Abstract
Introduction: Resistance exercise training (RET) can increase muscle mass and strength, and this adaptation is optimized when dietary protein is consumed to enhance muscle protein synthesis. Dairy milk has been endorsed for this purpose; however, allergy and lactose intolerance affect two-thirds of the global population making dairy milk unsuitable for many. Plant-based alternatives such as soy milk have gained popularity and exhibit comparable protein content. However, concerns regarding soy phytoestrogens potentially influencing circulating sex hormones and diminishing the anabolic response to RET have been raised. This study therefore aimed to assess the acute effects of dairy and soy milk consumption on circulating sex hormones (total, free testosterone, free testosterone percentage, total estrogen, progesterone, and sex hormone binding globulin) after RET.
Materials and methods: Six male participants were recruited for a double-blinded, randomized crossover study with either dairy or soy milk provided post RET. Venous samples were collected before and after milk consumption across seven timepoints (0-120 minutes) where circulating sex hormones were analyzed. Two-way ANOVA analyses were applied for repeated measures for each hormone. The area under the curve (AUC) was also calculated between dairy and soy milk. Significance was set at p<0.05.
Results: No significant differences were observed in acute circulating serum for free (p=0.95), % free (p=0.56), and total testosterone (p=0.88), progesterone (p=0.67), or estrogen (p=0.21) between milk conditions. Likewise, no significant differences in AUC were observed between any hormones.
Conclusion: These findings suggest that consumption of dairy milk and soy milk have comparable acute effects on circulating sex hormones following RET. Further investigations with expanded sample sizes are needed to strengthen and broaden these initial findings.
Keywords: lactose intolerant, plant-based milk, progesterone, estrogen, testosterone, hormones, strength training, soy milk, dairy milk
Introduction
Resistance exercise training (RET) is widely adopted by recreational exercisers and competitive athletes to improve skeletal muscle strength and mass, as well as overall exercise performance. During the acute period following RET (within one hour), protein consumption is recommended to facilitate muscle adaptations and recovery [1]. Dairy milk has been endorsed in this context due to its considerably high protein concentrations, most of which are casein and whey [2]. However, dairy milk allergy is the most prevalent food allergy, affecting up to 17% of the global population [3] and lactose intolerance affects two-thirds of the global population [4], making dairy unsuitable for many individuals. Dairy milk consumption has been steadily declining in Europe and the United States [5]. Plant-based milks provide an alternative to dairy milk and have been recently gaining popularity, and one of the most commonly used substitutes is soy milk, especially in exercising individuals [6]. Regular exercise increases protein requirements, and dairy and soy milk exhibit comparable total protein contents [7]. Studies have also shown comparable effects on muscle strength adaption for whey and soy protein [8,9]. However, perceived risks surrounding the effect of soy on sex hormones and their links to ‘feminization’ in men may discourage consumption [10].
The sex hormones testosterone, progesterone, and estrogen can influence anabolic pathways, adaptations to RET, and body composition. Testosterone activates satellite cells and promotes myonuclear accretion, facilitating muscle fiber hypertrophy [11]. Exercise-induced testosterone elevations have been shown to translate into increased muscle hypertrophy and strength development [12], while suppression of endogenous testosterone production diminishes the anabolic response to RET [13]. Elevated progesterone levels have been shown to depress serum testosterone levels and reduce thigh muscle cross-sectional area, exerting an anti-anabolic effect on muscle even when combined with exogenous testosterone in the context of RET [14]. Higher progesterone has also been shown to promote weight gain that is mostly or entirely fat in males and females [15]. Estrogen has been shown to improve muscle mass, strength, and collagen content, yet, in tendons and ligaments, estrogen can decrease stiffness, affecting performance and increasing injury rates [16]. While these hormones clearly influence anabolic pathways and body composition, research examining estrogen and progesterone levels post RET has predominantly been conducted in female cohorts [17,18]. Conversely, research analyzing anabolism following acute RET in men has typically focused on testosterone, growth hormone, insulin-like growth factor, and cortisol [19], thereby highlighting a gap in the body of evidence pertaining to estrogen and progesterone in men following RET.
Concerns about the effects of soy on sex hormones and the ensuing anabolic response to RET largely surround the isoflavones. These are a class of phytoestrogens found naturally in many plant foods and particularly concentrated in soy [20]. Isoflavones act as selective estrogen receptor modulators, exerting estrogen-like effects in some tissues and anti-estrogenic effects in others [21]. However, the effects of isoflavones are not equivalent to those of endogenous estrogens due to multiple biochemical differences [22]. For instance, while isoflavones share a similar chemical structure allowing them to bind to estrogen receptors, their effects vary and, in some instances, have been estimated to be some thousandfold weaker compared to 17-beta estradiol (E2), the primary female sex hormone [23]. In addition, their binding affinity to the two estrogen receptors differs when compared to estrogen resulting in divergent physiological outcomes such as transcriptional expression [24]. However, many athletes who engage in resistance training remain concerned about the potential effects of soy on sex hormones [21]. "Feminizing effects" such as gynecomastia are rare but have been documented when consuming very large quantities (2.8 liters per day) of soy milk [25].
While soy and isoflavones have come under scrutiny with respect to phytoestrogens, it is important to note that no estrogens or progesterone have been detected in soy [21,26]. The testosterone contents of soy are also unremarkable and comparable to levels detected in other plant foods [21]. In contrast, dairy milk and dairy products supply 60-80% of ingested female sex steroids [27]. Modern dairy milk production has undergone significant changes over the past decades, with the aim of maximizing efficiency and commercial output. Natural reproductive cycles in cows involve pregnancy followed by lactation to suppress ovulation and conception. To optimize commercial milk output, cows are artificially inseminated and kept pregnant for most of their lactation period, allowing for nearly continuous milk harvesting [28]. While this approach benefits farmers by increasing milk production, it leads to alterations in the composition of dairy milk available to consumers. As cows spend more time pregnant during lactation, sex-steroid-related pregnancy hormones accumulate in the milk. Concentrations of free and enzymatically deconjugated estrone (E1) rise significantly throughout the course of pregnancy, increasing from 7.9 ng/L in non-pregnant cows to 1266 ng/L during the third trimester of pregnancy [29]. Concentrations of 17 alpha-E2 and 17 beta-E2 also exhibit a notable increase during pregnancy, rising from 33 to 322 ng/L and from 18.6 to 51.2 ng/L, respectively [29]. Consequently, human consumers are exposed to these hormones when consuming dairy milk [28].
