Abstract
Human immunodeficiency virus type 1 (HIV-1) growth in lymphocyte cultures was increased when the virus inoculum was incubated in breast milk. The enhancing effect of milk was abolished by anti-cathepsin D antibody or by pepstatin A, a cathepsin D inhibitor. The cathepsin D-producing CD4-negative MCF7 mammary cells supported the growth of some HIV-1 isolates. An MCF7 line chronically producing HIV-1 IIIb was obtained. Cathepsin D may induce conformational modification of viral gp120, allowing direct interaction with a coreceptor. We demonstrated the presence of CXCR4 mRNA in MCF7 cells.
The cell source of prolonged production of human immunodeficiency virus type 1 (HIV-1) in breast milk (8, 11, 21–23, 26, 27, 29) is not yet clear, since lymphocyte counts in milk decline rapidly after delivery (15). The risk of mother-to-child transmission through breast-feeding has been reemphasized recently, when discontinuation of antiretroviral therapy was followed by an increase in HIV-1 RNA load (10, 13). Therapy withdrawal in a mother after childbirth might also increase viral load in milk, although a short course of oral zidovudine during the peripartum period provided an important protection of the child despite breast-feeding (7).
We have previously shown (12) that HIV-1 variants incubated in vaginal wash samples showed increased infectivity for lymphocyte cultures and acquired the ability to grow in a CD4-negative epithelial cell line obtained from a cervical epidermoid carcinoma. This effect was attributed to cathepsin D, which we purified from vaginal secretions. This protease might react with the viral gp120 and modify affinities for coreceptors.
In the present work, we found that human breast milk also enhanced the infectivity of some HIV-1 variants. Two experiments showed that the enhancing factor was susceptible to cathepsin D inhibitors.
Enhancing effect of milk is destroyed by inhibitors of cathepsin D.
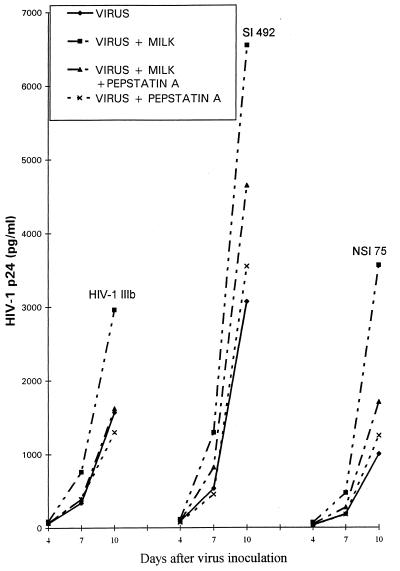
To demonstrate the effect of pepstatin A, an experiment was run in two steps. Milk sample 2 or buffer was first preincubated for 10 min at 37°C with 20 μg of pepstatin A per ml or with buffer, and these mixtures were further incubated with HIV-1 variants before inoculation into primary lymphocyte cultures. Milk preincubated with pepstatin A lost its enhancing effect on HIV-1 IIIb, but enhancement was not completely lost when the same milk sample was assayed on fresh syncytium-inducing isolates (SI), 492 isolates, or non-syncytium-inducing isolates (NSI), 75 isolates (Fig. 1). Chymotrypsin and trypsin inhibitors did not modify the enhancing effect (not shown). A similar experimental protocol was applied to study the effect of a polyclonal anti-cathepsin D antibody on enhancing properties of milk, vaginal wash, or cathepsin D (Table 1). Enhancement of SI 121 and NSI 114 isolates was decreased by treatment with the anti-cathepsin D antibody, although it was not completely abolished in some of the mixtures. Table 2 summarizes data obtained in various experiments and shows that the enhancement effect of milk sample 2 on the three isolates simultaneously tested was greater than that of milk sample 1, although this difference did not appear in the assay of the laboratory strain, HIV-1 IIIb. A survey of the whole table does not disclose a general trend distinguishing a difference between SI and NSI isolates regarding their susceptibility to milk enhancement. This table also shows the effect of various concentrations of milk sample 2. It was striking that the final dilution of 1:2 showed a lower enhancing effect than did the dilution of 1:10. At higher concentrations, there may be a balance with HIV-1 inhibition caused by lactoferrin (16). In women with a higher risk of vertical transmission of HIV to child, the serum lactoferrin concentration is decreased (9, 25). In these cases, the enhancing effect of the protease might dominate.
