a significant benefit for improving select PASC symptoms in a mostly vaccinated cohort with protracted symptom duration. Further studies are needed to determine the role of antivirals in the treatment of PASC.
Trial Registration
ClinicalTrials.gov Identifier: NCT05576662
This randomized clinical trial evaluates the efficacy of oral nirmatrelvir-ritonavir for treatment of moderate to severe postacute sequelae of SARS-CoV-2 infection of 3 months or longer duration.
Introduction
Postacute sequelae of SARS-CoV-2 infection (PASC), also known as long COVID or post−COVID-19 condition, has affected millions of people worldwide and encompasses a variety of conditions and symptoms that can persist months to years with impact on quality of life and function.1,2,3,4,5,6 Evolving definitions, growing mechanistic understanding, and clinical heterogeneity present challenges to the diagnosis and treatment of PASC.7,8,9 There is an urgent need for evidence-based treatments for PASC but currently a paucity of published trials testing interventions that target underlying pathophysiology.10,11,12,13
SARS-CoV-2 virus or viral particle persistence is one of several proposed casual mechanisms for PASC.14,15,16,17 Prolonged SARS-CoV-2 viral RNA shedding for months in the upper respiratory tract and in the stool has been observed.18,19,20,21 Although no reservoir of live replicating virus has been identified in individuals with PASC, SARS-CoV-2 RNA and/or protein has been found to persist in various tissues such as blood,22,23,24 periodontal pockets,25 gastrointestinal tract,26,27 the central nervous system,28 and other anatomic sites.28,29,30,31,32 Residual viral presence may trigger ongoing inflammation and immune dysregulation, resulting in a diverse array of symptoms.17 Thus, antiviral agents against SARS-CoV-2 present a therapeutic avenue for investigation to address a potential root cause of PASC.
Some studies suggest that antivirals such as nirmatrelvir, molnupiravir, and remdesivir taken during the acute infection period may reduce the risk of select post−COVID-19 sequelae,33,34,35,36 while others demonstrate mixed results in different cohorts.37,38,39 Nirmatrelvir is a peptidomimetic inhibitor of SARS-CoV-2 main protease (Mpro) preventing viral replication. Nirmatrelvir, in combination with low-dose ritonavir that slows nirmatrelvir metabolism via inhibition of CYP3A4 (nirmatrelvir-ritonavir), was approved by the US Food and Drug Administration for the treatment of mild to moderate COVID-19 in adults at high risk for progression to severe COVID-19.40 We and others have reported anecdotal cases of patients with PASC who noted improvement of symptoms after taking nirmatrelvir-ritonavir for SARS-CoV-2 reinfection,41,42 but there are no published randomized clinical trials testing nirmatrelvir-ritonavir for treatment of PASC.
The landscape of PASC research is dynamic. Smaller studies that are more focused and agile can scout the terrain ahead of larger and more definitive studies. The objectives of the Selective Trial of Paxlovid for PASC (STOP-PASC) were to assess the effect of a 15-day course of NMV/r vs PBO/r in improving PASC symptoms and other patient-reported outcomes. The secondary and exploratory objectives were to explore the potential biologic and digital wearable biomarkers of PASC and to collect multidimensional data to inform future research.
Methods
This randomized clinical trial was approved by the Stanford Institutional Review Board. All participants gave written informed consent. A Community Advisory Board that included patients with PASC provided input on the study. A data and safety monitoring board provided independent oversight. The study followed the Consolidated Standards of Reporting Trials (CONSORT) guidelines.
Study Design
STOP-PASC was a double-blind randomized clinical trial to investigate orally administered nirmatrelvir-ritonavir (NMV/r) compared with placebo-ritonavir (PBO/r) in outpatient adult participants with PASC of 3 or more months’ duration. The trial was conducted from November 8, 2022, to September 12, 2023, at Stanford University (California). The full trial protocol, statistical plan, and trial schematic are available in Supplements 1 and 2 and in eFigure 1 in Supplement 3, respectively.
Trial Participants
Participant inclusion criteria were being 18 years or older; weight greater than 40 kg; estimated glomerular filtration rate of 60 mL/min or higher; history of confirmed COVID-19 infection from early 2020 to more than 90 days before the end of enrollment; and PASC symptoms, as determined by clinician, persisting more than 90 days after the initial (index) COVID-19 infection and with at least 2 self-reported moderate or severe core symptoms or symptom clusters defined as fatigue, brain fog, body aches, cardiovascular symptoms, shortness of breath, and gastrointestinal symptoms. Key exclusion criteria included pregnancy or breastfeeding, severe liver disease, SARS-CoV-2 infection, and use of SARS-CoV-2−specific treatment within 30 days of randomization, SARS-CoV-2 vaccination within 28 days, or other vaccine within 14 days of randomization, or medications that interact with study drug. Full eligibility criteria are available in Supplement 1.
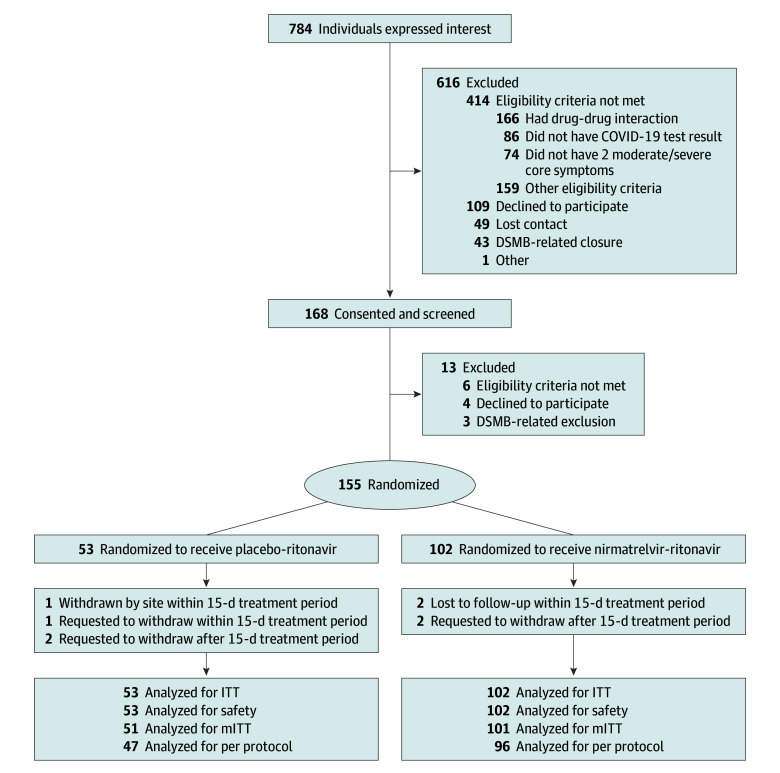
Between November 2022 and May 2023, a total of 784 individuals were prescreened of whom 168 proceeded to consent and screening (Figure 1). Eligible participants were randomized 2:1 to NMV/r and PBO/r and included in the intent-to-treat analyses. Enrollment was stopped early in June 2023 when the prespecified threshold for futility had been met (conditional power <10%).
Figure 1. CONSORT Diagram.
Eligibility exclusion reasons are not mutually exclusive. DSMB, indicates data and safety monitoring board; ITT, intent-to-treat, and mITT, modified intent-to-treat.