As dairy milk contains sex hormones and soy milk contains molecules that are structurally similar to sex hormones, these beverages have the potential to influence circulating sex hormones and subsequently muscle adaptations to exercise. The purpose of this study was to examine and compare the acute effects of dairy milk and soy milk consumption on serum sex hormone concentrations following an acute bout of RET. It was hypothesized that dairy milk consumption following resistance exercise will result in higher circulating estrogen and progesterone levels and lower circulating free and total testosterone levels compared to soy milk.
Materials and methods
The study utilized a double-blinded, randomized, within-subject repeated measures, crossover design at the University of Wollongong, Wollongong, Australia. Consent was obtained from all participants before the study commencement. It was approved by the University of Wollongong’s Human Research Ethics Research Committee (2022/211). The study was registered with the Australian New Zealand Clinical Trials Registry (registration number: ACTRN 12622001363774). It was conducted in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Participants attended the laboratory at the University of Wollongong on three occasions The initial visit was a familiarization and maximal strength assessment. The maximal strength assessment occurred for the following exercises: barbell bench press, barbell squats, seated barbell shoulder press, standing barbell bicep curls, and pronated pull-ups and was used to determine one-repetition maximum (1-RM) of each participant. The crossover occurred during the second and third visits where three sets of each exercise were performed at 70% of participants 1-RM until voluntary failure. At least 48 hours separated visits two and three, which were both completed within a seven-day time frame. Blood was collected immediately following exercise at visits two and three. Participants were then randomly allocated to consume dairy milk or soy milk at visit two and the alternate milk at visit three. Blood collection occurred at six timepoints after milk consumption.
Participants
Male participants aged between 18 and 30 years were recruited for this study. Invitations to participate were posted to local relevant social media groups. The inclusion criteria for participants consisted of engagement in regular resistance exercise for at least two years with a frequency of at least three times per week, a body mass index (BMI) between 18.5 and 30 kg/m2, and a level of English proficiency suitable to provide informed consent. The exclusion criteria for participants included the presence of any lactose or soy intolerance or allergy, any cardiac, hepatic, pulmonary, renal, neurological, hematological, psychiatric, or gastrointestinal illness, the use of ergogenic aids (i.e. anabolic steroids), and participants who were taking any medications. Screening was conducted to ensure participant suitability prior to study commencement. Participant exercise readiness was obtained using a pre-existing exercise readiness questionnaire [30]. Participants were required to read a participant information sheet and were given the opportunity to discuss any concerns prior to their involvement in the study. Informed written consent was subsequently obtained.
Procedures
Visit One: 1-RM Assessment
At the initial visit, participant height (m) and body mass (kg) were recorded using a stadiometer (Seca 217 Stable Stadiometer; seca GmbH & Co. KG., Hamburg, Germany) and calibrated electronic scales (Seca 875 Flat Scale; seca GmbH & Co. KG.) under laboratory conditions. Each participant performed a warm-up of five minutes walking on a treadmill at 6 km/hour. Following this, participants performed a maximal strength assessment to determine their 1-RM for the following exercises: barbell bench press, seated barbell shoulder press, barbell squats, standing barbell bicep curls, and pull-ups. Initially, participants were asked for their anticipated 1-RM weight for each exercise. They proceeded to warm up by completing 5-10 repetitions at 50% of their anticipated 1-RM for each exercise. Following a rest period of two minutes, participants completed two to three repetitions at 70-80% of their anticipated 1-RM load. Weight was then increased gradually by approximately 5-10% until failure, with the 1-RM determined as the heaviest successful attempt out of four attempts at each exercise. Two-minute rest periods were provided between attempts. The determined 1-RM was then used to calculate the 70% workload for each participant for visits two and three.
Visits Two and Three: Acute RET and Milk Consumption
In the 48 hours prior to visits two and three, intake of dairy, soy, and their derivative products as well as alcohol and more than one cup of coffee was prohibited. Participants were asked to photograph all food and beverage intake to monitor adherence using their smartphones. Exercise in the 48 hours prior to visits two and three was also not permitted. In the 12 hours prior to visits two and three, participants were instructed to fast. Water consumption was allowed throughout the study including during fasting periods and during exercise. Outside of these time periods, participants could engage in their regular diet and exercise regimes. An accredited practicing dietitian was available to advise and answer questions related to diet throughout the entire study.
Training Sessions
All participants completed the RET sessions at the same time of day (between 0700 and 0900 hours) to control for diurnal hormonal fluctuations. On visits two and three to the laboratory, identical resistance exercise protocols were performed, which were adapted from Simao, et al. [31], the intervention that elicited the largest acute rise in free testosterone following physical exercise in a recent meta-analysis [32]. Participants warmed up by walking on a treadmill for five minutes at 6 km/hour. They proceeded to perform the same exercises completed in the 1-RM testing (barbell bench press, seated barbell shoulder press, barbell squats, standing barbell bicep curls, and pull-ups) at 70% of their 1-RM until voluntary failure. Two minutes of passive rest were provided between sets and different exercises. Pausing between the eccentric and concentric phases of each repetition was not permitted.
Nutritional Conditions: Dairy Milk and Soy Milk Consumption
Utilizing a double-blinded randomized (block) crossover design, participants underwent each treatment condition, consuming dairy milk and soy milk following RET. The dairy milk was organic, grass-fed, locally procured whole milk. The soy milk was also organic, made from whole soybeans and water. 600 mL/m2 (approximately 1100 mL total) of milk was provided, a quantity administered in previous research yielding 33-40 g protein [33]. This translated to 0.48-0.56 g/kg protein (for men weighing 59.0-83.0 kg), an amount that aligns with the recommendation to consume 0.4-0.55 g/kg protein per meal to maximize anabolism [34]. Milk was provided at +45 minutes post-RET and was consumed within 10 min to ensure intake within the “post-workout anabolic window of opportunity,” which is generally considered to be within an hour after completing RET [1].
Blood Collection and Hormone Analysis
At both RET sessions (visits two and three), 4 mL of blood was collected at each of seven time points: directly following exercise completion, and at 30, 45, 60, 80, 100, and 120 minutes following milk consumption. These time points were informed by previous research investigating dairy and soy milk intake on sex hormones and the included response [33]. Blood was collected by a trained phlebotomist and samples were held for 20-30 minutes before being centrifuged at 3500 rpm at 4 degrees Celsius for 10 minutes. Serum was then extracted from the separated blood sample and pipetted into Eppendorf tubes which were then stored at -80 degrees Celsius until analysis. Cardinal laboratory analyzed the serum for total, free testosterone, free testosterone percentage, total estrogen, progesterone, and sex hormone binding globulin (SHBG).
