Abstract
In this review article, a perspective on the immobilization of various hydrolytic enzymes onto magnetic nanoparticles for synthetic organic chemistry applications is presented. After a first part giving short overview on nanomagnetism and highlighting advantages and disadvantages of immobilizing enzymes on magnetic nanoparticles (MNPs), the most important hydrolytic enzymes and their applications were summarized. A section reviewing the immobilization techniques with a particular focus on supporting enzymes on MNPs introduces the reader to the final chapter describing synthetic organic chemistry applications of small molecules (flavour esters) and polymers (polyesters and polyamides). Finally, the conclusion and perspective section gives the author's personal view on further research discussing the new idea of a synergistic rational design of the magnetic and biocatalytic component to produce novel magnetic nano‐architectures.
In this critical review article, a perspective on the immobilization of various hydrolytic enzymes onto magnetic nanoparticles for synthetic organic chemistry applications is presented.
INTRODUCTION
Biocatalysis is an example of a sustainable interdisciplinary technology traditionally operating through the synergism between biochemistry, organic chemistry, microbiology and molecular biology. Recently, interesting new perspectives have been opened by the possibility to design suitable support for enzyme immobilization giving materials physical chemists the possibility to play a role in biocatalysis field. In this scenario, this review sheds light on the current state of art about magnetic nanoparticles as support for enzyme immobilization in the framework of biocatalysis of condensation reactions. Previous reviews on this topic focused on enzyme immobilization (Hanefeld et al., 2009; Sheldon, 2007; Sheldon & van Pelt, 2013), giving an overview of the recent development in enzyme immobilization strategies for medical devices and biosensor (Ansari & Husain, 2012; Bilal et al., 2018; Vaghari et al., 2016). Also, some reviews discuss about modification of the biocatalytic properties of enzymes for efficient biocatalysis in food, agriculture, environment and energy‐related industries (Bilal et al., 2019) and chemical industries (Basso & Serban, 2019). The present perspective review, after a general overview of nanomagnetism, focuses on both synthesis of magnetic support with optimized magnetic properties and strategies for enzyme immobilization. All these contents have been discussed from the perspective of potential application for condensation reactions due to the easy separation and recyclability of the systems over several cycles of operation. Finally, Future perspectives sheds light on the synergistic rational design of the magnetic and biocatalytic component to produce novel magnetic nano‐architectures for promised and novel application. To the best of our knowledge, this is the first review on this topic written by researchers coming from Biotechnology and materials physical chemistry. In this view, the overall scope of this paper is to highlight how the strong interconnection between biotechnology and materials physical chemistry can be exploited at the highest level, opening interesting new perspectives.
Magnetism at the nanoscale: An overview
Minimization of energy provides a basis for predicting the direction of events in the universe. This is why ferro (i) magnetic materials, to minimize the magnetostatic energy, organize themselves in a number of small regions called domains (i.e. uniformly magnetized regions having atomic magnetic moment oriented in the same direction) having different sizes and shapes (Blundell, 2001; Cannas et al., 2012). Generally speaking, the physical properties of an object are influenced by its size, particularly when its dimensions become comparable to those pertinent to the specific property under consideration (Trohidou, 2014). In the realm of magnetism, among pertinent dimensions are the magnetic domains that typically fall within the 10–1000 nm range, depending on the magnetic properties of the materials (Bedanta & Kleemann, 2008). This is a crucial reason why nanoscale magnetic particles exhibit peculiar features distinct from their bulk counterparts. As the size decreases below a critical value, the formation of a magnetic single‐domain structure becomes energetically favoured over a multidomain arrangement. A magnetic single‐domain nanoparticle (MNPs, i.e., a particle that is in a state of uniform magnetization at any field (Bean & Livingston, 1959)) can be considered as a superspin with a large magnetic moment (μ p) (Magnetic moment of nanoparticle, μ p, can be considered proportional to saturation magnetization, M s, of the material and particle's volume V p (μ p = M s × V p).) typically ranging from 103 to 105 Bohr magneton (μ B). When interactions between particles become important, the MNP ensemble eventually exhibits collective behaviour dominating the individual anisotropic properties of the particles. Under sufficiently strong interactions, a MNP ensemble can display properties akin to superspin glass (SSG), resembling the behaviour observed in atomic spin glass systems in bulk. As the interactions further increase, a superferromagnetic (SFM) order can be observed. (Rancourt & Daniels, 1984) On the other hand, in a system consisting of non‐interacting single‐domain particles, each magnetic supermoment acts independently. The energy profile of non‐interacting MNPs with uniaxial magnetic anisotropy (Figure 1A) is characterized by an energy barrier (ΔE a) that can be considered directly proportional to particle volume (V p) and magnetic anisotropy (K). The ΔEa two energy minima corresponding to parallel of antiparallel direction of μ p with respect to the anisotropy axis. Then, below a certain temperature (i.e. blocking temperature, T B), when k B T < <ΔE a, magnetic supermoments are localized in the two minima and macroscopically an irreversible field dependence of magnetization (i.e. hysteresis loop) is observed. For small particles (i.e. to ensure the coherent rotation of magnetic moments) this behaviour can be successfully described by Stoner and Wolfhart model (Stoner & Wohlfarth, 1991). As an example, Figure 1B shows field dependence of magnetization recorded at 5 K for a system of ~5 nm CoFe2O4 nanoparticles covered by a monolayer of oleic acid (OA) to ensure weak interparticle interactions (Vasilakaki et al., 2018).
FIGURE 1.

(A) Graphical representation of the anisotropy energy barrier of magnetic nanoparticles. (B) The key parameters characterizing the hysteretic behaviour: – saturation magnetization (M s) corresponding to the maximum value that can be reached by magnetization; − remanent magnetization (M r) corresponding to the residual magnetization a zero field after that the sample has been magnetized, ideally to the saturation. Often, the reduced remanent magnetization (i.e. M red: M r/M s) is used to describe the remanence state. M red can change from one, considering a perfect squared Hysteresis, to zero when completely reversible behaviour is observed; − coercive field or coercivity (H c) is the magnetic field necessary to bring to zero remanent magnetization. Generally speaking, H c is the macroscopy parameter that can be considered directly proportional to magnetic anisotropy (K) and inversely proportional to M s. (C) M vs. H curve typical of superparamagnetic behaviour with H c = 0 and zero M r = 0.
Interestingly, above T b (i.e. k B T >> ΔE a) and on a specific time scale, the particle's moment can undergo a thermally activated transition that for isolated spherical particle results in the flipping of magnetization between its antiparallel easy directions, separated by ∆E a. From a macroscopic point of view, this phenomenon is analogous to paramagnetism (i.e. H c = 0 and zero M r = 0) but involving superspins, it is characterized by different time and magnetization scale and for this it is called superparamagnetism (Dormann et al., 1997). The CoFe2O4 MNPs discussed above show T b ⋍ 150 K and Figure 1C shows field dependence of magnetization recorded at 300 K (i.e. T >> T b). M vs. H curve shows complete irreversibility (i.e. H c = 0 and zero M r = 0) with a quite high value M s (Dormann et al., 1997) (Figure 1C). It is worth to underline that magnetic properties of an MNP ensemble are strictly dependent on physical–chemical (i.e. structure, chemical composition, etc) and morphological (i.e. particle size and particle size distribution) features of the sample (Suber & Peddis, 2007).
In this scenario, single‐domain magnetic nanoparticle showing superparamagnetism at room temperature represent an interesting playground for fundamental study. Moreover, the wide presence of MNPs in nature and in living systems makes them of interest in different branches of science with a large spectrum of applications: paleomagnetism (Lagroix & Guyodo, 2017; Newell, 2017; Yan et al., 2012), geology (Morales et al., 1997), mineralogy, cultural heritage (archaeology, paintings) (Rao et al., 2015), catalysis (Arizzi et al., 2015; Cunha et al., 2018; Gawande et al., 2013; Skliri et al., 2018), surface chemistry (Lykaki et al., 2018; Simo et al., 2018; Turcheniuk et al., 2013), biomagnetic separation (Fields et al., 2016; Hofman et al., 2017), medicine theragnostic of tumours (Arora & Bandopadhyaya, 2018; Gazeau et al., 2008; Hameed et al., 2018) and tissue engineering (Cagil et al., 2018; Leferink et al., 2018; Pankhurst et al., 2003), magnetic recording (López‐Ortega et al., 2015; Malek et al., 2018; Manzoor et al., 2018), permanent magnets (Chahal & Samra, 2018; Li et al., 2003; Lu et al., 2007), sensors (Cao et al., 2018; Dong et al., 2018; Rocha‐Santos, 2014), ferrofluid‐based devices (Felicia & Philip, 2015; Kuzhir et al., 2017; Seol et al., 2017), high frequency power applications (Felicia & Philip, 2015; Hayashi et al., 2010; Helal et al., 2018; Mondal et al., 2016) and magneto optical devices (da Silva et al., 2019).
In the context of this review, applications related with the interaction of MNPs with an external static (DC) or alternating (AC) magnetic field appear more interesting. The interaction of MNPs with a DC magnetic field allows the remote manipulation of the magnetic entities; this is particularly interesting in the field of magnetic delivery/harvesting of molecule (e.g. bioseparation; drug delivery) (Omelyanchik, Lamura, et al., 2021; Slimani, 2022) and references herein quoted. The main advantage to deal with a superparamagnetic object is that when the external magnetic field is removed, the magnetic object itself is not anymore magnetic (M r = 0). This will prevent clustering phenomena and it will also avoid the presence of local magnetic fields that can be undesirable in specific working conditions. The force generated by a DC magnetic field acting on MNPs can be described by the following Equation (1):
| (1) |
where V p is the nanoparticle volume and χ is the susceptibility of material (χ = dM/dH) that for its definition includes a dependence on M s of the materials. H is the magnetic field intensity and grad (H) is the variation of the intensity of in‐plane gradient of H z measured at a fixed z. A detailed discussion of Equation 1 can be found in previous publications on the topic (Omelyanchik, Lamura, et al., 2021; Panina et al., 2022). The H and grad (H) quantities depend on the number and on the arrangement of magnets used to generate DC magnetic field. To optimize magnetic recovery performances, specific devices can be designed (Omelyanchik, Lamura, et al., 2021).
The interaction of MNPs with an AC magnetic field with suitable frequency induces an important local heating of the magnetic materials, related to the process of reversal of the magnetic moment (i.e. magnetic hyperthermia, MH). During this process, MNPs rapidly align their magnetic moment with the changing direction of the AC field. This continuous realignment leads to the relaxation of the magnetic moments through Néel–Brownian relaxation (Fatima et al., 2021; Obaidat et al., 2019). In Neel relaxation, the magnetic moments within individual nanoparticles realign as they attempt to minimize energy dissipation caused by the changing magnetic field. Conversely, Brownian relaxation involves the physical rotation of entire nanoparticles in response to the AC magnetic field, leading to further reorientation of magnetic moments. As this continuous realignment and relaxation occur, a significant portion of the magnetic energy is converted into heat, inducing a local increase of temperature at the particle surface. By carefully controlling the frequency, intensity and duration of the AC magnetic field exposure, the degree of heating can be precisely modulated. Although MH presents a promising technique for cancer treatment (Peiravi et al., 2022; Rajan & Sahu, 2020), it still shows basic problems associated with local hyperthermia; such problems include heterogeneous temperature dispensation in the targeted part and inability to prevent overheating. In this view, self‐regulating magnetic hyperthermia (SRMH) (Niraula et al., 2024; Zhang et al., 2019) has been suggested. The self‐regulating heating effect consists of a stable temperature increase of the magnetic nanoparticles when exposed to AC field up to a temperature below the Curie temperature (Tc) of the material. The main advantage of SRMH is related to the capability of MNPs to distribute heat within the temperature range of interest avoiding the risk of tissue overheating.
In MH applications, magnetic properties of the materials such as K and M s represent key factors to improve heating performance (Obaidat et al., 2015). If the role of interparticle interactions is still debated in literature, other factors such as nanoparticle concentration, magnetic field frequency and the duration of exposure determine the heat generation efficiency (de la Presa et al., 2012; Shah et al., 2015; Tong et al., 2017).
Starting from this landscape clearly appears that for remote manipulation and MH applications, the magnetic properties of the MNPs must be properly designed, carefully tuning μ p, χ (i.e. M s and V p) and magnetic anisotropy.
Magnetic nanoparticle‐based supports for enzymes
Over the past two decades, the field of nanotechnology has witnessed significant advancements, leading to the fabrication and application of a diverse range of nano‐carriers for enzyme immobilization. In particular, magnetic nanoparticles have emerged as highly versatile supports due to their distinctive physicochemical and magnetic properties. These MNPs possess several advantageous characteristics, including biodegradability, biocompatibility, low cost and the ability to be tailored with specific surface chemistries. One of the most notable features of MNPs is their green chemistry approach. Unlike non‐magnetic carriers, their responsiveness to external magnetic field enables facile, rapid and efficient biocatalyst separation from reaction media. This magnetic responsiveness significantly simplifies the purification steps in enzymatic reactions: reducing time, and costs with respect to traditional separation techniques. Furthermore, MNPs provide a high surface area‐to‐volume ratio, providing broad binding sites for enzyme immobilization. This increases the loading capacity of enzymes onto the nanocarrier, thus enhancing the overall catalytic efficiency of the immobilized biocatalysts. In addition, the surface chemistry of MNPs can be precisely designed to improve enzyme binding affinity, specificity and activity, further enhancing their performance in various applications. MNPs also exhibit excellent recyclability, allowing repeated use of immobilized enzymes over multiple reaction cycles without significant loss in activity. This not only enhances the sustainability of enzymatic processes but also contributes to cost‐effectiveness and scalability in industrial applications. In fact, MNPs used as a support for enzyme immobilization have been successfully demonstrated in many applications, such as substrate sensing (Cheon et al., 2019), immunoassays and organic synthesis (Abu‐Dief & Abdel‐Fatah, 2018; Yu et al., 2012). Several studies have shown that the activity and stability of enzymes immobilized on MNPs are often significantly better than unbounded enzymes (Hosseinipour et al., 2015; Khoshnevisan et al., 2011). If enzymes are immobilized on a surface, their biological activity strongly depends on how “crowded” the surface is (i.e. immobilization efficiency). As the density of enzymes increases on a support surface, the immobilization efficiency decreases: if the surface is not large enough since the protein molecules tend to maximize the contact among them, thereby undergoing conformational distortion and inducing a consequent loss of activity. Hence, having high surface areas usually leads to maximization of the enzyme loading and decreases the probability of conformational distortions. In addition, when solid supports are used for enzyme immobilization, centrifugation or filtration is the obvious choice for removing the biocatalyst from the reaction environment. Starting from this framework, superparamagnetic MNPs have been used for enzyme immobilization. As an example, Huang et al. (2003) reported that the direct immobilization of the lipase from Candida rugosa (CRL) on ~13 nm Fe3O4 led to an increased hydrolytic activity to 4‐nitrophenyl palmitate (pNPP) when used as model substrate. Compared to the free enzyme, the bound lipase exhibited a 1.41‐fold enhanced activity, a 31‐fold improved stability and better tolerance to the variation of the solution's pH. This result was attributed to the stabilization of lipase molecules on the MNP surface, preventing the thermal inactivation of enzyme. It should be considered that the particle mobility, which is determined by particle size and solvent viscosity (Stokes–Einstein equation) (Liu et al., 2008), may affect the reaction kinetics (collision theory). Hence, changes in the particle size and reaction medium viscosity will evidently alter the intrinsic activity of the supported enzymes (Wang, 2006).