Randomization and Interventions
Participants were randomized 2:1 to receive nirmatrelvir, 300 mg, with ritonavir, 100 mg, or placebo with ritonavir, 100 mg, taken orally twice daily for 15 days and followed up until 15 weeks from randomization, stratified by the number of moderate or severe core symptoms (2 or 3 vs >3) and vaccination status (completed primary series vs not completed).43 Additional details are included in the eMethods in Supplement 3. A schedule of events, including assessments and procedures, are detailed in Supplement 1 and eFigure 1 in Supplement 3.
Outcomes
The primary end point was core symptoms severity during the past 7 days based on Likert scale score (where 0 is none, 1 mild, 2 moderate, 3 severe) pooled at 10 weeks postrandomization in participants treated with NMV/r vs PBO/r (eMethods in Supplement 3). Core symptoms were selected based on mechanistic rationale, clinical experience, and reported PASC symptoms prevalence and severity.5,44,45,46,47 A 10-week time point was chosen to assess durability of response to treatment.
Secondary end points included individual core symptom severity at 10 weeks and other time points, proportion of participants reporting relief (defined as reduction of severity from moderate to none or severe to mild or none for at least 1 core symptom) or alleviation (improvement of all core symptoms from none or mild at baseline to none or moderate to severe to none or mild) at 10 weeks, severity of most bothersome symptom, time to relief of each core symptom, change from baseline to 10 weeks in Patient-Reported Outcomes Measurement Information System (PROMIS) SF v2.0 Physical Function 4a; SF v1.0 Fatigue 7a; SF v1.0 Dyspnea Severity 5a; SF v2.0 Cognitive Abilities 4a scores48; change in orthostatic vital signs (seated and standing blood pressure and heart rate); sit-to-stand test at 10 weeks49; and Patient Global Impression of Severity (PGIS) and Patient Global Impression of Change (PGIC) at day 15, week 5, week 10, and week 15 in NMV/r vs PBO/r groups.50 Exploratory stool reverse transcription polymerase chain reaction was performed on all available baseline samples (eMethods in Supplement 3).
Statistical Analysis
The primary analysis followed the intent-to-treat (ITT) principle. Sensitivity analyses were conducted excluding (1) participants with no follow-up (modified ITT) and (2) participants with no follow-up and those taking more than 80% of intervention doses (per protocol).
The primary analysis involved first fitting a proportional odds logistic regression model for severity level of each core symptom at week 10. Each model was adjusted for baseline severity of the corresponding symptom and fit using only participants who experienced the corresponding symptoms at baseline. If participants missed the week-10 survey, their week 9 survey during the week-10 visit window was used for the primary analysis. A test statistic measuring the overall efficacy was calculated as the weighted average of the regression coefficient for the treatment indicator in the proportional odds model for each core symptom with inverse variance weighting.51,52 The P value for testing the overall efficacy was obtained by a nonparametric permutation test.
We initially determined that a sample size of 200 would provide power of at least 77% at the 2-sided significance level of P = .05 to detect an odds ratio (OR) of 1.6 for having a better Likert scale score at week 10 (NMV/r vs PBO/r) across all 6 core symptoms, assuming 10% attrition. An interim analysis for futility and safety was preplanned after 50% of participants completed week 10 with enrollment to be stopped if conditional power for concluding efficacy was less than 10% assuming that the underlying treatment effect size was the same as that observed in the interim analysis.
Proportional odds models were used to compare ordinal secondary end points. Linear regression was used to compare PGIS, PGIC, PROMIS measures, 1 minute sit-to-stand test, orthostatic vital signs, and the sum of the Likert scale scores for the core symptoms (summative score). Logistic regression was used to compare the probability of experiencing relief and the proportion of weeks with mild or no symptoms. Cox proportional hazards models were used to compare the time to relief of the most bothersome symptom and the core symptoms. Participants who did not experience any relief were right censored at the time of their last observation. We used a cumulative link mixed model with a participant-specific random intercept to compare the trajectory of symptoms between study groups in an exploratory analysis. In a post hoc analysis, we repeated selected analyses (the primary outcome, the proportion of weeks with mild or no symptoms, and PGIC) separately in patients who had their index infection before December 2021 and in patients who were infected later when the Omicron variant was dominant.
All models were adjusted for the stratification factor, ie, the number of moderate to severe core symptoms at baseline (2 or 3 vs >3), except the primary analysis and the proportion of weeks where participants had mild or no symptoms, where we adjusted for baseline severity instead. All tests were performed at the 2-sided .05 significance level. Analyses were performed in R, version 4.2.1 (The Foundation for Statistical Computing).53
Additional analysis information is included in Supplement 2 and the eMethods in Supplement 3. Per prespecified analysis plan, P values for secondary and exploratory analyses were not adjusted for multiple comparisons because these end points were intended to provide a global picture of the treatment effect, and therefore, individual secondary outcomes should be interpreted as exploratory given potential inflation for type I error due to multiple comparisons.
Results
Study Population
Among the 155 participants randomized (median [IQR] age, 43 [34-54] years), there were 92 (59.4%) females and 63 (40.6%) males, with 20 (12.9%) Asian, 3 (1.9%) Black, 19 (12.3%) Hispanic, 1 (1%) Native Hawaiian or other Pacific Islander, 115 (74.2%) White, and 6 (3.9%) participants of more than one race; for 10 participants’ (6.5%) race was unknown. The 2 groups were similar with respect to baseline characteristics (Table 1). The mean (SD) time between index SARS-CoV-2 infection and randomization was 17.5 (9.1) months. Only 1 participant in each group had not received the initial COVID-19 vaccination series. Before enrollment, 41 participants (26.5%) had used SARS-CoV-2 acute antiviral medication including NMV/r (Table 1). The most common PASC symptoms at enrollment were fatigue, reported by all participants, and brain fog, reported by 148 (95.5%). Overall, baseline severity of symptoms similar in both groups with a slightly higher distribution of severity scores for body aches and lower for cardiovascular and gastrointestinal symptoms in NMV/r than in PBO/r groups (Table 1; Figure 2). No baseline stool specimens had detectable SARS-CoV-2 RNA (eFigure 2 in Supplement 3).