Statistical analyses
Analysis of the collected data was conducted using GraphPad Prism version 8.0 (Dotmatics, Boston, Massachusetts, United States) software. The Shapiro-Wilks test was used to test for normality. Paired t-tests were used to compare differences between the mean number of repetitions lifted and total mass moved between visits two and three. Two-way ANOVA analyses were applied for repeated measures (total testosterone, free testosterone, free testosterone percentage, total estrogen, progesterone, and SHBG) with milk condition and time as the two variables with Bonferroni post hoc analysis performed. The area under the curve (AUC) was determined between dairy and soy milk for each hormone. The data collected were expressed as mean and standard deviation (SD). Alpha was set at p<0.05.
Results
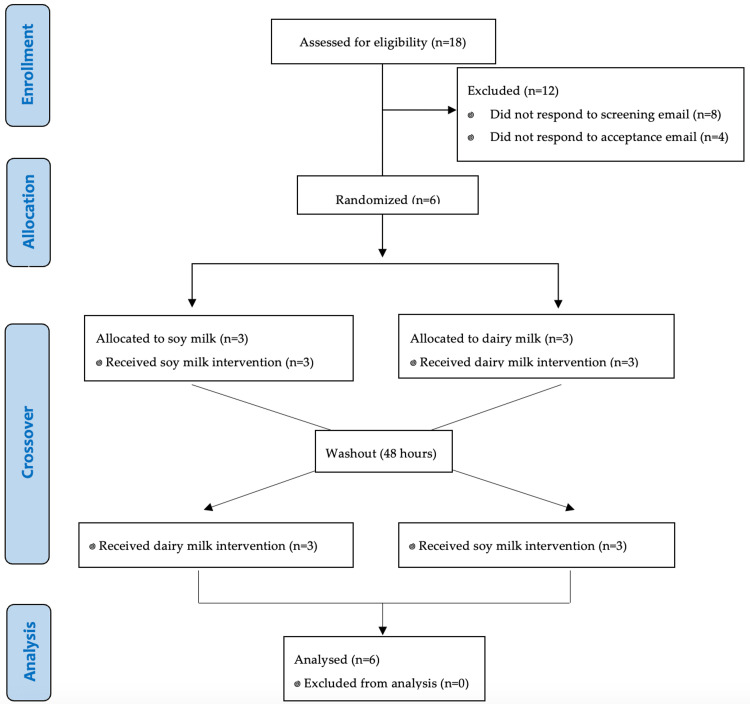
There were 18 individuals who expressed initial interest in participating in the study between November 2022 and April 2023. Of the 18 interested individuals, eight did not respond to the screening email. Among the remaining 10 eligible participants, four individuals did not respond to the acceptance email, resulting in a final enrollment of six participants who successfully completed the study (Figure 1). Participants were aged between 18 and 28 years, with a height range of 1.77-1.91 m and a body mass range of 68.1 kg to 107.4 kg (Table 1). BMI ranged from 19.9 to 29.7 kg/m2. Participants reported adherence to refraining from exercise in the 48 hours prior to visits two and three. Similarly, all participants had omitted soy and dairy in the 48 hours prior to visits two and three, which was confirmed by photographed meals reviewed by the accredited practicing dietitian.
Table 1. Participant data.
| Participants | Age (years) | Height (m) | Weight (kg) | BMI (kg/m2) | Training experience (years) |
| 1 | 28 | 1.91 | 107.4 | 29.4 | 10 |
| 2 | 21 | 1.87 | 103.7 | 29.7 | 2 |
| 3 | 24 | 1.77 | 73 | 23.3 | 3 |
| 4 | 21 | 1.79 | 72 | 22.5 | 3 |
| 5 | 18 | 1.87 | 86.7 | 24.8 | 3 |
| 6 | 18 | 1.85 | 68.1 | 19.9 | 3 |
| Mean ± standard deviation | 21.67±3.83 | 1.84±0.05 | 85.15±17.05 | 24.93±3.91 | 4±2.9 |
Figure 1. CONSORT flow diagram depicting the flow of participants through the randomized crossover trial.
CONSORT: Consolidated Standards of Reporting Trials
RET
There were no significant differences between total mass moved for participants between visits two and three for shoulder press (p=0.18), bicep curl (p=0.65), bench press (p=0.37), pull-ups (p=0.42), squats (p=0.21), or all exercised combined (p=0.94).