FIG. 1.
Inhibitory effect of pepstatin A on milk enhancement. As described in the text, milk sample 2 (diluted 1:10) or buffer was first incubated with pepstatin A or buffer, followed by a 2-h incubation with 1,000 pg of HIV-1 IIIb per ml or an SI 492 or NSI 75 isolate, and then by an inoculation into lymphocyte cultures. The mean level of HIV-1 p24 produced in an experiment in duplicate, with 5 to 10% variability, is shown.
TABLE 1.
Effect of anti-cathepsin D antibody on HIV-1 enhancementa
| HIV strain | Medium | Enhancement effect of:
|
|
|---|---|---|---|
| Buffer | Anti-cathepsin D antibody | ||
| HIV-1 IIIb | Vaginal fluid | 1.6 | 1.1 |
| Cathepsin D | 1.8 | 1 | |
| Milk | 1.4 | 1 | |
| SI 121 | Vaginal fluid | 2.6 | 1.4 |
| Cathepsin D | 1.5 | 1 | |
| Milk | 1.6 | 0.8 | |
| NSI 114 | Vaginal fluid | 2.5 | 1.7 |
| Cathepsin D | 2.1 | 1.5 | |
| Milk | 1.8 | 0.7 | |
Vaginal fluid diluted 1:10, milk diluted 1:10, and cathepsin D at 2 mg/ml were incubated for 10 min at 37°C with polyclonal anti-cathepsin D antibody (Biogenesis) diluted 1:500 or buffer. The mixtures were further incubated for 10 min with the three virus strains and then inoculated into lymphocyte cultures. After 10 days, levels of HIV p24 in the supernatant were measured, and enhancement was estimated by the ratio of results for treated viruses to nontreated viruses.
TABLE 2.
Summary of HIV-1 enhancement by two milk samples
HIV-1 growth in MCF7 mammary cancer cells.
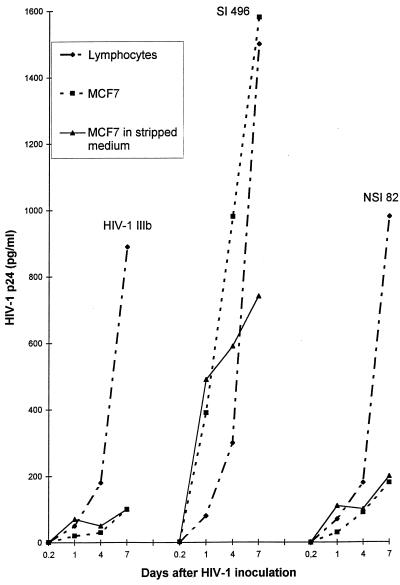
The SI 496 isolate grew in cathepsin D-producing MCF7 cells to an extent similar to that found in lymphocyte cultures not treated with the protease (Fig. 2). Virus growth in MCF7 cells decreased when the culture was grown in stripped medium deprived of steroid from fetal calf serum by treatment with dextran-coated charcoal. We verified that cell growth was not slowed down in this medium. Since estrogens have been shown to induce cathepsin D synthesis (14), our data indicate that replication of the SI 496 isolate in the CD4-negative MCF7 cells may depend on cathepsin D production. Curiously, however, virus infection in stripped medium resulted in an early burst of p24 production, followed by a decrease in production, an observation which will be discussed. In contrast to the SI 496 variant, HIV-1 IIIb and the NSI 82 isolate grew very poorly in MCF7 cells. In a second experiment (not shown), two of three isolates grew in MCF7 cells.
FIG. 2.