Table 1. Baseline Characteristics of Study Participants With Postacute Sequelae of SARS-CoV-2 Infection.
| Characteristic | No. (%) | ASDa | |
|---|---|---|---|
| NMV/r | PBO/r | ||
| Group participants, No. | 102 | 53 | NA |
| Age, median (IQR), y | 44.5 (35.25-56) | 41 (31-45) | 0.34 |
| Female | 61 (59.8) | 31 (58.5) | NA |
| Male | 41 (40.2) | 22 (41.5) | NA |
| Race | |||
| Asian | 11 (10.8) | 9 (17) | 0.37 |
| Black or African American | 1 (1) | 2 (3.8) | |
| Native Hawaiian or other Pacific Islander | 1 (1) | 0 | |
| White | 76 (74.5) | 39 (73.6) | |
| More than 1 race | 5 (4.9) | 1 (1.9) | |
| Unknown | 8 (7.8) | 2 (3.8) | |
| Hispanic ethnicity | 12 (11.8) | 7 (13.2) | 0.04 |
| Index COVID-19 infection dateb | |||
| Before May 2021 | 39 (38.2) | 22 (41.5) | 0.17 |
| May to December 2021 | 20 (19.6) | 7 (13.2) | |
| After December 2021 | 43 (42.2) | 24 (45.3) | |
| Hospitalized for index COVID-19 infection | 6 (5.9) | 3 (5.7) | 0.01 |
| Time from index infection to randomization, mean (SD), mo | 17.6 (9.1) | 17.3 (9.1) | 0.03 |
| Total COVID-19 infections, mean (SD)c | 1.45 (0.75) | 1.34 (0.55) | 0.17 |
| Prior use of SARS-CoV-2 acute medication | |||
| Prior use of medicationd | 27 (26.5) | 14 (26.4) | <0.01 |
| Prior use of Paxlovid | 18 (17.6) | 9 (17) | 0.02 |
| No prior use | 75 (73.5) | 39 (73.6) | NA |
| Vaccination status at randomization | |||
| Initial series completed | 101 (99) | 52 (98.1) | 0.08 |
| Initial series not completed | 1 (1) | 1 (1.9) | |
| BMI, mean (SD) | 27 (6.19) | 28 (6.66) | 0.18 |
| BMI group | |||
| Underweight (<18.5) | 4 (3.9) | 0 | 0.34 |
| Normal (18.5-24.9) | 39 (38.2) | 17 (32.1) | |
| Overweight (25.0-29.9) | 33 (32.4) | 18 (34) | |
| Obesity (≥30.0) | 26 (25.5) | 18 (34) | |
| Comorbiditiese | |||
| Depression | 24 (23.5) | 13 (24.5) | 0.02 |
| Allergies | 17 (16.7) | 12 (22.6) | 0.12 |
| Asthma | 15 (14.7) | 13 (24.5) | 0.22 |
| Anxiety | 15 (14.7) | 8 (15.1) | 0.02 |
| GERD | 15 (14.7) | 6 (11.3) | 0.13 |
| Moderate to severe post−COVID-19 symptoms, No. | |||
| 2-3 | 47 (46.1) | 25 (47.2) | 0.02 |
| >3 | 55 (53.9) | 28 (52.8 | |
| Moderate to severe symptom at baseline, % of participants | |||
| Fatigue | 95.1 | 96.2 | NA |
| Brain fog | 81.4 | 79.2 | NA |
| Body aches | 57.8 | 50.9 | NA |
| Cardiovascular | 49.0 | 60.4 | NA |
| Shortness of breath | 46.1 | 52.8 | NA |
| Gastrointestinal | 41.2 | 47.2 | NA |
Abbreviations: ASD, absolute standardized difference; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); GERD, gastroesophageal reflux disease; NA, not applicable; NMV/r, oral nirmatrelvir-ritonavir; PBO/r, placebo-ritonavir.
A larger ASD indicates a larger difference between the groups (eg, 0.2 = small difference, 0.5 = moderate difference, 0.8 = large difference).
Index COVID-19 infection was defined as the initial infection associated with subsequent onset of participant’s postacute sequelae of SARS-CoV-2 infection.
Total COVID-19 infections was the participant-reported total number of COVID-19 infections before enrollment.
Other medications for acute infection include remdesivir, molnupiravir, and bebtelovimab.
Participant-reported comorbidities included onset before and after the index COVID-19 infection.
Figure 2. Distribution of Core Symptom Severity Scores Over Time in Adults With Postacute Sequelae of SARS-CoV-2 Infection.
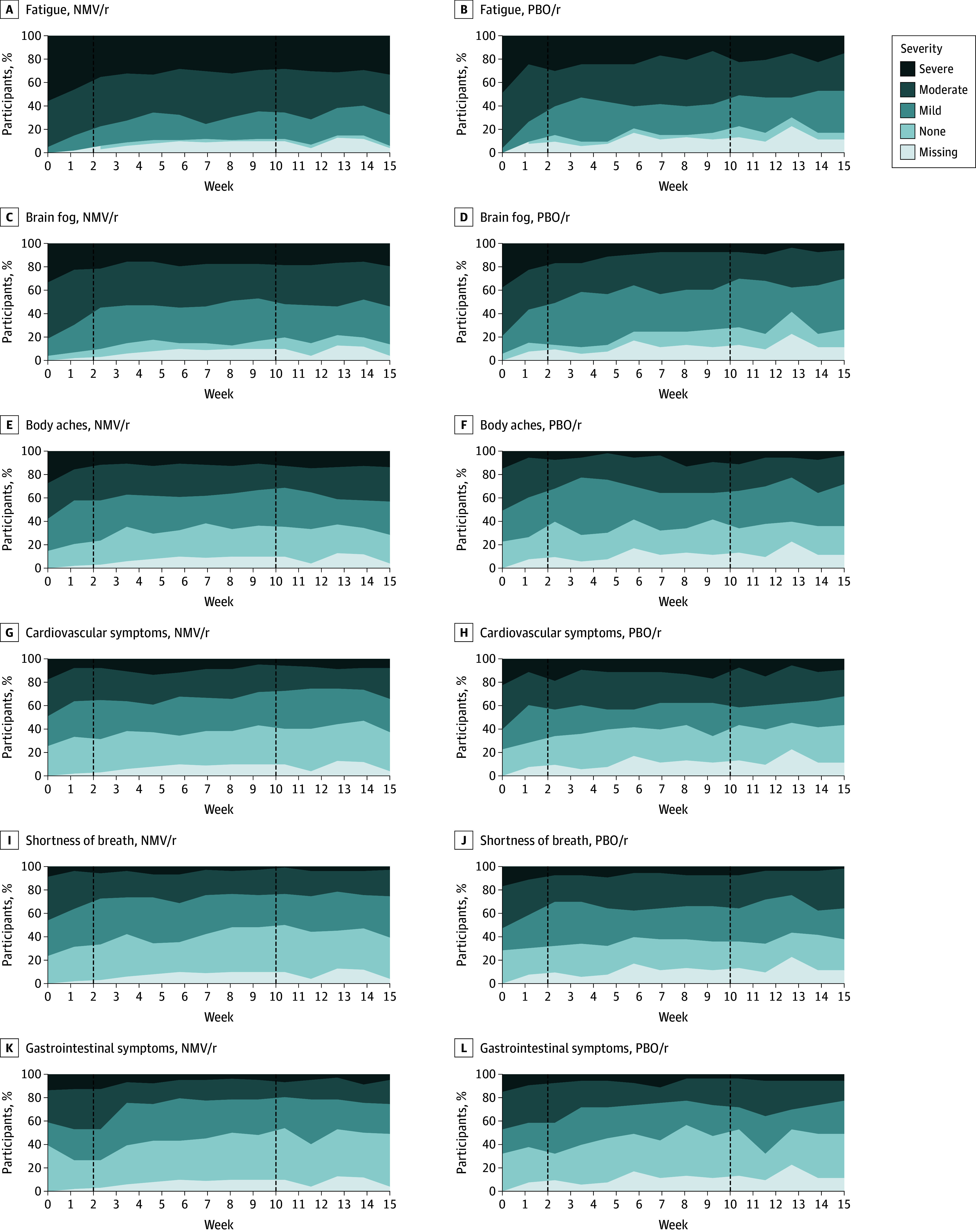
Dashed line on the left marks the end of the 15-day treatment period, and on the right, it marks the 10-week primary end point. NMV/r indicates nirmatrelvir-ritonavir, and PBO/r, placebo-ritonavir.