Even though magnetic supports have been used as a good candidates for enzyme immobilization, the biocatalyst's activity on the MNP surface might be strongly affected due to the orientation of the active sites and to mass transfer limitations (Hou et al., 2015). To overcome this issue, efforts are being made for the development and improvement of immobilization strategies, such as magnetic nanoparticle surface modification using biocompatible, natural molecules with specific functional groups. Similarly, some physical factors such as electromagnetic field (EMF), γ‐irradiation, supersonic wave and magnetic field (MF) to stimulate and alter enzyme activity also turned out to be an attractive alternative. In detail, MF can affect the molecular structure of a given enzyme, the kinetics of the chemical reaction and the biological processes. Numerous studies have reported the use of rotating magnetic field (RMF) obtained as the superposition of two or more in phase AC magnetic fields of identical frequency but spatially dispersed in phase with respect to each other. Generally, the use of RMF involves the induction of eddy currents in aqueous media, which creates their own MF being in co‐action with the applied one. This cooperation results in enhancing the mass transfer by reducing the diffusion mass transfer resistance (Wasak et al., 2019). The application of rotating magnetic fields (RMF) has emerged as a promising avenue for controlling enzyme‐based processes, as highlighted in a study by Wasak et al. (2019). Their findings reveal that exposure to RMF can significantly enhance enzyme activity, expanding its effectiveness across a broader pH range. Additionally, there is a noticeable shift in the optimum pH for enzyme activity, suggesting a potential for fine‐tuning enzymatic reactions. The same study reports the varied effects of different RMF frequencies on enzyme activity. A significant increase in enzyme activity, 11%, 11% and 9%, was observed for samples exposed to frequencies of 10, 40 and 50 Hz, respectively, indicating that any changes in activity were frequency dependent. Building upon this potential, recent research, such as the work by Kladko et al., 2020, has delved into leveraging RMF to accelerate whole‐cell bioprocesses. By inducing cell membrane permeabilization, RMF offers a means to modulate cell metabolism without compromising cell viability. This approach holds great promise for industrial biosynthesis, particularly in the production of beneficial products using genetically engineered cells, thereby enhancing biotechnological processes. However, studies, including one by Bertrand et al. (2018), indicate that while magnetic particle application also impacts cell metabolism, its effects appear to be comparatively smaller than those induced by RMF. This suggests that RMF may offer superior effectiveness in enhancing enzyme activity and controlling cell metabolism, underscoring its potential as a versatile tool in biotechnological applications.
Other studies have also reported an increase in the activity of immobilized enzymes using DC magnetic field (Büyükuslu et al., 2006; Liu et al., 2010). The increase in the enzyme activity prompted by an external magnetic field can be ascribed to the increased unpaired electron energy of metal ions (i.e. oriented in the same direction of the applied field) (Wagner et al., 2016). This energy can be transferred to the next molecules which produce more free radicals that in turn may affect the chemical reaction and the biological processes. From the above discussion, the application of an external (R) magnetic field leads to better immobilized enzyme activity and simple separation from the reaction medium. For efficient separation, best results could be obtained by increasing the MF gradient (Bhakdi et al., 2010). Changes in the gradient (Leong et al., 2016) leads to a change in the magnetic attractive force, which can significantly improve the separation process.
Hydrolytic enzymes
Hydrolytic enzymes are a class of more than 200 individual proteins. The enzyme classification by the Enzyme Commission number places hydrolases in the EC 3 category, which can be further subdivided in other subclasses according to the type of bond hydrolysed by the enzyme. Several families of hydrolases have been developed by Nature and each of them is characterized by a distinct biochemical role (Brocklehurst & Neuberger, 1988). Despite the specific protein structure and catalytic mechanism varying, the overall hydrolytic reaction can be summarized as follows in the Scheme 1A:
SCHEME 1.

(A) Cleavage of a chemical bond by hydrolytic enzymes in the presence of H2O and (B) condensation reaction of a generic diacid with a generic alcohol catalysed in the absence of H2O.
When used in vitro, hydrolytic enzymes can catalyse the reversed reaction (Scheme 1B) also called a condensation reaction in which two reagents are combined to form a single product, usually with the loss of a small molecule such as water or a short‐chain aliphatic alcohol.
Hydrolytic enzymes, which can be obtained from animals and plants and expressed using recombinant microorganism, are the most commonly used biocatalysts in many scientific fields ranging from biomass conversion to chemical degradation and fine chemical production (Manisha & Yadav, 2017; Sutay Kocabaş et al., 2022). α/β hydrolases in particular are a superfamily of enzymes that catalyse a wide range of hydrolytic reactions as a result of divergent evolution. Despite their divergence, these enzymes share a common fold conserving Ser‐His‐acid catalytic triad in the active site. The α/β hydrolase fold provides a stable scaffold for the active sites of a wide variety of enzymes. All these proteins have a core of mostly parallel β strands (typically 6–8 strands), surrounded by α‐helices. The catalytic residues that constitute the highly conserved triad are as follows: a nucleophile (typically serine, occasionally cysteine or aspartic acid) which is positioned on a “nucleophilic elbow” immediately after β sheet 5, (typically with the consensus sequence glycine‐xxx‐nucleophile‐xxx‐glycine, where xxx is any amino acid), an acidic residue positioned after strand 7 and a conserved histidine residue located after the last strand (Nardini & Dijkstra, 1999). Proteases (EC 3.4), lipases (EC 3.1.1.3), esterases (EC 3.1.1.1), epoxide hydrolases (EC 3.3.2.3) and dehalogenases (EC 3.8) are all biocatalysts that belong to the α/β hydrolase family.
As visible in Scheme 1, an enzymatic reaction is virtually reversible by controlling the equilibrium with appropriate conditions. On the basis of this version, many hydrolases can be employed as catalysts for the reverse reaction of hydrolysis, leading to a bond‐forming reaction (Kobayashi et al., 2001). Lipases (triacylglycerol lipases, triacylglycerol acyl hydrolases, E.C. 3.1.1.3) catalyse the hydrolysis of fatty acid esters when in aqueous media. On the other hand, some lipases, which are stable in organic solvents (e.g. toluene, benzene, diphenyl ether, cyclohexane, n‐hexane and so on) (Pugh et al., 2015) and in some green solvents (e.g. ionic liquids and supercritical CO2 (Fan & Qian, 2010), or the new emerging 2,2,5,5‐tetramethyloxolane (Byrne et al., 2019)), can be used as a catalyst for esterifications, transesterifications, amidation and transamidation reactions (Gotor, 1999). Among lipases, Candida antarctica lipase B (CaLB) is the most used biocatalyst for the synthesis of short molecules (e.g. flavour esters) and polymers (polyesters, polyamides). CaLB, which is a globular protein belonging to the family of α/β hydrolase fold, is composed of seven central β strands that are flanked on both sides by 10 α‐helices (Uppenberg et al., 1994). It is composed of 317 amino acids with a catalytic triad composed of Ser105‐His224‐Asp187 in its active site and two important amino acids for the enzyme reaction (Thr40 and Gln106) which are involved in what is called an oxyanion hole (Figure 2).
FIGURE 2.

3D structure of CaLB with the catalytic triad highlighted in blue and the oxyanion hole highlighted in red.
Lipases are a special class of esterases, but the presence of a movable lid is a peculiar feature able to explain the so‐called “interfacial activation” (Schrag & Cygler, 1997); in fact, the activity of lipases, differently from all the other esterases, is promoted by contact with a lipid–water interface, which is believed to enhance the opening of the lid. Since there are only a few cases of lipases that are active without showing a clear interfacial activation; however, a better distinction between lipases and esterases would be to consider lipases as esterases that act on long‐chain acylglycerols (Verger, 1997).
Another important hydrolase family is represented by proteases, which degrade proteins by breaking down the peptide (amide) bonds that link amino acids (Oda, 2012). Generally, there are six classes of proteases: (1) serine proteases (e.g. trypsin, chymotrypsin and subtilisin); (2) threonine proteases; (3) cysteine protease (such as papain and bromelain); (4) aspartate proteases (e.g. pepsin); (5) glutamate proteases and (6) metal proteases (e.g. thermolysin). Some proteases can be obtained from plants (like papain from Carica papaya) and animals (pancreatic trypsin, chymotrypsin, pepsin and chymosin), but due to the high enzyme demand in the industrial field, their production from microbial sources has become the main option. As seen for lipases, proteases catalyse not only the hydrolysis of peptide bonds but, in waterless conditions, also amide and ester bond formation, leading to the synthesis of peptides and production of polyesters. Peptide synthesis via protease catalysis proceeds through either thermodynamically (applicable to all proteases), for which synthesis is in equilibrium with hydrolysis, or kinetically controlled mechanism (limited only to serine, cysteine and threonine proteases), in which the success of peptide synthesis depends on the relative kinetic rates of the reaction (Cheng, 2010). Despite their catalytic properties, proteases are not optimal catalysts for the synthesis of high‐molecular‐weight polymers due to the fact that hydrolysis is always a limit that prevents peptide synthesis (Guzmán et al., 2007). Table 1 summarizes a list of enzymes belonging to the hydrolase family with their respective catalytic mechanism and the reverse synthetic applications.
TABLE 1.
Hydrolytic enzymes and their possible in vitro applications.
| Enzymes | Mechanism | Examples | Condensation reactions |
|---|---|---|---|
| Proteases (EC 3.4) | Hydrolyse peptide bonds between amino acids in protein structure | Chymotrypsin, Subtilisin, Trypsin, Papain | Synthesis of flavours, oligopeptide, oligoamides but also juice clarification (Mosafa et al., 2013) (papain) |
| Lipases (E.C. 3.1.1.3) | Hydrolyse lipids, the ester bonds in triglycerides, to produce fatty acids and glycerol | Porcine pancreatic lipase (PPL), Candida cylindracea (CCL), Candida rugosa (CRL), Aspergillus niger (Lipase A), Pseudomonas aeruginosa (PAL), Pseudomonas cepacia (PCL), lipase from Thermomyces lanuginose, lipase from Burkholderia cepacian, Candida antarctica (CaL), Candida antarctica lipase B (CaLB) | Synthesis of dipeptide, synthesis of polyamides and polyesters; Biodiesel production (Lipase from Burkholderia cepacian and Candida Antarctica Lipase B, Thermomyces lanuginosus; apple flavour synthesis; Sadighi et al., 2017) (Thermomyces lanuginose); oils esterification (Parashar et al., 2018) |
| Cutinases (EC 3.1.1.74) | In biological system, it catalyse the hydrolysis of cutin polymer resulting in the formation of alcohol and carboxylic acid monomer | Cutinase 1 from Thermobifida cellulosilytica (The_cut1), Cutinase from Humicola insolens (HiC) | Polycondensation reaction for the synthesis of oligoesters and polyesters |
| Glycosidases (EC 3.2.1) | Catalyse the cleavage of glycosidic linkage of glycan chains | Cellulase, amylases, xylanases, arabinases | Synthesis of natural and unnatural polysaccharide |
| Chitosanases (EC 3.2.1.132) | Catalyse the endohydrolysis reaction of beta‐bonds between D‐glucosamine residues in a partially acetylated chitosan | Bacterial Chitosanases (from Bacillus sp., Serratia sp., Streptomyces sp.), Fungal chitosanases (Aspergillus sp.), Cyanobacterial chitosanase | Production of chitosan oligosaccharides |
| Dehalogenases (EC 3.8) | Catalyse the removal of atom from a substrate without participation of oxygen or coenzymes | Haloalkane dehalogenases | Biodegradation of toxic halogenated compounds |
| Epoxide hydrolases (EC 3.3.2.3) | Catalyse the hydration of chemically reactive epoxides to their corresponding dihydrodiol | Epoxide hydrolases from Agrobacterium radiobacter AD1, from Aspergillus niger and from Mus musculus | Ring‐opening polymerization to produce oligomer |
IMMOBILIZATION
In the last three decades, biocatalysis has emerged as an important technology able to satisfy the growing demand of green and sustainable chemical manufacturing particularly in the synthesis of pharmaceuticals, flavours and other fine chemicals (Tao & Kazlauskas, 2011). The recent progress made for the isolation, engineering and production of novel enzymes enlarges the scope of the discipline allowing the synthesis of new compounds using mild and environmentally friendly conditions (Illanes et al., 2012). Nonetheless, enzyme applications are in most cases still limited due to the lack of long‐term stability, difficult recovery and lack of possibility of re‐use of the costly biocatalyst. These limitations could be partially (if not completely) overcome by utilizing enzyme immobilization strategies.
The immobilization of enzymes has as its main objective to preserve and even enhance some of the enzyme's properties such as activity, stability and substrate specificity. The immobilized preparations provide, in fact, a tool for an easy separation of the biocatalyst from the reaction product and facilitate the recovery and re‐use of the enzymes. Enzyme recyclability over several cycles of operation is, in fact, the main limiting step for the cost‐effective implementation of enzyme‐catalysed reactions in the chemical industry (Ansari & Husain, 2012). Another advantage that immobilization provides is the stability, under both storage and operational conditions, towards protein denaturation due to high temperature and possibility to use the biocatalyst in organic solvents (Sheldon, 2007). Nonetheless, immobilization of enzymes presents also some limitations including lowered activity compared to native enzymes due to the reduction of enzymatic kinetic and the change in the conformation and properties of the enzyme.
Immobilization methods
Over the years, many types of immobilization techniques such as binding to a support through physical adsorption or chemical attachment, entrapment in a matrix and enzyme crosslinking were developed in response to the needs manifested from various applications (e.g. synthesis of fine chemicals and pharmaceuticals but also in food and cosmetic sector) (Cantone et al., 2013) (Figure 3). Depending on the specific case, the matrix, or the support, immobilizes the enzyme permanently or for only a limited time. The performance of the immobilized enzyme system is related to the properties of both the biocatalyst and the support carrier; the specific biochemical and kinetic properties of the immobilized enzyme with the mechanical and chemical characteristics of the support can ensure physical resistance to compression and microbial attack, hydrophilicity, biocompatibility and availability at low cost. Classical supports or matrixes that are used for immobilization should be cheap and easily available and are divided into four main groups: (1) natural polymers (e.g. alginate, chitosan, dextrans, gelatine, cellulose, pectin, collagen); (2) synthetic polymers which are ion exchange resins or polymers and are insoluble supports with porous surface (e.g. diethylaminoethyl cellulose (DEAE cellulose), UV‐activated polyethylene glycol (PEG), polystyrene, polyacrylate, polymethacrylates, polyacrylamide, polyamides, vinyl and allyl‐polymers); (3) conductive polymer (e.g. PPy, PEDOT and derivatives of PPy and PEDOT) (Shen et al., 2022) and (4) inorganic materials (e.g. zeolites, ceramics, silica, activated carbon, glass). Nowadays an increasing interest for novel immobilization support is leading scientists towards the use of renewable biomasses having a low environmental fingerprint such as bamboo (G. Palma et al., 2021) and rice husks (Cespugli et al., 2018) or to inorganic systems with enhanced thermal, biocompatible and with a large surface area typical of magnetic nanoparticles (Vaghari et al., 2016) (discussed in paragraph 2.2).