Sex hormones
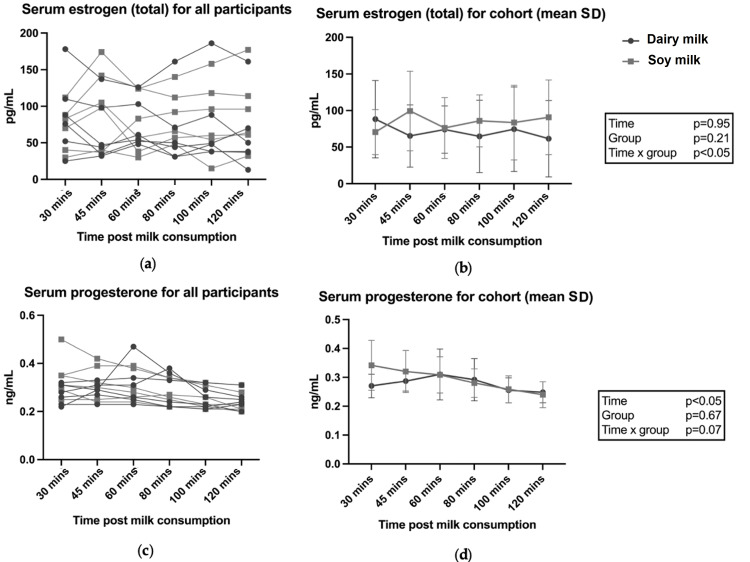
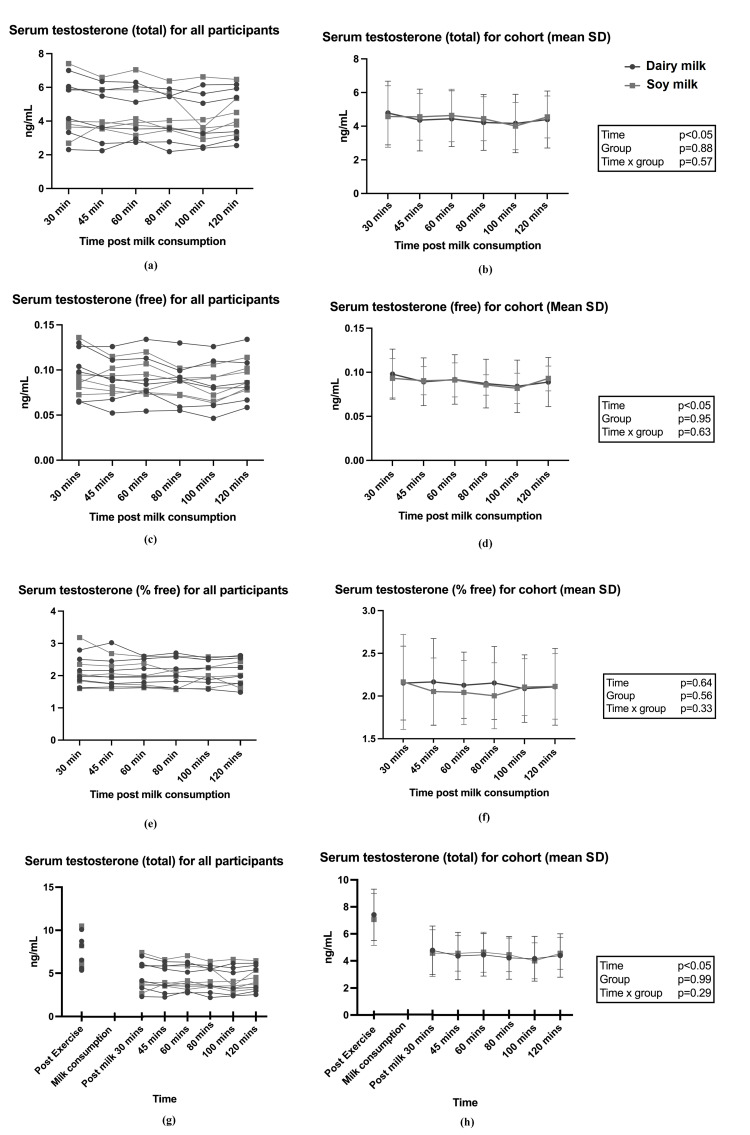
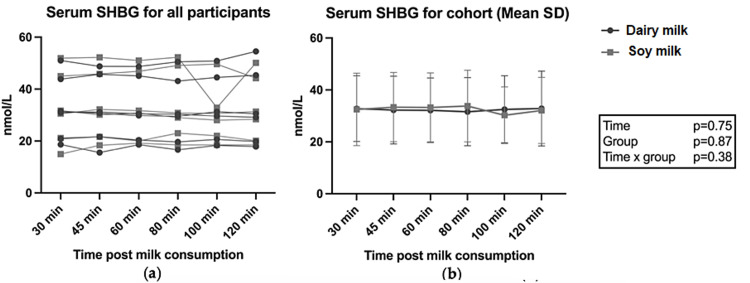
AUC for all hormones between conditions were not statistically different between the two milk groups (P>0.05; data not shown). Figure 2 shows the results for individual and group data for estrogen (total) and progesterone across milk conditions. There was a significant time x milk effect for total estrogen following soy milk consumption (p<0.05) (Figure 2b); however, the post-hoc analysis did not identify time-relevant differences between the groups. Testosterone results are shown in Figure 3. Total testosterone was elevated immediately following RET, with a significant time effect observed after both dairy milk and soy milk (p<0.05) (Figure 3h), but there were no differences between the two milk conditions. No differences were observed in SHBG following dairy and soy milk consumption (Figure 4). Descriptive statistics between milk consumption at each time point for total estrogen, free testosterone, total testosterone, free testosterone, progesterone, and SHBG can be seen in the Appendices.
Figure 2. Serum estrogen (total) and progesterone following dairy and soy milk consumption for individuals and the cohort (mean and SD).
P-values reported from two-way ANOVA analysis
Figure 3. (a-f) Testosterone (total, free, percentage free) following dairy and soy milk consumption for individuals and groups (mean and SD); (g, h) Testosterone directly after RET and following milk intake.
RET: resistance exercise training
P-values reported from two-way ANOVA analysis
Figure 4. Serum SHBG following dairy and soy milk consumption for individuals and groups (mean and SD).
SHBG: sex hormone binding globulin
P values reported from two-way ANOVA analysis
Discussion
To our knowledge, this is the first study that has compared the effects of dairy and soy milk consumption as a recovery beverage following RET on acute circulating sex hormones. Free and total testosterone levels were consistent with other studies reporting elevated serum concentrations immediately following exercise which provides context for investigating the presence of sex hormones in circulation [32]. There was an equivalence between total repetitions and total mass moved during RET for both conditions, providing a suitable model to compare levels of circulating sex hormones. The results of this pilot study suggest that there is no difference in circulating serum sex hormones following consumption of soy versus dairy milk. Given the pivotal role of testosterone in muscle protein synthesis (MPS), the finding that soy milk consumption after RET did not result in lower circulating testosterone levels compared with dairy milk is an important finding. While there was a group x time interaction effect for total estrogen, the post-hoc analysis did not identify time-relevant differences between the two milk conditions, and no differences in AUC for any hormones were observed. Overall, the findings suggest equivalence in acute circulating serum sex hormone concentrations after soy and dairy milk consumption.