Primary Outcome
At the 10-week primary end point, 99 of 102 participants in the NMV/r group (97.1%) and 49 of 53 participants in the PBO/r group (92.5%) had primary outcome data available. Considering the 6 core symptoms together (fatigue, brain fog, body aches, cardiovascular symptoms, shortness of breath, gastrointestinal symptoms), there was no statistically significant difference in the pooled symptom severity between NMV/r and PBO/r groups at 10 weeks, adjusted for baseline severity. The 6 core symptoms progressed toward lower severity in both groups (Table 2; Figure 2).
Table 2. Select Secondary Outcomes and Adverse Events in Nirmatrelvir-Ritonavir Use for Postacute Sequelae of SARS-CoV-2 Infection.
| Outcome or event | NMV/r | PBO/r | ||
|---|---|---|---|---|
| Participants in group | 102 | 53 | ||
| Moderate to severe symptoms, change from baseline at 10 wk, % of participants | ||||
| Fatigue | −23.5 | −43.4 | ||
| Brain fog | −28.4 | −47.2 | ||
| Body aches | −22.5 | −20.8 | ||
| Cardiovascular | −23.5 | −20.8 | ||
| Shortness of breath | −20.6 | −24.5 | ||
| Gastrointestinal | −19.6 | −11.3 | ||
| Mean (SD) | β (95% CI)a | P value | ||
| PROMIS, change from baseline to 10 wk | ||||
| Physical function | 2.73 (6.62) | 1.32 (5.75) | 0.57 (−1.96 to 3.10) | .66 |
| Fatigue | −3.92 (7.88) | −4.05 (5.90) | 0.38 (−2.40 to 3.15) | .79 |
| Dyspnea | −1.96 (7.90) | −2.38 (6.13) | 0.60 (−2.55 to 3.75) | .70 |
| Cognitive function | 4.84 (8.18) | 5.05 (7.56) | 0.03 (−3.21 to 3.28) | .98 |
| PGIC score at 10 wk | 3.38 (1.31) | 3.13 (1.03) | 0.10 (−0.48 to 0.67) | .74 |
| PGIS score at 10 wk | 4 (1.03) | 3.79 (1.06) | 0.19 (−0.25 to 0.62) | .40 |
| Summative score at 10 wk | 7.62 (3.75) | 7.69 (4.09) | −0.24 (−1.46 to 0.97) | .69 |
| HR (95% CI) | P value | |||
| Time to relief of most bothersome symptomb | 0.74 (0.40 to 1.38) | .33 | ||
| No. (%) | OR (95% CI) | P value | ||
| Experiencing relief at 10 wkb | 33 (32.4) | 22 (41.5) | 0.55 (0.27 to 1.09) | .09 |
| Experiencing alleviation at 10 wkc | 7 (6.86) | 5 (9.43) | 0.72 (0.21 to 2.44) | .60 |
| Median (IQR) | OR (95% CI) d | P value | ||
| Proportion of weeks 1-15 with mild or no symptoms | ||||
| Fatigue | 0.15 (0 to 0.39) | 0.15 (0 to 0.77) | 0.55 (0.33 to 0.92) | .02 |
| Brain fog | 0.31 (0 to 0.75) | 0.56 (0.15 to 0.85) | 0.50 (0.31 to 0.82) | .01 |
| Body aches | 0.54 (0.10 to 0.92) | 0.64 (0.29 to 0.83) | 1.32 (0.74 to 2.33) | .34 |
| Cardiovascular symptoms | 0.67 (0.19 to 0.92) | 0.46 (0 to 0.92) | 1.37 (0.76 to 2.48) | .29 |
| Shortness of breath | 0.769 (0.25 to 1) | 0.62 (0.09 to 0.89) | 1.32 (0.73 to 2.38) | .35 |
| Gastrointestinal symptoms | 0.63 (0.31 to 0.92) | 0.52 (0.28 to 0.90) | 1.40 (0.79 to 2.47) | .25 |
| No. (%) | ||||
| Participants with AEs | 101 (99) | 49 (92) | ||
| No. of AEs | 771 | 313 | ||
| Total SAEe | 3 (2.9) | 1 (1.9) | ||
| Participants with grade 3 or 4 AEsf | 5 (4.9) | 3 (5.7) | ||
| Fatalities | 0 | 0 | ||
Abbreviations: AE, adverse event; HR, hazard ratio; NA, not applicable; NMV/r, nirmatrelvir-ritonavir; OR, odds ratio; PBO/r, placebo-ritonavir; PGIC, Patient Global Impression of Change; PGIS, Patient Global Impression of Severity; PROMIS, Patient-Reported Outcomes Measurement Information System; SAE, serious adverse event.
Estimated coefficients (βs) for PGIC or PGIS can be interpreted as differences in PGIC and PGIS score values between groups, eg, an estimate of 0.3 means that, on average, those on NMV/r reported PGIC/PGIS 0.3 points higher than those on PBO/r. However, coefficients for PROMIS measures should be interpreted as differences (in NMV/r vs PBO/r) in change scores (baseline vs wk 10). A higher score value corresponds to reduced severity for PROMIS-physical and cognitive function; greater severity for PROMIS-fatigue and dyspnea; worsening status for PGIC; and greater severity for PGIS. Therefore, improvement from baseline to week 10 corresponds to positive change scores for PROMIS-physical and cognitive function, and negative change scores for PROMIS-fatigue and dyspnea.
Relief was defined as a reduction of severity from moderate to none or severe to mild or none for at least 1 core symptom; time to relief was measured in weeks.
Alleviation was defined as improvement of all core symptoms from none or mild at baseline to none or moderate to severe to none or mild.
An OR of 1.5 corresponds to a 50% increase in the odds of experiencing mild or no symptoms for those taking NMV/r compared to those taking PBO/r.
One participant each had blood-loss anemia, forearm fracture, and melanoma in the NMV/r group (all assessed as unrelated to intervention), and 1 participant had hepatitis in the PBO/r group (assessed as possibly related).
Graded per Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, version 2.1.
Secondary Outcomes
Evaluating individual symptoms at different time points during 15 weeks resulted in no consistent patterns to distinguish NMV/r from PBO/r groups (eFigures 3 and 4 in Supplement 3). The “most bothersome” core symptoms reported by participants most commonly were fatigue (n = 70; 45.2%) and brain fog (n =38; 24.5%), and there was no significant difference in severity of the most bothersome symptom between the 2 groups at 5 weeks but there were slightly higher odds of a more severe score for those in the NMV/r group compared with those in the PBO/r group at 10 weeks (OR, 1.99; 95% CI, 1.06-3.72; P = .03) and 15 weeks (OR, 2.42; 95% CI, 1.27-4.60; P = .01). There were no statistically significant differences in proportion of participants experiencing relief at 5, 10, and 15 weeks; alleviation at 10 weeks; or time to relief of each core symptom and the most bothersome symptom between the 2 groups (Table 2; eTable 1 in Supplement 3).