FIGURE 3.

Overview of the most used enzyme immobilization techniques: binding to a support, cross‐linking and entrapment.
Binding to a support
This immobilization technique can occur by physical (adsorption) or chemical (covalent bonding) interactions. In the physical binding method, enzymes can be attached to the support via reversible adsorption which is generally too weak (ionic interaction, hydrogen bonds, Van der Waal forces or hydrophobic interactions) to keep the enzyme fixed to the carrier under rigorous industrial conditions of high reactant and product concentrations and high ionic strength. On the other hand, the greatest advantage of adsorption method is that there will not be “pore diffusion limitations” since catalysts are immobilized externally on the support. For what concerns chemical binding, this method of immobilization prevents enzyme leaching from the support surface since the catalyst is tightly fixed on it through the formation of covalent bonds (Filho et al., 2019). An important limitation is related to possible chemical modifications to which is subjected the biocatalyst to potential changes in the conformation that affect its active site leading to irreversible enzyme inactivation.
Cross‐linking
For this method of immobilization, developed in the early 1960s, enzymes are linked by covalent bonds between chemical groups of amino acids present on the surface of the enzyme that are placed in a medium containing a bifunctional cross‐linking agent, among which the most commonly used are glutaraldehyde and diazonium salt, resulting in the formation of crosslinked enzymes (CLEs) with other variations of the technique such as those made from enzymes obtained by crystallization (cross‐linked enzymes crystal, CLECs) and aggregation (cross‐linked enzyme aggregates, CLEAs) (Sheldon & van Pelt, 2013). This technique guarantees a high enzymatic activity of the biocatalyst and low production costs due to the absence of additional supports or matrices, but the big drawback is that the bifunctional cross‐linking agent may denature or modify the structure of the enzyme leading to a low activity retention, poor reproducibility, low mechanical stability and difficulties in handling the gelatinous CLEs. To overcome these drawbacks, the attention focused on carrier‐bound enzymes, which became the most widely used methodology for enzyme immobilization (Sheldon & van Pelt, 2013).
Entrapment (encapsulation)
In the encapsulation techniques, enzymes are physically entrapped within porous polymeric network, typically organic or inorganic polymer matrices, such as polyacrylamide and silica sol–gel respectively. As the polymerization of the support proceeds, the enzyme is confined inside the matrix that protects it from direct contact with the reaction medium while the substrates and products are able to diffuse through the pores (Ji et al., 2017). Since the physical restraints are generally too weak, the addition of covalent attachments is sometimes required to stabilize the preparation and prevent enzyme leakage. The most common methods of entrapment are as follows: inclusion in gel by trapping the enzymes inside the gels, inclusion in fibres made of matrix material and inclusion in microcapsules formed by monomer mixtures such as polyamine and calcium alginate. This method of immobilization is fast, easy to practice at small scale and it can be used for biosensing applications (Naik et al., 2004). The greatest limitations of this method are the risk of leakage of enzymes from the matrix, due to the difficulties in controlling the size of pores, and mass transfer limitation through membranes or gels.
Immobilization on magnetic nanoparticles
To improve even further the separation of the biocatalyst preparation from the reaction product, new promising strategies of enzyme immobilization were developed (Bilal et al., 2019). Among them the application of MNPs as a support represents a promising approach to enable biocatalyst's immobilization satisfying both the need of preserving the enzyme's activity and the environmental requirements required to respect the “do not significant harm” principle that poses the basis of modern green chemistry (Sementsov & Golysheva, 2023; Vaillant et al., 2020). As already discussed in Introduction, MNPs of spinel ferrites (MeFe2O4, Me = Fe2+, Co2+, Mg2+, Mn2+, Zn2+, etc.) are the most used materials as support for biocatalyst. The ferrite spinel structure is based on a closed‐packed oxygen lattice, in which tetrahedral (A‐sites) and octahedral (B sites) interstices are occupied by the cations. The physical behaviour and the magnetic properties of the material depend on distribution of cations among A and B sites. It is worth to underline that the surface of spinel ferrites is preferentially formed by ions located in the octahedral sites and then, the chemical, catalytical and biological activity of these materials will strongly depend on their cationic distribution rather than on their chemical composition (Jacobs et al., 1994; Omelyanchik et al., 2020; Vozniuk et al., 2018). The structural properties and the rich crystal chemistry of spinels offer excellent opportunities for fine tuning the magnetic properties, opening very interesting perspectives for wide range of bio‐applications, such as biosensing (Kaushik et al., 2008), cell separation (Huang et al., 2010), magnetic hyperthermia (Latorre & Rinaldi, 2009), drug delivery (Guo et al., 2009), magnetic resonance imaging (MRI) as contrast agents (Chung et al., 2011) and magnetic particle imaging (MPI) (Du et al., 2013). Among the spinel ferrites, the most commonly used are magnetite (Fe3O4) and maghemite (γ‐Fe2O3) due to their good properties (i.e. high saturation magnetization and susceptibility) and for their low toxicity.67 However, the morpho‐structural features that are, in turn, connected to the magnetic properties of such materials are strongly dependent on the synthesis method and each application determines the implementation of a different protocol to obtain nanoparticles having the desired properties. In the past three decades, numerous synthetic strategies have been used within this context, encompassing diverse methodologies such as sol–gel (Brinker & Scherer, 2013; Cannas et al., 2001, 2002) and micellar (Moumen & Pileni, 1996; Vestal & Zhang, 2004), hydrothermal processing (Yoshimura & Byrappa, 2008), aerosol‐vapour methods (Ling & Hyeon, 2013), surfactant‐assisted high‐temperature decomposition techniques (Sun et al., 2004), along with their synergistic combinations. Notably, surfactant‐assisted solution‐phase techniques, including the micellar method and high‐temperature decomposition of organic precursors, have emerged as promising avenues for crafting innovative magnetic nanostructured materials, affording control over particle size, shape, structure and composition (Cannas et al., 2012). The micellar approach, in particular, has demonstrated versatility in generating magnetic nanoparticles with varied shapes, sizes and a narrow size distribution, showcasing its efficacy. Despite the advantages offered by micellar techniques, the synthesis at relatively low temperatures often leads to a reduced degree of crystallinity, with a consequent degradation of magnetic properties. Since 2002 (Sun & Zeng, 2002), a significant stride in controlling the microstructural features and crystallinity of nanoparticles has been achieved through a synthetic strategy based on high‐temperature decomposition (HTD) of metallorganic precursors (acetylacetonates). This approach incorporates surfactants (such as oleylamine and oleic acid) in an organic solvent with a high boiling point (e.g. octadecene, benzyl ether, phenyl ether). The key to achieving nanocrystals with a narrow size distribution lies in the separation of the nucleation step from the growth of existing nuclei. Consequently, the size of the nanoparticles becomes intricately linked to the choice of metal precursors, the thermal conditions, and the surfactant, which adsorbs and desorbs during the decomposition process, influencing nucleation and growth phenomena (Peddis et al., 2013).
This plethora of synthetic strategies allows a careful control of the morpho structural features and of the nanomaterial's magnetic properties. On the other hand, the direct use of bare magnetic nanoparticles as support for biomolecules could be limited by several factors such as colloidal instability of the particles that can form large clusters due to the reduction of their surface energy. In addition, MNPs have a high chemical reactivity due to their high surface‐to‐volume ratio that can induce a degradation of the magnetic properties. In this view, a key point in the proper design of solid magnetic support for biomolecules is surface functionalization which plays an important role in controlling colloidal and chemical stability of the MNPs. It is worth to underline that suitable molecular coating of MNPs can also provide specific sites to graft biological function such as catalysis through the further functionalization with specific enzymes (Figure 4). Nowadays, several routes are used as a strategy for surface modification: polymer coating is performed using biocompatible, bioactive and non‐toxic polymer like chitosan (Leal et al., 2018), polyvinyl alcohol (PVA) (Umut, 2013), polyethylene glycol (PEG) (Illés et al., 2015) and grafting with polyacrylic acid (PAA) (Mahdavian & Mirrahimi, 2010); another route is based on silica gel‐mediated organic synthesis since the formation of silica coating on the surface of MNPs not only prevent their aggregation in liquid and improve their chemical stability as well as biocompatibility but the hydrophilic surface of silica nanoparticles can also help the binding of enzymes in aqueous solution (Cannas et al., 2010; Lin & Haynes, 2009). Metal alkoxides, such as tetraethyl orthosilicate (TEOS), are widely used for silanization of magnetic nanoparticles with amino‐silanes obtaining a modified surface rich of amino groups (Rother et al., 2011). In this contest, for selective glycerol oxidation via enzymatic route, Bîtcan has reported how laccases from Trametes versicolor and Aspergillus sp. were immobilized by covalent binding onto magnetic particles, which were previously functionalized by using 3‐aminopropyl‐trimethoxysilane (NH2‐TMOS) or 3‐aminopropyl‐triethoxysilane (NH2‐TEOS) and activated with glutaraldehyde (Bîtcan et al., 2023).
FIGURE 4.

Configurations of bare nanoparticles and of the different organic or inorganic coatings highlighting the importance of MNP surface modification to provide specific surface sites with active groups which are used in enzyme immobilization by forming covalent bonds or physical adsorption, achieving reusable magnetic biocatalysts.
Another possibility is related to the application of a surface molecular coating focused on the use of α‐hydroxy acids like citric acid and dimercaptosuccinic acid (DMSA) that are biocompatible carboxylic acid, that can be absorbed into the surface of the particle using their carboxylate functionalities, leaving at least one of them exposed to be used for further functionalization (Hajdú et al., 2009).
APPLICATION OF HYDROLYTIC ENZYMES FOR SYNTHETIC REACTIONS
Biocatalysis is emerging as a fundamental tool within the chemical industry to produce fine chemicals. The scientific advances in this field have led to the development of suitable enzymes and reaction conditions for the synthesis of various macromolecular materials, which can become really interesting for several areas of industry and technology such as electronics, machinery, communications, transport, pharmacy and medicine as highly advanced materials (Kobayashi & Makino, 2009). New polymeric materials are being developed through novel production methods, including the polymerization catalyst, arrived at passing through several epoch‐making innovative works, such as the discovery of the Ziegler–Natta catalyst (Sinn & Kaminsky, 1980), the concept of living polymerization (Szwarc, 1956), the discovery of conducting polymers (Heeger & MacDiarmid, 1979) and the discovery of the metathesis catalyst (Schrock, 2002). Since the early stages of polymer chemistry (1920s), polymerization catalysts have used classical acid catalysts (Brønsted acids, Lewis's acids and various cations), bases (Lewis bases and various anions) and radical‐generating compounds. Subsequently, in the 1950s, polymer chemists started to use the transition metals in Ziegler–Natta catalyst and later in the metathesis catalysis, as well as using rare‐earth metals. Around the 1980s, a new approach to polymer synthesis employing enzymes as catalysts was developed, known as enzymatic polymerization (Kobayashi et al., 2001). Enzymatic polymerization is a reaction using enzyme as a catalyst, and it was defined as “chemical polymer synthesis in vitro via non biosynthetic pathways catalysed by an enzyme” (Kobayashi et al., 1995). Enzyme catalysis in polymer synthesis offers several advantages which are related to the chemical preparative routes. Enzymes, due to their high selectivity and high enantio‐ and regioselectivity, allow promising substrate conversion efficiency. Enzymes, which offer catalyst recyclability when immobilized, can be used in bulk reaction media avoiding organic solvents and the use of potentially toxic catalysts. In particular, enzymes from the class of hydrolases (e.g. lipases, cutinases and esterases) are nowadays among the most commonly used biocatalysts (together with other enzymes not belonging to hydrolase family like laccase), for polyester synthesis, since they are known to be versatile and well suited (Kobayashi & Makino, 2009).
Interest in enzyme catalysis for industrial applications has grown exponentially since Klibanov and Zaks pioneered the study of enzyme activity in organic media (Zaks & Klibanov, 1986). Today, enzymes are widely used for textile processing and in many other applications related to the degradation of polymers: they can hydrolyse polyesters but, at the same time, selected biocatalysts are able to catalyse the reverse reaction to produce aliphatic polyesters, oligomers with side chains and aromatic‐aliphatic polyesters. Most of these reactions are catalysed by lipases and among these, the most widely used biocatalyst for the synthesis of polyesters is Candida antarctica lipase B (CaLB), due to its commercial availability as a free and immobilized catalyst (Pellis et al., 2016). In 1984, Okumura and co‐workers published the first study in which a lipase from Aspergillus niger was able to catalyse the polycondensation of various dicarboxylic acids and polyols to obtain short oligomers (Okumura et al., 1984). Gutman et al. (1987) reported the first study on the synthesis of polyester by enzymatic polymerization of A‐B type monomers (Gutman et al., 1987). From that moment, lipases gained more interest as catalysts for the synthesis of polyesters and different classes of the same family were studied by Linko and co‐workers to explore their potential in this field. From the experimental evidence, Mucor Miehei lipase has emerged as the most promising biocatalyst for polyester synthesis and after all these studies conducted between 1980 and 1990s, CaLB, immobilized on acrylic resin (commercially known as Novozym 435), has become the most widely used biocatalytic enzyme for synthetic applications (Mahapatro et al., 2003).
In the first attempt, the attention of hydrolytic enzymes for synthetic applications was directed towards the synthesis of short molecules: in particular, Klibanov used a protease‐catalysed synthesis carried out in organic solvents like pyridine (Therisod & Klibanov, 1986). In the 1980s, research on enzyme‐catalysed synthesis of polypeptides and polyamides using proteases and related proteolytic enzymes was going strong with data that indicated that proteases were a good catalyst for the synthesis of oligomers, but their use was limited if high‐molecular‐weight polymers were desired. On the contrary, lipases have been used for the synthesis of both oligoamides and polyamides and, under suitable reaction conditions, lipases appear to be good catalysts for higher molecular weight polymers obtained via both polycondensation and ring‐opening polymerizations (Cheng, 2010). Therefore, the attention towards proteases gradually decreased and in the last two decades, the trend went more and more towards the use of lipases for the synthesis of polymers. Nowadays, it must be considered that just the specificity and a suitable activity of the enzyme are not sufficient to guarantee an efficient polymer synthesis. For this reason, as discussed in paragraph 2, the enzymes must be immobilized to ensure its recovery and reuse: this implies the development of suitable immobilization protocols to limit the detachment of the protein from the support avoiding the contamination of the product and the irreversible loss of activity of the immobilized biocatalyst.