Compared susceptibility of lymphocytes and MCF7 cells to infection of 106 cells by HIV-1 IIIb, an SI 496 isolate, or an NSI 82 isolate. Viruses were also inoculated into MCF7 cells which had been grown for 72 h in stripped medium devoid of estrogens. The cells remained in this medium after viral infection. The mean of an experiment in duplicate, with 3 to 10% variability, is shown.
Expression of HIV-1 mRNA in MCF7 cells.
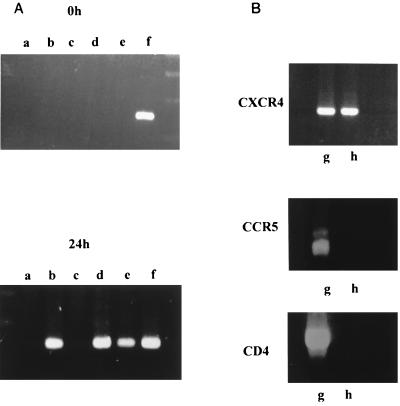
To ensure that HIV-1 p24 released in the culture supernatant resulted from de novo HIV-1 replication, we looked for intracellular HIV-1 mRNA (Fig. 3). Total mRNA was isolated from 106 cells with the Microfast Track kit (Invitrogen), and then reverse transcription was performed with a Subscript II kit (Gibco BRL). PCR on cDNA used the HIV-1 gp120-specific primers 5′-GAA TCT GTA GAA ATT AAT TGT ACA AGA CCC-3′ and 5′-TTT TGC TCT ACT AAT GTT ACA ATG TGC TTG-3′, according to Jones et al. (17). After a preliminary denaturing step at 94°C for 3 min, a series of 35 cycles performed denaturation at 94°C for 1 min, annealing at 63°C for 1 min, and extension at 72°C for 1.5 min. A final cycle lasted for 10 min at 72°C. PCR products run in a 2% agarose gel revealed a 141-bp DNA fragment. The sequence of this band showed homology with HIV sequences collected from data bank BIGBEN (accession no. AC U00367). Fig. 3A shows that viral mRNA was recovered when HIV-1 IIIb was inoculated into MCF7 cells grown in their cathepsin D-containing medium, but not when the virus was incubated with cells washed in phosphate-buffered saline (PBS) (lanes b and c). Infection of these washed cells did occur when the virus had been preincubated with 2 μg of cathepsin D per ml or with vaginal wash sample 22 (lanes d and e). Lane f shows the expression of HIV-1 mRNA in an MCF7 cell line which had been passed several times after viral infection and then frozen and subcultured again, indicating that a chronically infected cell line had been obtained.
FIG. 3.
(A) Conditions determining whether HIV-1 mRNA was expressed in MCF7 cells. Cultures were grown for 2 days, reached 80% confluence, and were incubated at 37°C with 1 ml of conditioned medium alone (lane a) or containing 1,000 pg of HIV-1 IIIb p24 (lane b). Three other cultures were incubated in estrogen-free medium plus HIV-1 IIIb pretreated for 10 min with either buffer (lane c), 2 μg of cathepsin D per ml (lane d), or vaginal wash sample 22 (lane e). After a 2-h incubation, cells were washed three times with phosphate-buffered saline. Samples of 106 cells were immediately harvested (time, 0 h) while the remaining cultures were grown for 24 h at 37°C and then collected with EDTA and centrifuged (time, 24 h). Cell pellets were treated with lysing buffer, and total mRNA was extracted and reverse transcribed. With the cDNA, a specific amplification of gp120 HIV-1 sequences was performed with primers described in the text. PCR products were run in a 2% agarose gel. MCF7 cells subcultured for several passages after HIV-1 inoculation were also tested (lane f). (B) Assay for receptor mRNA specific for CXCR4, CCR5, and CD4 in PBL (lane g) and in MCF7 cells (lane h).