Contrary to our hypothesis and other research showing dairy milk consumption can elevate circulating estrogen levels, this was not observed in the present study. It was hypothesized that dairy milk consumption following resistance exercise would produce a greater increase in circulating estrogen compared to soy milk due to the high concentrations of sex hormones typically found in commercially available dairy milk [27]. This hypothesis was also formulated with the understanding that hormones found in commercial dairy milk translate into elevated concentrations of circulating sex hormones in humans. For example, in a study involving a cohort of seven adult men and seven children, the consumption of 600 mL/m2 of whole milk resulted in significant increases in urinary levels of E1, E2, E3, and pregnanediol, measured by spot urine samples, and serum estrone and progesterone were also significantly elevated [33]. Similarly, Michels et al. examined urinary excretion of sex hormones following either skim (1.5% fat) or whole milk (3.5% fat) consumption in a crossover intervention of 109 postmenopausal women [27]. They observed that the consumption of dairy milk leads to a significant rise in E1 while ingestion of semi-skimmed milk appeared to significantly elevate E2 and E3. Furthermore, Carruba et al. conducted an interventional trial wherein 100 healthy postmenopausal women were assigned to follow either a Mediterranean diet or a Western diet, the latter being characterized by a high intake of milk and dairy products, meat, and animal fat [35]. The findings revealed that participants adhering to the Mediterranean diet experienced more than 40% reduction in urinary estrogen levels compared to those following the Western diet. However, while these studies all reported elevations in estrogens following dairy milk, they either did not have a control group or their comparator group consumed no milk, which was disparate to the present pilot study.
It was also hypothesized that testosterone would be lower following dairy milk versus soy milk consumption due to the high concentrations of progesterone typically found in commercially available dairy milk [36], which can depress serum testosterone levels [14]. A study by Maruyama et al. including seven men and seven children observed a significant reduction in serum testosterone concentration following consumption of 600ml/m2 dairy milk [33]. However, this was not observed in the present study whereby both free and total testosterone and progesterone were equivalent between milk conditions. This finding is noteworthy because testosterone is an androgenic anabolic hormone, exerting a wide range of biological effects including the stimulation of muscle growth [12]. Considering that free and total testosterone concentrations and free testosterone percentage were equivalent between groups, it is likely that the serum sex hormone profiles following consumption of both milks would produce a similar anabolic effect in response to RET.
Protein considerations between milk types
With regards to protein intake and its effect on the anabolic response, the "leucine threshold" theory suggests that the magnitude of the rise in blood leucine levels after a meal plays a crucial role in controlling the extent of the post-meal MPS response to the protein source consumed [37]. When a sufficient amount of total protein is consumed and leucine requirements are met, the type of protein may be inconsequential for anabolic and ergogenic outcomes [38]. Evidence in support of this theory can be observed in several studies comparing varying protein types (soy, whey, rice, pea), whereby providing a protein dose that sufficiently meets the "leucine threshold" results in equivalent increases in muscle mass and strength regardless of protein origin [39-42]. While there are limited studies that have directly compared the effects of dairy and soy milk on strength and MPS, two studies have demonstrated modest enhancements in hypertrophy in individuals consuming dairy milk compared to soy milk [26,43]. However, in both these studies, the leucine concentration was suboptimal (<1.8 g) with the protein administered in each study < 20 grams. When the leucine contents of dairy milk and soy milk were matched (2 g), a randomized trial including 48 participants following a 12-week RET program found that consumption of dairy milk or soy milk produced similar increases in lean body mass and strength [9]. Similarly, a recent meta-analysis conducted by Messina et al. concluded that soy protein supplementation produces similar gains in strength and lean body mass in response to RET as dairy milk and whey protein. In terms of dairy and soy milk consumption for post-exercise MPS and resultant changes in muscle mass and strength, the available evidence suggests both kinds of milk will evoke similar anabolic responses provided the leucine threshold is met [8].
Health
There are concerns that soy consumption is associated with an increased risk of breast cancer in women and "feminization" in males [10]; however, the literature does not support these notions. Indeed, in an umbrella review including 114 meta-analyses and systematic reviews examining multiple health outcomes, Li et al. identified beneficial associations for cancers, cardiovascular disease, and gynecological, metabolic, musculoskeletal, endocrine, neurological, and renal outcomes with soy and isoflavone consumption [44]. Considering the positive health associations observed with soy consumption, along with the similarities in protein content and influence on circulating sex hormones between milks, and probable equivalence in facilitating MPS between dairy and soy milk, soy milk consumption should be considered as a viable alternative to dairy milk as a recovery beverage.
Limitations
While this pilot study provides novel insights into the effects of soy and dairy milk on circulating sex hormones, it is not without limitations. The sample size was small, limiting statistical power to detect differences between milk conditions, and only included men in the age group of 18-30 years, thereby limiting the applicability to women as well as younger and older individuals. Further, the concentrations of sex hormones within the milk provisions were unknown. Assumptions were made relying on published data describing the concentrations of sex hormones within these milks, often obtained from countries outside of Australia, where the milk was sourced for this study. An analysis of the sex hormone constituents within the two types of milk would therefore have strengthened this study. Lastly, without a control group consuming neither soy or dairy milk (e.g. water), it is difficult to isolate the influence of either milk on circulating sex hormones. A third arm consisting of a control group should be considered for future studies.
Conclusions
This pilot study, the first to investigate the influence of dairy versus soy milk consumption as a post-exercise recovery beverage on circulating sex hormones following RET, revealed no significant differences in circulating serum sex hormones between the two milk consumption conditions. Soy milk consumption did not result in lower circulating testosterone or higher circulating total estrogen than dairy milk consumption. These findings are important since they imply that both dairy milk and soy milk can be considered equally in terms of their effects on sex hormones, with both types of milk providing viable options for promoting MPS and muscle hypertrophy in the context of post-exercise recovery. These results hold relevance for recreational exercisers and elite athletes pursuing increased muscle mass and strength, as they suggest that the choice between dairy milk and soy milk as a recovery beverage is unlikely to meaningfully impact circulating sex hormones. However, given the pilot nature of this study, further research with larger sample sizes is needed to corroborate and expand upon these preliminary findings.
Acknowledgments
The authors sincerely thank all participants for their involvement with completing this study.
Appendices
Table 2. Descriptive statistics between milk consumption at each time point for total estrogen, free testosterone, total testosterone, free testosterone, progesterone, and SHBG.