Synthesis of flavour esters
In the 1980s, scientific researchers faced with an increasing use of biocatalysts in organic media for new theoretical and practical applications (Carrea et al., 1995; Klibanov, 1986). Short‐chain acid alkyl organic esters are among the most important and versatile components of natural flavours and fragrances. The increasing demand for these esters is related to the fact that they are widely used in the food, beverage, cosmetic and pharmaceutical industries in a way to give a specific taste, preserve the flavour or minimize any unwanted aroma (Bicas et al., 2016). Esters are identified by their fruity odour; hence, they are generally employed as additives in candies, jams, beverages, baked items and dairy goods. The acetate esters give a unique flavour in alcoholic beverages, and wine originated from grapes while for food products such as cheese, the same goal is reached with ethyl or methyl ester (Rojas et al., 2001). The cyclic esters offer flavours like fruity, coconut, creamy, nutty, sweet and buttery (Fenaroli's handbook of flavour ingredients, 1976). Although these esters are commonly produced by chemical synthesis, there is an increasingly important preference for natural flavours, rather than chemically synthesized ones. In fact, the process leading to the synthesis of these latter is usually not environmentally favourable, not regio‐ and enantioselective and could be characterized by the further production of unwanted by‐products lowering the overall yield (Ben Akacha & Gargouri, 2015). However, similar to the natural flavours extracted directly from plants, the same flavour and fragrance esters prepared by enzymatic synthesis may be labelled as “natural” (Gillies et al., 1987). As an alternative, enzymatic synthesis (either by free or immobilized enzymes) is becoming of growing interest (Takahashi et al., 1985). Thus, after several studies which have identified lipase as a suitable biocatalyst for synthesis of short‐chain esters (Welsh et al., 1990), several researchers and industrial sector have focused the attention on the biocatalytic synthesis of these compounds as consumers prefer naturally derived or processed substances in respect to chemically synthesized ones. In particular, an immobilized lipase from Mucor miehei could be used to synthesize low‐molecular‐weight model esters made from propionic, butyric or hexanoic acids and ethyl or hexyl alcohols in organic media (Manjón et al., 1991). Therefore, as lipases are able to catalyse many synthetic organic reaction (Gotor, 2002), they can be employed in preparing many flavour and fragrance esters under conditions that are milder than those used industrially: an example is represented by CaLB which shows high catalytic efficiency in the resolution of chiral esters via esterification, transesterification and acidolysis reactions (Paiva et al., 2002) (Figure 5A–C). Nowadays, short‐chain flavour esters were successfully synthesized by a new multilayer magnetic biocatalyst containing immobilized CaLB. The design of this biocatalyst was obtained by considering different functionalization possibilities of the magnetic particles for an efficient covalent binding of the lipase and different combinations of silane precursors for the sol–gel entrapment (Vasilescu et al., 2019). More recently, other hydrolases such as Humicola insolens cutinase (HiC) and Thermobifida cellulosilytica cutinase 1 (Thc_Cut1), belonging to the cutinases subfamily, were immobilized via adsorption onto polypropylene beads and used for the synthesis of short flavour esters. Their activity and selectivity towards a wide array of substrates having a carbon chain length ranging from 2 to 8 were optimized using a full factorial experimental design (Fabbri et al., 2021).
FIGURE 5.

General reaction scheme of (A) esterification, (B) transesterification and (C) acidolysis.
Synthesis of polyesters
The enzymatic synthesis of polyesters occurs via two main reaction pathways, namely, polycondensations and ring‐opening polymerization (ROP), and among enzymes, lipases have been proved to be the most efficient for the in vitro polyester synthesis (Miletić et al., 2010). These latter are in widespread use in our modern life, ranging from bottles for carbonated soft drinks and water, fibres for shirts and other apparel, to photographic film and recording tapes. The most investigated polycondensation reactions are those between dicarboxylic acid diesters with polyols (Figure 6A), even though condensation polymerization of dicarboxylic acids and oxyacids and their esters were also reported (Figure 6B). Regarding ROP reactions, the polymerization of lactones (cyclic esters) is predominant over the investigation of other cyclic monomers (Figure 6C) (Kobayashi, 2010).
FIGURE 6.

The two basic pathways of enzyme‐catalysed polyester synthesis: (1) Polycondensation reaction of (A) dicarboxylic acid or their esters with alcohols or (B) oxyacids or their esters and (2) (C) Ring‐opening polymerization reactions.
Initially, several halogenated diesters were chosen together with toxic solvents (e.g. pyridine) for enzymatic polyester synthesis. In the early 2000s, the research interest shifted to the production of functional aliphatic polyesters carrying lateral hydroxy and epoxy groups that are suitable for further functionalization or cross‐linking reactions (Uyama, Inada, & Kobayashi, 1999). In the last decade, the field of enzymatic polymerization has been the subject of renewed interest from various research groups, with a focus on the production of bio‐based polyesters, in line with the desire to develop a more sustainable and environmentally friendly polymer industry using platform molecules derived from renewable resources. Currently, several companies are working in this direction to find bio‐based alternatives which are easier to (bio)degrade (or better to depolymerize) while keeping the same mechanical and barrier properties, like in the case of the substitution of poly(ethylene terephthalate) with poly(ethylene furanoate) (Weinberger et al., 2017). As mentioned before, there was also a strong interest in the possibility of recycling the enzyme leading to the development of several immobilization technologies (see paragraph 2) that allow the biocatalyst to be easy to handle, physically, thermally and hydrolytically stable, and to be stored for a longer time (Hanefeld et al., 2009). In this way, the biocatalyst could be recovered and reused for many cycles of synthesis. The most studied enzyme for this kind of reaction is CaLB in its commercially available immobilized form known as Novozym 435 (N435) that consists of the biocatalyst physically adsorbed within the macroporous resin poly (methyl methacrylate‐co‐butyl methacrylate) known as Lewatit VPOC 1600. Nowadays, a new promising strategy of enzyme immobilization has emerged due to the recent exploitation of nanoscience and thanks to the increasing use of magnetic nanoparticles. Thus, the immobilization of CaLB on magnetic Fe3O4 nanoparticles offers a simple way to separate and reuse the enzyme over a longer period than that for free enzymes alone and for enzymes which are immobilized by physical adsorption. This is due to the formation of covalent binding via a carbodiimide EDC activation used as a carboxyl activating agent for the coupling of functional groups present on Fe3O4 nanoparticles surface and the ones present in the biocatalyst: this mechanism does not permit the loss of enzyme by desorption from the support and protects the enzyme from denaturation by constraining it to the local environment of the nanoparticle. For the immobilized enzyme on MNPs, separation is facilitated by the use of a magnet where the product is removed while the immobilized enzyme is held in place with a magnetic field (Dyal et al., 2003).
The simplest type of polyesters that is possible to synthetize via enzymatic polycondensation and ROP is represented by aliphatic polyesters that can be divided into copolymeric linear chains composed by a dicarboxylic acid and a diol or homopolymeric chains formed from the ROP of a lactone (Pellis et al., 2019).
Polyesters can be obtained starting from hydroxy acids or, more generally, A‐B type monomers, where the groups A and B can react with other B and A groups respectively. Also, the alkyl esters of these compounds have been used as monomers for lipase‐catalysed polycondensation reactions. Condensations of the A‐B type generate a leaving group that, in most of the cases, must be efficiently removed in order to obtain high molecular weight polyesters (Miletić et al., 2010). Okumara and co‐workers were the first to experience the lipase‐catalysed synthesis of oligoesters from reactions between diols (AA) and dicarboxylic acids (BB) (Okumura et al., 1984). Polymerization via condensation of adipic acid and 1,4‐butanediol using Novozym 435 was studied by Binns et al. (1998). These two monomers, under solvent‐free conditions, were mixed and heated at 40°C for 4 h, followed by heating at 60°C for 10 h under reduced pressure. Polycondensation of 1,4‐butanediol and adipic acid for 4 h gave a product with a discrete array of hydroxy‐terminated oligomers and, after 14 h, a narrow dispersity oligoester (Ð =1.5) having a M w of around 2200 Da is obtained. Analogous coupling reactions performed in toluene at 60°C using the same monomers revealed apparently lower reactivity, probably associated with the poor solubility of adipic acid in the solvent. When compared to the solvent‐free system, another significant difference together with the poorer efficiency was the higher dispersity of the product (M w = 1500 Da and Ð = 2.3) (Binns et al., 1998). Thus, it is relevant not just to the enzyme but also to the choice of monomers. Early reports on enzymatic polycondensation of dicarboxylic acids and polyols indicate the formation of only low‐molecular‐weight products (Uyama, Yaguchi, & Kobayashi, 1999). To produce polyesters of high molecular weight, it is necessary to remove the by‐products (water or alcohol in the case of diesters) formed during the reaction in order to shift the equilibrium to the polymerization reaction. Equilibrium can be shifted towards polyesterification by using diesters instead of dicarboxylic acids, since the volatility of the by‐product (alcohol) is higher than that of water (by‐product from dicarboxylic acids) (Kobayashi, 1999) leading to a higher monomer conversion and molecular weight of the products performing the reaction under vacuum conditions. Recently CaLB () was tested for both bulk polymerization reaction and reactions in organic media (toluene) between dimethyl adipate (DMA) (the dimethyl ester of adipic acid) and diols having linear aliphatic chains of 4 and 8 carbon atoms (like 1,4‐butanediol, BDO; or 1,8‐octanediol, ODO) respectively (Figure 7).
FIGURE 7.

Lipase‐catalysed polycondensation of adipic acid ester with 1,4‐butanediol.
Besides the synthesis of aliphatic polyesters, the enzymatic synthesis of bio‐based polyesters focuses also on the possibility to produce, via polycondensation, a wide array of aliphatic‐aromatic polyesters, which mimic some of the polyesters currently used for beverage bottling, food packaging and automotive parts. Due to the exploit of furan‐based compounds, one of the most reported reactions involves the poly‐transesterification of dimethyl furan‐2,5‐dicarboxylate and linear α, ω‐aliphatic diols with chain length ranging from C2 to C12 (Figure 8A). Jiang et al., confirmed how CaLB is more active on longer chain aliphatic linear diols and that the reaction temperature (increased from 80 to 140°C) is a key point for obtaining high‐molecular‐weight furan‐based polyesters reaching 90–95% substrate conversions when C4–C12 aliphatic diols were used (Jiang et al., 2015). Alternatively, 2,5‐bis(hydroxymethyl) furan could be used in combination with several aliphatic diacid ethyl esters with chain length ranging from C4 to C12 to produce aliphatic‐aromatic polyesters, but in this case, the furan moiety derives from the diol component (Figure 8B) (Pellis et al., 2019). This synthetic approach was relatively unsuccessful since only low M n products of around 2000 Da were obtained. The likely cause of these low chain lengths was etherification that occurs during the enzymatic polymerization; for this reason, a different approach could be necessary based on lower reaction temperature, shorter reaction time and using different organic media (Jiang et al., 2014).
FIGURE 8.

Polycondensation of aliphatic‐aromatic polyesters based on (A) dimethyl‐2,5‐furan dicarboxylate (B) 2,5‐bis(hydroxymethyl)furan.
Differently from what is seen for polycondensation reaction, ring‐opening polymerization of lactones and carbonates does not produce a leaving group during the reaction. ROP is regularly reported as one of the most successful biocatalyzed polymerizations which was investigated for the first time in the 1993 by two independent groups (Knani et al., 1993). A representative example of ROP is given by the synthesis of poly(caprolactone) (PCL) starting from ε‐caprolactone (ε‐CL), which was studied using several ranges of conditions including in bulk and organic media system (Öztürk Düşkünkorur et al., 2014). For example, Gutman et al. investigated the enzymatic‐catalysed ROP of ε‐CL in n‐hexane in order to produce PCL using porcine pancreatic lipase (PPL) as a catalyst (Knani et al., 1993). Simultaneously, the enzymatic ROP of lactones was performed in bulk by Kobayashi et al, using Pseudomonas fluorescens lipase as a catalyst and PCL was formed in 92% yield with molecular weight M n of 7700 and Ð =2.4 (Uyama & Kobayashi, 1993) until arriving in the late 1990s, when the use of N435 in the enzymatic ROP of lactones was introduced by Bisht et al. (1997). N435 has become the most used biocatalyst for polyester synthesis starting from monomer substrates such as cyclic monomers like lactones, cyclic diesters and cyclic ketene acetals.
Synthesis of polyamides
Polyamides are polymers in which the monomeric units are linked together by amide bonds. Examples of polyamides include naturally occurring polyamides like proteins and synthetic polyamides that, similar to polyesters, can be classified as aliphatic, semi‐aromatic and aromatic polyamides.
As discussed for polyester synthesis, hydrolases, and in particular proteases with other proteolytic enzymes and lipases, are suitable enzymes for the in vitro polypeptide and polyamide synthesis through the formation of amide bonds (Cheng, 2010) and similar to the biocatalytic polyester synthesis, the enzyme‐catalysed polyamide synthesis occurs via (1) polycondensation of diacid/diesters and diamines or ω‐amino carboxylic acids/esters (Figure 9A) and (2) ring‐opening polymerization of lactams (Figure 9B). Lipase‐catalysed polymerization of polyamides is not so easy for two reasons: first, polyamides like nylons possess high T m (above 100°C) and the catalytic reactivity of lipases could be significantly decreased at those temperatures because enzymes are subjected to denaturation processes that lead to their inactivation. Secondly, several polyamides can be only dissolved in some aggressive solvents such as formic acid, concentrated H2SO4 and trifluoroacetic acid, in which lipases are subjected to a complete activity inhibition (Jiang & Loos, 2016). For these reasons, to investigate the optimal thermodynamic and kinetic conditions, several studies on different classes of lipases were conducted (Margolin & Klibanov, 1987; West & Wong, 1987). Thanks to these pioneer studies, Cheng et al. arrived to select commercial lipases to synthesize polyamides from diesters and diamines (Gu et al., 2008) until 2005, when the use of Novozym 435 () becomes the working horse as catalyst for the formation of amide bonds between dialkyl esters and diamines (Azim et al., 2005). In 2008, the synthesis of poly (β‐alanine) via ROP of 2‐azetidinone was also reported (Schwab et al., 2008) (Figure 9B).
FIGURE 9.

(A) Polycondensationof aliphatic polyamides and (B) ring‐opening polymerization of 2‐azetidinone.