Culture samples (conditions were as those for lanes b to e, described above) collected just after virus absorption (time 0) did not show any HIV-1 mRNA expression. Moreover, purified mRNA treated with RNase before reverse transcriptase PCR (RT-PCR) did not show any band on agarose gel (data not shown). These results demonstrate the absence of a significant amount of residual cell DNA and HIV genomic RNA.
Receptor expression in MCF7 cells.
We first assayed whether HIV-1 infection of MCF7 cells could be blocked by anti-CD4 antibody. Cell monolayers at 80% confluency were grown for 24 h in the presence of 1 μg of OKT4a monoclonal antibody per ml and then were infected with HIV-1 IIIb or an SI 496 isolate in the presence of the same amount of antibody. This treatment did not modify virus growth, although our previous data showed that it did abolish virus replication in CD4-positive CEM cells (12). We then explored the nature of the coreceptor that HIV-1 isolates might use to penetrate MCF7 cells. Aliquots of total mRNA from peripheral blood lymphocytes (PBL) and from MCF7 cells were reverse transcribed and cDNA sequences were amplified by PCR with primers specific for CD4, CCR5, and CXCR4, according to a technique modified from Raport et al. (24) and previously described (12). cDNA fragments specific for the three receptors were found in PBL, while only a CXCR4 fragment was detected in MCF7 cells (Fig. 3B).
Finally, since galactosylceramides have been shown to function as receptors in some CD4-negative mucosal epithelial cell lines (33), we treated MCF7 cells with a monoclonal anti-galactosylceramide antibody, which did not modify HIV growth (data not shown).
The present data, together with those obtained with vaginal secretions, indicate that niches in the organism other than blood may influence the local HIV-1 population, not only by selection of genetic variants (34) but also by phenotypic modifications. The importance of the cleavage of influenza virus hemagglutinin by respiratory proteases is well documented (1).
The implication of cathepsin D in the enhancing effect of milk was inferred here by the inhibition of the effect by anti-cathepsin D antibody and by pepstatin A, but we could only demonstrate the presence of procathepsin D in human milk, by affinity chromatography on pepstatin A agarose (data not shown), which is in agreement with published data (30). In the bovine milk, procathepsin D was the major form present and showed protease activity (20). Regarding the cell source of the protease in milk, in vitro cathepsin D production does not occur in stationary noncancerous mammary epithelial cells (3), but it might be induced in vivo in cells of lactating glands.
It has been shown (28) that some cultures of human mammary epithelial cells support the growth of HIV-1, but factors involved in permissiveness were not disclosed. We have identified cathepsin D as one of these factors. The finding that the cathepsin D-producing MCF7 cells could continuously replicate HIV-1 raises the possibility that proliferating cells from the lactating gland may be a continuous source of HIV-1 in milk.
When MCF7 cells cultured in steroid-free medium ceased to produce cathepsin D, the arrest of virus growth was preceded by a brief burst of HIV-1 p24 production. This burst may have been due to the fact that in the absence of estrogens, synthesis of the cathepsin D receptor is reduced (2, 5, 31), and thus cathepsin D may transiently persist in the free form.
In the mammary epithelial cell line studied here, the receptor used by HIV-1 isolates could be CXCR4. This would imply that MCF7 cells might preferentially replicate lymphotropic or dualtropic isolates. There are no data characterizing isolates from breast milk in the literature. We found that three of five SI isolates obtained from blood replicated in MCF7 while one NSI isolate did not. Although HIV-1 IIIb produced only low levels of p24 in the medium, viral mRNA was detected 24 h after infection.
We present here the hypothesis that cathepsin D interaction with HIV-1 gp120 may induce a conformational change similar to that caused by CD4 (18) that allows subsequent interaction with coreceptor molecules. On the virion, it has been shown that the binding site to the second receptor is masked by a V3 loop and V1/V2 stem (32). Rearrangement during gp120-CD4 interaction appears to expose the binding site to coreceptors. On the other hand, it has been shown that the V3 loop of HIV-1 is cleaved by cathepsin E, which shares cleavage sites with cathepsin D (4). Such a cleavage might produce deployment similar to that in a gp120-CD4 complex.