Total summative severity scores for all core symptoms at 5, 10, and 15 weeks were similar between the intervention and control groups (eFigure 5 in Supplement 3). Mean severity scores for all core symptoms in both groups generally improved (eFigure 6 in Supplement 3); the difference between groups for the change in severity scores across 15 weeks was statistically significant for brain fog only. A post hoc analysis found no statistically significant difference between the 2 groups in the proportion of total postrandomization weeks with mild or no symptoms for each core symptom when adjusted for baseline symptom severity, except for brain fog for which the NMV/r group had decreased odds of experiencing mild or no symptoms (Table 2). Heatmaps of symptom severity scores and change from baseline over time showed week-to-week variability and heterogeneity within both groups (eFigures 7 and 8 in Supplement 3).
Changes from baseline in PGIS and PGIC scores at 2, 5, 10, and 15 weeks and PROMIS scales for physical function, fatigue, dyspnea, and cognitive abilities showed no statistically significant between-group difference at 10 weeks (Table 2; Figure 3). One minute sit-to-stand test and orthostatic vital signs also showed no significant between-group differences from baseline at 10 weeks (eTable 1 in Supplement 3).
Figure 3. Patient Global Impression Scores Over Time in Adults With Postacute Sequelae of SARS-CoV-2 Infection.
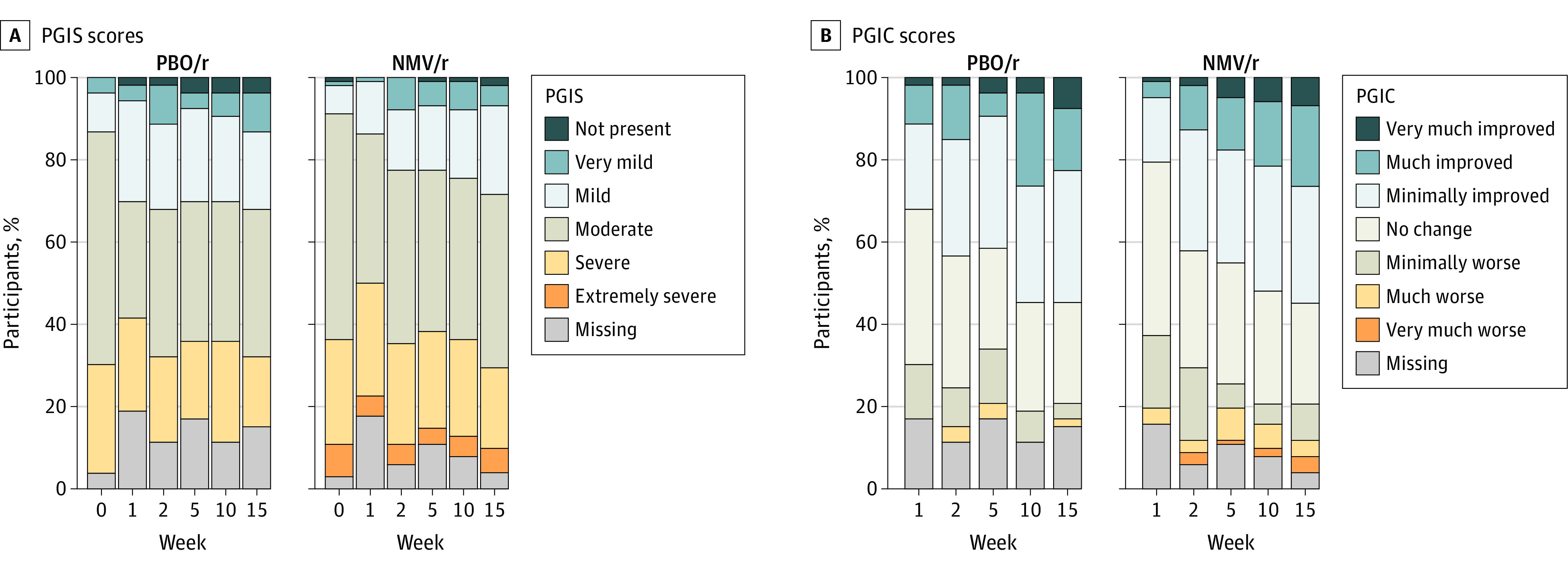
Likert plots depict the distribution of PGIS (A) and PGIC (B) scores at each week, by treatment group. NMV/r indicates nirmatrelvir-ritonavir; PBO/r, placebo-ritonavir; PGIC, Patient Global Impression of Change; and PGIS, Patient Global Impression of Severity.
Sensitivity and Subgroup Analyses
Sensitivity analyses excluding participants with no follow-up were similar to the ITT analyses. The results of the subgroup analyses of the pre- and post-Omicron subgroups were similar to the overall results (eTable 2 in Supplement 3).
Adverse Events
Throughout the 15-week study, 101 of 102 participants (99%) in the NMV/r group and 49 of 53 participants (92.5%) in the PBO/r reported at least 1 adverse event (AE), almost all of which were grade 1 or 2 (Table 2). Four serious AEs were reported: 3 in the NMV/r group (blood loss anemia, forearm fracture, and melanoma), assessed to have been unrelated to intervention; and 1 in the PBO/r group (hepatitis), assessed to have been possibly related to the intervention. The most common AEs reported during the 15-day treatment period were dysgeusia (63 [61.8%] in NMV/r group and 4 [7.5%] in the PBO/r group) and diarrhea (44 [43.1%] in NMV/r group and 19 [35.8%] in the PBO/r group). Six participants (3 [2.9%] in NMV/r group and 3 [5.7%] in PBO/r group) discontinued the intervention due to intolerability or AE. In the NMV/r group, 12 participants (11.8%) and in the PBO/r group, 5 (9.4%) reported COVID-19 reinfections during the study period. One reinfection in the PBO/r group occurred within the first 15 days; all others occurred after the 15-day treatment period. Additional information on AEs is reported in (eFigure 9 in Supplement 3).
Discussion
STOP-PASC is the first randomized clinical trial testing NMV/r for the treatment of PASC, to our knowledge. We found that a 15-day course of NMV/r had a safety profile similar to the 5-day acute treatment course and was generally tolerated; however, when compared to placebo-ritonavir, it did not improve select PASC symptoms (fatigue, brain fog, body aches, cardiovascular symptoms, shortness of breath, and gastrointestinal symptoms) or other health outcomes as measured by the PROMIS scales, global impression scales, and clinical measures of physical function and vital signs. Notably, both the intervention and control groups exhibited improvements in PASC symptoms over time. It is important to underscore that this study alone does not rule out NMV/r as a potential therapy for PASC. There are multiple reasons that would explain why this trial did not detect a benefit for the selected outcomes, and several key themes warrant further discussion to inform future trials in PASC.
PASC is likely not a single entity, and therefore, treatment will likely differ among PASC subtypes. Six core symptoms and symptom clusters were included in this exploratory study, but future trials—especially any smaller studies that are not well powered to detect subgroup differences—may benefit from targeting a specific phenotype. Our study cohort had protracted PASC illness averaging more than 16 months, and antivirals may need to be administered earlier in the illness, before downstream and possibly less reversible adverse effects occur. Our mostly vaccinated outpatient cohort likely differs from unvaccinated and previously hospitalized cohorts that often comprise older patients with multiple comorbidities, ie, risk factors for PASC.54,55,56
The natural history of PASC is varied and is still under investigation.57 We found that many participants with PASC in the PBO/r group improved over time, as did a control group in another trial in PASC.11 Therefore, an effective intervention needs to substantially accelerate that process to see a meaningful difference. There were week-to-week variations in symptoms severity in some participants, consistent with fluctuating patterns that have been described for PASC elsewhere.58,59,60 The heterogeneity and fluctuations of symptoms severity may mask signals, especially smaller ones. Thus, global trajectory assessments should be considered in addition to individual time points.