FUTURE PERSPECTIVES
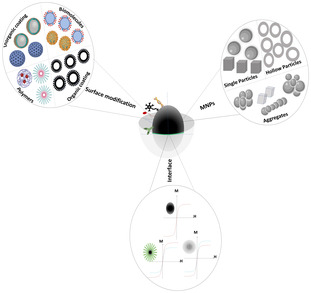
Due to the growing interest in stable biocatalysts, the development of enzymes immobilized on a magnetic support will continue to expand due to the easy separation and reusability of the preparation. In addition, this immobilization strategy not only can provide an active and stable biocatalyst, but also it is a relatively simple and low‐cost immobilization procedure that does not require an expensive support (Sheldon & van Pelt, 2013). In this new and exciting research line, most of the results have been obtained anchoring biocatalyst directly on the bare spherical surface of spinel iron oxide MNPs (i.e. maghemite and magnetite nanoparticles) (Conte et al., 2018; Romero et al., 2018). To improve particle's stability and ensure enzyme activity preservation and enhancement, MNPs can be functionalized with biocompatible polymers that are ideal substrates for biocatalyst anchoring (Fortes et al., 2017; Herdt et al., 2007). In this very promising scenario, some critical issues such as optimization of the magnetic properties, mass transfer limitations and low efficacy against insoluble substrates still need to be overcome. These improvements will lead to a synergistic rational design of the magnetic and biocatalytic component to produce novel magnetic nano architectures such as magnetic nano (hetero)‐structure coated by suitable molecular and/or biocatalytic functionalization (MNA). This novel MNA will exploit not only the performance of the biocatalyst (Figure 10A) and magnetic material (Figure 10B) but most of all their synergistic effect (Figure 10C,D) due to the interaction between the two components. Starting from biocatalytic component, it should be underlined that nowadays, genetic engineering (e.g. recombinant DNA technology coupled with computational biology) has made giant leaps forward towards the development of tailor‐made de novo enzymes, constructs multifunctional chimeric enzymes or incorporates unnatural amino acids and design artificial enzymes with desirable features (e.g. high catalytic activity, reaction selectivity, stability in specific organic solvents) based on relationships between the structure and function of enzymes (Fasim et al., 2021). These developments will broaden the scope of enzymatic synthesis of numerous kinds of polymers, in particular polyesters via polycondensation reactions. A big advantage in the polymer field will be the development of solventless synthesis processes where functional polymers could be obtained due to the mild operating temperatures (T < 90°C) of the immobilized biocatalyst. Through enzyme immobilization, MNPs serve as stable supports, ensuring efficient catalysis while offering the benefits of easy separation and reusability. Their magnetic properties facilitate thorough mixing, crucial for homogeneous reaction conditions, especially in complex multistep condensation reactions. Post‐reaction, MNPs' easy separation using external magnets simplifies product purification, contributing to cost‐effectiveness and environmental friendliness. Moreover, MNPs accommodate a wide range of enzymes, broadening the scope of biocatalytic condensation reactions and enabling tailored applications. By providing a controlled microenvironment for enzyme immobilization, MNPs enhance stability and prevent enzyme leaching, ensuring sustained catalytic activity. Their scalability renders them suitable for industrial‐scale production, facilitating the transition from lab to manufacturing settings. MNPs not only augment enzyme catalytic activity but also enable tandem reactions, where multiple enzymes on the same support drive sequential condensation reactions. In pharmaceutical synthesis, MNPs offer an eco‐friendly alternative, minimizing the environmental footprint and cost of chemical processes. Aligned with green chemistry principles, MNPs reduce hazardous reagent usage and waste generation, promoting sustainable synthesis pathways. Furthermore, MNPs excel in fine chemical synthesis, producing flavours, fragrances and natural products efficiently and sustainably. Their versatility extends to biotransformation, enabling the synthesis of valuable compounds from diverse substrates. MNPs also find utility in biomolecule synthesis, expanding their application in diverse scientific domains. With compatibility in flow chemistry systems, MNPs facilitate continuous‐flow reactions, ideal for large‐scale production. Acting as nanoreactors, MNPs provide confined spaces for reactions, enhancing condensation reaction selectivity. Additionally, MNPs contribute to biosensing applications, detecting condensation reaction products for various analytical purposes. Overall, MNPs represent a powerful tool in biocatalytic condensation reactions, offering efficient, sustainable and versatile pathways for chemical synthesis. To improve the performance of magnetic component (Figure 10B), as discussed in Introduction, a precise tuning of μ p (i.e. M s and V p) and magnetic anisotropy is mandatory. Even if from a synthetic point of view tuning particles' volume represents an easy task, it is worth to underline that a strong increase of V p will bring the magnetic component out of the superparamagnetic regime at working temperatures. In this view, an interesting alternative scenario is given by the possibility to build up a 3D MNP superstructure that represents a new generation of materials where a new physics emerges (Pileni, 2001). As an example, Cannas et al. (2008) reported that the synthesis of porous iso‐oriented magnetic aggregates (50–10 nm) constitutes ~7 nm primary particles. A detailed magnetic study shows that reversal process of magnetization can be ascribed to the primary particle ensuring superparamagnetic behaviour at room temperature (Laureti et al., 2010). Then, magnetic response, high specific surface area and superparamagnetic behaviour at room temperature make these materials excellent potential candidate as solid supports for enzyme immobilization. To tune K and M s chemical engineering of spinel ferrites (Muscas et al., 2015), as well as the possibility to create (bi)/−magnetic heterostructures (i.e. core shell, hollow particles, particles with controlled shape) (Omelyanchik, Villa, et al., 2021) represent an elegant and effective strategy (Figure 10B). In particular, the rich and flexible crystal chemistry of ferrites (see Immobilization on magnetic nanoparticles) allows relatively easy chemical engineering (Figure 10C) of these compounds and then a fine‐tuning of their magnetic properties. In this contest, particularly interesting are the Zn‐based (Me1‐XZnXFe2O4; Me: Ni2+; Co2+; Fe2+) spinel ferrites with complex stoichiometry that allows to increase the saturation magnetization (M s higher than ~170 emu/g) and a decrease of magnetic anisotropy (Omelyanchik et al., 2020). This strong increase of M s can be generally scribed to the tendency of Zn to accommodate itself in interstitial sites with tetrahedral symmetry. Then, depending on the stoichiometry of the compounds, the insert of Zn in the structure will force the other divalent cations to be in octahedral sites that, as already discussed in Immobilization methods, are mainly located in the particle' surface. In other words, tuning Zn content in spinel ferrite with complex stoichiometry not only will allow to optimize magnetic properties but it will allow to control the chemical composition of the nanoparticle's surface, optimizing the binding site for the biocatalyst (Figure 10D). In addition, recently, it has been demonstrated that binding specific functional group on the nanoparticle surface can drastically change the magnetic properties of the materials (Figure 10D). As particular examples, Vasilakaki et al. (2018) show that magnetic properties of ~5 nm CoFe2O4 nanoparticles change dramatically if a coating of di‐ethylene glycol has been replaced by oleic acid. In particular, the interaction of −OH group with atoms in the particle surface induces a strong increase of M s and a reduction of coercivity (i.e. anisotropy constant). Careful magnetic, Mössbauer characterization and multiscale modelling (i.e. DFT and Montecarlo calculations) correlate this behaviour to the magnetic and electronic structure of the materials. From the perspective of using MNA functionalized by biocatalysis for condensation reaction, MH represents an exciting and elegant solution to locally increase the temperature, improving the efficiency of the reaction. If MH has been already explored for catalytic applications (Mourdikoudis et al., 2021; Tatarchuk et al., 2022), few efforts have been made to the application of this technique to reaction catalysed by enzymes. Indeed, in these systems, the control of temperature must be very precise to avoid degradation of biocatalytic component itself. In this view, application of SRMH represents a very interesting possibility, allowing to carefully control the local temperature (Kraus et al., 2022; Torres‐Herrero et al., 2023).
FIGURE 10.

(A) Material classification for coating of particle surfaces (B) Examples of magnetic nanoheterostructures (C) chemical engineering of the surface (D) Molecular control of magnetism.
AUTHOR CONTRIBUTIONS
F. Papatola: Resources; validation; methodology; investigation; writing – original draft; writing – review and editing. S. Slimani: Writing – original draft; validation; visualization. D. Peddis: Supervision; resources; project administration; funding acquisition; conceptualization; writing – review and editing. A. Pellis: Supervision; resources; project administration; funding acquisition; conceptualization; writing – review and editing.
FUNDING INFORMATION
Mini curiosity driven grant from the Department of Chemistry and Industrial Chemistry of the University of Genova. Concession Decree No. 1561 of 11.10.2022 adopted by “Ministero dell'Università e della Ricerca (MUR),” according to attachment E of Decree No. 1561/2022, Project title “Network4 Energy Sustainable Transition‐NEST” (Grant / Award Number: ‘PE0000021’).
CONFLICT OF INTEREST STATEMENT
The authors declare no competing financial interest.
ACKNOWLEDGEMENTS
A.P. thanks the Department of Chemistry and Industrial Chemistry of the University of Genova for the Mini Curiosity Driven funding and DP and SS would like to thank Project code PE0000021, Concession Decree No. 1561 of 11.10.2022 adopted by “Ministero dell'Università e della Ricerca (MUR)”, according to attachment E of Decree No. 1561/2022, Project title “Network4 Energy Sustainable Transition‐NEST.”
Papatola, F. , Slimani, S. , Peddis, D. & Pellis, A. (2024) Biocatalyst immobilization on magnetic nano‐architectures for potential applications in condensation reactions. Microbial Biotechnology, 17, e14481. Available from: 10.1111/1751-7915.14481
Contributor Information
D. Peddis, Email: davide.peddis@unige.it.
A. Pellis, Email: alessandro.pellis@unige.it.
REFERENCES
- Abu‐Dief, A.M. & Abdel‐Fatah, S.M. (2018) Development and functionalization of magnetic nanoparticles as powerful and green catalysts for organic synthesis. Beni‐Suef University Journal of Basic and Applied Sciences, 7, 55–67. [Google Scholar]
- Ansari, S.A. & Husain, Q. (2012) Potential applications of enzymes immobilized on/in nano materials: a review. Biotechnology Advances, 30, 512–523. [DOI] [PubMed] [Google Scholar]
- Arizzi, A. , Gomez‐Villalba, L.S. , Lopez‐Arce, P. , Cultrone, G. & Fort, R. (2015) Lime mortar consolidation with nanostructured calcium hydroxide dispersions: the efficacy of different consolidating products for heritage conservation. European Journal of Mineralogy, 27, 311–323. [Google Scholar]
- Arora, G. & Bandopadhyaya, G. (2018) Paradigm shift in theranostics of neuroendocrine tumors: conceptual horizons of nanotechnology in nuclear medicine. Annals of Nuclear Medicine, 32, 151–164. [DOI] [PubMed] [Google Scholar]
- Azim, A. , Azim, H. , Sahoo, B. & Gross, R.A. (2005) Lipase‐catalyzed oligoamide synthesis. Polymeric Materials Science and Engineering, 93, 1730. [Google Scholar]
- Basso, A. & Serban, S. (2019) Industrial applications of immobilized enzymes—a review. Molecular Catalysis, 479, 110607. [Google Scholar]
- Bean, C.P. & Livingston, J.D. (1959) Superparamagnetism. Journal of Applied Physics, 30, S120–S129. [Google Scholar]
- Bedanta, S. & Kleemann, W. (2008) Supermagnetism. Journal of Physics D: Applied Physics, 42, 013001. [Google Scholar]
- Ben Akacha, N. & Gargouri, M. (2015) Microbial and enzymatic technologies used for the production of natural aroma compounds: synthesis, recovery modeling, and bioprocesses. Food and Bioproducts Processing, 94, 675–706. [Google Scholar]
- Bertrand, E. , Pasquier, C. , Duchez, D. , Girard, S. , Pons, A. , Bonnet, P. et al. (2018) High‐frequency, high‐intensity electromagnetic field effects on Saccharomyces cerevisiae conversion yields and growth rates in a reverberant environment. Bioresource Technology, 260, 264–272. [DOI] [PubMed] [Google Scholar]