SHBG: sex hormone binding globulin
| Dairy Milk | Soy Milk | |||||||||||||
| Mean | SD | Min | Max | Mean | SD | Min | Max | |||||||
| Total Estrogen (pg/mL) | Time 0 minutes | 87 | 43 | 34 | 161 | 94 | 42 | 42 | 166 | |||||
| Time 30 minutes | 88 | 53 | 25 | 178 | 71 | 31 | 30 | 112 | ||||||
| Time 45 minutes | 65 | 43 | 32 | 137 | 99 | 54 | 37 | 174 | ||||||
| Time 60 minutes | 74 | 32 | 48 | 126 | 76 | 42 | 30 | 125 | ||||||
| Time 80 minutes | 65 | 49 | 48 | 140 | 86 | 36 | 48 | 140 | ||||||
| Time 100 minutes | 75 | 58 | 38 | 186 | 84 | 51 | 15 | 158 | ||||||
| Time 120 minutes | 62 | 52 | 13 | 161 | 91 | 51 | 32 | 177 | ||||||
| Free testosterone (ng/mL) | Time 0 minutes | 0.15 | 0.033 | 0.11 | 0.19 | 0.15 | 0.024 | 0.12 | 0.19 | |||||
| Time 30 minutes | 0.098 | 0.028 | 0.064 | 0.13 | 0.093 | 0.022 | 0.073 | 0.14 | ||||||
| Time 45 minutes | 0.089 | 0.027 | 0.052 | 0.13 | 0.09 | 0.016 | 0.074 | 0.12 | ||||||
| Time 60 minutes | 0.092 | 0.028 | 0.054 | 0.13 | 0.091 | 0.019 | 0.073 | 0.12 | ||||||
| Time 80 minutes | 0.087 | 0.028 | 0.055 | 0.13 | 0.086 | 0.012 | 0.072 | 0.1 | ||||||
| Time 100 minutes | 0.084 | 0.03 | 0.046 | 0.13 | 0.082 | 0.017 | 0.063 | 0.11 | ||||||
| Time 120 minutes | 0.089 | 0.028 | 0.059 | 0.13 | 0.093 | 0.014 | 0.078 | 0.11 | ||||||
| Total testosterone (ng/mL) | Time 0 minutes | 7.4 | 1.9 | 5.3 | 10 | 7.1 | 1.9 | 5.6 | 11 | |||||
| Time 30 minutes | 4.8 | 1.8 | 2.3 | 7 | 4.6 | 1.7 | 2.7 | 7.4 | ||||||
| Time 45 minutes | 4.4 | 1.8 | 2.2 | 6.4 | 4.6 | 1.3 | 3.5 | 6.6 | ||||||
| Time 60 minutes | 4.4 | 1.6 | 2.7 | 6.3 | 4.6 | 1.5 | 3.2 | 7 | ||||||
| Time 80 minutes | 4.2 | 1.6 | 2.2 | 5.9 | 4.4 | 1.2 | 3.5 | 6.4 | ||||||
| Time 100 minutes | 4.2 | 1.7 | 2.4 | 6.2 | 4 | 1.3 | 2.9 | 6.6 | ||||||
| Time 120 minutes | 4.4 | 1.6 | 2.6 | 6.2 | 4.6 | 1.2 | 3.2 | 6.5 | ||||||
| Free Testosterone (%) | Time 0 minutes | 2.1 | 0.42 | 1.5 | 2.7 | 2.1 | 0.43 | 1.6 | 2.7 | |||||
| Time 30 minutes | 2.2 | 0.43 | 1.6 | 2.8 | 2.2 | 0.56 | 1.6 | 3.2 | ||||||
| Time 45 minutes | 2.2 | 0.51 | 1.7 | 3 | 2.1 | 0.39 | 1.6 | 2.7 | ||||||
| Time 60 minutes | 2.1 | 0.39 | 1.7 | 2.6 | 2 | 0.38 | 1.6 | 2.6 | ||||||
| Time 80 minutes | 2.2 | 0.43 | 1.6 | 2.7 | 2 | 0.39 | 1.6 | 2.6 | ||||||
| Time 100 minutes | 2.1 | 0.39 | 1.6 | 2.5 | 2.1 | 0.33 | 1.6 | 2.6 | ||||||
| Time 120 minutes | 2.1 | 0.45 | 1.5 | 2.6 | 2.1 | 0.38 | 1.6 | 2.6 | ||||||
| Progesterone (ng/mL) | Time 0 minutes | 0.35 | 0.064 | 0.24 | 0.41 | 0.35 | 0.1 | 0.25 | 0.51 | |||||
| Time 30 minutes | 0.27 | 0.041 | 0.22 | 0.32 | 0.34 | 0.086 | 0.25 | 0.5 | ||||||
| Time 45 minutes | 0.29 | 0.034 | 0.23 | 0.33 | 0.32 | 0.073 | 0.24 | 0.42 | ||||||
| Time 60 minutes | 0.31 | 0.088 | 0.23 | 0.47 | 0.31 | 0.063 | 0.24 | 0.39 | ||||||
| Time 80 minutes | 0.29 | 0.073 | 0.22 | 0.38 | 0.22 | 0.049 | 0.22 | 0.34 | ||||||
| Time 100 minutes | 0.26 | 0.043 | 0.21 | 0.32 | 0.26 | 0.047 | 0.21 | 0.32 | ||||||
| Time 120 minutes | 0.25 | 0.037 | 0.2 | 0.31 | 0.24 | 0.045 | 0.2 | 0.31 | ||||||
| SHBG (nmol/L) | Time 0 minutes | 35 | 15 | 19 | 59 | 35 | 16 | 19 | 56 | |||||
| Time 30 minutes | 33 | 13 | 19 | 51 | 33 | 14 | 15 | 52 | ||||||
| Time 45 minutes | 32 | 13 | 16 | 49 | 33 | 13 | 18 | 52 | ||||||
| Time 60 minutes | 32 | 12 | 19 | 49 | 33 | 13 | 19 | 51 | ||||||
| Time 80 minutes | 32 | 13 | 17 | 51 | 34 | 14 | 19 | 52 | ||||||
| Time 100 minutes | 33 | 13 | 18 | 51 | 30 | 11 | 19 | 50 | ||||||
| Time 120 minutes | 33 | 14 | 18 | 55 | 32 | 13 | 19 | 50 | ||||||
The authors have declared financial relationships, which are detailed in the next section.
David M Goldman declare(s) personal fees from Metabite, Inc. David M. Goldman consults for Metabite, Inc.