To date, there are no validated clinical end points or biomarkers of PASC established for clinical trials, to our knowledge. The symptoms selected for this study were based on mechanistic rationale and prevalence and severity in patients.5,44,45,46,47 Other symptoms or clinical end points that were not captured in this study may be responsive to the intervention. The PASC symptoms survey developed and used in this study shares similarities with other patient-informed surveys used in clinical practice and by other studies,46,61,62 and the findings in this study are consistent across a variety of different measures. With the urgent need to find therapies for PASC, exploratory studies such as ours have pushed forward to simultaneously assess efficacy and safety while investigating biomarkers. We underscore the need to establish validated clinical and biological end points for PASC.
This trial’s results do not reject the hypothesis that viral persistence may lead to PASC but they will help inform further studies in this area.63,64,65,66,67 None of the participant baseline stool specimens had detectable SARS-CoV-2 RNA; other tissues were not assessed. As assays to detect SARS-CoV-2 reservoirs become optimized and validated, they could help to identify individuals who may benefit from antiviral therapy.17 Longer treatment durations, dose variations, optimal timing, and different phenotypes of PASC should be investigated in larger studies.63 Additionally, multiple pathways may contribute to PASC pathogenesis; therefore, in addition to testing single therapies, combination therapies (eg, antivirals with immunomodulators) warrant exploration.13,14 Adaptive platform trials would allow randomized controlled comparisons of multiple interventions simultaneously, with the flexibility to adapt key design features of the study in response to accumulating information, thereby maximizing efficiency and prioritizing more promising interventions.68
Strengths and Limitations
The strengths of this study include longitudinal follow-up with a high retention rate and multidimensional data collection with clinical, biospecimen, and digital wearable data that will be integrated in future analyses. The study’s limitations include enrollment at a single academic center, which impacts generalizability, and a smaller sample size than originally planned due to early enrollment closure. The high rate of exclusion due to eligibility criteria, such as drug-drug interaction, also limits generalizability and potentially misses subgroups of patients who could be responders. Although PASC symptoms were assessed as not being attributable to another cause for eligibility, it is still possible that non-PASC factors impacted some participants’ symptoms over the course of the study, which may bias outcomes. Co-interventions, such as concomitant medications, may also influence outcomes. Ritonavir is known to be associated with dysgeusia and was therefore part of the control intervention to minimize unmasking, but the higher rate of dysgeusia reported in the NMV/r group may have impacted self-reported outcomes if unintended unmasking occurred. Severity of the acute COVID-19 infection may impact outcomes and was not captured in depth aside from hospitalization status.
Conclusions
This randomized clinical trial demonstrated the overall safety of a 15-day course of NMV/r in patients with PASC but did not find a significant benefit of this therapy for a subset of PASC symptoms among a mostly vaccinated cohort with prolonged PASC symptoms. Ancillary analyses and evaluation for molecular and digital biomarkers from the STOP-PASC trial are forthcoming. Findings from this and other randomized clinical trials of NMV/r will collectively determine whether this antiviral is beneficial for treating PASC.
Trial Protocol
Statistical Plan
eMethods
eFigure 1. Trial schematic
eFigure 2. Stool RT-PCR on baseline samples
eFigure 3. Forest plots of core symptoms severity at different time points
eFigure 4. Forest plots of expanded symptoms severity at different time points
eFigure 5. Density plots of summative severity scores for core symptoms at 5, 10, and 15 weeks by group
eFigure 6. Mean core symptoms severity score over time by group
eFigure 7. Heatmaps of raw severity scores for each core symptom over time
eFigure 8. Heatmaps of change from baseline severity for each core symptom over time
eFigure 9. Percentage of participants experiencing aes for each system organ class by severity and group
eTable 1. Additional secondary outcomes
eTable 2. Post-hoc subgroup analyses for select outcomes
eReferences
Data Sharing Statement
References
- 1.Wulf Hanson S, Abbafati C, Aerts JG, et al. ; Global Burden of Disease Long COVID Collaborators . Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA. 2022;328(16):1604-1615. doi: 10.1001/jama.2022.18931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Mahoney LL, Routen A, Gillies C, et al. The prevalence and long-term health effects of long COVID among hospitalised and non-hospitalised populations: a systematic review and meta-analysis. EClinicalMedicine. 2022;55:101762. doi: 10.1016/j.eclinm.2022.101762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.US Centers for Disease Control and Prevention . Long COVID-19—Household Pulse Survey—COVID-19. Published October 11, 2023. Accessed October 18, 2023. https://www.cdc.gov/nchs/covid19/pulse/long-covid.htm
- 4.Malesevic S, Sievi NA, Baumgartner P, et al. Impaired health-related quality of life in long-COVID syndrome after mild to moderate COVID-19. Sci Rep. 2023;13(1):7717. doi: 10.1038/s41598-023-34678-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang L, Yao Q, Gu X, et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021;398(10302):747-758. doi: 10.1016/S0140-6736(21)01755-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Hare AM, Vig EK, Iwashyna TJ, et al. ; VA COVID Observational Research Collaboratory (CORC) . Complexity and challenges of the clinical diagnosis and management of long COVID. JAMA Netw Open. 2022;5(11):e2240332. doi: 10.1001/jamanetworkopen.2022.40332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phillips S, Williams MA. Confronting our next national health disaster: long-haul COVID. N Engl J Med. 2021;385(7):577-579. doi: 10.1056/NEJMp2109285 [DOI] [PubMed] [Google Scholar]
- 9.Mantovani A, Morrone MC, Patrono C, et al. ; COVID-19 Commission of the Accademia Nazionale dei Lincei . Long COVID: where we stand and challenges ahead. Cell Death Differ. 2022;29(10):1891-1900. doi: 10.1038/s41418-022-01052-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diseases TLI; The Lancet Infectious Diseases . Where are the long COVID trials? Lancet Infect Dis. 2023;23(8):879. doi: 10.1016/S1473-3099(23)00440-1 [DOI] [PubMed] [Google Scholar]
- 11.Hansen KS, Mogensen TH, Agergaard J, et al. High-dose coenzyme Q10 therapy versus placebo in patients with post COVID-19 condition: a randomized, phase 2, crossover trial. Lancet Reg Health Eur. 2023;24:100539. doi: 10.1016/j.lanepe.2022.100539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lau RI, Su Q, Lau ISF, et al. . A symbiotic preparation (SIM01) for post-acute COVID-19 syndrome in Hong Kong (RECOVERY): a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2023:S1473-3099(23)00685-0. doi: 10.1016/S1473-3099(23)00685-0 [DOI] [PubMed] [Google Scholar]