- Bhakdi, S.C. , Ottinger, A. , Somsri, S. , Sratongno, P. , Pannadaporn, P. , Chimma, P. et al. (2010) Optimized high gradient magnetic separation for isolation of plasmodium‐infected red blood cells. Malaria Journal, 9, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicas, J.L., Pastore, G.M. & Maróstica, M.R., Jr. (Eds.). (2016) Biotechnological production of natural ingredients for food industry. Sharjah: Bentham Science Publishers. [Google Scholar]
- Bilal, M. , Zhao, Y. , Noreen, S. , Shah, S.Z.H. , Bharagava, R.N. & Iqbal, H.M. (2019) Modifying bio‐catalytic properties of enzymes for efficient biocatalysis: a review from immobilization strategies viewpoint. Biocatalysis and Biotransformation, 37, 159–182. [Google Scholar]
- Bilal, M. , Zhao, Y. , Rasheed, T. & Iqbal, H.M. (2018) Magnetic nanoparticles as versatile carriers for enzymes immobilization: a review. International Journal of Biological Macromolecules, 120, 2530–2544. [DOI] [PubMed] [Google Scholar]
- Binns, F. , Harffey, P. , Roberts, S.M. & Taylor, A. (1998) Studies of lipase‐catalyzed polyesterification of an unactivated diacid/diol system. Journal of Polymer Science, Part A: Polymer Chemistry, 36, 2069–2080. [Google Scholar]
- Bisht, K.S. , Henderson, L.A. , Gross, R.A. , Kaplan, D.L. & Swift, G. (1997) Enzyme‐catalyzed ring‐opening polymerization of ω‐pentadecalactone. Macromolecules, 30(9), 2705–2711. [Google Scholar]
- Bîtcan, I. , Petrovici, A. , Pellis, A. , Klébert, S. , Károly, Z. , Bereczki, L. et al. (2023) Enzymatic route for selective glycerol oxidation using covalently immobilized laccases. Enzyme and Microbial Technology, 163, 110168. [DOI] [PubMed] [Google Scholar]
- Blundell, S. (2001) Magnetism in condensed matter. Oxford: Oxford University Press. [Google Scholar]
- Brinker, C.J. & Scherer, G.W. (2013) Sol‐gel science: the physics and chemistry of sol‐gel processing. London: Academic Press. [Google Scholar]
- Brocklehurst, K. & Neuberger, A. (1988) Hydrolytic enzymes. Amsterdam: Elsevier. [Google Scholar]
- Burdock, G.A. (2009) Fenaroli's handbook of flavor ingredients, 6th edition. London: Routledge. [Google Scholar]
- Büyükuslu, N. , Çelik, Ö. & Atak, Ç. (2006) The effect of magnetic field on the activity of superoxide dismutase. Journal of Molecular Cell Biology, 5, 57–62. [Google Scholar]
- Byrne, F.P. , Hodds, W.M. , Shimizu, S. , Farmer, T.J. & Hunt, A.J. (2019) A comparison of the solvation power of the green solvent 2,2,5,5‐tetramethyloxolane versus toluene via partition coefficients. Journal of Cleaner Production, 240, 118175. [Google Scholar]
- Cagil, E.M. , Ozcan, F. & Ertul, S. (2018) Fabrication of calixarene based protein scaffold by electrospin coating for tissue engineering. Journal of Nanoscience and Nanotechnology, 18, 5292–5298. [DOI] [PubMed] [Google Scholar]
- Cannas, C. , Ardu, A. , Musinu, A. , Peddis, D. & Piccaluga, G. (2008) Spherical nanoporous assemblies of iso‐oriented cobalt ferrite nanoparticles: synthesis, microstructure and magnetic properties. Chemistry of Materials, 20, 6364–6371. [Google Scholar]
- Cannas, C. , Concas, G. , Gatteschi, D. , Falqui, A. , Musinu, A. , Piccaluga, G. et al. (2001) Superparamagnetic behaviour of γ‐Fe 2 O 3 nanoparticles dispersed in a silica matrix. Physical Chemistry Chemical Physics, 3, 832–838. [Google Scholar]
- Cannas, C. , Concas, G. , Gatteschi, D. , Musinu, A. , Piccaluga, G. & Sangregorio, C. (2002) How to tailor maghemite particle size in γ‐Fe 2 O 3–SiO 2 nanocomposites. Journal of Materials Chemistry, 12, 3141–3146. [Google Scholar]
- Cannas, C. , Musinu, A. , Ardu, A. , Orru, F. , Peddis, D. , Casu, M. et al. (2010) CoFe2O4 and CoFe2O4/SiO2 core/shell nanoparticles: magnetic and spectroscopic study. Chemistry of Materials, 22, 3353–3361. [Google Scholar]
- Cannas, C. , Peddis, D. & Geologiche, C. (2012) Design of magnetic spinel oxide nanoarchitectures. La Chim e l'industria, 3, 109–117. [Google Scholar]
- Cantone, S. , Ferrario, V. , Corici, L. , Ebert, C. , Fattor, D. , Spizzo, P. et al. (2013) Efficient immobilisation of industrial biocatalysts: criteria and constraints for the selection of organic polymeric carriers and immobilisation methods. Chemical Society Reviews, 42, 6262–6276. [DOI] [PubMed] [Google Scholar]
- Cao, F. , Huang, Y. , Wang, F. , Kwak, D. , Dong, Q. , Song, D. et al. (2018) A high‐performance electrochemical sensor for biologically meaningful L‐cysteine based on a new nanostructured L‐cysteine electrocatalyst. Analytica Chimica Acta, 1019, 103–110. [DOI] [PubMed] [Google Scholar]
- Carrea, G. , Ottolina, G. & Riva, S. (1995) Role of solvents in the control of enzyme selectivity in organic media. Trends in Biotechnology, 13(2), 63–70. [Google Scholar]
- Cespugli, M. , Lotteria, S. , Navarini, L. , Lonzarich, V. , Del Terra, L. , Vita, F. et al. (2018) Rice husk as an inexpensive renewable immobilization carrier for biocatalysts employed in the food, cosmetic and polymer sectors. Catalysts, 8(10), 471. Available from: 10.3390/catal8100471 [DOI] [Google Scholar]
- Chahal, K. & Samra, K.S. (2018) Magnetic and dielectric behavior of praseodymium substituted barium hexaferrite. Journal of Alloys and Compounds, 737, 387–391. [Google Scholar]
- Cheng, H.N. (2010) Enzyme‐catalyzed synthesis of polyamides and polypeptides. In: Loos, K. (Ed.) Biocatalysis in polymer chemistry. Hoboken, NJ: Wiley. [Google Scholar]
- Cheon, H.J. , Adhikari, M.D. , Chung, M. , Tran, T.D. , Kim, J. & Kim, M. (2019) Magnetic nanoparticles‐embedded enzyme‐inorganic hybrid Nanoflowers with enhanced peroxidase‐like activity and substrate channeling for glucose biosensing. Advanced Healthcare Materials, 8, 1–8. [DOI] [PubMed] [Google Scholar]
- Chung, H.J. , Lee, H. , Bae, K.H. , Lee, Y. , Park, J. , Cho, S.W. et al. (2011) Facile synthetic route for surface‐functionalized magnetic nanoparticles: cell labeling and magnetic resonance imaging studies. ACS Nano, 5(6), 4329–4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte, M.P. , Sahoo, J.K. , Abul‐Haija, Y.M. , Lau, K.H.A. & Ulijn, R.V. (2018) Biocatalytic self‐assembly on magnetic nanoparticles. ACS Applied Materials & Interfaces, 10, 3069–3075. [DOI] [PubMed] [Google Scholar]
- Cunha, G.D.C. , Dos Santos, B.T. , Alves, J.R. , Silva, I.A.A. , De Souza Cruz, D.R. & Romao, L.P. (2018) Applications of magnetic hybrid adsorbent derived from waste biomass for the removal of metal ions and reduction of 4‐nitrophenol. Journal of Environmental Management, 213, 236–246. [DOI] [PubMed] [Google Scholar]
- da Silva, F.G. , Depeyrot, J. , Campos, A.F. , Aquino, R. , Fiorani, D. & Peddis, D. (2019) Structural and magnetic properties of spinel ferrite nanoparticles. Journal of Nanoscience and Nanotechnology, 19, 4888–4902. [DOI] [PubMed] [Google Scholar]
- de la Presa, P. , Luengo, Y. , Multigner, M. , Costo, R. , Morales, M.P. , Rivero, G. et al. (2012) Study of heating efficiency as a function of concentration, size, and applied field in γ‐Fe2O3 nanoparticles. The Journal of Physical Chemistry C, 116(48), 25602–25610. [Google Scholar]
- Dong, M. , Wang, Y. , Li, Z. , Weng, Z. & Yu, N. (2018) Simple fabrication of homogeneous ZnO core/shell nanorod arrays for ultraviolet photodetectors. Journal of Nanoscience and Nanotechnology, 18, 5686–5691. [DOI] [PubMed] [Google Scholar]
- Dormann, J.‐L. , Fiorani, D. & Tronc, E. (1997) Magnetic relaxation in fine‐particle systems. Advances in Chemical Physics, 98, 283–494. [Google Scholar]
- Du, Y. , Lai, P.T. , Leung, C.H. & Pong, P.W.T. (2013) Design of superparamagnetic nanoparticles for magnetic particle imaging (MPI). International Journal of Molecular Sciences, 14, 18682–18710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyal, A. , Loos, K. , Noto, M. , Chang, S.W. , Spagnoli, C. , Shafi, K.V.P.M. et al. (2003) Activity of Candida rugosa lipase immobilized on γ‐Fe2O3 magnetic nanoparticles. Journal of the American Chemical Society, 125, 1684–1685. [DOI] [PubMed] [Google Scholar]
- Fabbri, F. , Bertolini, F.A. , Guebitz, G.M. & Pellis, A. (2021) Biocatalyzed synthesis of flavor esters and polyesters: a design of experiments (DoE) approach. International Journal of Molecular Sciences, 22(16), 8493. Available from: 10.3390/ijms22168493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, Y. & Qian, J. (2010) Lipase catalysis in ionic liquids/supercritical carbon dioxide and its applications. Journal of Molecular Catalysis B: Enzymatic, 66, 1–7. [Google Scholar]
- Fasim, A. , More, V.S. & More, S.S. (2021) Large‐scale production of enzymes for biotechnology uses. Current Opinion in Biotechnology, 69, 68–76. [DOI] [PubMed] [Google Scholar]
- Fatima, H. , Charinpanitkul, T. & Kim, K.‐S. (2021) Fundamentals to apply magnetic nanoparticles for hyperthermia therapy. Nanomaterials, 11, 1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felicia, L.J. & Philip, J. (2015) Effect of hydrophilic silica nanoparticles on the magnetorheological properties of ferrofluids: a study using opto‐magnetorheometer. Langmuir, 31, 3343–3353. [DOI] [PubMed] [Google Scholar]
- Fields, C. , Li, P. , O'Mahony, J.J. & Lee, G.U. (2016) Advances in affinity ligand‐functionalized nanomaterials for biomagnetic separation. Biotechnology and Bioengineering, 113, 11–25. [DOI] [PubMed] [Google Scholar]
- Filho, D.G. , Silva, A.G. & Guidini, C.Z. (2019) Lipases: sources, immobilization methods, and industrial applications. Applied Microbiology and Biotechnology, 103(18), 7399–7423. [DOI] [PubMed] [Google Scholar]
- Fortes, C.C.S. , Daniel‐da‐Silva, A.L. , Xavier, A.M.R.B. & Tavares, A.P.M. (2017) Optimization of enzyme immobilization on functionalized magnetic nanoparticles for laccase biocatalytic reactions. Chemical Engineering and Processing: Process Intensification, 117, 1–8. [Google Scholar]
- Gawande, M.B. , Branco, P.S. & Varma, R.S. (2013) Nano‐magnetite (Fe3O4) as a support for recyclable catalysts in the development of sustainable methodologies. Chemical Society Reviews, 42, 3371–3393. [DOI] [PubMed] [Google Scholar]
- Gazeau, F. , Lévy, M. & Wilhelm, C. (2008) Optimizing magnetic nanoparticle design for nanothermotherapy. Nanomedicine, 3(6), 831–844. [DOI] [PubMed] [Google Scholar]
- Gillies, B. , Yamazaki, H. & Armstrong, D.W. (1987) Production of flavor esters by immobilized lipase. Biotechnology Letters, 9, 709–714. [Google Scholar]
- Gotor, V. (1999) Non‐conventional hydrolase chemistry: amide and carbamate bond formation catalyzed by lipases. Bioorganic and Medicinal Chemistry, 7, 2189–2197. [DOI] [PubMed] [Google Scholar]
- Gotor, V. (2002) Biocatalysis applied to the preparation of pharmaceuticals. Organic Process Research and Development, 6, 420–426. [Google Scholar]
- Gu, Q.M. , Maslanka, W.W. & Cheng, H.N. (2008) Chapter 12: Enzyme‐catalyzed polyamides and their derivatives. In: ACS symposium series, Vol. 999. Polymer Biocatalysis and Biomaterials II , pp. 309–319.
- Guo, S. , Li, D. , Zhang, L. , Li, J. & Wang, E. (2009) Monodisperse mesoporous superparamagnetic single‐crystal magnetite nanoparticles for drug delivery. Biomaterials, 30, 1881–1889. [DOI] [PubMed] [Google Scholar]
- Gutman, A.L. , Zuobi, K. & Boltansky, A. (1987) Enzymatic lactonisation of γ‐hydroxyesters in organic solvents. Synthesis of optically pure γ‐methylbutyrolactones and γ‐phenylbutyrolactone. Tetrahedron Letters, 28(33), 3861–3864. [Google Scholar]
- Guzmán, F. , Barberis, S. & Illanes, A. (2007) Peptide synthesis: chemical or enzymatic. Electronic Journal of Biotechnology, 10(2), 279–314. Available from: 10.2225/vol10-issue2-fulltext-13 [DOI] [Google Scholar]
- Hajdú, A. , Illés, E. , Tombácz, E. & Borbáth, I. (2009) Surface charging, polyanionic coating and colloid stability of magnetite nanoparticles. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 347(1‐3), 104–108. [Google Scholar]
- Hameed, S. , Bhattarai, P. & Dai, Z. (2018) Cerasomes and bicelles: hybrid bilayered nanostructures with silica‐like surface in cancer theranostics. Frontiers in Chemistry, 6, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanefeld, U. , Gardossi, L. & Magner, E. (2009) Understanding enzyme immobilisation. Chemical Society Reviews, 38, 453–468. [DOI] [PubMed] [Google Scholar]
- Hayashi, K. , Ono, K. , Suzuki, H. , Sawada, M. , Moriya, M. , Sakamoto, W. et al. (2010) High‐frequency, magnetic‐field‐responsive drug release from magnetic nanoparticle/organic hybrid based on hyperthermic effect. ACS Applied Materials & Interfaces, 2, 1903–1911. [DOI] [PubMed] [Google Scholar]
- Heeger, A.J. & MacDiarmid, A.G. (1979) Semiconducting and metallic organic polymers: Chemically doped polyacetylene, (CH)x. In: Quasi‐One‐Dimensional Conductors II: Proceedings of the International Conference Dubrovnik, SR Croatia, SFR Yugoslavia, 1978 , pp. 359–383.