Viral envelope-CD4 coreceptor structures are potentially good immunogens, which may lead to vaccines mimicking the natural in vivo presentation of the viral gp120 (19). It would be interesting to test whether envelope-cathepsin D-coreceptor structures could represent similar immunogens.
Finally, it should be stressed that our data suggest a possible source of HIV-1 in the mammary gland but do not deal with the route of HIV-1 penetration into the infant, which has been recently discussed (6).
Acknowledgments
We thank J. Fantini for providing anti-galactosylceramide antibody.
REFERENCES
- 1.Barbey-Morel C L, Oeltman T N, Edwards K M. Role of respiratory proteases in infectivity of influenza A virus. J Infect Dis. 1987;155:667–672. doi: 10.1093/infdis/155.4.667. [DOI] [PubMed] [Google Scholar]
- 2.Capony F, Garcia M, Capdevielle J, Rougeot C, Ferrata P, Rochefort H. Purification and first characterization of the secreted and cellular 52-kDa proteins regulated by estrogens in human-breast cancer cells. Eur J Biochem. 1986;161:505–512. doi: 10.1111/j.1432-1033.1986.tb10471.x. [DOI] [PubMed] [Google Scholar]
- 3.Capony F, Rougeot C, Montcourrier P, Cavailles V, Salazar G, Rochefort H. Increased secretion, altered processing, and glycolysation of pro-cathepsin D in human mammary cancer cells. Cancer Res. 1989;49:3904–3909. [PubMed] [Google Scholar]
- 4.Clements G J, Price-Jones M J, Stephens P E, Sutton C, Schultz T F, Clapham P R, McKeating J A, McClure M O, Thomson S, Marsh M, Kay J, Weiss R A, Moore J P. The V3 loops of the HIV-1 and HIV-2 surface glycoproteins contain proteolytic cleavage sites: a possible function in viral fusion. AIDS Res Hum Retrovir. 1991;7:3–16. doi: 10.1089/aid.1991.7.3. [DOI] [PubMed] [Google Scholar]
- 5.Confort C, Rochefort H, Vignon F. Insulin-like growth factors (IGFs) stimulate the release of a1-antichymotrypsin and soluble IGF-II/mannose 6-phosphate receptor from MCF7 breast cancer cells. Endocrinology. 1995;136:3759–3766. doi: 10.1210/endo.136.9.7649082. [DOI] [PubMed] [Google Scholar]
- 6.Coutsoudis A, Pillay K, Spooner E, Kuhn L, Coovadia H M. Influence of infant-feeding patterns on early mother-to-child transmission of HIV-1 in Durban, South Africa: a prospective cohort study. Lancet. 1999;354:471–476. doi: 10.1016/s0140-6736(99)01101-0. [DOI] [PubMed] [Google Scholar]
- 7.Dabis F, Msellat P, Meda N, Welffens-Ekra C, You B, Manigart O, Leroy V, Simonon A, Cartoux M, Combe P, Ouangré A, Ramon R, Ky-Zerbo O, Montoho C, Salamon R, Rouzioux C, Vandeperre P, Mandelbrot L. 6-month efficacy, tolerance, and acceptability of a short regimen of oral zidovudine to reduce vertical transmission of HIV in breastfed children in Côte d'Ivoire and Burkina Faso: a double-blind placebo-controlled multicentre trial. Lancet. 1999;353:786–792. doi: 10.1016/s0140-6736(98)11046-2. [DOI] [PubMed] [Google Scholar]
- 8.Datta P, Embree J E, Kreiss J K, Ndinya-Achola J O, Braddick M, Temmerman M, Nagelkerke N J D, Maitha G, Holmes K K, Piot P, Pamba H O, Plummer F A. Mother-to-child transmission of human immunodeficiency virus type 1 report from the Nairobi study. J Infect Dis. 1994;170:1134–1140. doi: 10.1093/infdis/170.5.1134. [DOI] [PubMed] [Google Scholar]
- 9.Defer M C, Dugas B, Picard O, Damas C. Impairment of circulating lactoferrin in HIV-1 infection. Cell Mol Biol. 1995;41:417–421. [PubMed] [Google Scholar]