- 13.Bonilla H, Peluso MJ, Rodgers K, et al. Therapeutic trials for long COVID-19: A call to action from the interventions taskforce of the RECOVER initiative. Front Immunol. 2023;14:1129459. doi: 10.3389/fimmu.2023.1129459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21(3):133-146. doi: 10.1038/s41579-022-00846-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Proal AD, Van Elzakker MB. Long COVID or Post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front Microbiol. 2021;12:698169. doi: 10.3389/fmicb.2021.698169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohandas S, Jagannathan P, Henrich TJ, et al. ; RECOVER Mechanistic Pathways Task Force . Immune mechanisms underlying COVID-19 pathology and post-acute sequelae of SARS-CoV-2 infection (PASC). Elife. 2023;12:e86014. doi: 10.7554/eLife.86014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Proal AD, Van Elzakker MB, Aleman S, et al. SARS-CoV-2 reservoir in post-acute sequelae of COVID-19 (PASC). Nat Immunol. 2023;24(10):1616-1627. doi: 10.1038/s41590-023-01601-2 [DOI] [PubMed] [Google Scholar]
- 18.Fontana LM, Villamagna AH, Sikka MK, McGregor JC. Understanding viral shedding of severe acute respiratory coronavirus virus 2 (SARS-CoV-2): review of current literature. Infect Control Hosp Epidemiol. 2021;42(6):659-668. doi: 10.1017/ice.2020.1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi B, Choudhary MC, Regan J, et al. Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med. 2020;383(23):2291-2293. doi: 10.1056/NEJMc2031364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cevik M, Tate M, Lloyd O, Maraolo AE, Schafers J, Ho A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe. 2021;2(1):e13-e22. doi: 10.1016/S2666-5247(20)30172-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Natarajan A, Zlitni S, Brooks EF, et al. Gastrointestinal symptoms and fecal shedding of SARS-CoV-2 RNA suggest prolonged gastrointestinal infection. Med. 2022;3(6):371-387.e9. doi: 10.1016/j.medj.2022.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schultheiß C, Willscher E, Paschold L, et al. Liquid biomarkers of macrophage dysregulation and circulating spike protein illustrate the biological heterogeneity in patients with post-acute sequelae of COVID-19. J Med Virol. 2023;95(1):e28364. doi: 10.1002/jmv.28364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swank Z, Senussi Y, Manickas-Hill Z, et al. Persistent circulating severe acute respiratory syndrome coronavirus 2 spike is associated with post-acute coronavirus disease 2019 sequelae. Clin Infect Dis. 2023;76(3):e487-e490. doi: 10.1093/cid/ciac722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peluso MJ, Deeks SG, Mustapic M, et al. SARS-CoV-2 and mitochondrial proteins in neural-derived exosomes of COVID-19. Ann Neurol. 2022;91(6):772-781. doi: 10.1002/ana.26350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Badran Z, Gaudin A, Struillou X, Amador G, Soueidan A. Periodontal pockets: a potential reservoir for SARS-CoV-2? Med Hypotheses. 2020;143:109907. doi: 10.1016/j.mehy.2020.109907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zollner A, Koch R, Jukic A, et al. Postacute COVID-19 is characterized by gut viral antigen persistence in inflammatory bowel diseases. Gastroenterology. 2022;163(2):495-506.e8. doi: 10.1053/j.gastro.2022.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheung CCL, Goh D, Lim X, et al. Residual SARS-CoV-2 viral antigens detected in GI and hepatic tissues from five recovered patients with COVID-19. Gut. 2022;71(1):226-229. doi: 10.1136/gutjnl-2021-324280 [DOI] [PubMed] [Google Scholar]
- 28.Stein SR, Ramelli SC, Grazioli A, et al. ; NIH COVID-19 Autopsy Consortium . SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature. 2022;612(7941):758-763. doi: 10.1038/s41586-022-05542-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vibholm LK, Nielsen SSF, Pahus MH, et al. SARS-CoV-2 persistence is associated with antigen-specific CD8 T-cell responses. EBioMedicine. 2021;64:103230. doi: 10.1016/j.ebiom.2021.103230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trypsteen W, Van Cleemput J, Snippenberg WV, Gerlo S, Vandekerckhove L. On the whereabouts of SARS-CoV-2 in the human body: a systematic review. PLoS Pathog. 2020;16(10):e1009037. doi: 10.1371/journal.ppat.1009037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roden AC, Boland JM, Johnson TF, et al. Late complications of COVID-19. Arch Pathol Lab Med. 2022;146(7):791-804. doi: 10.5858/arpa.2021-0519-SA [DOI] [PubMed] [Google Scholar]
- 32.Goh D, Lim JCT, Fernaíndez SB, et al. Case report: persistence of residual antigen and RNA of the SARS-CoV-2 virus in tissues of two patients with long COVID. Front Immunol. 2022;13:939989. doi: 10.3389/fimmu.2022.939989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie Y, Choi T, Al-Aly Z. Association of treatment with nirmatrelvir and the risk of post-COVID-19 condition. JAMA Intern Med. 2023;183(6):554-564. doi: 10.1001/jamainternmed.2023.0743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie Y, Choi T, Al-Aly Z. Molnupiravir and risk of post-acute sequelae of COVID-19: cohort study. BMJ. 2023;381:e074572. doi: 10.1136/bmj-2022-074572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boglione L, Meli G, Poletti F, et al. Risk factors and incidence of Long-COVID syndrome in hospitalized patients: does remdesivir have a protective effect? QJM. 2022;114(12):865-871. doi: 10.1093/qjmed/hcab297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Badenes Bonet D, Caguana Vélez OA, Duran Jordà X, et al. ; MAR Post-COVID-19 Unit . Treatment of COVID-19 during the acute phase in hospitalized patients decreases post-acute sequelae of COVID-19. J Clin Med. 2023;12(12):4158. doi: 10.3390/jcm12124158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ioannou GN, Berry K, Rajeevan N, et al. Effectiveness of nirmatrelvir-ritonavir against the development of post-COVID-19 conditions among US veterans: a target trial emulation. Ann Intern Med. 2023;176(11):1486-1497. doi: 10.7326/M23-1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nevalainen OPO, Horstia S, Laakkonen S, et al. ; Solidarity Finland Investigators . Effect of remdesivir post hospitalization for COVID-19 infection from the randomized SOLIDARITY Finland trial. Nat Commun. 2022;13(1):6152. doi: 10.1038/s41467-022-33825-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Durstenfeld MS, Peluso MJ, Lin F, et al. Association of nirmatrelvir for acute SARS-CoV-2 infection with subsequent long COVID symptoms in an observational cohort study. J Med Virol. 2024;96(1):e29333. doi: 10.1002/jmv.29333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hammond J, Leister-Tebbe H, Gardner A, et al. ; EPIC-HR Investigators . Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19. N Engl J Med. 2022;386(15):1397-1408. doi: 10.1056/NEJMoa2118542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geng LN, Bonilla HF, Shafer RW, Miglis MG, Yang PC. The use of nirmatrelvir-ritonavir in a case of breakthrough long COVID. Exploratory Research and Hypothesis in Medicine. 2023;8(4):394-396. doi: 10.14218/ERHM.2022.00045 [DOI] [Google Scholar]