- Helal, E. , Pottier, C. , David, E. , Fréchette, M. & Demarquette, N.R. (2018) Polyethylene/thermoplastic elastomer/zinc oxide nanocomposites for high voltage insulation applications: dielectric, mechanical and rheological behavior. European Polymer Journal, 100, 258–269. [Google Scholar]
- Herdt, A.R. , Kim, B.‐S. & Taton, T.A. (2007) Encapsulated magnetic nanoparticles as supports for proteins and recyclable biocatalysts. Bioconjugate Chemistry, 18, 183–189. [DOI] [PubMed] [Google Scholar]
- Hofman, J. , Maher, B.A. , Muxworthy, A.R. , Wuyts, K. , Castanheiro, A. & Samson, R. (2017) Biomagnetic monitoring of atmospheric pollution: a review of magnetic signatures from biological sensors. Environmental Science & Technology, 51, 6648–6664. [DOI] [PubMed] [Google Scholar]
- Hosseinipour, S.L. , Khiabani, M.S. , Hamishehkar, H. & Salehi, R. (2015) Enhanced stability and catalytic activity of immobilized α‐amylase on modified Fe3O4 nanoparticles for potential application in food industries. Journal of Nanoparticle Research, 17, 382. [Google Scholar]
- Hou, C. , Zhu, H. , Li, Y. , Li, Y. , Wang, X. , Zhu, W. et al. (2015) Facile synthesis of oxidic PEG‐modified magnetic polydopamine nanospheres for Candida rugosa lipase immobilization. Applied Microbiology and Biotechnology, 99, 1249–1259. [DOI] [PubMed] [Google Scholar]
- Huang, S.H. , Liao, M.H. & Chen, D.H. (2003) Direct binding and characterization of lipase onto magnetic nanoparticles. Biotechnology Progress, 19, 1095–1100. [DOI] [PubMed] [Google Scholar]
- Huang, Y.F. , Wang, Y.F. & Yan, X.P. (2010) Amine‐functionalized magnetic nanoparticles for rapid capture and removal of bacterial pathogens. Environmental Science and Technology, 44, 7908–7913. [DOI] [PubMed] [Google Scholar]
- Illanes, A. , Cauerhff, A. , Wilson, L. & Castro, G.R. (2012) Recent trends in biocatalysis engineering. Bioresource Technology, 115, 48–57. [DOI] [PubMed] [Google Scholar]
- Illés, E. , Tombácz, E. , Szekeres, M. , Tóth, I.Y. , Szabó, Á. & Iván, B. (2015) Novel carboxylated PEG‐coating on magnetite nanoparticles designed for biomedical applications. Journal of Magnetism and Magnetic Materials, 380, 132–139. [Google Scholar]
- Jacobs, J.‐P. , Maltha, A. , Reintjes, J.G. , Drimal, J. , Ponec, V. & Brongersma, H.H. (1994) The surface of catalytically active spinels. Journal of Catalysis, 147, 294–300. [Google Scholar]
- Ji, X. , Su, Z. , Liu, C. , Wang, P. & Zhang, S. (2017) Regulation of enzyme activity and stability through positional interaction with polyurethane nanofibers. Biochemical Engineering Journal, 121, 147–155. [Google Scholar]
- Jiang, Y. & Loos, K. (2016) Enzymatic synthesis of biobased polyesters and polyamides. Polymers, 8(7), 243. Available from: 10.3390/polym8070243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Y. , Woortman, A.J.J. , Alberda Van Ekenstein, G.O.R. & Loos, K. (2015) A biocatalytic approach towards sustainable furanic‐aliphatic polyesters. Polymer Chemistry. [Google Scholar]
- Jiang, Y. , Woortman, A.J.J. , Alberda Van Ekenstein, G.O.R. & Loos, K. (2015) A biocatalytic approach towards sustainable furanic‐aliphatic polyesters. Polymer Chemistry, 6, 5198–5211. [Google Scholar]
- Kaushik, A. , Khan, R. , Solanki, P.R. , Pandey, P. , Alam, J. , Ahmad, S. et al. (2008) Iron oxide nanoparticles‐chitosan composite based glucose biosensor. Biosensors and Bioelectronics, 24(4), 676–683. Available from: 10.1016/j.bios.2008.06.032 [DOI] [PubMed] [Google Scholar]
- Khoshnevisan, K. , Bordbar, A.‐K. , Zare, D. , Davoodi, D. , Noruzi, M. , Barkhi, M. et al. (2011) Immobilization of cellulase enzyme on superparamagnetic nanoparticles and determination of its activity and stability. Chemical Engineering Journal, 171, 669–673. [Google Scholar]
- Kladko, D.V. , Zakharzhevskii, M.A. & Vinogradov, V.V. (2020) Magnetic field‐mediated control of whole‐cell biocatalysis. The Journal of Physical Chemistry Letters, 11, 8989–8996. [DOI] [PubMed] [Google Scholar]
- Klibanov, A.M. (1986) Enzymes that work in organic solvents. ChemTech, 16, 354–359. [Google Scholar]
- Knani, D. , Gutman, A.L. & Kohn, D.H. (1993) Enzymatic polyesterification in organic media. Enzyme‐catalyzed synthesis of linear polyesters. I. Condensation polymerization of linear hydroxyesters. II. Ring‐opening polymerization of ϵ‐caprolactone. Journal of Polymer Science Part A: Polymer Chemistry, 31(5), 1221–1232. [Google Scholar]
- Kobayashi, S. (1999) Enzymatic polymerization: a new method of polymer synthesis. Journal of Polymer Science, Part A: Polymer Chemistry, 37, 3041–3056. [Google Scholar]
- Kobayashi, S. (2010) Lipase‐catalyzed polyester synthesis – a green polymer chemistry. Proceedings of the Japan Academy. Series B, Physical and Biological Sciences, 86(4), 338–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi, S. & Makino, A. (2009) Enzymatic polymer synthesis: an opportunity for green polymer chemistry. Chemical Reviews, 109, 5288–5353. [DOI] [PubMed] [Google Scholar]
- Kobayashi, S. , Shoda, S.I. & Uyama, H. (1995) Enzymatic polymerization and oligomerization. Advances in Polymer Science, 121, 1–30. [Google Scholar]
- Kobayashi, S. , Uyama, H. & Kimura, S. (2001) Enzymatic polymerization. Chemical Reviews, 101, 3793–3818. [DOI] [PubMed] [Google Scholar]
- Kraus, S. , Rabinovitz, R. , Sigalov, E. , Eltanani, M. , Khandadash, R. , Tal, C. et al. (2022) Self‐regulating novel iron oxide nanoparticle‐based magnetic hyperthermia in swine: biocompatibility, biodistribution, and safety assessments. Archives of Toxicology, 96, 2447–2464. [DOI] [PubMed] [Google Scholar]
- Kuzhir, P. , Magnet, C. , Ezzaier, H. , Zubarev, A. & Bossis, G. (2017) Magnetic filtration of phase separating ferrofluids: from basic concepts to microfluidic device. Journal of Magnetism and Magnetic Materials, 431, 84–90. [Google Scholar]
- Lagroix, F. & Guyodo, Y. (2017) A new tool for separating the magnetic mineralogy of complex mineral assemblages from low temperature magnetic behavior. Frontiers in Earth Science, 5, 61. [Google Scholar]
- Latorre, M. & Rinaldi, C. (2009) Applications of magnetic nanoparticles in medicine: magnetic fluid hyperthermia. Puerto Rico Health Sciences Journal, 28, 227–238. [PubMed] [Google Scholar]
- Laureti, S. , Varvaro, G. , Testa, A.M. , Fiorani, D. , Agostinelli, E. , Piccaluga, G. et al. (2010) Magnetic interactions in silica coated nanoporous assemblies of CoFe2O4 nanoparticles with cubic magnetic anisotropy. Nanotechnology, 21, 315701. [DOI] [PubMed] [Google Scholar]
- Leal, E. , Dantas, J. , dos Santos, P.T.A. , Bicalho, S.M.C.M. , Kiminami, R.H.G.A. , Da Silva, M.R. et al. (2018) Effect of the surface treatment on the structural, morphological, magnetic and biological properties of MFe2O4 iron spinels (M=Cu, Ni, Co, Mn and Fe). Applied Surface Science, 455, 635–645. [Google Scholar]
- Leferink, A.M. , Reis, D.S. , Van Blitterswijk, C.A. & Moroni, L. (2018) An antibody based approach for multi‐coloring osteogenic and chondrogenic proteins in tissue engineered constructs. Biomedical Materials, 13, 044102. [DOI] [PubMed] [Google Scholar]
- Leong, S.S. , Yeap, S.P. & Lim, J.K. (2016) Working principle and application of magnetic separation for biomedical diagnostic at high‐ and low‐field gradients. Interface Focus, 6(6), 20160048. Available from: 10.1098/rsfs.2016.0048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Wang, Z.L. , Zeng, H. , Sun, S. & Ping Liu, J. (2003) Interface structures in FePt/Fe3 Pt hard‐soft exchange‐coupled magnetic nanocomposites. Applied Physics Letters, 82, 3743–3745. [Google Scholar]
- Lin, Y.‐S. & Haynes, C.L. (2009) Synthesis and characterization of biocompatible and size‐tunable multifunctional porous silica nanoparticles. Chemistry of Materials, 21, 3979–3986. [Google Scholar]
- Ling, D. & Hyeon, T. (2013) Chemical design of biocompatible iron oxide nanoparticles for medical applications. Small, 9, 1450–1466. [DOI] [PubMed] [Google Scholar]
- Liu, J. , Cao, D. & Zhang, L. (2008) Molecular dynamics study on nanoparticle diffusion in polymer melts: a test of the stokes−Einstein law. Journal of Physical Chemistry C, 112, 6653–6661. [Google Scholar]
- Liu, Y. , Jia, S. , Ran, J. & Wu, S. (2010) Effects of static magnetic field on activity and stability of immobilized a‐amylase in chitosan bead. Catalysis Communications, 11, 364–367. [Google Scholar]
- López‐Ortega, A. , Estrader, M. , Salazar‐Alvarez, G. , Roca, A.G. & Nogués, J. (2015) Applications of exchange coupled bi‐magnetic hard/soft and soft/hard magnetic core/shell nanoparticles. Physics Reports, 553, 1–32. [Google Scholar]
- Lu, H.M. , Zheng, W.T. & Jiang, Q. (2007) Saturation magnetization of ferromagnetic and ferrimagnetic nanocrystals at room temperature. Journal of Physics D: Applied Physics, 40, 320–325. [Google Scholar]
- Lykaki, M. , Pachatouridou, E. , Carabineiro, S.A. , Iliopoulou, E. , Andriopoulou, C. , Kallithrakas‐Kontos, N. et al. (2018) Ceria nanoparticles shape effects on the structural defects and surface chemistry: implications in CO oxidation by Cu/CeO2 catalysts. Applied Catalysis B: Environmental, 230, 18–28. [Google Scholar]
- Mahapatro, A. , Kalra, B. , Kumar, A. & Gross, R.A. (2003) Lipase‐catalyzed polycondensations: effect of substrates and solvent on chain formation, dispersity, and end‐group structure. Biomacromolecules, 4, 544–551. [DOI] [PubMed] [Google Scholar]
- Mahdavian, A.R. & Mirrahimi, M.A.‐S. (2010) Efficient separation of heavy metal cations by anchoring polyacrylic acid on superparamagnetic magnetite nanoparticles through surface modification. Chemical Engineering Journal, 159, 264–271. [Google Scholar]
- Malek, T.J. , Chaki, S.H. , Tailor, J.P. & Deshpande, M.P. (2018) Nonisothermal decomposition kinetics of pure and Mn‐doped Fe3O4 nanoparticles. Journal of Thermal Analysis and Calorimetry, 132, 895–905. [Google Scholar]
- Manisha & Yadav, S.K. (2017) Technological advances and applications of hydrolytic enzymes for valorization of lignocellulosic biomass. Bioresource Technology, 245, 1727–1739. [DOI] [PubMed] [Google Scholar]
- Manjón, A. , Iborra, J.L. & Arocas, A. (1991) Short‐chain flavour ester synthesis by immobilized lipase in organic media. Biotechnology Letters, 13, 339–344. [Google Scholar]
- Manzoor, A. , Khan, M.A. , Khan, M.Y. , Akhtar, M.N. & Hussain, A. (2018) Tuning magnetic and high frequency dielectric behavior in Li‐Zn ferrites by Ho doping. Ceramics International, 44, 6321–6329. [Google Scholar]
- Margolin, A.L. & Klibanov, A.M. (1987) Peptide synthesis catalyzed by lipases in anhydrous organic solvents. Journal of the American Chemical Society, 109, 3802–3804. [Google Scholar]
- Miletić, N. , Loos, K. & Gross, R.A. (2010) Enzymatic polymerization of polyester. In: Biocatalysis in polymer chemistry. Berlin: Wiley‐VCH Verlag GmbH & Co. KGaA. [Google Scholar]
- Mondal, M. , Kumar Rai, V. , Srivastava, C. , Sarkar, S. & Akash, R. (2016) Enhanced frequency upconversion in Ho3+/Yb3+/Li+: YMoO4 nanophosphors for photonic and security ink applications. Journal of Applied Physics, 120, 233101. [Google Scholar]
- Morales, M.P. , Serna, C.J. , Bødker, F. & Mørup, S. (1997) Spin canting due to structural disorder in maghemite. Journal of Physics: Condensed Matter, 9, 5461–5467. [Google Scholar]
- Mosafa, L. , Moghadam, M. & Shahedi, M. (2013) Papain enzyme supported on magnetic nanoparticles: preparation, characterization and application in the fruit juice clarification. Cuihua Xuebao/Chinese Journal of Catalysis, 34, 1897–1904. [Google Scholar]
- Moumen, N. & Pileni, M.P. (1996) New syntheses of cobalt ferrite particles in the range 2–5 nm: comparison of the magnetic properties of the nanosized particles in dispersed fluid or in powder form. Chemistry of Materials, 8, 1128–1134. [Google Scholar]
- Mourdikoudis, S. , Kostopoulou, A. & LaGrow, A.P. (2021) Magnetic nanoparticle composites: synergistic effects and applications. Advanced Science, 8, 2004951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscas, G. , Yaacoub, N. , Concas, G. , Sayed, F. , Hassan, R.S. , Greneche, M. et al. (2015) Evolution of the magnetic structure with chemical composition in spinel iron oxide nanoparticles. Nanoscale, 7, 13576–13585. [DOI] [PubMed] [Google Scholar]
- Naik, R.R. , Tomczak, M.M. , Luckarift, H.R. , Spain, J.C. & Stone, M.O. (2004) Entrapment of enzymes and nanoparticles using biomimetically synthesized silica. Chemical Communications, 15, 1684–1685. [DOI] [PubMed] [Google Scholar]
- Nardini, M. & Dijkstra, B.W. (1999) α/β hydrolase fold enzymes: the family keeps growing. Current Opinion in Structural Biology, 9, 732–737. [DOI] [PubMed] [Google Scholar]
- Newell, A.J. (2017) Frequency dependence of susceptibility in magnets with uniaxial and triaxial anisotropy. Journal of Geophysical Research: Solid Earth, 122, 7544–7561. [Google Scholar]
- Niraula, G. , Wu, C. , Yu, X. , Malik, S. , Verma, D.S. , Yang, R. et al. (2024) The curie temperature: a key playmaker in self‐regulated temperature hyperthermia. Journal of Materials Chemistry B, 12, 286–331. [DOI] [PubMed] [Google Scholar]
- Obaidat, I. , Issa, B. & Haik, Y. (2015) Magnetic properties of magnetic nanoparticles for efficient hyperthermia. Nanomaterials, 5, 63–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obaidat, I.M. , Narayanaswamy, V. , Alaabed, S. , Sambasivam, S. & Muralee Gopi, C.V.V. (2019) Principles of magnetic hyperthermia: a focus on using multifunctional hybrid magnetic nanoparticles. Magnetochemistry, 5, 67. [Google Scholar]
- Oda, K. (2012) New families of carboxyl peptidases: serine‐carboxyl peptidases and glutamic peptidases. Journal of Biochemistry, 151, 13–25. [DOI] [PubMed] [Google Scholar]
- Okumura, S. , Iwai, M. & Tominaga, Y. (1984) Synthesis of Ester oligomer by aspergillus Niger lipase. Agricultural and Biological Chemistry, 48, 2805–2808. [Google Scholar]
- Omelyanchik, A. , Lamura, G. , Peddis, D. & Canepa, F. (2021) Optimization of a NdFeB permanent magnet configuration for in‐vivo drug delivery experiments. Journal of Magnetism and Magnetic Materials, 522, 167491. [Google Scholar]
- Omelyanchik, A. , Levada, K. , Pshenichnikov, S. , Abdolrahim, M. , Baricic, M. , Kapitunova, A. et al. (2020) Green synthesis of Co‐Zn spinel ferrite nanoparticles: magnetic and intrinsic antimicrobial properties. Materials, 13, 5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelyanchik, A. , Villa, S. , Vasilakaki, M. , Singh, G. , Ferretti, A.M. , Ponti, A. et al. (2021) Interplay between inter‐and intraparticle interactions in bi‐magnetic core/shell nanoparticles. Nanoscale Advances, 3, 6912–6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öztürk Düşkünkorur, H. , Pollet, E. , Phalip, V. , Güvenilir, Y. & Avérous, L. (2014) Lipase catalyzed synthesis of polycaprolactone and clay‐based nanohybrids. Polymer, 55, 1648–1655. [Google Scholar]