- 10.de Jong M D, de Boer R J, de Wolf F, Foudraine N A, Boucher C A B, Goudsmit J, Lange J M A. Overshoot of HIV-1 viraemia after early discontinuation of antiretroviral treatment. AIDS. 1997;11:F79–F84. doi: 10.1097/00002030-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Ekpini E R, Wiktor S Z, Satten G A, Adjorlolo-Johnson G T, Sibailly T S, Ou C Y, Karon J M, Brattegaard K, Whittaker J P, Gnaore E, De Cock K M, Greenberg A E. Late postnatal mother-to-child transmission of HIV-1 in Abidjan, Côte d'Ivoire. Lancet. 1997;349:1054–1059. doi: 10.1016/s0140-6736(96)06444-6. [DOI] [PubMed] [Google Scholar]
- 12.El Messaoudi K, Thiry L, Van Tieghem N, Liesnard C, Englert Y, Moguilevsky N, Bollen A. HIV-1 infectivity and host range modification by cathepsin D present in human vaginal secretions. AIDS. 1999;13:333–339. doi: 10.1097/00002030-199902250-00005. [DOI] [PubMed] [Google Scholar]
- 13.Garcia F, Plana M, Vidal C, Cruceta A, O'Brien W A, Pantaleo G, Pumarola T, Gallart T, Miro J M, Gatell J M. Dynamics of viral load rebound and immunological changes after stopping effective antiretroviral therapy. AIDS. 1999;13:F79–F86. doi: 10.1097/00002030-199907300-00002. [DOI] [PubMed] [Google Scholar]
- 14.Garcia M, Capony F, Derocq D, Simon D, Pau B, Rochefort H. Characterization of monoclonal antibodies to the estrogen-regulated M. 52,000 glycoprotein and their use in MCF7 cells. Cancer Res. 1985;45:709–716. [PubMed] [Google Scholar]
- 15.Goldman A S, Garza C, Nichols B L, Goldblum R M. Immunological factors in human milk during the first year of lactation. J Pediatr. 1982;100:563–567. doi: 10.1016/s0022-3476(82)80753-1. [DOI] [PubMed] [Google Scholar]
- 16.Harmsen M C, Swart P J, de Béthune M P, Pauwels R, De Clercq E, The T H, Meijer D K F. Antiviral effects of plasma and milk proteins lactoferrin show potent activity against both human immunodeficiency virus and human cytomegalovirus replication in vitro. J Infect Dis. 1995;172:380–388. doi: 10.1093/infdis/172.2.380. [DOI] [PubMed] [Google Scholar]
- 17.Jones J A E, Fiscus S A, Sawanstrom R. Evolutionary variants of the human immunodeficiency virus type 1 V3 region characterized by using a heteroduplex tracking assay. J Virol. 1997;71:8750–8758. doi: 10.1128/jvi.71.11.8750-8758.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwong P D, Wyatt R, Robinson J, Sweet R W, Sodroski J. Structure of an HIV gp 120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.La Casse R A, Follis K E, Trahey M, Scarborough J D, Littman D R, Nunberg J H. Fusion-competent vaccines: broad neutralization of primary isolates of HIV. Science. 1999;283:357–362. doi: 10.1126/science.283.5400.357. [DOI] [PubMed] [Google Scholar]
- 20.Larsen L B, Benfeldt C, Rasmussen L K, Petersen T E. Bovine milk procathepsin D and cathepsin D: coagulation and milk protein degradation. J Dairy Res. 1996;63:119–130. doi: 10.1017/s0022029900031599. [DOI] [PubMed] [Google Scholar]