Funding Statement
This work was supported by funding from ‘Switch4good Inc.’, a Registered 501(c) 3 non-profit corporation with the United States IRS. EIN # 81-3396276. The funder ‘Switch4Good’ was not involved with any part of the data collection, data analysis, interpretation, or production of this research
Author Contributions
Concept and design: David M. Goldman, Joel C. Craddock, Amelia Wakefield, Gregory E. Peoples, Theresa A. Larkin
Drafting of the manuscript: David M. Goldman, Joel C. Craddock, Amelia Wakefield
Critical review of the manuscript for important intellectual content: David M. Goldman, Joel C. Craddock, Amelia Wakefield, Gregory E. Peoples, Theresa A. Larkin
Acquisition, analysis, or interpretation of data: Joel C. Craddock, Amelia Wakefield, Gregory E. Peoples, Theresa A. Larkin
Supervision: Joel C. Craddock, Gregory E. Peoples, Theresa A. Larkin
Human Ethics
Consent was obtained or waived by all participants in this study. University of Wollongong Human Research Ethics Research Committee issued approval 2022/211
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Is there a postworkout anabolic window of opportunity for nutrient consumption? Clearing up controversies. Schoenfeld BJ, Aragon AA. J Orthop Sports Phys Ther. 2018;48:911–914. doi: 10.2519/jospt.2018.0615. [DOI] [PubMed] [Google Scholar]
- 2.Cow's milk as a post-exercise recovery drink: implications for performance and health. James LJ, Stevenson EJ, Rumbold PL, Hulston CJ. Eur J Sport Sci. 2019;19:40–48. doi: 10.1080/17461391.2018.1534989. [DOI] [PubMed] [Google Scholar]
- 3.The prevalence of food allergy: a meta-analysis. Rona RJ, Keil T, Summers C, et al. J Allergy Clin Immunol. 2007;120:638–646. doi: 10.1016/j.jaci.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 4.Country, regional, and global estimates for lactose malabsorption in adults: a systematic review and meta-analysis. Storhaug CL, Fosse SK, Fadnes LT. Lancet Gastroenterol Hepatol. 2017;2:738–746. doi: 10.1016/S2468-1253(17)30154-1. [DOI] [PubMed] [Google Scholar]
- 5.Consumption of milk and dairy products: facts and figures. Zingone F, Bucci C, Iovino P, Ciacci C. Nutrition. 2017;33:322–325. doi: 10.1016/j.nut.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 6.Soy milk consumption in the United States of America: an NHANES data report. Storz MA, Brommer M, Lombardo M, Rizzo G. Nutrients. 2023;15:2532. doi: 10.3390/nu15112532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Comparison of nutritional composition between plant-based drinks and cow's milk. Walther B, Guggisberg D, Badertscher R, et al. Front Nutr. 2022;9:988707. doi: 10.3389/fnut.2022.988707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.No difference between the effects of supplementing with soy protein versus animal protein on gains in muscle mass and strength in response to resistance exercise. Messina M, Lynch H, Dickinson JM, Reed KE. Int J Sport Nutr Exerc Metab. 2018;28:674–685. doi: 10.1123/ijsnem.2018-0071. [DOI] [PubMed] [Google Scholar]
- 9.No significant differences in muscle growth and strength development when consuming soy and whey protein supplements matched for leucine following a 12 week resistance training program in men and women: a randomized trial. Lynch HM, Buman MP, Dickinson JM, Ransdell LB, Johnston CS, Wharton CM. Int J Environ Res Public Health. 2020;17:3871. doi: 10.3390/ijerph17113871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soy foods and supplementation: a review of commonly perceived health benefits and risks. D'Adamo CR, Sahin A. https://pubmed.ncbi.nlm.nih.gov/24473985/ Altern Ther Health Med. 2014;20 Suppl 1:39–51. [PubMed] [Google Scholar]
- 11.Cellular and molecular mechanisms responsible for the action of testosterone on human skeletal muscle. A basis for illegal performance enhancement. Kadi F. Br J Pharmacol. 2008;154:522–528. doi: 10.1038/bjp.2008.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Exercise-induced hormone elevations are related to muscle growth. Mangine GT, Hoffman JR, Gonzalez AM, et al. J Strength Cond Res. 2017;31:45–53. doi: 10.1519/JSC.0000000000001491. [DOI] [PubMed] [Google Scholar]
- 13.Suppression of endogenous testosterone production attenuates the response to strength training: a randomized, placebo-controlled, and blinded intervention study. Kvorning T, Andersen M, Brixen K, Madsen K. Am J Physiol Endocrinol Metab. 2006;291:0–32. doi: 10.1152/ajpendo.00143.2006. [DOI] [PubMed] [Google Scholar]
- 14.Effects of testosterone replacement and/or resistance exercise on the composition of megestrol acetate stimulated weight gain in elderly men: a randomized controlled trial. Lambert CP, Sullivan DH, Freeling SA, Lindquist DM, Evans WJ. J Clin Endocrinol Metab. 2002;87:2100–2106. doi: 10.1210/jcem.87.5.8505. [DOI] [PubMed] [Google Scholar]
- 15.Body-composition changes in patients who gain weight while receiving megestrol acetate. Loprinzi CL, Schaid DJ, Dose AM, Burnham NL, Jensen MD. J Clin Oncol. 1993;11:152–154. doi: 10.1200/JCO.1993.11.1.152. [DOI] [PubMed] [Google Scholar]
- 16.Effect of estrogen on musculoskeletal performance and injury risk. Chidi-Ogbolu N, Baar K. Front Physiol. 2018;9:1834. doi: 10.3389/fphys.2018.01834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Endogenous anabolic hormone responses to endurance versus resistance exercise and training in women. Consitt LA, Copeland JL, Tremblay MS. Sports Med. 2002;32:1–22. doi: 10.2165/00007256-200232010-00001. [DOI] [PubMed] [Google Scholar]
- 18.Hormonal responses to resistance exercise during different menstrual cycle states. Nakamura Y, Aizawa K, Imai T, Kono I, Mesaki N. Med Sci Sports Exerc. 2011;43:967–973. doi: 10.1249/MSS.0b013e3182019774. [DOI] [PubMed] [Google Scholar]