- Paiva, A.L. , Van Rossum, D. & Malcata, F.X. (2002) Kinetics of lipase‐mediated synthesis of butyl butyrate in n‐hexane. Biocatalysis and Biotransformation, 20, 43–51. [Google Scholar]
- Palma, G. , Raquel, B. , Rodrigo, R.O. & Pandoli, G. (2021) Immobilization of lipases on lignocellulosic bamboo powder for biocatalytic transformations in batch and continuous flow. Catalysis Today, 381, 280–287. [Google Scholar]
- Panina, L.V. , Gurevich, A. , Beklemisheva, A. , Omelyanchik, A. , Levada, K. & Rodionova, V. (2022) Spatial manipulation of particles and cells at micro‐and nanoscale via magnetic forces. Cells, 11, 950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankhurst, Q.A. , Connolly, J. , Jones, S.K. & Dobson, J. (2003) Applications of magnetic nanoparticles in biomedicine. Journal of Physics D: Applied Physics, 36, R167–R181. [Google Scholar]
- Parashar, S.K. , Srivastava, S.K. , Dutta, N.N. & Garlapati, V.K. (2018) Engineering aspects of immobilized lipases on esterification: a special emphasis of crowding, confinement and diffusion effects. Engineering in Life Sciences, 18, 308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peddis, D. , Cannas, C. , Musinu, A. , Ardu, A. , Orrù, F. , Fiorani, D. et al. (2013) Beyond the effect of particle size: influence of CoFe2O4 nanoparticle arrangements on magnetic properties. Chemistry of Materials, 25, 2005–2013. [Google Scholar]
- Peiravi, M. , Eslami, H. , Ansari, M. & Zare‐Zardini, H. (2022) Magnetic hyperthermia: potentials and limitations. Journal of the Indian Chemical Society, 99(1), 100269. [Google Scholar]
- Pellis, A. , Herrero Acero, E. , Gardossi, L. , Ferrario, V. & Guebitz, G.M. (2016) Renewable building blocks for sustainable polyesters: new biotechnological routes for greener plastics. Polymer International, 65, 861–871. [Google Scholar]
- Pellis, A. , Nyanhongo, G.S. & Farmer, T.J. (2019) Recent advances on enzymatic catalysis as a powerful tool for the sustainable synthesis of bio‐based polyesters. In: Bastidas‐Oyanedel, J.R. & Schmidt, J. (Eds.) Biorefinery. Cham: Springer. [Google Scholar]
- Pileni, M.P. (2001) Nanocrystal self‐assemblies: fabrication and collective properties. The Journal of Physical Chemistry. B, 105, 3358–3371. [Google Scholar]
- Pugh, C. , Liu, T. , Yan, J. & Kobayashi, S. (2015) Enzymatic polymerizations. In: Encyclopedia of polymeric nanomaterials. Heidelberg: Springer Berlin. [Google Scholar]
- Rajan, A. & Sahu, N.K. (2020) Review on magnetic nanoparticle‐mediated hyperthermia for cancer therapy. Journal of Nanoparticle Research, 22, 319. [Google Scholar]
- Rancourt, D.G. & Daniels, J.M. (1984) Influence of unequal magnetization direction probabilities on the Mössbauer spectra of superparamagnetic particles. Physical Review B, 29, 2410–2414. [Google Scholar]
- Rao, K.S. , Choudary, G. , Rao, K.H. & Sujatha, C. (2015) Structural and magnetic properties of ultrafine CoFe2O4 nanoparticles. Procedia Materials Science, 10, 19–27. [Google Scholar]
- Rocha‐Santos, T.A. (2014) Sensors and biosensors based on magnetic nanoparticles. TrAC Trends in Analytical Chemistry, 62, 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas, V. , Gil, J.V. , Piñaga, F. & Manzanares, P. (2001) Studies on acetate ester production by non‐saccharomyces wine yeasts. International Journal of Food Microbiology, 70(3), 283–289. [DOI] [PubMed] [Google Scholar]
- Romero, C.M. , Spuches, F.C. , Morales, A.H. , Perotti, N.I. , Navarro, M.C. & Gómez, M.I. (2018) Design and characterization of immobilized biocatalyst with lipase activity onto magnetic magnesium spinel nanoparticles: a novel platform for biocatalysis. Colloids and Surfaces B: Biointerfaces, 172, 699–707. [DOI] [PubMed] [Google Scholar]
- Rother, D. , Sen, T. , East, D. & Bruce, I.J. (2011) Silicon, silica and its surface patterning/activation with alkoxy‐and amino‐silanes for nanomedical applications. Nanomedicine, 6, 281–300. [DOI] [PubMed] [Google Scholar]
- Sadighi, A. , Motevalizadeh, S.F. , Hosseini, M. , Ramazani, A. , Gorgannezhad, L. , Nadri, H. et al. (2017) Metal‐chelate immobilization of lipase onto polyethylenimine coated MCM‐41 for apple flavor synthesis. Applied Biochemistry and Biotechnology, 182, 1371–1389. [DOI] [PubMed] [Google Scholar]
- Schrag, J.D. & Cygler, M. (1997) Lipases and α/β hydrolase fold. Methods in Enzymology, 284, 85–107. Available from: 10.1016/s0076-6879(97)84006-2 [DOI] [PubMed] [Google Scholar]
- Schrock, R.R. (2002) High oxidation state multiple metal‐carbon bonds. Chemical Reviews, 102, 145–180. [DOI] [PubMed] [Google Scholar]
- Schwab, L.W. , Kroon, R. , Schonten, A.J. & Loos, K. (2008) Enzyme‐catalyzed ring‐opening polymerization of unsubstituted β‐lactam. Macromolecular Rapid Communications, 29, 794–797. [Google Scholar]
- Sementsov, S. & Golysheva, A. (2023) Green finance in Eurasian union: should we expect a common solution? In: Devezas, T.C., Leitão, J.C.C., Yegorov, Y. & Chistilin, D. (Eds.) Global challenges of climate change, Vol. 2: risk assessment, political and social dimension of the green energy transition. Cham: Springer International Publishing, pp. 223–250. [Google Scholar]
- Seol, M.‐L. , Jeon, S.‐B. , Han, J.‐W. & Choi, Y.‐K. (2017) Ferrofluid‐based triboelectric‐electromagnetic hybrid generator for sensitive and sustainable vibration energy harvesting. Nano Energy, 31, 233–238. [Google Scholar]
- Shah, R.R. , Davis, T.P. , Glover, A.L. , Nikles, D.E. & Brazel, C.S. (2015) Impact of magnetic field parameters and iron oxide nanoparticle properties on heat generation for use in magnetic hyperthermia. Journal of Magnetism and Magnetic Materials, 387, 96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon, R.A. (2007) Enzyme immobilization: the quest for optimum performance. Advanced Synthesis & Catalysis, 349, 1289–1307. [Google Scholar]
- Sheldon, R.A. & van Pelt, S. (2013) Enzyme immobilisation in biocatalysis: why, what and how. Chemical Society Reviews, 42, 6223–6235. [DOI] [PubMed] [Google Scholar]
- Shen, F. , Arshi, S. , Magner, E. , Ulstrup, J. & Xiao, X. (2022) One‐step electrochemical approach of enzyme immobilization for bioelectrochemical applications. Synthetic Metals, 291, 117205. [Google Scholar]
- Simo, A. , Sibanyoni, J. , Fuku, X. , Numan, N. , Omorogbe, S. & Maaza, M. (2018) Shape control VO2 nanorods prepared by soft chemistry and electrochemical method. Applied Surface Science, 446, 145–150. [Google Scholar]
- Sinn, H. & Kaminsky, W. (1980) Ziegler‐Natta catalysis. Advances in Organometallic Chemistry, 18, 99–149. [Google Scholar]
- Skliri, E. , Miao, J. , Xie, J. , Liu, G. , Salim, T. , Liu, B. et al. (2018) Assembly and photochemical properties of mesoporous networks of spinel ferrite nanoparticles for environmental photocatalytic remediation. Applied Catalysis B: Environmental, 227, 330–339. [Google Scholar]
- Slimani, S. (2022) Design magnetic hybrid nanomaterials for molecules harvesting/delivery. PhD thesis. Genova: Universita’ degli Studi di Genova. Available from: https://hdl.handle.net/11567/1089350 [Accessed 19th January 2024]. [Google Scholar]
- Stoner, E.C. & Wohlfarth, E.P. (1991) A mechanism of magnetic hysteresis in heterogeneous alloys. IEEE Transactions on Magnetics, 27, 3475–3518. [Google Scholar]
- Suber, L. & Peddis, D. (2007) Approaches to synthesis and characterization of spherical and anisometric metal oxide magnetic nanomaterials. Nanotechnologies for the Life Sciences. Available from: 10.1002/9783527610419.ntls0174 [DOI] [Google Scholar]
- Sun, S. & Zeng, H. (2002) Size‐controlled synthesis of magnetite nanoparticles. Journal of the American Chemical Society, 124, 8204–8205. [DOI] [PubMed] [Google Scholar]
- Sun, S. , Zeng, H. , Robinson, D.B. , Raoux, S. , Rice, P.M. , Wang, S.X. et al. (2004) Monodisperse mfe2o4 (m= fe, co, mn) nanoparticles. Journal of the American Chemical Society, 126, 273–279. [DOI] [PubMed] [Google Scholar]
- Sutay Kocabaş, D. , Lyne, J. & Ustunol, Z. (2022) Hydrolytic enzymes in the dairy industry: applications, market and future perspectives. Trends in Food Science and Technology, 119, 467–475. [Google Scholar]
- Szwarc, M. (1956) “Living” polymers. Nature, 178, 1168–1169. [Google Scholar]
- Takahashi, K. , Kodera, Y. , Yoshimoto, T. , Ajima, A. , Matsushima, A. & Inada, Y. (1985) Ester‐exchange catalyzed by lipase modified with polyethylene glycol. Biochemical and Biophysical Research Communications, 131, 532–536. [DOI] [PubMed] [Google Scholar]
- Tao, J. & Kazlauskas, R. (2011) Biocatalysis for green chemistry and chemical process development. Hoboken, NJ: Wiley. [Google Scholar]
- Tatarchuk, T. , Danyliuk, N. , Lapchuk, I. , Shyichuk, A. & Kotsyubynsky, V. (2022) Catalytic activity of magnetite and its magnetic heating properties. Materials Today Proceedings, 62, 5805–5811. [Google Scholar]
- Therisod, M. & Klibanov, A.M. (1986) Facile enzymatic preparation of Monoacylated sugars in pyridine. Journal of the American Chemical Society, 108, 5638–5640. [Google Scholar]
- Tong, S. , Quinto, C.A. , Zhang, L. , Mohindra, P. & Bao, G. (2017) Size‐dependent heating of magnetic iron oxide nanoparticles. ACS Nano, 11, 6808–6816. [DOI] [PubMed] [Google Scholar]
- Torres‐Herrero, B. , Armenia, I. , Alleva, M. , Asín, L. , Correa, S. , Ortiz, C. et al. (2023) Remote activation of enzyme Nanohybrids for cancer prodrug therapy controlled by magnetic heating. ACS Nano, 17, 12358–12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trohidou, K.N. (2014) Magnetic nanoparticle assemblies. Boca Raton, FL: CRC Press. [Google Scholar]
- Turcheniuk, K. , Tarasevych, A.V. , Kukhar, V.P. , Boukherroub, R. & Szunerits, S. (2013) Recent advances in surface chemistry strategies for the fabrication of functional iron oxide based magnetic nanoparticles. Nanoscale, 5, 10729–10752. [DOI] [PubMed] [Google Scholar]
- Umut, E. (2013) Surface modification of nanoparticles used in biomedical applications. Modern Surface Engineering Treatments, 20, 185–208. [Google Scholar]
- Uppenberg, J. , Hansen, M.T. , Patkar, S. & Jones, T.A. (1994) The sequence, crystal structure determination and refinement of two crystal forms of lipase B from Candida Antarctica. Structure, 2, 293–308. [DOI] [PubMed] [Google Scholar]
- Uyama, H. , Inada, K. & Kobayashi, S. (1999) Regioselective polymerization of divinyl sebacate and triols using lipase catalyst. Macromolecular Rapid Communications, 20, 171–174. [Google Scholar]
- Uyama, H. & Kobayashi, S. (1993) Enzymatic ring‐opening polymerization of lactones catalyzed by lipase. Chemistry Letters, 22, 1149–1150. [Google Scholar]
- Uyama, H. , Yaguchi, S. & Kobayashi, S. (1999) Enzymatic synthesis of aromatic polyesters by lipase‐catalyzed polymerization of dicarboxylic acid divinyl esters and glycols. Polymer Journal, 31, 380–383. [Google Scholar]
- Vaghari, H. , Jafarizadeh‐Malmiri, H. , Mohammadlou, M. , Berenjian, A. , Anarjan, N. , Jafari, N. et al. (2016) Application of magnetic nanoparticles in smart enzyme immobilization. Biotechnology Letters, 38, 223–233. [DOI] [PubMed] [Google Scholar]
- Vaillant, L. , Andresz, S. & Schneider, T. (2020) Environmental impacts associated with radioactive waste management: a review of standards and practices according to the do not significant harm approach of the European taxonomy . Report No. 326.
- Vasilakaki, M. , Ntallis, N. , Yaacoub, N. , Muscas, G. , Peddis, D. & Trohidou, K. (2018) Optimising the magnetic performance of Co ferrite nanoparticles via organic ligand capping. Nanoscale, 10, 21244–21253. [DOI] [PubMed] [Google Scholar]
- Vasilescu, C. , Todea, A. , Nan, A. , Circu, M. , Turcu, R. , Benea, I.‐C. et al. (2019) Enzymatic synthesis of short‐chain flavor esters from natural sources using tailored magnetic biocatalysts. Food Chemistry, 296, 1–8. [DOI] [PubMed] [Google Scholar]
- Verger, R. (1997) “Interfacial activation” of lipases: facts and artifacts. Trends in Biotechnology, 15(1), 32–38. [Google Scholar]
- Vestal, C.R. & Zhang, Z.J. (2004) Magnetic spinel ferrite nanoparticles from microemulsions. International Journal of Nanotechnology, 1, 240–263. [Google Scholar]
- Vozniuk, O. , Tabanelli, T. , Tanchoux, N. , Millet, J.‐M.M. , Albonetti, S. , Di Renzo, F. et al. (2018) Mixed‐oxide catalysts with spinel structure for the valorization of biomass: the chemical‐loop reforming of bioethanol. Catalysts, 8, 332. [Google Scholar]
- Wagner, W. , Albuquerque, C. , Marcos, R. , Brand, P. , Salazar, T. , Lúcia, A. et al. (2016) Evidences of the static magnetic field influence on cellular systems. Progress in Biophysics and Molecular Biology, 121, 16–28. [DOI] [PubMed] [Google Scholar]
- Wang, P. (2006) Nanoscale biocatalyst systems. Current Opinion in Biotechnology, 17, 574–579. [DOI] [PubMed] [Google Scholar]
- Wasak, A. , Drozd, R. , Jankowiak, D. & Rakoczy, R. (2019) Rotating magnetic field as tool for enhancing enzymes properties‐laccase case study. Scientific Reports, 9, 3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger, S. , Haernvall, K. , Scaini, D. , Ghazaryan, G. , Zumstein, M.T. , Sander, M. et al. (2017) Enzymatic surface hydrolysis of poly(ethylene furanoate) thin films of various crystallinities. Green Chemistry, 19, 5381–5384. [Google Scholar]
- Welsh, F.W. , Williams, R.E. & Dawson, K.H. (1990) Lipase mediated synthesis of low molecular weight flavor esters. Journal of Food Science, 55, 1679–1682. [Google Scholar]
- West, J.B. & Wong, C.H. (1987) Use of nonproteases in peptide synthesis. Tetrahedron Letters, 28, 1629–1632. [Google Scholar]
- Yan, L. , Zhang, S. , Chen, P. , Liu, H. , Yin, H. & Li, H. (2012) Magnetotactic bacteria, magnetosomes and their application. Microbiological Research, 167, 507–519. [DOI] [PubMed] [Google Scholar]
- Yoshimura, M. & Byrappa, K. (2008) Hydrothermal processing of materials: past, present and future. Journal of Materials Science, 43, 2085–2103. [Google Scholar]
- Yu, C.C. , Kuo, Y.Y. , Liang, C.F. , Chien, W.T. , Wu, H.T. , Chang, T.C. et al. (2012) Site‐specific immobilization of enzymes on magnetic nanoparticles and their use in organic synthesis. Bioconjugate Chemistry, 23, 714–724. [DOI] [PubMed] [Google Scholar]
- Zaks, A. & Klibanov, A.M. (1986) Substrate specificity of enzymes in organic solvents vs. water is reversed. Journal of the American Chemical Society, 108, 2767–2768. [Google Scholar]
- Zhang, W. , Yu, X. , Li, H. , Dong, D. , Zuo, X. & Wu, C. (2019) Magnetic nanoparticles with low curie temperature and high heating efficiency for self‐regulating temperature hyperthermia. Journal of Magnetism and Magnetic Materials, 489, 165382. [Google Scholar]