- 42.Peluso MJ, Anglin K, Durstenfeld MS, et al. Effect of oral nirmatrelvir on long COVID symptoms: 4 cases and rationale for systematic studies. Pathog Immun. 2022;7(1):95-103. doi: 10.20411/pai.v7i1.518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.US Centers for Disease Control and Prevention . COVID-19 Vaccination: Clinical & Professional Resources. Published October 4, 2023. Accessed February 1, 2024. https://www.cdc.gov/vaccines/covid-19/index.html
- 44.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11(1):16144. doi: 10.1038/s41598-021-95565-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Subramanian A, Nirantharakumar K, Hughes S, et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med. 2022;28(8):1706-1714. doi: 10.1038/s41591-022-01909-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonilla H, Quach TC, Tiwari A, et al. Myalgic encephalomyelitis/chronic fatigue syndrome is common in post-acute sequelae of SARS-CoV-2 infection (PASC): results from a post-COVID-19 multidisciplinary clinic. Front Neurol. 2023;14:1090747. doi: 10.3389/fneur.2023.1090747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Larsen NW, Stiles LE, Shaik R, et al. Characterization of autonomic symptom burden in long COVID: a global survey of 2,314 adults. Front Neurol. 2022;13:1012668. https://www.frontiersin.org/articles/10.3389/fneur.2022.1012668 doi: 10.3389/fneur.2022.1012668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.HealthMeasures . Patient-Reported Outcomes Measurement Information System. Accessed February 1, 2024. https://www.healthmeasures.net/explore-measurement-systems/promis
- 49.Bohannon RW, Crouch R. 1-Minute Sit-to-Stand Test: systematic review of procedures, performance, and clinimetric properties. J Cardiopulm Rehabil Prev. 2019;39(1):2-8. doi: 10.1097/HCR.0000000000000336 [DOI] [PubMed] [Google Scholar]
- 50.eProvide Mapi Research Trust . Patient Global Impressions scale: Change, Improvement, Severity. Published October 5, 2023. Accessed February 1, 2024. https://eprovide.mapi-trust.org/instruments/patient-global-impressions-scale-change-improvement-severity
- 51.Wei LJ. Johnson WE. Combining dependent tests with incomplete repeated measurements. Biometrika. 1985;72(2):359-364. doi: 10.1093/biomet/72.2.359 [DOI] [Google Scholar]
- 52.Xu X, Tian L, Wei LJ. Combining dependent tests for linkage or association across multiple phenotypic traits. Biostatistics. 2003;4(2):223-229. doi: 10.1093/biostatistics/4.2.223 [DOI] [PubMed] [Google Scholar]
- 53.The R Project for Statistical Computing. R software. Accessed October 31, 2023. https://www.r-project.org/
- 54.Su Y, Yuan D, Chen DG, et al. ; ISB-Swedish COVID-19 Biobanking Unit . Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185(5):881-895.e20. doi: 10.1016/j.cell.2022.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Byambasuren O, Stehlik P, Clark J, Alcorn K, Glasziou P. Effect of covid-19 vaccination on long COVID: systematic review. BMJ Med. 2023;2(1):e000385. doi: 10.1136/bmjmed-2022-000385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsampasian V, Elghazaly H, Chattopadhyay R, et al. Risk factors associated with post-COVID-19 condition: a systematic review and meta-analysis. JAMA Intern Med. 2023;183(6):566-580. doi: 10.1001/jamainternmed.2023.0750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tran VT, Porcher R, Pane I, Ravaud P. Course of post COVID-19 disease symptoms over time in the COMPARE long COVID prospective e-cohort. Nat Commun. 2022;13(1):1812. doi: 10.1038/s41467-022-29513-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O’Brien KK, Brown DA, McDuff K, et al. Conceptualising the episodic nature of disability among adults living with long COVID: a qualitative study. BMJ Glob Health. 2023;8(3):e011276. doi: 10.1136/bmjgh-2022-011276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ballouz T, Menges D, Anagnostopoulos A, et al. Recovery and symptom trajectories up to two years after SARS-CoV-2 infection: population based, longitudinal cohort study. BMJ. 2023;381:e074425. doi: 10.1136/bmj-2022-074425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Servier C, Porcher R, Pane I, Ravaud P, Tran VT. Trajectories of the evolution of post-COVID-19 condition, up to two years after symptoms onset. Int J Infect Dis. 2023;133:67-74. doi: 10.1016/j.ijid.2023.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thaweethai T, Jolley SE, Karlson EW, et al. ; RECOVER Consortium . Development of a definition of postacute sequelae of SARS-CoV-2 infection. JAMA. 2023;329(22):1934-1946. doi: 10.1001/jama.2023.8823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hughes SE, Haroon S, Subramanian A, et al. Development and validation of the symptom burden questionnaire for long COVID (SBQ-LC): rasch analysis. BMJ. 2022;377:e070230. doi: 10.1136/bmj-2022-070230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zimmerman KO. RECOVER-VITAL: a platform protocol for evaluation of interventions for viral persistence, viral reactivation, and immune dysregulation in post-acute sequelae of SARS-CoV-2 infection (PASC). 2024. Accessed December 31, 2023. https://clinicaltrials.gov/study/NCT05595369
- 64.Krumholz HM. An Interventional decentralized phase 2, randomized, double-blind, 2-arm study to investigate the efficacy and safety of orally administered nirmatrelvir/ritonavir compared with placebo/ritonavir in participants with long COVID. 2023. Accessed December 31, 2023. https://clinicaltrials.gov/study/NCT05668091
- 65.Brodin P. An interventional, double-blinded, 2-arm study to investigate the efficacy of orally administered nirmatrelvir/ritonavir compared with placebo/ritonavir in non-hospitalized adult participants suffering from post-COVID. 2023. Accessed December 31, 2023. https://clinicaltrials.gov/study/NCT05823896
- 66.Henrich T. placebo-controlled, randomized trial of ensitrelvir (s-217622) for viral persistence and inflammation in people experiencing long COVID (PREVAIL-LC). 2023. Accessed December 31, 2023. https://clinicaltrials.gov/study/NCT06161688
- 67.Peluso M. An exploratory, randomized, double-blind placebo-controlled study to assess the safety of an anti-SARS-CoV-2 monoclonal antibody and response to treatment in individuals with long COVID (OutSMART-LC). 2024. Accessed December 31, 2023. https://clinicaltrials.gov/study/NCT05877508
- 68.Adaptive Platform Trials Coalition . Adaptive platform trials: definition, design, conduct and reporting considerations. Nat Rev Drug Discov. 2019;18(10):797-807. doi: 10.1038/s41573-019-0034-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Plan
eMethods
eFigure 1. Trial schematic
eFigure 2. Stool RT-PCR on baseline samples
eFigure 3. Forest plots of core symptoms severity at different time points
eFigure 4. Forest plots of expanded symptoms severity at different time points
eFigure 5. Density plots of summative severity scores for core symptoms at 5, 10, and 15 weeks by group
eFigure 6. Mean core symptoms severity score over time by group
eFigure 7. Heatmaps of raw severity scores for each core symptom over time
eFigure 8. Heatmaps of change from baseline severity for each core symptom over time
eFigure 9. Percentage of participants experiencing aes for each system organ class by severity and group
eTable 1. Additional secondary outcomes
eTable 2. Post-hoc subgroup analyses for select outcomes
eReferences
Data Sharing Statement