- 19.Growth hormone(s), testosterone, insulin-like growth factors, and cortisol: roles and integration for cellular development and growth with exercise. Kraemer WJ, Ratamess NA, Hymer WC, Nindl BC, Fragala MS. Front Endocrinol (Lausanne) 2020;11:33. doi: 10.3389/fendo.2020.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The biochemistry, chemistry and physiology of the isoflavones in soybeans and their food products. Barnes S. Lymphat Res Biol. 2010;8:89–98. doi: 10.1089/lrb.2009.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soy and health update: evaluation of the clinical and epidemiologic literature. Messina M. Nutrients. 2016;8:754. doi: 10.3390/nu8120754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isoflavones: estrogenic activity, biological effect and bioavailability. Vitale DC, Piazza C, Melilli B, Drago F, Salomone S. Eur J Drug Metab Pharmacokinet. 2013;38:15–25. doi: 10.1007/s13318-012-0112-y. [DOI] [PubMed] [Google Scholar]
- 23.Reprint of The nature and utility of the phytoestrogens: a review of the evidence. Albertazzi P, Purdie DW. Maturitas. 2008;61:214–226. doi: 10.1016/j.maturitas.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 24.Interaction of phytoestrogens with estrogen receptors alpha and beta. Morito K, Hirose T, Kinjo J, et al. Biol Pharm Bull. 2001;24:351–356. doi: 10.1248/bpb.24.351. [DOI] [PubMed] [Google Scholar]
- 25.An unusual case of gynecomastia associated with soy product consumption. Martinez J, Lewi JE. Endocr Pract. 2008;14:415–418. doi: 10.4158/EP.14.4.415. [DOI] [PubMed] [Google Scholar]
- 26.Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Hartman JW, Tang JE, Wilkinson SB, Tarnopolsky MA, Lawrence RL, Fullerton AV, Phillips SM. Am J Clin Nutr. 2007;86:373–381. doi: 10.1093/ajcn/86.2.373. [DOI] [PubMed] [Google Scholar]
- 27.Urinary excretion of sex steroid hormone metabolites after consumption of cow milk: a randomized crossover intervention trial. Michels KB, Binder N, Courant F, Franke AA, Osterhues A. Am J Clin Nutr. 2019;109:402–410. doi: 10.1093/ajcn/nqy279. [DOI] [PubMed] [Google Scholar]
- 28.The possible role of female sex hormones in milk from pregnant cows in the development of breast, ovarian and corpus uteri cancers. Ganmaa D, Sato A. Med Hypotheses. 2005;65:1028–1037. doi: 10.1016/j.mehy.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 29.Naturally occurring estrogens in processed milk and in raw milk (from gestated cows) Malekinejad H, Scherpenisse P, Bergwerff AA. J Agric Food Chem. 2006;54:9785–9791. doi: 10.1021/jf061972e. [DOI] [PubMed] [Google Scholar]
- 30.Norton K, Norton L. Pre-Exercise Screening: Guide to the Australian Adult Pre-Exercise Screening System. Canberra: Exercise and Sports Science Australia / Fitness Australia /Sports Medicine Australia; 2011. Pre-exercise screening: Guide to the Australian adult pre-exercise screening system exercise and sports science Australia. [Google Scholar]
- 31.Influence of upper-body exercise order on hormonal responses in trained men. Simão R, Leite RD, Speretta GF, et al. Appl Physiol Nutr Metab. 2013;38:177–181. doi: 10.1139/apnm-2012-0040. [DOI] [PubMed] [Google Scholar]
- 32.Endogenous transient doping: physical exercise acutely increases testosterone levels-results from a meta-analysis. D'Andrea S, Spaggiari G, Barbonetti A, Santi D. J Endocrinol Invest. 2020;43:1349–1371. doi: 10.1007/s40618-020-01251-3. [DOI] [PubMed] [Google Scholar]
- 33.Exposure to exogenous estrogen through intake of commercial milk produced from pregnant cows. Maruyama K, Oshima T, Ohyama K. Pediatr Int. 2010;52:33–38. doi: 10.1111/j.1442-200X.2009.02890.x. [DOI] [PubMed] [Google Scholar]
- 34.How much protein can the body use in a single meal for muscle-building? Implications for daily protein distribution. Schoenfeld BJ, Aragon AA. J Int Soc Sports Nutr. 2018;15:10. doi: 10.1186/s12970-018-0215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.A traditional Mediterranean diet decreases endogenous estrogens in healthy postmenopausal women. Carruba G, Granata OM, Pala V, et al. Nutr Cancer. 2006;56:253–259. doi: 10.1207/s15327914nc5602_18. [DOI] [PubMed] [Google Scholar]
- 36.Naturally produced steroid hormones and their release into the environment. Shore LS, Shemesh M. http://publications.iupac.org/pac/2003/pdf/7511x1859.pdf Pure Appl Chem. 2003;75:1859–1871. [Google Scholar]
- 37.Nutrient interaction for optimal protein anabolism in resistance exercise. Breen L, Phillips SM. Curr Opin Clin Nutr Metab Care. 2012;15:226–232. doi: 10.1097/MCO.0b013e3283516850. [DOI] [PubMed] [Google Scholar]
- 38.Role of ingested amino acids and protein in the promotion of resistance exercise-induced muscle protein anabolism. Reidy PT, Rasmussen BB. J Nutr. 2016;146:155–183. doi: 10.3945/jn.114.203208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Effect of whey and soy protein supplementation combined with resistance training in young adults. Candow DG, Burke NC, Smith-Palmer T, Burke DG. Int J Sport Nutr Exerc Metab. 2006;16:233–244. doi: 10.1123/ijsnem.16.3.233. [DOI] [PubMed] [Google Scholar]
- 40.The effects of 8 weeks of whey or rice protein supplementation on body composition and exercise performance. Joy JM, Lowery RP, Wilson JM, et al. Nutr J. 2013;12:86. doi: 10.1186/1475-2891-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Evaluating the leucine trigger hypothesis to explain the post-prandial regulation of muscle protein synthesis in young and older adults: a systematic review. Zaromskyte G, Prokopidis K, Ioannidis T, Tipton KD, Witard OC. Front Nutr. 2021;8:685165. doi: 10.3389/fnut.2021.685165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pea proteins oral supplementation promotes muscle thickness gains during resistance training: a double-blind, randomized, placebo-controlled clinical trial vs. whey protein. Babault N, Païzis C, Deley G, Guérin-Deremaux L, Saniez MH, Lefranc-Millot C, Allaert FA. J Int Soc Sports Nutr. 2015;12:3. doi: 10.1186/s12970-014-0064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Wilkinson SB, Tarnopolsky MA, Macdonald MJ, Macdonald JR, Armstrong D, Phillips SM. Am J Clin Nutr. 2007;85:1031–1040. doi: 10.1093/ajcn/85.4.1031. [DOI] [PubMed] [Google Scholar]
- 44.Soy and isoflavone consumption and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomized trials in humans. Li N, Wu X, Zhuang W, et al. Mol Nutr Food Res. 2020;64:0. doi: 10.1002/mnfr.201900751. [DOI] [PubMed] [Google Scholar]