- 21.Nduati R W, John J C, Richardson B A, Overbaugh J, Welch M, Ndinya-Achola J, Moses S, Holmes K, Onyango F, Kreiss J K. Human immunodeficiency virus type-1 infected cells in breast milk: association with immunodepression and vitamin A deficiency. J Infect Dis. 1995;172:1461–1468. doi: 10.1093/infdis/172.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newell M L. Mechanisms and timing of mother-to-child transmission of HIV-1. AIDS. 1998;12:831–837. doi: 10.1097/00002030-199808000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Oxtoby M J. Human immunodeficiency virus and other viruses in human milk: placing the issue on broader perspectives. Pediatr Infect Dis J. 1988;7:825–835. [PubMed] [Google Scholar]
- 24.Raport C L, Gosling J, Sceickart V L, Gray P W, Charo I F. Molecular cloning and functional characterization of a novel CC chemokine receptor (CCR5) for Rantes, MIP-1b and MIP-1a. J Biol Chem. 1996;271:17161–17166. doi: 10.1074/jbc.271.29.17161. [DOI] [PubMed] [Google Scholar]
- 25.Semba R D, Miotti P G, Lan Y, Chiphangwi J D, Hoover D R, Dallabetta G A, Yang L P, Saah A J. Maternal serum lactoferrin and vertical transmission of HIV. AIDS. 1998;12:331–332. [PubMed] [Google Scholar]
- 26.Simonon A, Lepage F, Karita E. An assessment of the timing of mother-to-child transmission of human immunodeficiency virus type 1 by means of polymerase chain reaction. J Acquir Immune Defic Syndr. 1994;4:952–957. [PubMed] [Google Scholar]
- 27.Thiry L, Sprecher-Goldberger S, Jonckheer T, Levy J, Van de Perre P, Henrivaux P, Cogniaux-Le Clerc J, Clumeck N. Isolation of AIDS virus from cell-free breast milk of three healthy virus carriers. Lancet. 1985;ii:891–892. doi: 10.1016/s0140-6736(85)90156-4. [DOI] [PubMed] [Google Scholar]
- 28.Tonolio A, Serra C, Conaldi P G, Basolo F, Falcone V, Dolei A. Productive HIV-1 infection of normal human mammary epithelial cells. AIDS. 1995;9:859–866. doi: 10.1097/00002030-199508000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Vandeperre P, Meda N, Cartoux M, Leroy V, Dabis F. Late post natal transmission of HIV-1 and early weaning. Lancet. 1997;350:221. doi: 10.1016/S0140-6736(05)62390-2. [DOI] [PubMed] [Google Scholar]
- 30.Vetvicka V, Vagner J, Baudys M, Tang J, Foundling S I, Fusek M. Human breast milk contains procathepsin D—detection by specific antibodies. Biochem Mol Biol Int. 1993;30:921–928. [PubMed] [Google Scholar]
- 31.Vignon F, Rochefort H. Interactions of pro-cathepsin D and IGF-II on the mannose-6-phosphate/IGF-II receptor. Breast Cancer Res Treat. 1992;22:47–57. doi: 10.1007/BF01833333. [DOI] [PubMed] [Google Scholar]
- 32.Wyatt R, Kwong P D, Desjardins E, Sweet R W, Robinson J, Hendrickson W A, Sodroski J G. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature. 1998;393:705–711. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 33.Yahi N, Sabatier J-M, Baghdiguian S, Gonzalez-Scarano F, Fantini J. Synthetic multimeric peptides derived from the principal neutralization domain (V3 loop) of human immunodeficiency virus type 1 (HIV-1) gp120 bind to galactosylceramide and block HIV-1 infection in a human CD4-negative mucosal epithelial cell line. J Virol. 1995;69:320–325. doi: 10.1128/jvi.69.1.320-325.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu T, Wang N A, Carr A. Genetic characterization of human immunodeficiency virus type 1 in blood and genital secretions: evidence for viral compartmentalization and selection during sexual transmission. J Virol. 1996;70:3098–3107. doi: 10.1128/jvi.70.5.3098-3107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]